Abstract
Intervertebral disc degeneration is a well-known cause of disability, the result of which includes neck and back pain with associated mobility limitations. The purpose of this article is to provide an overview of the known molecular mechanisms through which intervertebral disc degeneration occurs as a result of complex interactions of exogenous and endogenous stressors. This review will focus on some of the identified molecular changes leading to the deterioration of the extracellular matrix of both the annulus fibrosus and nucleus pulposus. In addition, we will provide a summation of our current knowledge supporting the role of associated DNA and intracellular damage, cellular senescence's catabolic effects, oxidative stress, and the cell's inappropriate response to damage in contributing to intervertebral disc degeneration. Our current understanding of the molecular mechanisms through which intervertebral disc degeneration occurs provides us with abundant insight into how physical and chemical changes exacerbate the degenerative process of the entire spine. Furthermore, we will describe some of the related molecular targets and therapies that may contribute to intervertebral repair and regeneration.
Keywords: Intervertebral disc, nucleus pulposus, annulus fibrosus, degeneration, cellular senescence, oxidative stress, inflammation, DNA damage
Introduction
Over the past century, life expectancy has continued to increase, with an estimated 0.5 billion people worldwide aged 65 or older, and is projected to reach 1.5 billion people by 20501). It is well documented that low back and neck pain increase with age, and are the 1st and 4th leading causes of disability, respectively2-4). Back pain is one of the most common orthopaedic conditions, and it accounts for 149 million missed worked days annually with an estimated loss of $90 billion in the United States, secondary to related disability5). Specifically, intervertebral disc degeneration (IDD) plays a substantial role in the generation of back pain, involving more than 50% of all cases6-8). In the elderly, decreased mobility is a confirmed predictor of loss of independence and increase in mortality. This makes the preservation and understanding the degeneration of healthy joints, especially intervertebral discs (IVDs), vital in our aging population9-11).
IDD refers specifically to the functional as well as the structural failure of the disc related to its cellular pathogenesis and extracellular matrix (ECM) modifications12). Causes of disc degeneration include aging, injury, genetics, and environmental factors such as smoking, or a combination13-19). IDD is not exclusive to the older population, although the aged population and cases with degenerated disc appear to share similar changes18-22). The IVD is one of the first tissues to undergo degeneration in adults, with an average onset in the second decade of life and is known to be influenced by a combination of genetic, biological, aging, and physical chemical changes20,21,23). The exact pathophysiology of degeneration has not yet been completely delineated, but this review will focus on some of the known cellular changes and molecular pathways leading to IDD.
Characteristics of IVD
IVD are situated between two cartilaginous end plates of adjacent vertebra of the spine. They help in constructing a polyaxial cartilaginous joint that allows for flexibility, while also providing support24,25). The IVD is made of two major components: an annulus fibrosus (AF) and a nucleus pulposus (NP). The AF is composed of highly organized rings known as lamella, rich in collagen type I that surround the NP. These collagen fibers of the AF arranged in alternating angles of approximately 30-60 degrees serve to restrain the NP's circumferential stress during bending and twisting, while preventing lateral displacement and collapse of the NP24,26). The NP that is centrally located, is composed of approximately 80% water caused by the osmotic gradient of proteoglycans, primarily aggrecan, which consists of highly anionic glycosaminoglycan side chains of chondroitin and keratin sulfate. The NP also contains a loose and randomly arranged framework of sparse collagen type II and elastin fibers, which in combination with the proteoglycan, serves as a primary shock absorber defusing compressive stresses into the end plates and the annulus fibrosus24,27). In addition, the cartilage end plates, composed primarily of cells with a morphology similar to that of chondrocytes that produce a hyaline matrix, serve as an interface between the adjacent vertebral body and IVD28). As discs are predominantly avascular, the cartilaginous end plates are particularly important for nutrient and metabolite delivery to the disc by diffusion through its rich blood supply. This tenuous perfusion and low cellularity makes the disc susceptible to injury, in addition to the limited repair and accumulation of dysfunctional tissue29,30).
