Abstract
Background
We provide the first multicenter analysis of patients cared for by eight Pulmonary Embolism Response Teams (PERTs) in the United States (US); describing the frequency of team activation, patient characteristics, pulmonary embolism (PE) severity, treatments delivered, and outcomes.
Methods
We enrolled patients from the National PERT Consortium™ multicenter registry with a PERT activation between 18 October 2016 and 17 October 2017. Data are presented combined and by PERT institution. Differences between institutions were analyzed using chi-squared test or Fisher's exact test for categorical variables, and ANOVA or Kruskal-Wallis test for continuous variables, with a two-sided P value < 0.05 considered statistically significant.
Results
There were 475 unique PERT activations across the Consortium, with acute PE confirmed in 416 (88%). The number of activations at each institution ranged from 3 to 13 activations/month/1000 beds with the majority originating from the emergency department (281/475; 59.3%). The largest percentage of patients were at intermediate–low (141/416, 34%) and intermediate–high (146/416, 35%) risk of early mortality, while fewer were at high-risk (51/416, 12%) and low-risk (78/416, 19%). The distribution of risk groups varied significantly between institutions (P = 0.002). Anticoagulation alone was the most common therapy, delivered to 289/416 (70%) patients with confirmed PE. The proportion of patients receiving any advanced therapy varied between institutions (P = 0.0003), ranging from 16% to 46%. The 30-day mortality was 16% (53/338), ranging from 9% to 44%.
Conclusions
The frequency of team activation, PE severity, treatments delivered, and 30-day mortality varies between US PERTs. Further research should investigate the sources of this variability.
Keywords: assessing and improving clinician behavior, cardiopulmonary pharmacology and therapeutics, cardiovascular diseases, pulmonary embolism, registries
Introduction
Acute pulmonary embolism (PE) is a common disease with an estimated incidence of 1–2/1000 adults per year.1,2 PE is the third most common cardiovascular cause of death after heart attack and stroke.3 In recent years, novel therapies have shown promise in the treatment of intermediate- and high-risk PE. These include reduced doses of systemic thrombolysis, catheter-based embolectomy, catheter-directed thrombolysis (CDT), optimized surgical techniques, and extracorporeal membrane oxygenation (ECMO). Unfortunately, comparative clinical data on efficacy and safety are lacking, and clinicians are forced to determine the optimal treatment for their PE patients on a case by case basis.4–7
To organize and improve the efficiency of PE care, and to harmonize the approach to PE across specialties, hospitals have developed a new paradigm for the evaluation and treatment of patients with acute PE: the Pulmonary Embolism Response Team (PERT).8 The PERT model involves rapid consultation and treatment by a multidisciplinary team of specialists. Since 2012, the number of PERTs have risen throughout the United States (US) and across the world.9,10 PERTs are diverse in their structure and resources.11,12 However, it is not known whether this diversity extends to treatments and outcomes.13
We provide the first national, multicenter analysis of patients cared for by eight US PERTs; describing the frequency of team activation, patient characteristics, PE severity, treatments delivered, and outcomes.
Methods
Structure of PERTs
The PERT approach has been described in detail previously.14 In short, referring physicians activate the PERT via a 24-h telephone number. Relevant clinical information is gathered by a PERT representative, and the team is notified of a multidisciplinary meeting. Data and radiologic images are reviewed and discussed in real time by PERT members. A consensus opinion is reached and diagnostic and treatment recommendations are communicated to the referring physician. PERTs may also mobilize resources and staff needed for advanced treatments.
PERT Consortium™ multicenter registry
We include data from the PERT Consortium™ multicenter registry (pertconsortium.org). The PERT Consortium™ is a not-for-profit organization that works to expand and support PERTs around the world with the overall aim of improving care of patients suffering from acute PE.10,11 At the time this study was performed, the PERT Consortium did not provide specific guidelines for the clinical work up, decision making or treatment of patients. Most PERTs focus on the treatment of intermediate and high-risk PE, where advanced treatment might be indicated, so patients treated by PERTs are likely to undergo certain tests (e.g. echocardiography). However, the performance of specific diagnostic tests is at the discretion of the treating physician. The PERT Consortium™ also serves as a platform for multicenter research, and recently established an ongoing, multicenter registry of PERT patients. In this report, we included data from the initial year of the multicenter registry, with eight US PERTs contributing data: Massachusetts General Hospital, Cleveland Clinic, Emory Clinic, Lancaster General Hospital, Medical University of South Carolina, Saint Louis University Care, Northwestern Medicine, Penn Medicine. All institutions are large tertiary academic centers with the logistical framework and clinical experience needed to deliver timely advanced diagnostic and interventional care as needed. As this paper is not intended for qualitative comparisons of institutions, data is presented anonymously under numbers 1–8.
Enrollment and data collection
We enrolled all patients with a PERT activation between 18 October 2016 and 17 October 2017, the first full year of the PERT multicenter registry. The PERT Consortium™ asks that sites enter all PERT activations in the database. Site monitoring visits or review of screening logs for conformation are, however, not performed. Registry data were entered into a HIPPA-compliant web-based application (www.project-redcap.org) by study team members at their respective institutions. Patient data describing time and origin of PERT activation, time of PERT meeting, demographics, comorbid and concurrent illness or illnesses, PE risk factors, medication use, presenting signs/symptoms and vital signs, imaging, and electrocardiographic and laboratory findings are recorded at the time of PERT activation. After PERT activation, study staff record follow-up data from medical records describing clinical progress, diagnostic test results, treatments, and outcomes at 30 days.
