Abstract
BACKGROUND:
Functional status, an important predictor of health outcomes in older patients, has not been studied in an IPF population. This study aimed to determine the prevalence of frailty and geriatric conditions in older patients with IPF.
METHODS:
IPF patients age ≥65 years were identified prospectively at the University of Michigan. Frailty was assessed using the Fried frailty phenotype. Questionnaires addressing functional status, geriatric conditions and symptoms were administered. Quantitative measurement of pectoralis muscle area was performed. Patient variables were compared among different frailty groups.
RESULTS:
Of the 50 participants, 48% were found to be frail and 40% had ≥2 geriatric conditions. Frailty was associated with increased age, lower lung function, shorter 6-minute walk distance, higher symptom scores and a greater number of comorbidities, geriatric conditions and functional limitations (p<0.05). Pectoralis muscle area was nearly significant (p=0.08). Self-reported fatigue score (odds ratio [OR]=2.13, confidence interval [CI] 95% 1.23–3.70, p=0.0068) and diffusion capacity (OR=0.54 CI 95% 0.35–0.85, p=0.0071) were independent predictors of frailty.
CONCLUSIONS:
Frailty and geriatric conditions are common in older patients with IPF. The presence of frailty was associated with objective (diffusion capacity) and subjective (self-reported fatigue score) data. Longitudinal evaluation is necessary to determine impact of frailty on disease-related outcomes in IPF.
Keywords: idiopathic pulmonary fibrosis, frailty, geriatric conditions, body composition
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive fibrosing interstitial pneumonia of unknown etiology that occurs primarily in older adults and is associated with the histopathologic and/or radiologic pattern of usual interstitial pneumonia (1). The incidence and prevalence of IPF increase almost exponentially with each decade of life and two thirds of patients are >60 at time of presentation (2). Furthermore, IPF has been shown to share common pathophysiologic mechanisms with the aging process itself (3). While the pathologic process in IPF is thought to be limited to the lungs, IPF may have extra-pulmonary impact and comorbidities are common at diagnosis and as disease progresses.
Frailty is characterized as a generalized vulnerability to stressors, resulting from cumulative declines across multiple physiologic systems, causing susceptibility to adverse outcomes (4). Frailty is highly prevalent with increasing age and confers higher risk for adverse health outcomes. Markers of frailty include age-associated declines in lean body mass, strength, endurance, balance, walking performance and low activity (4). Frailty and deconditioning have been suggested as important comorbidities associated with IPF in elderly patients (age ≥65) specifically (5). Geriatric conditions are a collection of symptoms and signs common in older adults not necessarily related to a specific disease that are precipitated by a variety of acute insults (often episodic) and are often followed by functional decline (6, 7). Functional status is one of the most important predictors of health outcomes in older patients and becomes more predictive than specific diagnoses as people age (8). Clinical frailty has been associated with decreased survival in chronic obstructive pulmonary disease (COPD) (9); however, this outcome has not been studied in an IPF population and the prevalence in IPF is unknown.
Our goal was to determine the prevalence of clinical frailty and geriatric conditions in older patients with IPF. We hypothesized that geriatric conditions and frailty, as manifestations of aging, are frequent in persons with IPF, with higher prevalence than published controls, elderly patients in the general population (4, 7, 9). To explore the association between frailty and body composition we also performed quantitative assessment of thoracic muscles in persons with IPF.
MATERIAL & METHODS
Patient Selection & Data Collection
Patients age ≥65 years with IPF based on the American Thoracic Society international consensus definition were identified prospectively through the University of Michigan pulmonary clinics for inclusion (1). All disease severities were included with no limitations based on treatment. Non-ambulatory patients were excluded. The study was organized as a pilot study to address the utility of assessing frailty in a new population, IPF patients ≥65, therefore a target of 50 patients was selected. Patients were recruited from March 14, 2016, to July 22, 2016. Participants provided written informed consent for participation. Very few subjects approached regarding the study ultimately declined to participate due to time constraints (i.e. subsequent appointments, travel). The institutional review board at the University of Michigan approved this study (HUM00110674).
Frailty Assessment
Several validated measures of frailty have been described in the literature, which have been derived from distinct theoretical views on how frailty develops and manifests in older adults (10). Models address frailty as deficiencies in functional domains, an index of cumulative health burden, a biological syndrome, and a battery of lower extremity performance measures (4, 11–14). The Fried Biologic Syndrome model is commonly used (4, 13, 15–18), and was chosen for this evaluation based on its emphasis on physical functioning with both objective and subjective measures that can be rapidly assessed in the clinical setting. The Fried Frailty Phenotype (FFP) is an aggregate score of five components: shrinking, weakness, exhaustion, slowness and low physical activity (4). A categorical scoring system identifies participants with FFP score ≥3 as frail, 1–2 as pre-frail, and 0 as not frail (4). Self-reported performance measures have been used in place of physical performance measures with replicable results (10, 13, 19). These self-reported measures were chosen to simplify frailty assessment for ease of implementation in the clinical setting and to decrease patient burden. Use of hand-grip strength testing was maintained. Details on frailty measures and scoring are provided in Table E1 in the online supplement.