Degenerative changes lead to an altered spinal mechanical function with loss of elasticity and an increased stiffness31). Maintaining stability in the multiaxial motion of the spine is a complex interaction between all components of the spine working together counteracting compressive and tensile stress32-39). Degenerative changes of the spine that exacerbate the IDD process include facet cartilage erosion, weakening of supportive ligaments such as posterior and anterior spinal ligaments, and physiological muscle atrophy such as fatty infiltration40-43). Cells isolated from annular-puncture induced degenerative discs have shown unfavorable altered responses to mechanical loading and exaggerated response to inflammatory stimulus44). Studies in rats have indicated an increased IDD when they are imposed to an upright stance, which presumably alters the disc loading45). Other analyses have also made associations with long-term loading leading to loss of spinal mobility and decrease in disc height46-49). This loss of anatomical structure is the end result of damage-induced apoptosis, cellular senescence, and pathologic alterations to metabolism with the subsequent loss of standard cellular function and ECM structural support50-53).
It is known that the ECM of the NP and AF are vital to the biomechanical function and physiological state of the IVD54,55). The ECM of the NP contains only approximately 1% cells, based on its total volume. These cells produce structural matrix proteins, cytokines, growth factors, and proteases. All these products serve to maintain the balance between ECM production and degradation, while preserving biomechanical function54,55). Progressive proteoglycan loss with coinciding changes of low oxygen tension, free radical formation, changes in pH, and increased activity of aberrant proteolytic enzymes, leads to loss of disc height and compressive resistance. These changes result in a progressive disruption of the spinal motion leading to spinal instability and mechanical stress, compounding the degenerative process56,57).
Pathology of IDD
IDD has been defined by the loss of biologic structural support and function through the accumulation of degenerated molecules leading to inappropriate cellular reactions that exacerbate the pathogenesis58). These changes lead to a fibrous disc with dehydration of the NP, loss of disc height, and accumulation of granular debris. In addition, there is neovascularization in the peripheral AF and an increase in the number and size of fissures20,59,60). The cartilaginous end plates also undergo ossification and thinning with subsequent microfractures, bone sclerosis, and reduction in the blood supply. The reduction in perfusion followed by a decrease in nutrient supply and accumulation of cellular waste, all lead to an increase in the acidic environment that can negatively affect cell function61-65). The molecular mechanisms contributing to IDD that have been elaborated below result in cellular dysfunction through the accumulation of damaged proteins, altered intercellular communication, deregulated nutrient sensing, mitochondrial dysfunction, DNA damage, and interruption of tissue regenerative capacity with loss of progenitor cells (Fig. 1)66-70).
Figure 1.
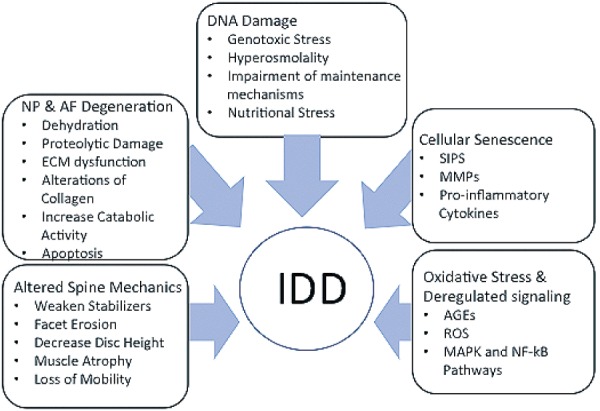
Factors Contributing to Intervertebral Disc Degeneration.
Demonstration of the multifactorial contributions to Intervertebral Disc Degeneration (IDD) including alterations of spine mechanics, Nucleus Pulposus (NP) & Annulus Fibrosus (AF) degeneration, DNA damage, Cellular Senescence and Oxidative Stress & Deregulated signaling. Extracellular Matrix (ECM), Stress-induced Premature Senescence (SIPS), Matrix Metalloproteinases (MMPs), Advanced Glycation End Productions (AGEs), and Reactive Oxygen Species (ROS).
