Abstract
Introduction
We describe 5 patients who underwent operative treatment for arachnoid web (AW) and discuss the postoperative clinical outcome in each case. AW is an extremely rare disease that causes cord compression and syringomyelia in the thoracic spine. To date, 14 cases only of AW have been reported, and the effect of surgical intervention on clinical and radiologic outcomes is unknown.
Methods
Five patients who underwent surgical treatment for AW were retrospectively reviewed. The clinical outcomes were evaluated using the thoracic Japanese Orthopaedic Association (T-JOA) score. Preoperative and postoperative images were reviewed.
Results
All the patients presented with spastic gait and numbness in the lower extremities. Two patients also presented with bladder-bowel dysfunction (BBD). AW, or the so-called “scalpel” sign, was seen dorsally in the thoracic spine on magnetic resonance imaging in all the patients. Syringomyelia adjacent to the web was observed in 4 patients. Fenestration and web resection without instrumentation was performed in all the cases. Overall, significant improvement was seen in locomotion and the total T-JOA score postoperatively. However, numbness in the lower extremities improved in 2 patients but was unchanged in 3 cases. BBD was ameliorated in 1 patient but remained unchanged in the other patient.
Conclusions
Our experience suggests that surgical treatment, including the another patient and resection of the web, can correct the flow dynamics of cerebrospinal fluid and allow neurologic recovery, in particular locomotion, in patients with AW.
Keywords: arachnoid web, syringomyelia, thoracic spine, surgical treatment, scalpel sign
Introduction
The prevalence of myelopathy caused by a compressive lesion at the thoracic spine is low when compared with that at the cervical spine. Arachnoid web (AW) is an extremely rare lesion causing the compression of the thoracic spinal cord. AW has been shown to be a focal adhesive lesion that can result in the compression of the spinal cord concurrent with syringomyelia. Here, we report on five patients who developed myelopathy caused by AW.
Methods
The study was approved by the ethics review board at our institution. Five patients with a diagnosis of AW were included. Patients with idiopathic syringomyelia or arachnoiditis without a definitive diagnosis of AW were excluded. All the five patients underwent radiologic examinations, including magnetic resonance imaging (MRI) of the thoracic spine and computed tomography (CT) myelography, before surgery. In all the patients, laminectomy was performed at the level of the compressive lesion caused by AW along with separation of the AW membrane. All membranes and scar tissue forming the web were carefully removed as much as was possible without damaging the spinal cord. No patient had spinal fusion. Brain stimulating-muscle evoked potential (Br-MEP) testing was performed in all patients. The neurologic findings were assessed before and after surgery using the thoracic Japanese Orthopaedic Association (T-JOA) score1) (Table 1). MRI was performed postoperatively to confirm recovery of the cerebrospinal fluid (CSF) dynamics.
Table 1.
Scoring System for Thoracic Myelopathy (T-JOA Score).
| I Motor function in the lower extremities | ||
| 0: | Unable to stand up and walk by any means | |
| 0.5: | Able to stand up but unable to walk | |
| 1: | Unable to walk without a cane or other support on level ground | |
| 1.5: | Able to walk without support but with a clumsy gait | |
| 2: | Walks independently on level ground but needs support ascending stairs | |
| 2.5: | Walks independently when going upstairs, but needs support descending stairs | |
| 3: | Capable of walking fast but clumsily | |
| 4: | Normal | |
| II Sensory function | ||
| A. Trunk | ||
| 0: | Complete loss of touch and pain sensation | |
| 0.5: | 50% or less normal sensation and/or severe pain or numbness | |
| 1: | More than 60% normal sensation and/or moderate pain or numbness | |
| 1.5: | Slight subjective numbness without any objective sensory deficit | |
| 2: | Normal | |
| B. Lower extremities | ||
| Same as (A) | ||
| III Bladder function | ||
| 0: | Urinary retention and/or incontinence | |
| 1: | Sensation of incomplete voiding, dribbling, thin stream, and/or incontinence | |
| 2: | Urinary continence regression and/or pollakiuria | |
| 3: | Normal | |
T-JOA, Thoracic Japanese Orthopaedic Association
Results
Table 2 shows the patient demographics. Mean age was 70.6 (range 59-78) years. No patient had a history of spinal trauma. One patient (case 3) had previously undergone spinal surgery for a pyogenic epidural abscess before onset of the AW. The corresponding segment was T3-4 in 3 cases and T7 in 2 cases. Four patients had coexisting syringomyelia; the syrinx was located above the AW in 2 patients and below it in the other 2 cases. The patients were followed up for a mean of 24.8 (range 12-36) months. Mean preoperative T-JOA score was 6.9 (3.5-8.5) points. No change in Br-MEP in the lower extremities occurred during surgery in any patient. The mean postoperative T-JOA score was 8 (range 5.5-9.5) points, with a recovery rate of 32% (range 20%-40%) at the final visit. There were significant improvements in locomotion and in the total T-JOA scoreA (Fig. 1). However, the numbness in the lower extremities improved in 2 patients but did not change in 3. Bowel-bladder dysfunction was ameliorated in 1 patient but remained unchanged in the other.