Geriatric Conditions, Functional Limitations and Measures of Disability & Dependence
The instruments and measures of geriatric conditions, functional limitations, activities of daily living (ADL) and instrumental-ADL (IADL) utilized in our evaluation were based on those used in the Health and Retirement Study, which are available in public domain and have been extensively validated (7, 20–23). Geriatric conditions assessed include incontinence, dizziness, vision impairment, hearing impairment and falls. The specific ADL, IADL and functional limitations assessed are outlined in Table E2 of the online supplement.
Quantitative Pectoralis Muscle Mass Determination
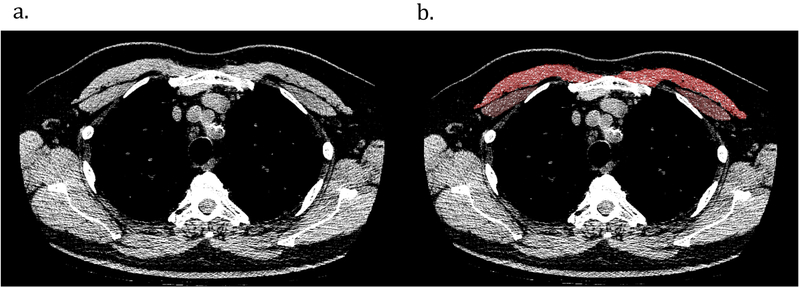
Computerized tomography (CT) based assessments of muscle area have been increasingly performed in COPD populations to assess disease-related outcomes (24–26), but have not been evaluated in IPF. Quantitative assessment of pectoralis muscle area (PMA) was performed on a single axial slice of the CT scan above the aortic arch using Slicer body composition software (www.slicer.org), a slice chosen due to ease of identification and replicability (Figure 1) (26, 27). Muscles were manually shaded, using a predefined attenuation range of −50 to 90 Hounsfield units (26, 27). PMA was presented as the aggregate area (in mm2) of the right and left pectoralis major and minor assessed in this axial plane. The last available CT for each patient was used for analysis. Chest CTs completed within 6 months of study inclusion were prioritized for evaluation if available (n=22).
Figure 1.
Sample computed tomography (CT) scans used to determine muscle area in idiopathic pulmonary fibrosis patients. (a) CT image used for pectoralis muscle imaging. (b) Pectoralis muscles shaded in red and brown.
Other Measures
Clinical and physiologic data obtained as part of routine evaluation were recorded. Comorbidities were based on documented history and active medications. The University of California San Diego Shortness of Breath questionnaire (UCSD-SOBQ) was used to assess the level of dyspnea or breathlessness (28, 29). The Fatigue Severity Scale (FSS) was administered to assess fatigue (30).
Statistical Analysis
Binary and ordinal comparisons were performed among different frailty groups (frail, pre-frail or not frail). Descriptive statistics were used to describe patient variables. For associations of frailty with other factors, the Cochran-Armitage trend test p-value was used. A p-value of 0.05 was used as the threshold for statistical significance. Univariate analysis was performed using binary logistic regression to identify odds ratio for frailty for each individual variable. Best-fit multivariable logistic regression models that were age-adjusted were utilized to identify factors that were independent predictors of frailty.
RESULTS
Of the 50 participants enrolled, age ranged from 65 to 86 years. Complete demographic data are presented in Table 1. Frailty was more common in our elderly IPF cohort, occurring in 48% (n=24), compared to 6.9% in the general population over age 65 in the Cardiovascular Health Study (4). The prevalence of individual components of the FFP is shown in Table E3 in the online supplement. Unintentional weight loss was the most common frailty criteria met in pre-frail participants and the only category without a statistically significant difference among frailty group comparisons.
Table1.