Degeneration of ECM in the NP
Typical ECM molecules in NP are proteoglycan aggregates, consisting of core protein, link protein, sulfated glycosaminoglycan (S-GAG), and hyaluronan, all encased within a collagenous fiber network. This ECM provides higher osmotic pressure to create swelling pressure capable of withstanding compressive loading stresses71). As discs degenerate and simultaneously dehydrate, the majority of the matrix evolves with decreased amount of GAG, aggrecan and elastin, and increased amounts of collagen and collagen crosslinking, fragmented aggrecan, and advanced glycation end productions (AGEs)73). This decreased amount of GAG chain lengths and link protein levels, while hyaluronan levels increase, are all thought to be a result of proteolytic and glycolytic damage71-73). Versican is another hyaluronan-binding protein that undergoes deterioration with the degeneration of discs. These non-aggregating proteoglycans appear to have a reduced functional ability compared to intact aggregates based on their size, charge density, spatial rigidity, and matrix interactions71-76). Syndecan-4 is a transmembrane heparan sulfate proteoglycan that also plays a significant role in IDD through intracellular signaling77-79). Specifically, dysregulated activities of syndecan-4 mediate matrix and aggrecan degradation by a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5) function and matrix metalloproteinases (MMPs) expression e.g., MMP377-79). The small leucine-rich repeat family of proteoglycans (SLRPs) typically play a significant role in structural support of the ECM. But with degeneration, SLRPs appear to undergo proteolytic damage leading to a loss of their GAG content, negatively affecting the structural support of the ECM71). Specifically, biglycan and decorin, dermatan sulfate proteoglycans, interact with collagen type IV and type I/II respectively71). Others include disc collagen fibril associated SLRPs, feratin sulfate-containing fibromodulin and lumicans, which decrease and increase with age respectively, are thought to have an effect on disc matrix structure71,80). As mentioned above, discs contain a rich collagen network containing 80% of Type I and II, and 10-20% of type IV71). This collagen structure also undergoes proteolytic damage from dysregulated collagenase activity, resulting in weakened mechanical strength and the formation of non-enzymatic crosslinks between basic amino acids of collagen and reducing sugars81,82). As elaborated below, this proteolytic damage results in increased oxidative stress through AGEs, which increase throughout the disc with degeneration impairing collagen fibril formation83,84). To further support this concept, oxidative stress has been shown to decrease disc elasticity and alter the formation of secondary and tertiary structure of collagen molecules in mice models31,83-85). This increases their susceptibility to cleavage by MMPs, resulting in a deterioration of the biomechanical strength and structural integrity31).
Degeneration of ECM in the AF
Although the exact mechanism is not completely delineated, it is assumed that mechanical load, oxidative stress, genetics, inflammation, and DNA damage also contribute to a rupture of the AF through apoptosis and altered integrity with herniation of the NP86,87). Homeostasis in ECM, through regulation of catabolic and anabolic functions, have been shown to play an essential role in maintaining the integrity of the AF structure88). Specifically, with disc degeneration, this homeostasis is disrupted with excessive matrix catabolic activity89,90). Two apoptotic pathways in mammalian cells, the mitochondrial pathway and Fas/FasLigand pathway have both been shown to be involved in AF cell apoptosis regulation91-93). Islet amyloid polypeptide (IAPP), identical in composition to amyloid protein, participates in the regulation of glucose metabolism, reactive oxygen species, apoptosis, and inflammation94-98). It has been shown that the expression of IAPP, the calcitonin receptor, and receptor activity modifying protein decrease in AF cells with IDD99). This decrease in IAPP induces an increase in reactive oxygen species and intracellular calcium concentration along with a decrease in MMPs, all leading to cellular death99). Human samples, mouse models, and AF culture experiments have demonstrated that mechanical overload-induced IDD is mediated through the mitochondrial apoptotic pathway in AF cells92). In addition, abnormal loading on intervertebral disc resulted in a thickening and stiffening of collagen fibrils of the AF at the microscale and alteration of the collagen fibrils at the nanoscale. These changes likely lead to a change in the mechanical and physiological homeostasis100).