Table 2.
Patient Demographics.
| Patient | Age (years)/Sex | History of trauma | History of spine surgery | Level of web | Presence of syrinx | Follow-up period (months) | Preoperative/Postoperative T-JOA score (Locomotion/trunk sensation/LE sensation/BBD) | Rate of recovery of T-JOA score |
|---|---|---|---|---|---|---|---|---|
| 1 | 73/M | No | No | T4 | Above | 24 | 8.5 (2.5/2/1/3)/9.5 (3/2/1.5/3) | 40% |
| 2 | 59/M | No | No | T3-4 | Above | 12 | 8 (3/1/1/3)/9 (4/1/1/3) | 33.3% |
| 3 | 71/F | No | Yes | T7 | Below | 28 | 3.5 (1/1/0.5/1)/5.5 (1.5/1/1/2) | 26.7% |
| 4 | 78/M | No | No | T3 | Below | 36 | 6 (1/2/1/2)/7 (2/2/1/2) | 20% |
| 5 | 72/F | No | No | T7 | No | 24 | 8.5 (2/2/1.5/3)/9.5 (3/2/1.5/3) | 40% |
Recovery rate of T-JOA score, (Post-T-JOA -Pre- T-JOA)×100 / (11 - Pre- T-JOA) (%). BBD, bladder-bowel dysfunction; F, female; LE, lower extremities; M, male; T-JOA, Thoracic Japanese Orthopaedic Association
Figure 1.
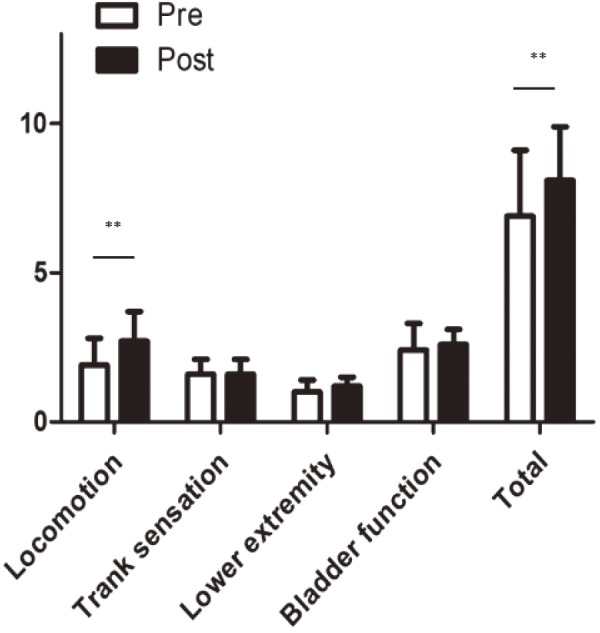
Preoperative and postoperative thoracic Japanese Orthopedic Association scores. There are significant differences between the preoperative and postoperative scores for locomotion and in the total score (**p<0.01).