Patient characteristics overall and by the presence of frailty*
| All Patients N=50 |
Not Frail N=6 (12%) |
Pre-frail N=20 (40%) |
Frail N=24 (48%) |
Trend P-value |
|
|---|---|---|---|---|---|
| Age, years | 73.8 (5.4) | 70.2 (4.4) | 72.2 (5.6) | 76.0 (4.5) | 0.0032 |
| Male | 33 (66.0%) | 5 (83.3%) | 14 (70.0%) | 14 (58.3%) | 0.2100 |
| Smoking | 0.4366 | ||||
| Current | 1 (2.0%) | 0 | 1 (5.0%) | 0 | |
| BMI, kg/m2 | 30.2 (4.4) | 30.7 (3.8) | 30.0 (4.8) | 30.2 (4.3) | 0.9041 |
| FVC, % predicted | 67.6 (15.8) | 77.0 (6.3) | 71.6 (15.0) | 61.9 (16.3) | 0.0102 |
| FVC, L | 2.5 (0.9) | 3.1 (0.6) | 2.67 (0.7) | 2.2 (0.9) | 0.0045 |
| FEV1, % predicted | 81.7 (17.3) | 95.0 (9.6) | 85.7 (16.3) | 75.1 (17.1) | 0.0035 |
| FEV1, L | 2.0 (0.6) | 2.6 (0.6) | 2.1 (0.5) | 1.7 (0.6) | 0.0008 |
| Ratio | 82.2 (8.0) | 83.5 (4.2) | 81.5 (9.0) | 82.3 (8.1) | 0.9200 |
| DLCO, % predicted | 51.8 (18.8) | 73.3 (10.7) | 56.7 (16.4) | 42.4 (16.5) | <0.0001 |
| 6MWD, feet | 1117.7 (477.0) | 1579 (494.3) | 1150.9 (486.4) | 989.7 (420.1) | 0.0299 |
| Oxygen use | 12 (24.5%) | 0 | 3 (15.0%) | 9 (39.1%) | 0.0193 |
| Number of Comorbidities | 6.0 (2.2) | 4.3 (1.5) | 5.5 (2.0) | 6.8 (2.2) | 0.0050 |
| Antifibrotic Agent | 30 (60%) | 3 (50%) | 11 (55%) | 16 (66.7%) | 0.3545 |
| UCSD SOB | 37.4 (23.4) | 6.5 (5.7) | 33.2 (18.3) | 48.7 (22.1) | <0.0001 |
| FSS | 37.5 (15.3) | 17.5 (7.8) | 34.2 (13.3) | 45.2 (12.7) | <0.0001 |
| Pectoralis muscle area, mm2 | 2921.8 (1118.3) | 3194.3 (833.9) | 3271.7 (1396.8) | 2592.0 (867.5) | 0.0836 |
| Functional limitations | 4.2 (2.2) | 1.5 (1.1) | 3.4 (1.8) | 5.6 (1.5) | <0.0001 |
| Geriatric conditions† | 1.3 (1.2) | 0.2 (0.4) | 1.1 (1.2) | 1.8 (1.1) | 0.0014 |
| ADL dependence | 0.1 (0.4) | 0 | 0.1 (0.2) | 0.2 (0.5) | 0.2164 |
| ADL difficulty | 0.5 (0.7) | 0 | 0.3 (0.6) | 0.7 (0.9) | 0.0128 |
| IADL dependence | 0.3 (0.8) | 0.2 (0.4) | 0.1 (0.2) | 0.6 (1.1) | 0.0592 |
| IADL difficulty | 0.5 (0.9) | 0.2 (0.4) | 0.1 (0.3) | 0.9 (1.1) | 0.0069 |
Frailty categories based on number of criteria met: not frail = 0; pre-frail = 1–2; frail = ≥3 (Fried frailty phenotype). Continuous variables are summarized by mean (standard deviation); categorical variables are summarized by count (percentage).
Geriatric conditions include incontinence, vision impairment, hearing impairment, dizziness, falls.
Abbreviations: 6MWD=6-minute walk distance; ADL=activities of daily living; BMI=body mass index; DLCO=diffusion capacity; FEV1=forced expiratory volume; FSS=fatigue severity scale; FVC=forced vital capacity; IADL=instrumental activities of daily living; UCSD SOBQ=University of California San Diego shortness of breath questionnaire.
Geriatric conditions were also more prevalent in our cohort with 40% reporting ≥2 geriatric conditions, compared to 19.5% with ≥2 geriatric conditions in the 2000 wave of the Health and Retirement (7). Of the 20 participants with ≥2 geriatric conditions, 45% were found to be frail. Of the 30 participants with <2 geriatric conditions, 50% were found to be frail.
We explored the frequency of frailty based on the severity of other individual variables, including FVC, DLCO and the number of functional limitations and comorbidities (Table 2). Severity cut-offs for FVC and DLCO were based on median values as well as phase III clinical trial inclusion criteria identifying mild-to-moderate disease (FVC ≥50% predicted, DLCO ≥35% predicted) (31–33). The median value was used for functional limitations. For comorbidities, ≥4 comorbid conditions were chosen based on previously published data (34).
Table 2.