PE diagnosis and severity
For analysis of PERT activations and patient characteristics, we report all PERT activations including both confirmed and non-confirmed PE. For PE severity, treatment, and outcomes, analyses were restricted to patients with PE confirmed by imaging performed prior to or within 3 days of PERT activation (i.e. positive computed tomography pulmonary angiography (CTPA) or high probability ventilation/perfusion (V/Q) scan). If a patient had echocardiography performed, we used that to define RV strain. If no echocardiogram was performed, but RV strain was evaluated on CT, we used CT to define RV strain. If RV strain was not evaluated by either echocardiogram or CT, we categorized patients as having no RV strain on imaging—reflecting that the PERT members had no imaging information on RV strain at the time of team activation. We characterized confirmed PE as low-, intermediate-low-, intermediate-high- or high-risk in accordance with published guidelines from the European Society of Cardiology.15 High-risk PE was defined as confirmed acute PE with sustained hypotension or pulselessness. Intermediate- to high-risk PE was defined as confirmed acute PE without hypotension but with right ventricular (RV) strain confirmed by both imaging (CTPA or echocardiography) and biomarkers (elevated Troponin or brain natriuretic peptides (BNP or NT-proBNP), as defined by institution-specific cut-offs). Intermediate-low risk was defined as confirmed acute PE without hypotension but with right ventricular (RV) strain confirmed either by imaging or biomarkers. Low-risk PE was defined as confirmed acute PE with none of the above criteria. We did not include the PESI or sPESI scores in the algorithm due to feasibility.
Treatment and outcomes
We recorded if the patient received any of the following treatments within 3 days of PERT activation: anticoagulation alone, systemic intravenous thrombolysis, catheter-directed thrombolysis, catheter-directed embolectomy/fragmentation, surgical thromboembolectomy, extracorporeal membrane oxygenation (ECMO), and inferior vena cava (IVC) filter placement. Three days was chosen to capture decisions made by the PERT in the acute phase of treatment. To compare with previous studies, we evaluated outcomes occurring ≤30 days after PERT activation, including all-cause mortality, recurrent venous thromboembolism (VTE), and major bleeding complications (based on International Society on Thrombosis and Hemostasis criteria).16,17
Statistical analysis
Data were exported from REDCap™ to SAS® version 9.4 (SAS Institute, Cary, NC) for analysis. Continuous variables are presented as means and standard deviations (SD) and categorical variables as frequency with proportions. Data were analyzed in their totality as well as stratified by hospital. Analyses comparing hospitals were limited to institutions with 40 or more activations during the study period. To provide comparable estimates, the number of PERT activations was adjusted for the time contributing to the registry and size of each hospital (number of registered beds). Differences between PERT institutions were analyzed using chi-squared test or Fisher's exact test for categorical variables, and ANOVA or Kruskal-Wallis test for continuous variables. We limited analyses of PE severity, treatment, and outcomes to patients with confirmed PE; follow-up analyses were limited to subjects for whom 30-day follow-up was complete. Due to the small number of patients receiving advanced therapies at each institution, we dichotomized treatments into anticoagulation alone versus any advanced therapy. A two-sided P value < 0.05 was considered statistically significant.
Results
PERT activations
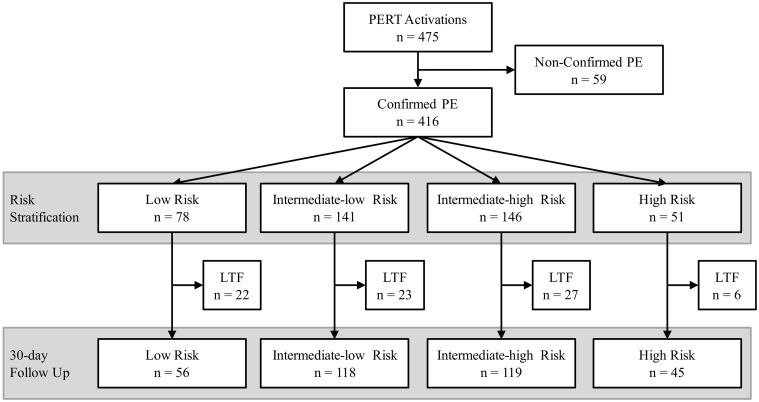
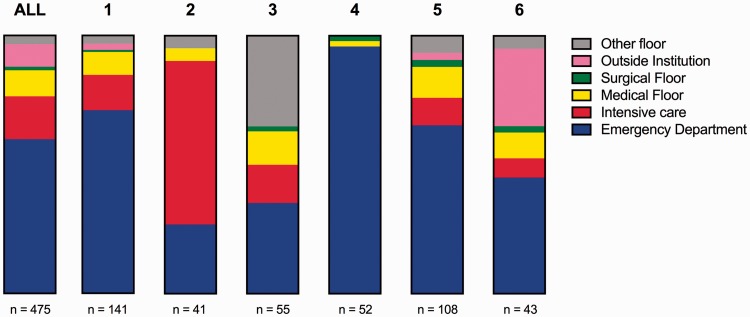
In the year of inclusion there were 475 unique PERT activations across all eight institutions (Fig. 1). The number of PERT activations at each institution are reported in Table 1. Adjusting for time contributing to the registry and hospital size, the activations ranged from 3 to 13 activations/month/1000 beds with a mean of 8 activations/month/1000 beds. The origin of activations is presented in Fig. 2. The majority of activations came from the Emergency Department (281/475; 59.3%). The remaining activations were mainly divided between the intensive care unit (78/475; 16.5%), medical floor (50/475; 10.5 %), and outside institutions (37/475; 7.8%). The origin of activation varied between institutions (P < 0.0001). While institution 2 received the majority of activations from the Intensive Care Unit, institution 4 was activated almost exclusively from the Emergency Department.