Case 2
History and examination
A 59-year-old man presented with a spastic gait, a 1-year history of allodynia on the plantar surface of both feet, and bilateral numbness of the lower extremities without intermittent claudication. He had previously received chiropractic treatment but gradually developed difficulty walking without a cane. On examination, he had slight muscle weakness in the proximal lower extremities and hypesthesia on the left trunk below T7 and in the left lower extremity. The deep tendon reflexes were almost normal in the lower extremities bilaterally. The T-JOA score was 8 points (3/1/1/3).
Imaging findings
An MRI of the cervical and thoracic spine showed extensive syringomyelia at the C6-T4 levels. The scalpel sign was observed below the syrinx (Fig. 2A). These findings were consistent with those on the CT myelography (Fig. 2B). There was no filling defect in the spinal cord.
Figure 2.
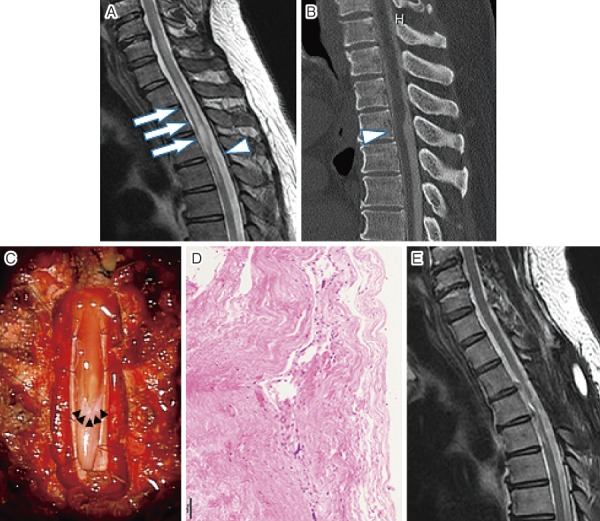
(A) Preoperative mid-sagittal view on T2-weighted magnetic resonance image showing the scalpel sign at T7 level (arrow head) and diffuse syringomyelia at C6-T4 levels (arrow). (B) Preoperative computed tomography myelogram showing flattering and anterior migration of the spinal cord (arrow head). (C) Intraoperative photograph showing a transverse band adherent to the posterior portion of the spinal cord (arrow head). (D) Hematoxylin and eosin staining of the web. (E) A postoperative T2-weighted magnetic resonance image showing a reduction in the size of the syrinx and adequate decompression of the spinal cord.
Surgical treatment
The patient underwent laminectomy at the T2-5 levels and resection of the AW. After durotomy, the AW was exposed and adhesion of the membrane was identified (Fig. 2C). The lesion could be separated and was resected atraumatically from the spinal cord. Histologic examination revealed that the web consisted of fibrous tissue that had accumulated in layers. No lymphocytes or dyskaryotic cells were detected (Fig. 2D).
Postoperative course
The allodynia in the lower extremities had gradually improved by 3 months after surgery. The numbness in the lower extremities remained but had decreased. The gait disturbance disappeared. The T-JOA score was 9 points (4/1/1/3) at the final follow-up visit. The postoperative MRI showed that the syrinx had disappeared and the spinal cord had shifted posteriorly (Fig. 2E).
Case 5
History and examination
A 72-year-old woman presented with gait disturbance, bilateral numbness of the lower extremities without intermittent claudication, and mild low back pain. She also had pain in the left lower extremity attributable to radiculopathy caused by degenerative listhesis at L4. On examination, she had full strength and normal sensation in both of the lower extremities. The deep tendon reflexes were increased in the lower extremities bilaterally. The T-JOA score was 8.5 points (2/2/1.5/3).
Imaging findings
An MRI of the thoracic spine revealed the scalpel sign at the T7 level (Fig. 3A, 3B). There was no syringomyelia adjacent to the AW in this patient.
Figure 3.