Frequency of frailty based on the severity of individual variables
| Variable (n, %) |
Not Frail n (%) |
Frail n (%) |
|
|---|---|---|---|
| FVC (median 67.5% predicted) |
FVC ≥67.5% predicted (25, 50) | 17 (68) | 8 (32) |
| FVC ≥50% predicted (43, 86) | 25 (58.1) | 18 (41.9) | |
| DLCO (median 52% predicted) |
DLCO ≥52% predicted (26, 52) | 16 (61.5) | 10 (38.5) |
| DLCO ≥35% predicted (40, 80) | 25 (62.5) | 15 (37.5) | |
| Functional limitations (median 4.5) |
<4.5 (25, 50) |
15 (60) | 10 (40) |
| Number of Comorbidities (median 6) |
<4 (7, 14) |
3 (42.9) | 4 (57.1) |
Abbreviations: DLCO=diffusion capacity; FVC=forced vital capacity
Overall, there was a high burden of comorbid conditions, with a mean of 6.0 (SD 2.2) amongst all participants, with 86% having ≥4 comorbid conditions. Comorbidities identified in our cohort were similar to those reported in publications investigating impact of comorbidities in IPF (34), including cardiovascular diseases, gastroesophageal reflux disease, COPD, psychiatric conditions, diabetes, obstructive sleep apnea, hyperlipidemia, cancer, thromboembolic disease and obesity. No participants were undergoing active therapy for malignancy at time of study inclusion. Gastroesophageal reflux, hyperlipidemia and arterial hypertension were most frequently observed, documented in 68–76% of participants. Obesity, defined as BMI ≥30, was found in 46%. Please refer to online supplement Table E4 for full breakdown of comorbid conditions as well as comorbidity-associated therapies.
The presence of frailty was associated with statistically significant (p<0.05) differences in age, FVC, forced expiratory volume, DLCO, 6-minute walk distance (6MWD), dyspnea/fatigue scores and the number of comorbidities, geriatric conditions and functional limitations (Table 1). There was no difference in sex, smoking status, BMI or the use of antifibrotic agents. While there was a trend towards a difference in PMA between frailty groups (frail participants with lower PMA), this did not reach statistical significance (p=0.08). Four participants (3 pre-frail, 1 frail) were excluded from PMA analysis due to inadequate CT. The average time between CT and frailty assessment was 12 months (median 9 months prior, range 7.5 years prior to 6 months post inclusion). There was a significant difference in self-reported difficulty with ADLs and IADLs, but not dependency. The minimal clinically important difference for the UCSD-SOBQ in IPF has been estimated as 5–11 points (28, 29, 35). For the FSS, a score of >36 is indicative of severe fatigue or need for further evaluation (30). Therefore, the difference in symptom scores across frailty groups also met clinical significance (Table 1).
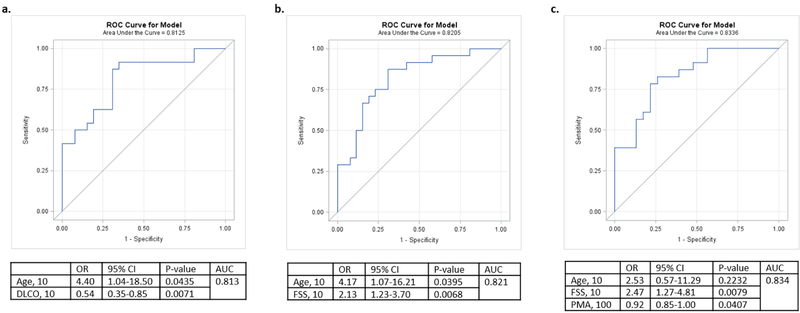
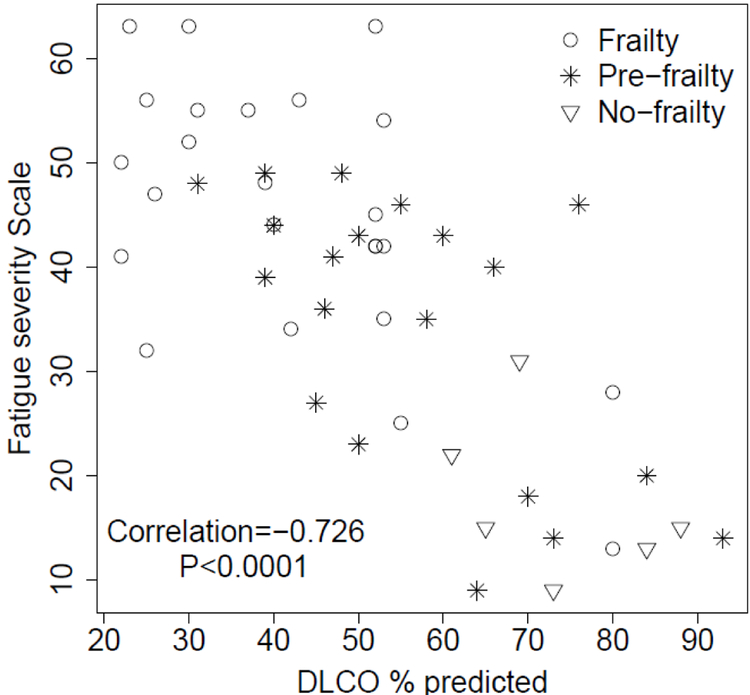
Univariate analysis using binary logistic regression (frail versus not frail/pre-frail) determined odds ratio for the presence of frailty using each individual parameter listed in table 1 (Table 3). Best-fit multivariable logistic regression models, adjusted for age, identified both self-reported fatigue score (OR=2.13, CI 95% 1.23–3.70, p=0.0068) and DLCO (OR=0.54 CI 95% 0.35–0.85, p=0.0071) as independent predictors of frailty (AUC=0.82 and 0.81, respectively) (Figure 2). DLCO & self-reported fatigue score were negatively correlated, r=−0.73 p<0.0001, with clustering of frail patients in area of low DLCO and high fatigue score (Figure 3). This strong correlation, limited the ability of these two factors to be utilized in the same model. When PMA was incorporated into multivariable models with age and self-reported fatigue score, we found a statistically significant association between PMA and frailty status with higher overall AUC (0.83) (Figure 2).