DNA Damage
Proteins and other macromolecules can be degraded and replaced; however, this is not the case with DNA66). DNA requires repair mechanisms, and despite these rigorous mechanisms, cells still accumulate damaged DNA over time. It has been shown that inherited defects in genome maintenance mechanisms can lead to a variety of diseases with accelerated degeneration67). For example, DNA repair-deficient Ercc1/D mice exhibit early onset of key IDD features, including loss of matrix proteoglycan, reduction in disc height, and increased cellular senescence57,69). DNA damage as a cause of IDD is further supported by exposure to genotoxic stress in humans and mice, including ionizing radiation and tobacco smoking that accelerate disc degeneration101-104).
Hyperosmolality has also been shown to induce DNA damage, through activation of the ATM/p53/p21WAF1 pathway leading to the hypophosphorylation of the pRb protein and cell cycle arrest in the G1 phase of the cell cycle105). An increase in osmolality has been shown to lead to chromatin changes and DNA damage106). Although it is still unclear what level of hyperosmolality in NP is needed to induce DNA damage, cells of the NP are exposed to hyperosmolality levels up to 500 mOsm/kg H2O in vivo compared to < 300 mOsm/kg H2O in the majority of other tissues105).
Nutritional stress is another factor that can affect disc tissue leading to degeneration and cellular damage. Because discs are mostly avascular, the nutritional environment resides narrowly above the cellular requirements in the NP with low oxygen and glucose107-109). In addition, the disc maintains an acidic environment with high concentrations of lactate110). It has been shown that low O2 and low pH conditions can cause DNA damage and reduce proteoglycan and collagen synthesis111). This extreme environment puts the disc at risk to any additional stresses112,113).
Cellular Senescence
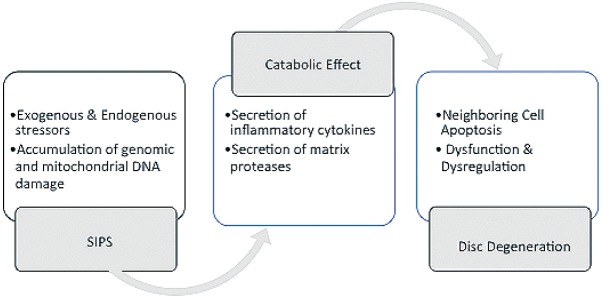
Cellular senescence, an important mechanism for the limiting of proliferation of potential cancer cells, has been described by cessation of cell proliferation due to the critical shortening of telomere length following successive replicative cell cycles114,115). Another type of cell senescence, stress-induced premature senescence (SIPS), results from accumulation of genomic and mitochondrial DNA damage. In addition, SIPS cells also acquire a senescence associated secretory phenotype leading to high amounts of secreted inflammatory cytokines and matrix proteinases causing a catabolic effect on neighboring cells and on the ECM, promoting degeneration (Fig. 2)116-120). This pathologic disc matrix process is further supported by the observed amount of senescent cells which have been measured by an increased expression of senescent markers including senescence-associated b-galactosidase, p16INK4A, and decreased telomere length. In addition, p16INK4A has been shown to have a positive correlation with the expression of matrix metalloproteases e.g., MMP-13 and ADAMTs-5121-125). Further supporting DNA impairment can lead to disc cellular senescence is the observation of elevated cellular senescence in the disc of DNA repair-deficient Erccl_/D genotoxin exposed mice14,101,102). Also, further supporting SIPS as a potential cause of disc degeneration are in vitro cell culture studies using H2O2 to simulate oxidative stress leading to DNA damage with a transformed, catabolic phenotype126-128). This leads to increased matrix degradation with high levels of MMPs and pro-inflammatory cytokines, with IL-1 as a predominant cytokine in the pathogenesis of IDD126-128).