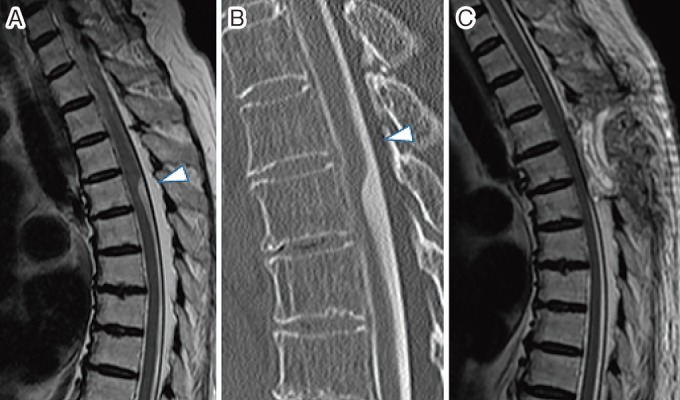
(A) Preoperative mid-sagittal view on T2-weighted magnetic resonance image showing the scalpel sign at T7 level (arrow head) without a syrinx. (B) Preoperative computed tomography myelogram demonstrates flattening and anterior migration of the spinal cord (arrow head) without a filling lesion in the cord above the web. (C) Postoperative T2-weighted magnetic resonance image showing disappearance of the scalpel sign14).
Surgical treatment
The patient underwent laminectomy at the T6-8 levels and separation of the AW as well as posterior lumbar interbody fusion at L4-5. After a midline durotomy at T7, a membrane was observed to be slightly adherent to the cord in the most compressed region. The membrane was removed easily and adequate posterior migration of the spinal cord was seen.
Postoperative course
Although the patient's numbness in the lower extremities was unchanged, she was able to walk without a cane by 1 year after surgery; by this time, the T-JOA score had improved to 9.5 points (3/2/1.5/3). The postoperative MRI showed that the scalpel sign had disappeared and that the spinal cord was shifted posteriorly (Fig. 3C).
Discussion
Since Klekamp et al.2) first reported arachnoid scarring as a cause of syringomyelia, AW3,4) has been described as intradural transverse extramedullary bands of arachnoid tissue connecting the dorsal surface of the spinal cord. Although the prevalence of this pathology remains unclear, it is considered to be one of the arachnopathies that also include the more common arachnoid cysts. It has been suggested that AW is an arachnoid cyst that is in the process of forming or collapsing5). Given the propensity for arachnoid cysts to form dorsal to the spinal cord, Perret et al.6) suggested that these cysts derive from the diverticula of the septum posticum. However, this hypothesis would not account for the presence of ventral arachnoid cysts. Therefore, the etiology of the arachnopathies remains controversial.
AW may occur secondary to the disruption or inflammation of the arachnoid tissue. To date, there have been 7 reports of AW caused by inflammation following trauma, prior surgery, or hemorrhage3-5,7,8). A preceding spinal infection might have caused the AW in patient 3 in this series. The focal abnormality of the arachnoid membrane alters the CSF flow dynamics, resulting in the formation of syringomyelia. Like AW, spinal cord herniation (SH) is also an uncommon entity that affects the thoracic spinal cord. The pathologic states of SH are focal ventral or, less commonly, lateral protrusion of part of the cord through the dural defect. Randall et al.9) reviewed the findings on MRI and CT myelography for both AW and SH and found that the scalpel sign was positive in all the patients with AW and that a C-shaped deformity was present in 5 of 6 patients with SH. They also mentioned that the ventral subarachnoid space was preserved in all the patients with AW. The findings reported by Randall et al. and our present results indicate that the presence of the scalpel sign and the preservation of the ventral CSF space are strongly suggestive of AW. It is very important for spine surgeons to be able to differentially diagnose arachnoid cyst, AW, and SH (Table 3).
Table 3.