Table 3.
Univariate regression analysis predicting the presence of frailty*
| Frail vs. No/Pre-binary logistic | |||
| OR | 95% CI | P-value | |
|
Age
(per 10-year increase) |
5.623 | 1.555–20.328 | 0.0085 |
| Male | 0.516 | 0.157–1.691 | 0.2744 |
| Smoking | 1.286 | 0.389–4.247 | 0.6801 |
| BMI | 1.003 | 0.882–1.141 | 0.9614 |
|
FVC, % predicted
(per 10% increase) |
0.610 | 0.404–0.921 | 0.0187 |
| FVC, L | 0.381 | 0.176–0.827 | 0.0147 |
|
FEV1, % predicted
(per 10% increase) |
0.608 | 0.409–0.904 | 0.0140 |
| FEV1, L | 0.226 | 0.076–0.670 | 0.0073 |
|
DLCO, % predicted
(per 10% increase) |
0.516 | 0.338–0.787 | 0.0021 |
| 6MWD, feet (per 100 ft increase) | 0.870 | 0.735–1.031 | 0.1074 |
| Oxygen use | 4.929 | 1.138–21.347 | 0.0329 |
| Number of comorbidities | 1.465 | 1.064–2.016 | 0.0192 |
|
UCSD SOB (per 10 point increase) |
1.644 | 1.189–2.274 | 0.0027 |
|
FSS (per 10 point increase) |
2.252 | 1.347–3.763 | 0.0019 |
| Functional limitations | 2.483 | 1.522–4.051 | 0.0003 |
| Geriatric conditions† | 2.016 | 1.157–3.515 | 0.0134 |
| Pectoralis muscle area, mm2 (per 100 mm2 increase) | 0.94 | 0.89–1.00 | 0.0525 |
Odds ratio (OR) presented for each variable is per 1-unit increase, unless otherwise listed
Geriatric conditions include incontinence, vision impairment, hearing impairment, dizziness, falls.
Abbreviations: 6MWD=6-minute walk distance; BMI=body mass index; DLCO=diffusion capacity; FEV1=forced expiratory volume; FSS=fatigue severity scale; FVC=forced vital capacity; UCSD SOBQ=University of California San Diego shortness of breath questionnaire.
Figure 2.
Multivariate models of frailty (yes versus no/pre). DLCO: diffusion capacity; FSS: fatigue severity scale; OR: odds ratio; PMA: pectoralis muscle area.
Figure 3.
Scatter plot between diffusion capacity (DLCO) and fatigue severity scale.
DISCUSSION
In our pilot study of fifty patients aged 65 or older with IPF, we found that frailty as defined by the Fried Frailty Phenotype and geriatric conditions (incontinence, dizziness, vision impairment, hearing impairment and/or falls) are common comorbidities. The presence of frailty is associated with both objective & subjective disease measures, including low DLCO and high self-reported fatigue scores, with possible association with PMA, a novel measure in patients with IPF. Although frailty appears to be associated with patient characteristics indicative of more severe disease or a higher number of comorbid conditions, it is also a recognizable parameter in patients with more preserved pulmonary function and less comorbidity.
When compared to published controls, frailty and geriatric conditions are more prevalent in patients with IPF over age 65 than the general population. Frailty as defined by multiple different models has been found to be predictive of falls, hospitalization, disability, institutionalization, death and reduced health-related quality of life (HRQOL) in geriatric populations (4, 9, 36). Frailty and respiratory impairment have been shown to have a strong association with increased risk of death in the elderly when both are present (37). In a lung transplant population, frailty was common and independently associated with patient-reported disability and subsequent delisting or death before transplant (18). Furthermore, among patients with IPF awaiting lung transplant, the 6MWD has been shown to be more predictive of survival at 6 months than lung function (38), suggesting that walk distance is capturing more than just lung function. Distinguishing frail from fit elderly in an aging population with chronic lung disease such as IPF may enhance risk assessment of a vulnerable population.