Figure 2.
Stress-Induced Premature Senescence Catabolic Effects.
Illustrating the stepwise formation and subsequent catabolic effects of stress-induced premature senescence (SIPS).
Oxidative Stress and Deregulated Signaling
Oxidative inflammation is thought to be one of the main causes of molecular damage through exogenous and endogenous stressors (Fig. 3)103,104). The advanced glycation end productions (AGEs) including pentosidine and carboxymethl-lysine, further support oxidative damage as a source of molecular damage80,129). In the example of pentosidine, which cross links collagen molecules, there is evidence to suggest that it may play a role in increased collagen stiffness and fragility affecting the biomechanics80,82,129,130). Also, oxidative post-translational modifications, i.e. protein carboxylation, have also shown an increase in protein fragmentation and aggregation leading to increased disc stiffness in a mice model21). Although disc cells reside in relative low oxygen tension environments, reactive oxygen species (ROS) are still generated through oxidative phosphorylation131). As disc degeneration and AF develop fissures with associated neovascularization, this leads to an increase in oxygen tension on otherwise hypoxic cells, further supporting the oxidative stress on the disc environment131).
Figure 3.
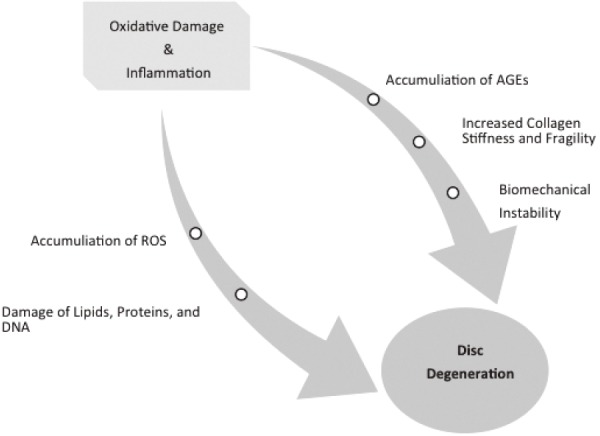
Pathways Contributing to Intervertebral Disc Degeneration.
Demonstrating two pathways - inflammation and oxidation - that contribute to intervertebral disc degeneration. Reactive Oxygen Species (ROS), Advanced Glycation End Productions (AGEs).
ROS is detrimental to the structural and functional homeostasis of the disc by causing the damage of lipids, DNA, and proteins132). Hydrogen peroxide, identified in human NP tissue, along with peroxisomes detected in AF cells in vitro, further support discs cells as ROS generators133,134). With mitochondrion dysfunction as a known source of excessive ROS production, the role of mitochondrion-dependent ROS has been described in various disc cells including human and rat NP and AF cells135-139). And more specifically, excessive ROS production has been reported in degenerative discs of rats140). Several studies have shown hydrogen peroxide to down regulate the expression of collagen type II and aggrecan in both human and rat disc cells.126,141-145) In addition, pro-inflammatory cytokines leading to ROS overproduction have been shown to suppress matrix synthesis and increase the expression of matrix degradation proteases in human and rat disc cells126,137,141,142,145,146). ROS is also known to form positive feedback loops that enhance ROS production in disc cells126,136,141,145-150).
Inappropriate and deregulated signaling (e.g. NF-κB and MAPK pathway) in addition to abnormal variations in cell fate (e.g., cellular senescence), dysregulated nutrient sensing, and mitochondrial dysfunction has also been reported in deteriorating tissues including IDD70). Elevated levels of the pro-inflammatory cytokine TNF-α, matrix proteoglycan degradative products, MMPs-including MMP-3, ADAMTS-5, have also been reported in degenerative disc suggesting an imbalance of matrix homeostasis151-154). Other phenotypic and functional changes that likely result from irregular responses to damage include depletion of disc matric proteoglycan, tissue dehydration, and altered disc load distribution. These distorted conditions lead to elevated necrosis, apoptosis, and senescence112,121,157,158).
