Abstract
Introduction
Patients with neuromuscular disorders sometimes show progressive spinal scoliosis. The surgery for neuromuscular scoliosis (NMS) has high rates of complications. In this study, we elucidated the perioperative complications in patients with NMS.
Methods
We included 83 patients with NMS (58 boys and 25 girls; 61 with muscular dystrophy, 18 with spinal muscular atrophy, and 4 others) who had undergone posterior fusion surgery for scoliosis. We evaluated the perioperative complications (within 3 months), age at time of surgery, operative time, blood loss, preoperative %VC and FEV1.0 (%) for pulmonary function, and preoperative ejection fraction (EF) for cardiac function.
Results
There were 5 (6%) major complications, including pneumonia and a cardiovascular complication requiring intensive care unit (ICU) care, and 15 (18%) minor complications including viral enteritis and a urinary tract infection. Overall, there were 20 (24%) complications. Three of the 5 major complications were pulmonary. The mean age at the time of surgery was 13.7 y, operative time was 304 min, and blood loss was 1530 ml. The mean preoperative %VC was 41%, FEV1.0 was 91%, and EF was 60%. When we separated the patients into a group with major complications (n = 5) and a group without major complications (n = 78), the preoperative %VC in the group with major complications (23%) was significantly lower than that in the group without (42%) (p < 0.05). However, operative time, blood loss, preoperative FEV1.0 (%) and EF between the two groups were not significantly different (p > 0.05).
Conclusions
Compared with the previous findings of the perioperative complication rate (45%-74%) for NMS, the complication rate was remarkably low in this case series. Because of advances in medical skills, including anesthesia and surgical instruments, surgery for NMS appears to be safe. However, patients with NMS with complications demonstrated severe restrictive ventilatory impairment preoperatively. Therefore, we should be vigilant for perioperative pulmonary complications especially in patients with NMS and preoperative severe restrictive ventilatory impairment.
Keywords: Neuromuscular scoliosis, perioperative complications, posterior spinal fusion surgery
Introduction
Patients with muscular dystrophy including Duchenne muscular dystrophy and spinal muscular atrophy develop systemic and progressive muscle weakness, and often exhibit scoliosis. Posterior corrective spinal fusion surgery on patients with neuromuscular scoliosis (NMS) is widely performed to improve the quality of life (QOL) of the patients13), and surgery is expected to inhibit the progression of scoliosis and improve sitting balance8). Furthermore, in patients with NMS, the large Cobb angle is correlated with low pulmonary function including %VC and FEV1.0 (%)9). Therefore, spinal fusion surgery for NMS is recommended.
However, in many cases, patients with NMS exhibit decreased cardiac and respiratory functions before surgery, and there is concern about the high occurrence of perioperative complications. The aim of this study was to elucidate the incidence of perioperative complications in patients with NMS who have undergone posterior spinal fusion surgery.
Materials and Methods
Ethical approval from our Institutional Review Board (IRB) was obtained for this study, which was conducted in accordance with the ethical principles specified in the 1964 Declaration of Helsinki and its later amendments.
Patient population
We considered 100 patients who had undergone posterior spinal corrective fusion surgery for NMS from 2006 to 2016. We excluded 17 patients who were able to walk. The remaining 83 patients were included in the present study. There were 58 boys and 25 girls, including 62 with muscular dystrophies (Duchenne muscular dystrophy, 46; Fukuyama type congenital muscular dystrophy, 11; other type of muscular dystrophy, 3), 18 with spinal muscular atrophies, and 3 with other myopathies. Most of the patients could communicate with others without trouble, but some had mental retardation, especially those with the Fukuyama type congenital muscular dystrophy. However, all the patients underwent pulmonary function evaluation via spirometry and cardiac function evaluation via echocardiography preoperatively. All the patients had sitting difficulty and back pain associated with scoliosis. No patients were excluded because of their preoperative physical status.