Fig. 1.
Flow chart of all patients included in the study. LTF: Lost to follow-up.
Table 1.
Number of PERT activations.
| Institution | Number of PERT activations1 | Number of PERT activations /month/1000 beds2 |
|---|---|---|
| 1 | 141 | 11 |
| 2 | 41 | 3 |
| 3 | 55 | 13 |
| 4 | 52 | 7 |
| 5 | 108 | 13 |
| 6 | 43 | 6 |
| 7 | 10 | 3 |
| 8 | 25 | 6 |
| Total/mean | 475 | 8 |
Total number of unique PERT activations from each institution and the total combined number.
Number of PERT activations corrected for the time of contribution to the registry (from date of first activation to end of study) and the National Institutes of Health (NIH) registered beds at institutions. Presented for each institution and the mean for all institutions combined.
Fig. 2.
Origin of all unique PERT activations depicted as parts of a whole for all sites combined, and stratified by institutions with ≥40 activations.
PERTs can be activated for patients with either suspected or confirmed PE, so 416/475 (88%) of all PERT activations had a PE diagnosis confirmed. Of the 59/475 (12%) with non-confirmed PE, 37/59 (63%) had PE ruled out by imaging, and, for 22/59 (37%), a PE diagnosis was not confirmed nor excluded (e.g. due to hemodynamic instability).
Characteristics of patients
Demographics, comorbid illnesses and PE risk factors among all PERT activations (PE confirmed and non-confirmed) are described in Table 2. For all PERT activations, the mean patient age was 61.7 ± 17.7 years and slightly more than half were female (51.4%). Mean body mass index (BMI) was 31.9 (SD = 10.0) kg/cm2 and mean Charlson Comorbidity Index (CCI) score was 1.9 (SD = 2.4). Patients with confirmed PE were younger, had higher CCI, had more malignancy and other risk factors for VTE: recent hospitalization, recent surgery, previous PE, previous DVT, and family history of VTE compared with patients with no PE diagnosis (Table 2).
Table 2.
Demographics, Comorbidity and risk factors among all PERT activations.
| All PERT activations |
Non-confirmed PE |
Confirmed PE |
||||
|---|---|---|---|---|---|---|
| n | %/SD | n | %/SD | n | %/SD | |
| PERT Activations | 475 | 100.0% | 59 | 12.4% | 416 | 87.6% |
| Demographics | ||||||
| Age in years (mean, SD) | 61.7 | 17.7 | 67.1 | 15.9 | 61.2* | 17.8 |
| Male | 231 | 48.6% | 24 | 40.7% | 207 | 49.8% |
| Race/ethnicity | ||||||
| Asian | 7 | 1.5% | 1 | 1.7% | 6 | 1.4% |
| Black or African American | 120 | 25.3% | 14 | 23.7% | 106 | 25.5% |
| Hispanic or Latino Ethnicity | 19 | 4.0% | 3 | 5.1% | 16 | 3.8% |
| White | 285 | 60.0% | 37 | 62.7% | 248 | 59.6% |
| Other/unknown | 42 | 8.8% | 3 | 5.1% | 39 | 9.4% |
| Height in cm (mean/SD) | 170.3 | 23.8 | 172.2 | 8.4 | 170.2 | 24.5 |
| Weight in kg (mean/SD) | 93.6 | 29.5 | 92.6 | 27.6 | 93.6 | 29.7 |
| Body mass index (mean/SD) | 31.9 | 10.0 | 31.1 | 8.4 | 31.9 | 10.1 |
| Primary insurance | ||||||
| None/self pay | 16 | 3.4% | 2 | 3.4% | 14 | 3.4% |
| Private insurance | 151 | 31.8% | 8 | 13.6% | 143 | 34.4% |
| Medicare | 171 | 36.0% | 10 | 16.9% | 161 | 38.7% |
| Medicaid | 44 | 9.3% | 3 | 5.1% | 41 | 9.9% |
| Military | 2 | 0.4% | 0 | 0% | 2 | 0.5% |
| Other | 7 | 1.5% | 0 | 0% | 7 | 1.7% |
| Unknown | 39 | 11.5% | 31 | 8.4% | 39 | 11.8% |
| Comorbid illness | ||||||
| Chronic obstructive pulmonary disease | 48 | 10.1% | 6 | 10.2% | 42 | 10.1% |
| Connective tissue disease | 11 | 2.3% | 1 | 1.7% | 10 | 2.4% |
| Congestive heart failure | 26 | 5.5% | 3 | 5.1% | 23 | 5.5% |
| Coronary heart disease | 66 | 13.9% | 6 | 10.2% | 60 | 14.4% |