Potential sources of oxidative stress and DNA damage include inflammation and high glucose induced stress, as in diabetes159). NF-kB signaling is known to play a critical role in a cell's response to inflammation and damage, and an increase in its activity has been connected to IDD with accumulated oxidative stress129,139,159,160). Pharmacologic and genetic systemic inhibition of NF-kB activity has been shown to reduce associated IDD in a mouse model141). Symptomatic discs have been shown to have higher levels of pro-inflammatory cytokines, TNF-α, IL1β, IL-6, and IL-8, which are considered be associated with the NF-kB pathway127,128,156,162,163). Mitogen-Activated Protein Kinases (MAPKs), a family of signal transduction pathways, allow cells to respond to extracellular inputs, including inflammatory cytokines and environmental stress164,165). Specifically, the expression of p38 MAPK, a subfamily of MAPK, has been described in senescent AF cells166). Many components of the catabolic process (ex. MMPs, ADAMTSs, COX-2, PEGE2, iNOS, etc.) are dually regulated by MAPK and NF-kB, showing the overlap between the two signaling pathways155). As mentioned above, pro-inflammatory cytokines (IL-1β and TNF-α) activate MAPK pathways leading to catabolic molecules such as ADAMTs-4, MMP-3, and syndecan-4146,167-169). This relationship may represent a tool to ease disc degeneration by blocking MAPK activation either with synesthetic or natural compounds such as glucosamine (Fig. 4)146,170). Another characteristic feature of disc degeneration is the formation of cell proliferation clusters in damaged areas, which is thought to be partly due to the overexpression of growth factor and receptors171,172). Indicating another role of MAPK in disc denegation is the fact that growth factors such as PDGF, IGF-1, and bFGF stimulate cell proliferation through extracellular signal-related kinases, another subfamily of MAPKs173,174).
Figure 4.
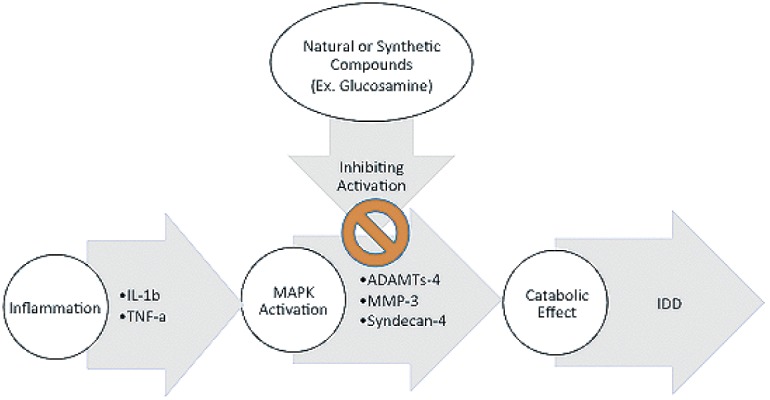
Activation of MAPKs Pathway.
Illustrating one of the roles that inflammation and MAPK pathway play in contributing to intervertebral disc degeneration (IDD), and a possible method of hindering IDD by preventing the activation of the MAPK pathway.