The usual perioperative treatment of NMS consisted of the following: NMS patients were admitted 1 week prior to the operation, and their motor and respiratory functions were evaluated by a physical therapist. An anesthesiologist and pediatrician reviewed also carefully all of the patients' data including the physical findings and preoperative pulmonary and cardiac function, and prepared the perioperative management system for use in the operation room and in the pediatric intensive care unit. We attempted extubation as soon as possible after the operation when the patients could ventilate their lungs spontaneously to prevent respirator dependency. Furthermore, we tried also to execute the management system, including early detection and early treatment, according to the perioperative communication among the orthopedic surgeon, anesthesiologist, and pediatrician.
Surgical procedure
All the surgeries were performed under general anesthesia. The spinal cord function was monitored using motor-evoked potentials. Autotransfusion was performed using preoperative storage, while intraoperative collection was used during the surgical procedure. An incision was made on the midline of the back, and the spinal structure was exposed from the upper thoracic spine to the sacrum. After the removal of all soft tissues, posterior instrumentation was performed with pedicle screws, hooks, and sublaminar cables (Nesplon Cable System, Alfresa, Tokyo, Japan). The fusion levels were usually from T4 to L5 but were sometimes determined according to the flexibility or apex of the scoliosis curve. After placement of the instruments, a local autograft bone was obtained from the spinous processes, laminae, and transverse processes of the vertebrae. To obtain scoliosis flexibility, Ponte osteotomy was performed on several (usually four or five) segments around the apex of the scoliosis. The spinal deformities in all the cases were corrected using two combined techniques, including a cantilever technique and a rod rotation technique. After the correction, all laminae and facet joints were decorticated and local autograft bone mixed with bioresorbable bone graft were placed. The wound was sutured in three layers and two drainage tubes were placed. We attempted extubation as soon as possible after the operation when the patients could ventilate their lungs spontaneously to prevent respirator dependency.
Clinical Endpoint
We evaluated the perioperative complications, age at operation, the operative time, blood loss, the preoperative pulmonary function, the preoperative cardiac function, and the preoperative Cobb angle. Perioperative complications of grade III or higher using the Clavien-Dindo classification were defined as major complications. Clavien-Dindo classification grade III or above indicates a complication requiring some kind of surgical intervention and life-threatening complications requiring intensive care management7). For the pulmonary function, %VC and FEV1.0 (%) were evaluated, and then for the cardiac function, ejection fraction (EF) was evaluated.
Statistical analysis
We divided the patients into two groups: those with a major complication (+) and those without (−). The operative time, blood loss, the preoperative pulmonary function, the preoperative cardiac function, and the preoperative Cobb angle between those with (+) and without (−) a major complication were compared using a Mann-Whitney U test. A p < 0.05 was considered significant.
Results
Patient characteristics are described in Table 1. Their mean age at time of surgery was 13.7 y, operative time was 304 min, blood loss was 1,530 ml, preoperative %VC was 41%, FEV1.0 was 91%, EF was 60%, preoperative Cobb angle was 82°, and postoperative Cobb angle was 39° (Table 1).
Table 1.
Patient Characteristics.
| Average | Range | |
|---|---|---|
| Age | 13.7 | 9-21 |
| Operative time | 304 min | 145-410 min |
| Blood loss | 1530 ml | 480-3860 ml |
| Pre-ope %VC | 40.7% | 8.4-82.0% |
| Pre-ope FEV1.0 | 90.6% | 57.1-100% |
| Pre-ope EF | 60.2% | 39-87.9% |
| Pre-ope Cobb angle | 81.7° | 14.7-165° |
| Post-ope Cobb angle | 39.3° | 2.6-115° |
Perioperative complications were observed in 20 patients (24%), of which five (6%) were major complications including CO2 narcosis, pneumonia, and heart failure; there were 15 patients (18%) with minor complications including infections and delayed wound healing (Table 2). Three of the five major complications (4%) were respiratory complications including pneumonia, CO2 narcosis, and respiratory failure. The patient with respiratory failure required re-intubation and management with an artificial ventilator. The patients with pneumonia or CO2 narcosis required additional ICU treatment. However, all the patients recovered fully after the additional treatment. The other two of the five major complications (2%) were cardiac complications including acute heart failure and hemodynamical instability, both of which required additional ICU care. However, all the patients recovered fully after the additional treatment. A majority of the minor complications were infections including urinary tract infections and wound surface infections, which were found in seven cases (8%). Although most of the patients required antibiotics and wound treatments, the infections did not develop into major complications.