| Depression/anxiety | 78 | 16.4% | 4 | 6.8% | 74 | 17.8% |
| Diabetes | 107 | 22.5% | 7 | 11.9% | 100 | 24.0% |
| Gastrointestinal bleeding | 13 | 2.7% | 1 | 1.7% | 12 | 2.9% |
| Hypertension | 255 | 53.7% | 13 | 22.0% | 242 | 58.2% |
| Malignancy | 118 | 24.8% | 7 | 11.9% | 111 | 26.7% |
| Hematologic | 10 | 2.1% | 1 | 1.7% | 9 | 2.2% |
| Solid | 106 | 22.3% | 6 | 10.2% | 100 | 24.0% |
| Known solid tumor metastases | 49 | 10.3% | 3 | 5.1% | 46 | 11.1% |
| Active treatment | 35 | 7.4% | 3 | 5.1% | 32 | 7.7% |
| Renal insufficiency/failure | 39 | 8.2% | 3 | 5.1% | 36 | 8.7% |
| Stroke/neurovascular disease | 30 | 6.3% | 2 | 3.4% | 28 | 6.7% |
| Charlson comorbidity index (mean SD) | 1.9 | 2.4 | 1.0 | 2.0 | 2.0* | 2.4 |
| Other PE Risk Factors | ||||||
| Smoking | 141 | 29.7% | 13 | 22.0% | 128 | 30.8% |
| Recent hospitalization | 102 | 21.5% | 5 | 8.5% | 97 | 23.3% |
| Reduced mobility | 69 | 14.5% | 4 | 6.8% | 65 | 15.6% |
| Recent surgery or invasive procedure | 88 | 18.5% | 4 | 6.8% | 84 | 20.2% |
| Prior PE | 69 | 14.5% | 2 | 3.4% | 67 | 16.1% |
| Prior DVT | 75 | 15.8% | 3 | 5.1% | 72 | 17.3% |
| Family history of VTE | 30 | 6.3% | 0 | 0% | 30 | 7.2% |
| Hormone use | 29 | 6.1% | 1 | 1.7% | 28 | 6.7% |
| Recent trauma | 22 | 4.6% | 3 | 5.1% | 19 | 4.6% |
| Indwelling catheter | 17 | 3.6% | 0 | 0% | 17 | 4.1% |
Demographics, comorbidity, and risk factors among all PERT activations presented for all activations, confirmed PE and non-confirmed PE. SD: Standard deviation. *P < 0.05 (confirmed vs. non-confirmed).
Characteristics of PE
Table 3 describes the markers of PE severity among patients with confirmed PE. More than half of patients with confirmed PE had signs of RV strain on CT 214/416 (51%) and echocardiography 231/416 (55.5%), while 113/416 (27%) had elevated troponin, and 153/416 (37%) had elevated BNP/NT-proBNP. DVT was present in 191/416 (46%) patients with confirmed PE. 245/416 (59%) of patients with confirmed PE were admitted to the ICU and 52/416 (13%) were intubated.
Table 3.
PE risk factors among 416 patients with confirmed PE.
| Confirmed PE |
||
|---|---|---|
| PE category | n | % |
| Low-Risk | 78 | 18.8 |
| Intermediate-low | 141 | 33.9 |
| Intermediate-high | 146 | 35.1 |
| High | 51 | 12.3 |
| PE location | ||
| Saddle | 85 | 20.4 |
| Main pulmonary artery | 184 | 44.2 |
| Lobar pulmonary artery | 64 | 15.4 |
| Segmental pulmonary artery | 53 | 12.7 |
| Unknown | 30 | 7.2 |
| Extra-pulmonary thrombus | ||
| Intracardiac | 21 | 5.0 |
| PE Severity | ||
| Right heart strain on echocardiogram | 231 | 55.5 |
| Right heart strain on CT | 214 | 51.4 |
| Troponin ( > institutional cut-off) | 113 | 27.2 |
| NT-proBNP ( > institutional cut-off) | 153 | 36.8 |
| DVT present | 191 | 45.9 |
| Proximal to knee | 149 | 35.8 |
| Distal to knee | 40 | 9.6 |
| Upper extremity | 2 | 0.5 |
| Clinical severity | ||
| Endotracheally intubated | 52 | 12.5 |
| Admitted to ICU | 245 | 58.9 |
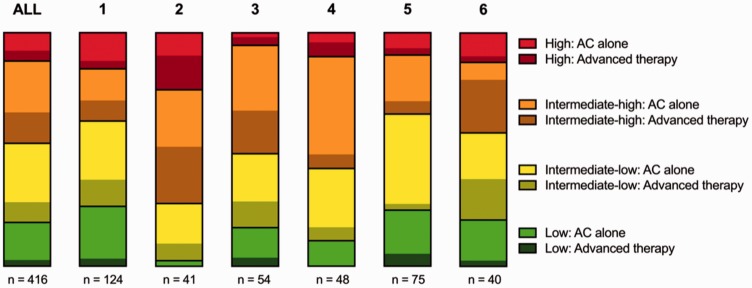
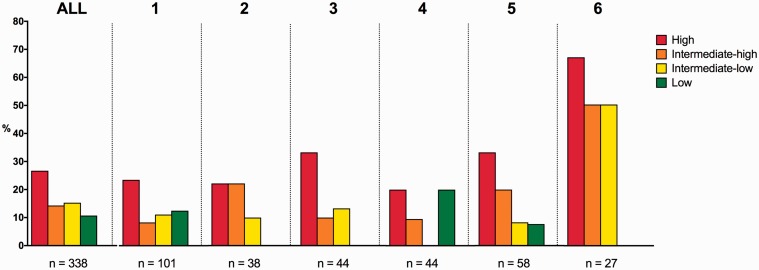
The distribution of patients with confirmed PE into low-, intermediate- to low-, intermediate- to high- and high-risk of early mortality according to ESC guidelines is shown in Fig. 3.15 The majority of patients were intermediate–low (141/416, 34%) and intermediate–high (146/416, 35%) risk of early mortality, while (51/416, 12%) were at high risk and (78/416 19%) were at low risk. The distribution of risk groups varied significantly between institutions (P = 0.002).