Frailty correlates with both objective and subjective variables including low DLCO and high self-reported fatigue scores. While there was a statistically significant difference in FVC across different frailty groups and on univariate analysis, the association was lost when this variable was incorporated in multivariate models adjusted for age, DLCO and fatigue score. Adverse symptoms commonly reported with antifibrotic therapy, such as nausea, diarrhea and fatigue (31–33, 39), could also impact frailty; however, there was no difference in antifibrotic use among frailty groups. While the presence of frailty correlates with objective markers of disease severity, such as reduced lung function and supplemental oxygen use, patients were identified that met frailty criteria but had relatively preserved pulmonary function. Conversely, patients with severe lung disease were not uniformly frail. These data suggest that while patients with more severe disease may be at increased risk of being frail, frailty may also be an independent marker with the potential to be modifiable at all disease stages.
Unintentional weight loss, an important component of the Fried frailty model, may be missed in overweight or obese patients. BMI does not address low muscle mass and sarcopenia, which still can be present in obese patients. Quantitative assessment of thoracic muscles represents a new potential target for evaluation in patients with IPF. Our investigation of PMA demonstrated a possible association with frailty status, with increased significance when incorporated into multivariable models. As our evaluation was limited by small sample size and timing of CT scans (completed an average of one year prior to frailty assessment), further analyses were not performed. CT is critical in the diagnostic algorithm of ILD, particularly IPF (1). Furthermore, there has been recent interest in using quantitative and semi-quantitative measures of CT features to predict outcomes in IPF. PMA represents a new measure, outside of usual parenchymal evaluation and spirometric measures, that could be applied to existing clinically acquired CT scans of the chest and may provide additional clinically relevant insight into patients with IPF.
The impact of multiple comorbidities on mortality represents an evolving area of interest in IPF. A patient’s total number of comorbidities has been significantly associated with survival in IPF (34). Although valuable, geriatric conditions and frailty specifically were not addressed. Geriatric conditions have been shown to have similar prevalence to chronic diseases in older adults and in some cases are as strongly associated with disability (7). These conditions are clinically relevant as they can be prevented or delayed (7), and are not current targets of IPF management. Frailty is conventionally considered secondary to age-related decline, however, chronic disease(s) can accelerate the rate of decline and precipitate a frail state (40). The modified FFP as applied here has the potential for easy applicability in a clinical setting to rapidly identify frail patients that may benefit from further evaluation and intervention.
In a disease where treatment options are limited, establishing interventions that improve symptoms and function can have a positive impact on HRQOL. Prior investigations have highlighted the ability to improve frailty status with a multifactorial interdisciplinary approach utilizing targeted exercise and nutrient based interventions in both general geriatrics and COPD (40–42). However, the impact of these interventions on long-term outcomes is unknown and their impact in IPF has not been addressed. Pulmonary rehabilitation (PR) may represent a specific modality within chronic lung disease to address clinical frailty. Two controlled trials of PR in IPF have demonstrated improvement in 6MWD and symptoms or quality of life (43, 44), with benefits more pronounced in patients with worse baseline functional status (45). Frailty measures may identify more patients who are likely to benefit from PR. However, the ability to improve frailty status with PR is unknown.
Our study is the first prospective evaluation of the prevalence of frailty and geriatric conditions in an IPF-specific population. However, there were some notable limitations to our evaluation. First, this was a pilot study with a small sample size, at a single center. With the cross-sectional study design, we cannot infer causal mechanisms between IPF and frailty. Symptom scores were utilized as a surrogate for HRQOL, though direct HRQOL measures would likely add to assessment. Our frailty model depended on patient report for 4 out of 5 frailty measures. While similar methods were used in prior evaluations, there is opportunity for further validation of our adapted frailty model. Patients were also identified at various time points in disease course for inclusion; some shortly after diagnosis, others having survived several years following diagnosis with minimal change in pulmonary function. Older patients with long standing disease may represent a “survivor” population, less apt to have functional limitation and disability. Given the social and psychological issues IPF patients may have, it is possible that an even greater percentage would be found to be frail if we were to look further outside of physical characteristics. It has been argued that some frailty components, such as low activity or exhaustion in the FFP may be confounded by lung disease itself. However, lung disease could promote frailty, thus placing this population at increased risk.