Table 2.
Summary of Perioperative Complications.
| Complication | No. (n) | |
|---|---|---|
| Major | Respiratory complications | |
| Pneumonia | 1 | |
| CO2 narcosis | 1 | |
| Respiratory failure | 1 | |
| Cardiac complications | ||
| Acute heart failure | 1 | |
| Hemodynamically unstable | 1 | |
| Minor | Infection complications | |
| Urinary tract infection | 3 | |
| Wound surface infections | 3 | |
| Transmissible gastroenteritis | 1 | |
| Other complications | ||
| Wound Dehiscence | 5 | |
| Meralgia paresthetica | 1 | |
| Hepatic disorders | 1 | |
| Anemia | 1 | |
| Total | 20 |
The mean preoperative %VC, FEV1.0, and EF in patients with major complications were 23%, 92%, and 56%, respectively. All the five patients with major complications showed severe preoperative restrictive ventilator impairment (%VC < 30%). In addition, 30 out of 83 patients in this series showed severe preoperative restrictive ventilator impairment (%VC < 30%) and 5 out of 30 patients with severe preoperative restrictive ventilator impairment showed major complications perioperatively.
When we classified the patients into a group with major complications (+) and a group without major complications (−), the preoperative %VC in the (+) group (23%) was significantly lower than that in the (−) group (42%) (p < 0.05) (Fig. 1a). The preoperative Cobb angle tended to be higher in the (+) group than in the (−) group without major complications, but the difference was not significant (p > 0.05) (Fig. 1d). Furthermore, there were no significant differences in the preoperative FEV1.0 (%), the preoperative EF, the operative time, or blood loss between the two groups (Fig. 1b, c, e, f).
Figure 1.
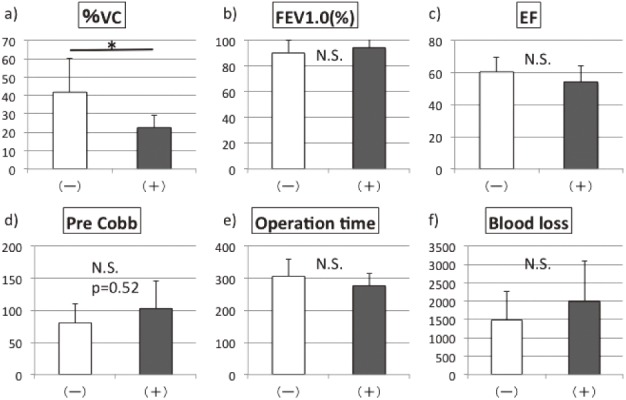
Comparison of (a) preoperative %VC, (b) preoperative FEV1.0 (%), (c) preoperative ejection fraction (EF), (d) preoperative Cobb angle, (e) operative time, (f) blood loss between the group with major complications (+) and the group without major complications (-).
Discussion
Patients with NMS often exhibit decreased respiratory and cardiac functions before surgery and there is concern regarding the perioperative complications in these patients. In the present study, the perioperative complication rate was 24% including major complications 6% and minor complications 18%, although the preoperative respiratory and cardiac functions were decreased in most cases. According to the previous reports, the overall complication rates are between 22.5% and 68%, and the major complication rates are between 6% and 49% (Table 3)2-5,7,10-12,14,18). Although the criteria for major complications between the studies were different, the perioperative complication rate in the present study was remarkably low. Not only the improvements in surgical instruments and techniques, and the advancement of anesthetic techniques in recent years, but also our perioperative supporting system of anesthesiologist and pediatrician might have contributed to the reduced rates of complications. We reported previously that spinal fusion surgery for scoliosis in a case of Duchenne muscular dystrophy successfully improved the patient's sitting difficulty and back pain16). In addition, Velasco et al. reported spinal fusion surgery for NMS decreased the rate of respiratory decline18). Galasko et al. reported spinal fusion surgery improved survival of patients with NMS6). Therefore, spinal fusion surgery for NMS is strongly recommended.