Radiologic Characteristics of Arachnoid Web, Arachnoid Cyst, and Spinal Cord Herniation.
| Entity | Presence of scalpel sign | Presence of C-shaped deformity | Preservation of ventral CSF space | Presence of cysts | Presence of syrinx |
|---|---|---|---|---|---|
| Arachnoid web | Yes | No | Yes | No | Mostly yes |
| Arachnoid cyst | No | No | Yes | Yes | Sometimes yes |
| Spinal cord herniation | No | Yes | No | No | Mostly no |
CSF, cerebrospinal fluid
To the best of our knowledge, there have been 19 documented cases of symptomatic AW treated with surgical resection3-5,7,8), including the cases in the present series (Table 4). Our case series includes the largest number of patients to date. A syrinx occurred adjacent to the AW in 16 (84.2%) of these 19 patients. AW is more closely associated with the onset of syringomyelia than arachnoid cyst (in approximately one third of cases)10). Systolic pressure drives the CSF from high-pressure regions to low-pressure regions. If there is an obstruction to the flow of the CSF, a syrinx may form from the transmission of CSF pressure through the spinal cord or by the reflection of the pressure onto the cord. Interestingly, in the 16 cases reported to have syringomyelia, the syrinx existed immediately rostral to the AW in 8 cases and immediately caudal to the AW in 8 cases; this suggests that an obstruction of the AW could lead to a 1-way valve mechanism either in the rostral or caudal direction. However, the direction of this 1-way mechanism has yet to be determined. Viswanathan et al.11) reported that neurologic recovery in patients with arachnoid cyst was not different between those without and with syringomyelia. However, in our series, 1 patient (case 5) had no syrinx and her preoperative neurologic symptoms tended to be milder than in the patients with syringomyelia. It is possible that early-stage AW that does not result in the development of a syrinx adjacent to the epicenter may not impair neurologic function as badly as AW with syringomyelia.
Table 4.
Summary of the Patient Demographics, Pathology, and Treatment in the 19 Cases Reported in the Literature to Date.
| Patient | Age (years) /sex | History of trauma | History of spine surgery | Level of web | Location of syrinx |
|---|---|---|---|---|---|
| 1 | 73/M | No | No | T4 | Above |
| 2 | 59/M | No | No | T3-4 | Above |
| 3 | 71/F | No | Yes | T7 | Below |
| 4 | 78/M | No | No | T3 | Below |
| 5 | 72/F | No | No | T7 | No |
| 6 | 54/M | No | Yes | T4 | Below |
| 7 | 45/M | No | No | T7 | Below |
| 8 | 34/M | No | Yes | T6 | Below |
| 9 | 73/M | No | No | T1 | Below |
| 10 | 39/M | No | No | T6 | Above |
| 11 | 43/M | Yes | No | T4 | Above |
| 12 | 51/F | No | No | T4 | Above |
| 13 | 56/F | No | No | T3 | Above |
| 14 | 56/F | No | No | T6 | Below |
| 15 | 45/M | Yes | Yes | T5 | No |
| 16 | 56/M | No | No | T7 | No |
| 17 | 45/M | No | No | T3 | Above |
| 18 | 63/F | Yes | No | T3 | Above |
| 19 | 62/F | No | Yes | T8 | Below |
M, male; F, female; cases 1-5 are presented in the present report
In each documented case, the AW has occurred dorsal to the spinal cord at the upper or middle thoracic level. Hakky et al.12) reviewed the CT myelograms for 7 patients with AW and suggested that the web might be derived from a derangement of the septum posticum in the thoracic spine. Freund et al.13) evaluated the flow of CSF in the whole spine using an optimized MRI protocol and concluded that its velocity was highest in the thoracic spine. These findings suggest that the flow of CSF may be turbulent in the thoracic spine, resulting in an AW.
The previous reports and the findings of our present series indicate that surgical treatment, including fenestration and resection of the web, is the optimal method for amelioration of neurologic impairment in patients with AW. Locomotion improved by an average of 0.8 ± 0.3 points and the numbness in the lower extremities and bladder-bowel dysfunction resolved partially in our patients. Furthermore, postoperative MRI scans confirmed the disappearance of the scalpel sign and syrinx adjacent to the web. Therefore, it is suggested that surgical separation and resection of the web may improve the CSF flow dynamics and reduce the syrinx and compression of the spinal cord, particularly the dorsal cord. This could explain why the gait disturbance resulting from the posterior column disorder improved in all our cases. However, there has been no report of the recurrence of AW, possibly because there have been no long-term follow-up studies of patients with AW. Careful radiologic follow-up is needed in these patients to check for the recurrence of AW.