Fig. 3.
Risk stratification of patients with confirmed PE. Distribution of patients with confirmed PE into groups of risks of early mortality. Within each risk group the proportion of patients receiving advanced therapies (any other therapy than anticoagulation alone) is depicted as darkened. Data is presented as parts of a whole for all sites combined and institutions with ≥40 activations. AC: Anticoagulation.
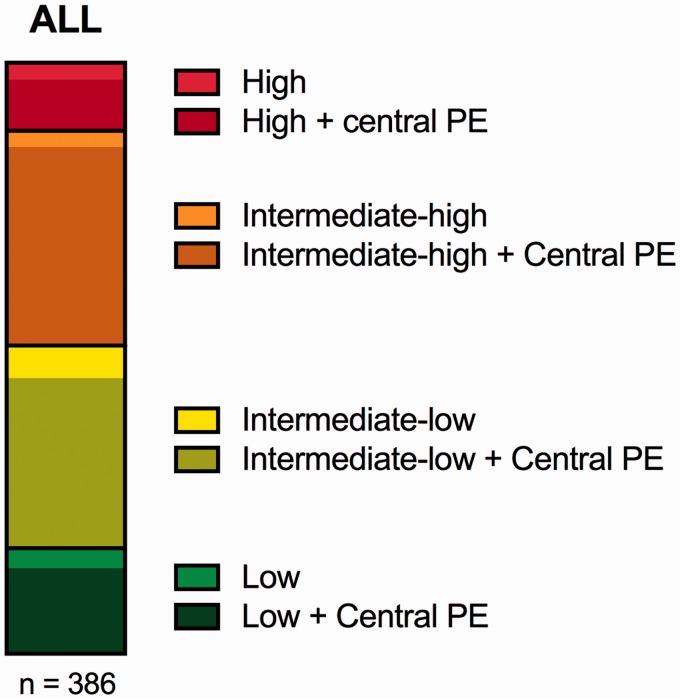
The proportion of patients with central PE (intracardiac, saddle, main PA, right/left PA, or lobar) versus distal PE (segmental or smaller) among patients with confirmed PE and imaging available is shown in Fig. 4. The large majority of patients (330/386; 86%) had a central PE. The proportion of central PE was 76% (34/45) in the high-risk patients and 80% (56/70) in the low-risk patients.
Fig. 4.
Proportion of central PE in risk groups. Distribution of patients with confirmed PE into groups of risks of early mortality. Within each risk group the proportion of patients with a central PE (intracardiac, saddle, main PA, left/right PA, lobar PA) is depicted as darkened. Data is presented as parts of a whole. PE: Pulmonary embolism.
Treatment of patients with PE
Anticoagulation alone was the most common therapy, delivered to 289/416 (70%) patients with confirmed PE. The proportion of patients receiving anticoagulation alone versus any advanced therapy is shown in Fig. 3, stratified by institution and PE risk group. The proportion of patients receiving advanced therapy increased with the risk groups: low (11/78, 14%), intermediate–low (36/141, 26%), intermediate–high (55/146, 38%), and high (19/51, 37%). The proportion of patients receiving advanced therapy varied between institutions (P = 0.0003), ranging from 16% at institution 5 to 46% at institution 2.
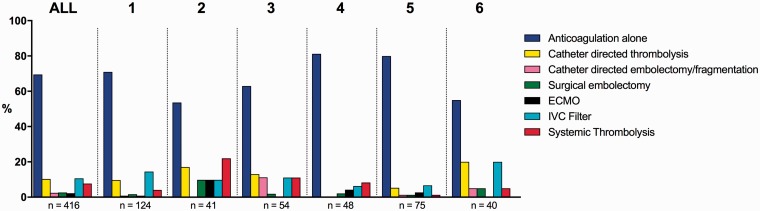
Treatments delivered to patients with confirmed PE stratified by institution are shown in Fig. 5. Overall, 127/416 (31%) patients treated received at least one advanced therapy: inferior vena cava (IVC) filters (44/416, 11%), CDT (43/416, 10%), and systemic thrombolysis (32/416, 8%) were the most common. Due to the limited numbers, we were not able to perform an adjusted statistical analysis for variation in the use of individual treatments across institutions. However, there did appear to be some variation. For example, CDT was used in 20% of patients with confirmed PE in institution 6, while no patients received CDT in institution 4. Comparing institutions 2 and 5, ECMO (10% vs. 3%), surgical embolectomy (10% vs. 1%) and systemic thrombolysis (22% vs. 1%) also appeared to differ.