Table 3.
Previous Reports of Perioperative Complication Rate for NMS.
| Major complication rate | Overall complication rate | Diagnosis | |
|---|---|---|---|
| Boachie-Adjei et al. 1989 | N/A | 48% | CP: 22; others: 34 |
| Gau et al. 1991 | N/A | 62% | CP: 34; others: 34 |
| Ramirez et al. 1997 | 27% | 43% | DMD: 30 |
| Benson et al. 1998 | 6% | 28% | CP: 20; others: 30 |
| Sarwashi et al. 2001 | 21.6% | 22.5% | Spinal dysraphism: 58; CP: 25; others: 28 |
| Mohamad et al. 2007 | N/A | 33.1% | CP: 129; others: 46 |
| Modi et al. 2009 | 24% | 68% | DMD: 18; CP: 18; SMA: 8; others: 6 |
| Rummalla et al. 2016 | N/A | 40.1% | N/A |
| Bendon et al. 2016 | 49% | N/A | CP: 19 |
| Present study | 6% | 24% | DMD: 46; SMA: 18; others: 19 |
N/A: not available
CP: cerebral palsy
DMD: Duchenne muscular dystrophy
SMA: Spinal muscular atrophy
Three of the five major complications after NMS surgery in the present study were respiratory. Preoperative restrictive ventilatory impairment might lead to the development of perioperative complications. The previous reports showed also that most of the major complications were respiratory10,15). In the present study, the Cobb angle tended to be higher in the group with major complications than the group without major complications, although this tendency was not statistically significant. Kang et al. reported patients with low pulmonary function and a high Cobb angle were more likely to develop a postoperative pulmonary complication9). An increasing Cobb angle might lead to pulmonary dysfunction19). These possibilities indicate that we should be vigilant for perioperative pulmonary complications when we perform surgery especially in patients with NMS and preoperative severely restrictive ventilatory impairment.
We believe that the most important step for preventing perioperative complications is attempting extubation once the patients could ventilate their lungs spontaneously as soon as possible after the operation to prevent respirator dependency. We reported previously that extubation on the operative day for NMS surgery showed good outcomes without any respiratory complications17). However, optimal extubation timing remains controversial. Bach et al. recommended extubation for NMS surgery by the third postoperative day1). However, a future prospective study is needed to confirm our findings.
There are some limitations to this study. First, the pulmonary function test results were strongly dependent on the patient's understanding of the procedure. Some patients who could not understand the procedure completely, because of mental retardation, were included in this study. Second, we could not detect any factors on the multivariate analysis because of the low major complications rate. In the future, additional large studies are needed to enable the multivariate analysis. Third, we could not obtain the postoperative pulmonary and cardiac function in all cases. Thus, whether spinal fusion surgery for NMS improves pulmonary or cardiac function remains unclear. Fourth, we did not evaluate the postoperative clinical findings including SRS-22.
In conclusion, the rate of complications was remarkably low in the present case series. However, patients with NMS and major complications demonstrated severely restrictive ventilatory impairment preoperatively. Therefore, we should be vigilant for perioperative pulmonary complications in patients with NMS and preoperative severely restrictive ventilatory impairment.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Sources of Funding: This investigation was supported in part by the Intramural Research Grant (29-3) for Neurological and Psychiatric Disorders of NCNP.
Author Contributions: MiM drafted the manuscript and participated in the design of the study. MaM conceived this study and revised the manuscript. WS, TI, and GI participated in its design and coordination and drafted the manuscript. ES, KU, and TA helped to revise the manuscript and carried out statistical analysis. NT and MT conceived the study and participated in its design and coordination. All the authors have read and have approved the final manuscript.