AW is an extremely rare disease that arises dorsally in the thoracic spine and causes severe cord compression. Further radiologic examination of the thoracic spine with inclusion of AW in the differential diagnosis should be considered in a patient who presents with gait disturbance and numbness of the lower extremities without intermittent claudication.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Author Contributions: Takashi Hirai; study design, data acquisition, data analysis, drafting the manuscript
Takashi Taniyama: data acquisition, interpretation of the data, critical revision of the manuscript
Toshitaka Yoshii: data acquisition, interpretation of the data, critical revision of the manuscript
Koichi Mizuno: data acquisition, data analysis
Mikio Okamoto: data acquisition, data analysis
Hiroyuki Inose: data acquisition, data analysis
Masato Yuasa: data acquisition, data analysis
Kazuyuki Otani: data acquisition and critical revision of the manuscript
Shigeo Shindo: data acquisition and critical revision of the manuscript
Osamu Nakai: data acquisition and critical revision of the manuscript
Atsushi Okawa: study design, data acquisition, interpretation of the data, critical revision of the manuscript
References
- 1.Tomita K, Kawahara N, Baba H, et al. Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine (Phila Pa 1976). 1990;15(11):1114-20. [DOI] [PubMed] [Google Scholar]
- 2.Klekamp J, Batzdorf U, Samii M, et al. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86(2):233-40. [DOI] [PubMed] [Google Scholar]
- 3.Paramore CG. Dorsal arachnoid web with spinal cord compression: variant of an arachnoid cyst? Report of two cases. J Neurosurg. 2000;93(2 Suppl):287-90. [DOI] [PubMed] [Google Scholar]
- 4.Brodbelt AR, Stoodley MA. Syringomyelia and the arachnoid web. Acta Neurochir (Wien). 2003;145(8):707-11. [DOI] [PubMed] [Google Scholar]
- 5.Reardon MA, Raghavan P, Carpenter-Bailey K, et al. Dorsal thoracic arachnoid web and the “scalpel sign”: a distinct clinical-radiologic entity. AJNR Am J Neuroradiol. 2013;34(5):1104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perret G, Green D, Keller J. Diagnosis and treatment of intradural arachnoid cysts of the thoracic spine. Radiology. 1962;79(3):425-9. [DOI] [PubMed] [Google Scholar]
- 7.Sridharan A, Heilman CB. Transverse dorsal arachnoid web and syringomyelia: case report. Neurosurgery. 2009;65(1):E216-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D, Papavassiliou E. Spinal intradural arachnoid webs causing spinal cord compression with inconclusive preoperative imaging: a report of 3 cases and a review of the literature. World Neurosurg. 2017;99:251-8. [DOI] [PubMed] [Google Scholar]
- 9.Schultz R, Steven A, Wessell A, et al. Differentiation of idiopathic spinal cord herniation from dorsal arachnoid webs on MRI and CT myelography. J Neurosurg Spine. 2017;26(6):754-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang MY, Levi AD, Green BA. Intradural spinal arachnoid cysts in adults. Surg Neurol. 2003;60(1):49-55. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan VK, Manoharan SR, Do H, et al. Clinical and radiologic outcomes after fenestration and partial wall excision of idiopathic intradural spinal arachnoid cysts presenting with myelopathy. World Neurosurg. 2017;105:213-22. [DOI] [PubMed] [Google Scholar]
- 12.Hakky MM, Justaniah AI, David C, et al. The neuroimaging spectrum of septum posticum derangement and associated thoracic myelopathy. J Neuroimaging. 2015;25(5):818-23. [DOI] [PubMed] [Google Scholar]
- 13.Freund M, Adwan M, Kooijman H, et al. [Measurement of CSF flow in the spinal canal using MRI with an optimized MRI protocol: experimental and clinical studies]. Rofo. 2001;173(4):306-14. German. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto M, Hirai T, Yoshii Y, et al. Thoracic Arachnoidweb improved with surgical treatment: three case reports. Kantoseikeisaigaigekazasshi. 2019 in press [Google Scholar]