Fig. 5.
Treatment delivered by PERT in patients with confirmed PE. Distribution of treatments delivered by PERT in patients with confirmed PE. Treatments are not mutually exclusive with the exception of anticoagulation alone. Data is presented as percentages of patients receiving a given treatment for all sites combined and stratified by institutions with ≥40 activations. ECMO: Extra Corporal Membrane Oxygenation; IVC: Inferior Vena Cava.
Outcomes of patients with PE
Among the 416 patients with confirmed PE, 338 (81%) completed 30-day follow-up. In patients with complete follow-up, 30-day mortality was 16% (53/338). As above, due to the limited numbers, we were not able to perform an adjusted statistical analysis for variation in mortality across institutions, but unadjusted mortality was 9% (4/44) at institution 4 and 44% (12/27) at institution 6. Fig. 6 shows 30-day mortality for each institution, stratified by risk groups. Overall, 30-day mortality was highest in the high-risk group (12/45, 27%) and lowest in the low-risk group (6/56, 11%). The 30-day mortality was similar in the two intermediate groups (intermediate–low: 18/118 (15%), intermediate–high: 17/119 (14%)) and only slightly higher than the low-risk group. This pattern was seen in the majority of individual sites, with the exception of institution 4, where there was 0% (0/13) 30-day mortality in the intermediate- to low-risk patients but 20% (1/5) mortality in the low-risk group.
Fig. 6.
30-day all-cause mortality in patients with confirmed PE and complete 30-day follow up stratified by risk groups. Data presented as percentages for all sites combined and by institutions with ≥40 activations.
The major bleeding rate at 30 days was 13% (44/338) overall. Unadjusted bleeding rates were highest in high-risk patients (11/45, 24%) and lower in the other risk groups (low: 6/56, 11%, intermediate–low: 16/118, 14%, intermediate–high: 16/119, 9%). There was no significant difference in bleeding rates between patients receiving advanced therapies compared with patients receiving anticoagulation alone (advanced therapy: 18/110, (16%) vs. anticoagulation alone: 27/234, (12%), P = 0.23). Despite a weak tendency towards higher bleeding rates in patients receiving thrombolysis, the differences were not significant (systemic thrombolysis: 5/29, 17% vs. no systemic thrombolysis: 40/315, 13%, P = 0.56), (CDT 7/37, 19% vs. no CDT: 38/307, 12%, P = 0.29).
The overall rate of recurrent VTE at 30 days was 7% (24/338) and distributed evenly among risk groups (low: 3/56, 5%, intermediate–low: 9/118, 8%, intermediate–high: 8/119, 7 %, high: 4/45, 9%). Recurrent VTE rates were higher in patients receiving advanced therapies compared with patients receiving anticoagulation alone (advanced therapy: 12/110, 11% vs. anticoagulation alone 12/234, 5%, P = 0.049). Looking at individual treatments, there was significantly more VTE recurrence for patients receiving systemic thrombolysis (systemic thrombolysis: 5/29, 17% vs. no systemic thrombolysis: 19/315, 6%, P = 0.041) and a trend towards an increase in patients receiving CDT (CDT 5/37, 14% vs. no CDT: 19/307, 6%, P = 0.068).
Discussion
We performed the first multicenter analysis of patients cared for by PERTs, including 475 unique PERT activations from eight US institutions. We found that the frequency of team activation, patient characteristics, PE severity, treatments delivered, and outcomes varies between institutions.
Adjusting for the time contributing to the registry and size of each institution, the number of activations differed between sites, with a range of 3 to 13 activations/month/1000 beds. The majority of PERT activations in this report came from the Emergency Department (59%). This is in line with previous reports.8 However, the origin of activations varied between sites. At one institution, the majority of activations were from the ICU, while at another institution, activations were almost exclusively from the ED. This may be due to the variation of medical specialties of primary PERTs members at each institution.11 Awareness of PERT may be higher at departments with key PERT members, causing more frequent activations. We do note an apparent trend towards institutions with more activations from the ED having lower mortality. Whether this is true, and the reasons for this, should be explored further.
Overall, 81% of patients with confirmed PE had some degree of RV strain, putting them at intermediate- or high-risk for early mortality according to current guidelines.15 While activation of PERT for low-risk patients was not the original purpose of PERT, previously published reports from the National PERT Consortium™, found that 19%–30% of institutions consider PERT activation for low-risk patients appropriate.8,9 Low-risk patients, despite not having RV strain, may still be challenging due to comorbidity, contraindications to standard treatment or other complicating factors such as massive DVT or thrombus in transit. The tendency towards clinicians activating PERT for reasons other than their risk group, is further underlined by the fact that as many as 80% (56/70), of low-risk patients in this report had a central PE—a higher proportion than the high-risk patients 76% (34/45). Clinicians may worry about central PE on CTPA and activate PERT even though the patient has no signs of RV strain (low risk).