Age-related factors are infrequently assessed in pulmonary medicine, though may have significant impact, especially in diseases such as IPF, which are strongly associated with advanced age. While the exact physiologic effects of frailty in IPF are unknown, frailty and geriatric conditions are common in elderly patients with IPF. Results of our evaluation suggest utility of further study of frailty and geriatric conditions in a larger cohort to validate conclusions and to evaluate the contribution of frailty and geriatric conditions to markers of disease progression in IPF, including symptoms, pulmonary function, disability and ultimately mortality. If longitudinally validated, functional measures could enhance traditional measures in predicting outcomes and response to interventions among patients with IPF.
Supplementary Material
Acknowledgements
Funding Information: National Institutes of Health NHLBI grants R01 HL91743, T32 HL00749 and K24 HL111316 (Kevin R. Flaherty)
ABBREVIATIONS LIST
- 6MWD
6-minute walk distance
- ADL
Activities of daily living
- BMI
Body mass index
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- CT
Computed tomography
- DLCO
Diffusion capacity
- FEV1
Forced expiratory volume
- FFP
Fried frailty phenotype
- FSS
Fatigue severity scale
- FVC
Forced vital capacity
- HRQOL
Health-related quality of life
- IADL
instrumental activities of daily living
- IPF
Idiopathic pulmonary fibrosis
- OR
Odds ratio
- PMA
Pectoralis muscle area
- PR
Pulmonary rehabilitation
- UCSD SOBQ
University of California San Diego shortness of breath questionnaire
Footnotes
Prior Presentation: Abstract A1117, ATS International Conference, May 2017, Washington, D.C.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr., Kondoh, Myers, Muller, Nicholson, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ, Fibrosis AEJACoIP. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014; 2: 566–572. [DOI] [PubMed] [Google Scholar]
- 3.Thannickal VJ, Loyd JE. Idiopathic pulmonary fibrosis: a disorder of lung regeneration? Am J Respir Crit Care Med 2008; 178: 663–665. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–156. [DOI] [PubMed] [Google Scholar]
- 5.Meyer KC, Danoff SK, Lancaster LH, Nathan SD. Management of Idiopathic Pulmonary Fibrosis in the Elderly Patient: Addressing Key Questions. Chest 2015; 148: 242–252. [DOI] [PubMed] [Google Scholar]
- 6.Besdine R, Boult C, Brangman S, Coleman EA, Fried LP, Gerety M, Johnson JC, Katz PR, Potter JF, Reuben DB, Sloane PD, Studenski S, Warshaw G, American Geriatrics Society Task Force on the Future of Geriatric M. Caring for older Americans: the future of geriatric medicine. J Am Geriatr Soc 2005; 53: S245–256. [DOI] [PubMed] [Google Scholar]
- 7.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med 2007; 147: 156–164. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 2006; 295: 801–808. [DOI] [PubMed] [Google Scholar]
- 9.Galizia G, Cacciatore F, Testa G, Della-Morte D, Mazzella F, Langellotto A, Raucci C, Gargiulo G, Ferrara N, Rengo F, Abete P. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res 2011; 23: 118–125. [DOI] [PubMed] [Google Scholar]
- 10.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc 2009; 57: 830–839. [DOI] [PubMed] [Google Scholar]
- 11.Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53: S9–16. [DOI] [PubMed] [Google Scholar]
- 12.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007; 62: 738–743. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–94. [DOI] [PubMed] [Google Scholar]
- 15.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 2006; 61: 262–266. [DOI] [PubMed] [Google Scholar]
- 16.Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, Orwoll ES, Osteoporotic Fractures in Men Research G. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc 2007; 55: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 17.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, Tracy JK, Hochberg MC, Rodondi N, Cawthon PM, Study of Osteoporotic Fractures Research G. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2007; 62: 744–751. [DOI] [PubMed] [Google Scholar]
- 18.Singer JP, Diamond JM, Gries CJ, McDonnough J, Blanc PD, Shah R, Dean MY, Hersh B, Wolters PJ, Tokman S, Arcasoy SM, Ramphal K, Greenland JR, Smith N, Heffernan P, Shah L, Shrestha P, Golden JA, Blumenthal NP, Huang D, Sonett J, Hays S, Oyster M, Katz PP, Robbins H, Brown M, Leard LE, Kukreja J, Bacchetta M, Bush E, D’Ovidio F, Rushefski M, Raza K, Christie JD, Lederer DJ. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. Am J Respir Crit Care Med 2015; 192: 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsay SE, Arianayagam DS, Whincup PH, Lennon LT, Cryer J, Papacosta AO, Iliffe S, Wannamethee SG. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart 2015; 101: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cigolle CT, Lee PG, Langa KM, Lee YY, Tian Z, Blaum CS. Geriatric conditions develop in middle-aged adults with diabetes. J Gen Intern Med 2011; 26: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, Burke JR, Fisher GG, Fultz NH, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Weir DR, Willis RJ. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology 2005; 25: 181–191. [DOI] [PubMed] [Google Scholar]