References
- 1.Bach JR, Sabharwal S. High pulmonary risk scoliosis surgery. J Spinal Disord Tech. 2005;18(6):527-30. [DOI] [PubMed] [Google Scholar]
- 2.Bendon AA, George KA, Patel D. Perioperative complications and outcomes in children with cerebral palsy undergoing scoliosis surgery. Paediatr Anaesth. 2016;26(16):970-5. [DOI] [PubMed] [Google Scholar]
- 3.Benson ER, Thomson JD, Smith BG, et al. Results and morbidity in a consecutive series of patients undergoing spinal fusion for neuromuscular scoliosis. Spine. 1998;23(21):2308-17. [DOI] [PubMed] [Google Scholar]
- 4.Boachie-Adjei O, Lonstein JE, Winter RB, et al. Management of neuromuscular spinal deformities with Luque segmental instrumentation. J Bone Joint Surg Am. 1989;71(4):548-62. [PubMed] [Google Scholar]
- 5.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galasko CS, Delaney C, Morris P. Spinal stabilisation in Duchenne muscular dystrophy. J Bone Joint Surg Br. 1992;74(2):210-4. [DOI] [PubMed] [Google Scholar]
- 7.Gau YL, Lonstein JE, Winter RB, et al. Luque-Galveston procedure for correction and stabilization of neuromuscular scoliosis and pelvic obliquity: a review of 68 patients. J Spinal Disord. 1991;4(4):399-410. [DOI] [PubMed] [Google Scholar]
- 8.Hahn F, Hauser D, Espinosa N, et al. Scoliosis correction with pedicle screws in Duchenne muscular dystrophy. Eur Spine J. 2008;17(2):255-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang GR, Suh SW, Lee IO. Preoperative predictors of postoperative pulmonary complications in neuromuscular scoliosis. J Orthop Sci. 2011;16(2):139-47. [DOI] [PubMed] [Google Scholar]
- 10.Modi HN, Suh SW, Yang JH, et al. Surgical complications in neuromuscular scoliosis operated with posterior- only approach using pedicle screw fixation. Scoliosis. 2009;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamad F, Parent S, Pawelek J, et al. Perioperative complications after surgical correction in neuromuscular scoliosis. J Pediatr Orthop. 2007;27(4):392-7. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez N, Richards BS, Warren PD, et al. Complications after posterior spinal fusion in Duchenne's muscular dystrophy. J Pediatr Orthop. 1997;17(1):109-14. [PubMed] [Google Scholar]
- 13.Rideau Y, Glorion B, Delaubier A, et al. The treatment of scoliosis in Duchenne muscular dystrophy. Muscle Nerve. 1984;7(4):281-6. [DOI] [PubMed] [Google Scholar]
- 14.Rumalla K, Yarbrough CK, Pugely AJ, et al: Spinal fusion for pediatric neuromuscular scoliosis: national trends, complications, and in-hospital outcomes. J Neurosurg Spine. 2016;25(4):500-8. [DOI] [PubMed] [Google Scholar]
- 15.Sarwahi V, Sarwark JF, Schafer MF, et al. Standards in anterior spine surgery in pediatric patients with neuromuscular scoliosis. J Pediatr Orthop. 2001;21(6):756-60. [PubMed] [Google Scholar]
- 16.Takaso M, Nakazawa T, Imura T, et al. Surgical management of severe scoliosis with high-risk pulmonary dysfunction in Duchenne muscular dystrophy. Int Orthop. 2010;34(3):401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaso M, Nakazawa T, Imura T, et al. Two-year results for scoliosis secondary to Duchenne muscular dystrophy fused to lumbar 5 with segmental pedicle screw instrumentation. J Orthop Sci. 2010;15(2):171-7. [DOI] [PubMed] [Google Scholar]
- 18.Velasco MV, Colin AA, Zurakowski D, et al. Posterior spinal fusion for scoliosis in duchenne muscular dystrophy diminishes the rate of respiratory decline. Spine. 2007;32(4):459-65. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein SL, Zavala DC, Ponseti IV. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am. 1981;63:702-12. [PubMed] [Google Scholar]