Anticoagulation alone was the most common therapy delivered to more than two-thirds of patients with confirmed PE. This is not surprising as the majority of patients across institutions were at low- or intermediate-risk for whom guidelines recommend anticoagulation alone.15 On the other hand, advanced therapy was not delivered exclusively to patients at high-risk. We observed that the proportion of patients receiving advanced therapy increased with the risk groups. Even so, a considerable proportion of lower risk patients received advanced therapies—the most frequent being IVC filters and CDT, followed by systemic thrombolysis. While the proportion of patients receiving any advanced therapy varied greatly between institutions, we did not have the statistical power to test for variation in treatment. Meanwhile, it did appear that there were variations between institution in the use of ECMO (range: 3–10%), CDT (range: 0–20%), systemic thrombolysis (range: 1–22%), and surgical embolectomy (range: 1–10%). These findings must be viewed with caution, as we could not adjust for variations in comorbid illness or risk profiles between institutions. However, these data suggest that treatment of acute PE varies between US PERTs—both regarding established therapies such as anticoagulation, thrombolysis, and surgery, and also novel therapies such as CDT and ECMO. It is also important to recognize that the available data supporting the effectiveness of most invasive therapies provided by PERTs are still limited, and that more research is required to demonstrate improvements in patient-centered outcomes.
Overall 30-day mortality for patients with confirmed PE was 16%, and increased with increasing risk groups. The 30-day mortality was 31% in the high-risk group, which is in line with previous reports and underlines the severity of high risk PE.8,18,19 The mortality was lowest in the low-risk group (11%). While this is similar to previous PERT cohorts, it is significantly higher than the 0–3% 30-day mortality for low risk PE in previous non-PERT cohorts.20,21 The high mortality of low-risk patients in this and other PERT cohorts, is likely due to the increased comorbidity in the selected PERT population, and may not be related solely to acute PE.19 We observed rather high 30-day rates of major bleeding (13%) and recurrent VTE (7%) in this cohort. This is again comparable to a previously published PERT cohort, but higher than cohorts of unselected PE patients. We found that bleeding rate was not significantly different between patients receiving advanced therapy compared with anticoagulation alone (16% vs. 12%, P = 0.23). However, the number of events are low and the analysis may be under-powered. Furthermore, the definition of advanced therapies in this study includes a range of therapies with different risks of bleeding. When looking at patients receiving systemic and catheter-directed thrombolysis, however, we find bleeding rates to be similar to the overall advanced group (systemic thrombolysis: 17%, CDT: 19%). The high bleeding rate of 12% in patients treated with AC alone also demonstrates the high risk profile of this selected PERT population. We also found that patients receiving advanced therapy had a higher rate of recurrent VTE compared with patients receiving AC alone (11% vs 5%, P = 0.049). We acknowledge that these findings contradict prior studies and warrants further exploration. It is possible that PERT patients represent a selected population, and among PERT patients those who receive advanced therapies are further selected. This is supported by the high 30-day mortality both in patients treated with advanced therapies and AC alone in this report (advanced: 29/110, 27% vs. AC alone: 29/234, 12%, P = 0.0019). While the overall differences in VTE and bleeding rates may differ with PE risk groups, further stratification of the data was not possible due to the low number of events in our current data.
Lastly, we found substantial variation in the mortality across institutions. While this could be due to treatments provided, we did not have sufficient power to control for comorbid illness or other potential confounders. However, by demonstrating variability in the treatment of PE and outcomes across PERT institutions, these findings highlight the need for more data on quality and standardization in the care of PE patients. The variability we observed, with mortality and the other measures we studied, is particularly interesting considering that all of the participating centers are large, academic medical centers. While variation from large academic hospitals to small community hospitals might be expected, the reason for this variation among teaching hospitals of similar size and PE expertise warrants further exploration.
Limitations
This study has several limitations. The study is based data obtained from the PERT National Consortium™ registry. Registry data, such as ours, may include biases based on the selective inclusion and retrospective data entry. Although sites are expected to enter data on all PERT patients, we are not able to confirm that a consecutive sample of patients were entered at each site.
Data was ascertained primarily by review of the electronic medical record, supplemented by interview of clinical providers to obtain any missing data or clarify uncertainties. Data quality therefore depends on the individual entering data, quality of the medical records, and reliability of clinical providers. This database includes only patients with acute PE who warranted a PERT activation. Most PERT guidelines focus on patients with intermediate- or high risk PE or complicated low-risk patients. This selection bias should be taken into account when comparing patient characteristics, treatment, and outcomes with the general patient population of acute PE. The goal of our study was to describe clinical practice and determine whether variation existed across PERT institutions, but we acknowledge that we are unable to differentiate how our findings are affected by differing practices between institutions or different risk profiles of PE patients. Furthermore, we are not able to evaluate the effects of the implementation of PERTs on treatments or outcomes in this report, as we do not have pre-PERT data in the registry.