- 22.Rodgers W, Miller B. A comparative analysis of ADL questions in surveys of older people. J Gerontol B Psychol Sci Soc Sci 1997; 52 Spec No: 21–36. [DOI] [PubMed] [Google Scholar]
- 23.Spector WD, Fleishman JA. Combining activities of daily living with instrumental activities of daily living to measure functional disability. J Gerontol B Psychol Sci Soc Sci 1998; 53: S46–57. [DOI] [PubMed] [Google Scholar]
- 24.Guerri R, Gayete A, Balcells E, Ramirez-Sarmiento A, Vollmer I, Garcia-Aymerich J, Gea J, Orozco-Levi M. Mass of intercostal muscles associates with risk of multiple exacerbations in COPD . Respir Med 2010; 104: 378–388. [DOI] [PubMed] [Google Scholar]
- 25.Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166: 809–813. [DOI] [PubMed] [Google Scholar]
- 26.McDonald ML, Diaz AA, Ross JC, San Jose Estepar R, Zhou L, Regan EA, Eckbo E, Muralidhar N, Come CE, Cho MH, Hersh CP, Lange C, Wouters E, Casaburi RH, Coxson HO, Macnee W, Rennard SI, Lomas DA, Agusti A, Celli BR, Black-Shinn JL, Kinney GL, Lutz SM, Hokanson JE, Silverman EK, Washko GR. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 2014; 11: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz AA, Zhou L, Young TP, McDonald ML, Harmouche R, Ross JC, San Jose Estepar R, Wouters EF, Coxson HO, MacNee W, Rennard S, Maltais F, Kinney GL, Hokanson JE, Washko GR, investigators E. Chest CT measures of muscle and adipose tissue in COPD: gender-based differences in content and in relationships with blood biomarkers. Acad Radiol 2014; 21: 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998; 113: 619–624. [DOI] [PubMed] [Google Scholar]
- 29.Kupferberg DH, Kaplan RM, Slymen DJ, Ries AL. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil 2005; 25: 370–377. [DOI] [PubMed] [Google Scholar]
- 30.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 31.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE Jr., Lancaster L, Sahn SA, Szwarcberg J, Valeyre D, du Bois RM, Group CS. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 32.King TE Jr., Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW, Group AS. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 33.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR, Investigators IT. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 34.Kreuter M, Ehlers-Tenenbaum S, Palmowski K, Bruhwyler J, Oltmanns U, Muley T, Heussel CP, Warth A, Kolb M, Herth FJ. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS One 2016; 11: e0151425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swigris JJ, Han M, Vij R, Noth I, Eisenstein EL, Anstrom KJ, Brown KK, Fairclough D. The UCSD shortness of breath questionnaire has longitudinal construct validity in idiopathic pulmonary fibrosis. Respir Med 2012; 106: 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahousse L, Maes B, Ziere G, Loth DW, Verlinden VJ, Zillikens MC, Uitterlinden AG, Rivadeneira F, Tiemeier H, Franco OH, Ikram MA, Hofman A, Brusselle GG, Stricker BH. Adverse outcomes of frailty in the elderly: the Rotterdam Study. Eur J Epidemiol 2014; 29: 419–427. [DOI] [PubMed] [Google Scholar]
- 37.Vaz Fragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med 2012; 125: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lederer DJ, Arcasoy SM, Wilt JS, D’Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174: 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richeldi L, Cottin V, Flaherty KR, Kolb M, Inoue Y, Raghu G, Taniguchi H, Hansell DM, Nicholson AG, Le Maulf F, Stowasser S, Collard HR. Design of the INPULSIS trials: two phase 3 trials of nintedanib in patients with idiopathic pulmonary fibrosis. Respir Med 2014; 108: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 40.Maddocks M, Kon SS, Canavan JL, Jones SE, Nolan CM, Labey A, Polkey MI, Man WD. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016; 71: 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fairhall N, Langron C, Sherrington C, Lord SR, Kurrle SE, Lockwood K, Monaghan N, Aggar C, Gill L, Cameron ID. Treating frailty--a practical guide. BMC Med 2011; 9: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, Sherrington C, Lord SR, Kurrle SE. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med 2013; 11: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax 2008; 63: 549–554. [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama O, Kondoh Y, Kimura T, Kato K, Kataoka K, Ogawa T, Watanabe F, Arizono S, Nishimura K, Taniguchi H. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 2008; 13: 394–399. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira A, Garvey C, Connors GL, Hilling L, Rigler J, Farrell S, Cayou C, Shariat C, Collard HR. Pulmonary rehabilitation in interstitial lung disease: benefits and predictors of response. Chest 2009; 135: 442–447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.