All included centers are large tertiary academic centers located in US metropolitan areas with high volume EDs and all medical specialties represented, and the logistical framework and clinical experience to deliver all therapies available. However, our data do not currently allow us to answer detailed questions about why PERTs members chose the treatments they did. In particular, we are not able to definitively answer why some high-risk patients do not receive advanced therapy, while some low-risk PE patients do. Our data do suggest that some patients, while being in the ESC low-risk PE group, might still be very complicated due to comorbidities, etc. The concept of the “high-risk patient” with a low risk PE is novel, but logical. In the future, when the database has sufficient numbers, we hope to investigate how patients treated with advanced therapies differ from those treated with anticoagulation alone. Similarly, while the availability of an interventional specialist may have influenced the use of invasive therapies, all PERTs involved in this study (and all PERTs in general) have specialists capable of providing percutaneous and other interventions. It is therefore unlikely that the availability of interventional specialists influenced our outcomes. However, our data are not granular enough to know how the participation of particular specialists influenced the decision making at each institution. This requires future study. While being the largest multicenter report of PERT patients, this study is still limited by relatively small numbers. We have taken that into consideration by limiting the site-by-site analysis for institutions contributing more than 40 activations to the registry. Even so, we did not have sufficient statistical power to perform multivariable adjustment in our analysis of treatment and outcomes. The overall loss to follow up was 78/416 (19%), ranging across institutions from 8% (institution 4) to 33% (institution 6). This may introduce bias and should be taken into account when interpreting outcome data, especially data from institutions contributing smaller numbers of patients. The PERT Consortium registry is continuing to include centers and growing rapidly, so future analyses should adjust for patient comorbidity, PE severity, and other factors.
Conclusions
We provide the first national, multicenter analysis of patients cared for by eight US PERTs from the National PERT Consortium™ multicenter registry. The frequency of team activation, PE severity, treatments delivered, and 30-day mortality varies between institutions. This variation between expert sites highlights the challenges we face in the evolving field of acute PE.
Acknowledgments
The authors extent our acknowledgments to all members of the National PERT Consortium™ who have contributed with their hard work in the establishment of the National PERT Consortium™ multicenter registry. We especially thank all the people involved in entering data for their efforts. Data collection and analysis was approved by the Human Research Committee of Partners HealthCare (protocol number 2012P002257) as well as contributing sites.
Conflict of interest
The authors declare that there are no conflicts of interests.
Funding
This research received no grant funding from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007; 98: 756–764. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost 2005; 3: 1611–1617. [DOI] [PubMed] [Google Scholar]
- 3.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012; 379: 1835–1846. [DOI] [PubMed] [Google Scholar]
- 4.Schultz J, Andersen A, Kabrhel C, et al. Catheter-based therapies in acute pulmonary embolism. EuroIntervention 2017; 13: 1721–1727. [DOI] [PubMed] [Google Scholar]
- 5.Jaber WA, Fong PP, Weisz G, et al. Acute pulmonary embolism. J Am Coll Cardiol 2016; 67: 991–1002. [DOI] [PubMed] [Google Scholar]
- 6.Bloomer TL, El-Hayek GE, McDaniel MC, et al. Safety of catheter-directed thrombolysis for massive and submassive pulmonary embolism: Results of a multicenter registry and meta-analysis. Cathet Cardiovasc Intervent 2017; 89: 754–760. [DOI] [PubMed] [Google Scholar]
- 7.Merli GJ. Pulmonary embolism in 2017: How we got here and where are we going?. Tech Vasc Interv Radiol 2017; 20: 128–134. [DOI] [PubMed] [Google Scholar]
- 8.Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 2016; 150: 384–393. [DOI] [PubMed] [Google Scholar]
- 9.Barnes G, Giri J, Courtney DM, et al. Nuts and bolts of running a pulmonary embolism response team: results from an organizational survey of the National PERTTM Consortium members. Hosp Pract 2017; 45: 76–80. [DOI] [PubMed] [Google Scholar]
- 10.Zern EK, Young MN, Rosenfield K, et al. A Pulmonary Embolism Response Team: initial experiences and future directions. Expert Rev Cardiovasc Ther 2017; 15: 481–489. [DOI] [PubMed] [Google Scholar]
- 11.Barnes GD, Kabrhel C, Courtney DM, et al. Diversity in the pulmonary embolism response team model: an organizational survey of the national PERT Consortium members. Chest 2016; 150: 1414–1417. [DOI] [PubMed] [Google Scholar]
- 12.Galmer A, Weinberg I, Giri J, et al. The role of the pulmonary embolism response team: how to build one, who to include, scenarios, organization, and algorithms. Tech Vasc Interv Radiol 2017; 20: 216–223. [DOI] [PubMed] [Google Scholar]
- 13.Todoran TM, Giri J, Barnes GD, et al. Treatment of submassive and massive pulmonary embolism: a clinical practice survey from the second annual meeting of the Pulmonary Embolism Response Team Consortium. J Thromb Thrombolysis 2018; 46: 39–49. [DOI] [PubMed] [Google Scholar]
- 14.Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016; 133: 98–103. [DOI] [PubMed] [Google Scholar]
- 15.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–3073. [DOI] [PubMed] [Google Scholar]
- 16.Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 17.Schulman S, Angerås U, Bergqvist D, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010; 8: 202–204. [DOI] [PubMed] [Google Scholar]
- 18.Søgaard KK, Schmidt M, Pedersen L, et al. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation 2014; 130: 829–836. [DOI] [PubMed] [Google Scholar]
- 19.Mahar JH, Haddadin I, Sadana D, et al. A pulmonary embolism response team (PERT) approach: initial experience from the Cleveland Clinic. J Thromb Thrombolysis 2018; 46: 186–192. [DOI] [PubMed] [Google Scholar]
- 20.Elias A, Mallett S, Daoud-Elias M, et al. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open 2016; 6: e010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becattini C, Lankeit M, Masotti L, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 2016; 48(3): 780–786. [DOI] [PubMed] [Google Scholar]