Conclusion
The distinctive disc function that maintains stability and motion of a mechanically loaded structure in nutrient-poor, acidic, and hypoxic environment, offers an extraordinary opportunity to discover novel disc degeneration mechanisms. As noted above, disc degeneration is a systemic process that does not occur in isolation and is influenced by the degeneration of adjacent spinal structures and systemic factors. Therefore, degeneration research of the whole spine is an imperative for the future direction of spine degeneration and regeneration research. And more specifically, therapeutic interventions will need to target the early phases of disc and spine degeneration prior to functional failure. Several recent studies have strengthened our understanding of intervertebral repair and regeneration. The in vivo performance of an acellular disc-like angle ply structure that mimics the native intervertebral disc was studied in a rat tail model, and it was noteworthy that native cells were able to infiltrate the angle ply structure while promoting tissue formation and restoring some biomechanical behaviors175). Mesenchymal stem cell injections, another way to induce regeneration, have been shown to increase the amounts of GAG accumulation when injected early into injured rat intervertebral discs176). Inorganic polysphates have been shown to promote proteoglycan accumulation in NP cells, even under hypoxic conditions177). In addition, bone morphogenetic protein 2 (BMP-2) and BMP-7 have been shown to stimulate the NP to produce aggrecan and collagen II in vitro and in organ culture models178). And as mentioned above, NF-kB inhibition results in the largest reduction of IL-1β, a pro-inflammatory catabolic cytokine179). With disc degeneration being a significant risk factor for associated pain and disability, the prevalence will continue to rise with our growing and aging population. This should help stimulate further research to develop safe and effective treatments for intervertebral degeneration and pathology.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Author Contributions: Sean M. Rider wrote and prepared the manuscript. All authors have read, reviewed, and approved the article.
References
- 1.The Department of Economic and Social Affairs of the United Nations Secretariat. World Population Prospects The 2015 Revision. Available from: http://esa.un.org/unpd/wpp/Publications/FIles/Key_Findings_WPP_2015.pdf
- 2.Anderson GBJ, Bouchard J, Bozic KJ, et al. The Burden of Musculoskeletal Diseases in the United States, 1st ed. 2008. Available from: http://www.boneandjointburden.org/ docs/The Burden of Musculoskeletal Diseases in the United States (BMUS) 1st Edition (2008).pdf.
- 3.Derby R, Lee SH, Kim BJ. Discography. Interventional Spine: An algorithmic approach. Philadelphia: Elsevier; 2008. 291-302 p. [Google Scholar]
- 4.Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou X, Pietrodon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29(1):79-86. [DOI] [PubMed] [Google Scholar]
- 6.Dillon C, Rasch E, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-1994. J Rheumatol. 2006;33:2271-2279. [PubMed] [Google Scholar]
- 7.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626-34. [DOI] [PubMed] [Google Scholar]
- 8.Aigner T, Rose J, Martin J, et al. Aging theories of primary osteoarthritis: From epidemiology to Molecular Biology. Rejuvenation Res. 2004;7:134-45. [DOI] [PubMed] [Google Scholar]
- 9.Cesari M, Kritchevsky SB, Nicklas B, et al. Oxidative Damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J Gerontol Ser A Biol Sci Med Sci. 2012;67A:671-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493-8. [DOI] [PubMed] [Google Scholar]
- 11.Morone NE, Karp JF, Lynch CS, et al. Impact of chronic musculoskeletal pathology on older adults: a study of differences between knee OA and low back pain. Pain Med. 2015;10:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila. Pa. 1976). 2006;31:2151-2161. [DOI] [PubMed] [Google Scholar]
- 13.Nasto LA, Ngo K, Leme AS, et al. Investigating the role of DNA damage in tobacco smoking-induced spine degeneration. Spine J. 2014;14:416-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vo N, Seo HY, Robinson A, et al. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res. 2010;28:1600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan D, Song Y, Sham P, et al. Genetics of disc degeneration. Eur Spine J. 2006;15:S317-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon CH, Jacobs L, Kim JH, et al. Part 2: quantitative proton T2 and sodium magnetic resonance imaging to assess intervertebral disc degeneration in a rabbit model. Spine (Phila. Pa. 1976). 2012;37:E1113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samartzis D, Karppinen J, Chan D, et al. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheum. 2012;64:1488-96. [DOI] [PubMed] [Google Scholar]
- 18.Livshits G, Popham M, Malkin I, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70:1740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366-72. [DOI] [PubMed] [Google Scholar]
- 20.Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila. Pa. 1976). 2002;27:2631-44. [DOI] [PubMed] [Google Scholar]
- 21.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine (Phila. Pa. 1976). 1988;13:173-8. [PubMed] [Google Scholar]
- 22.Haschtmann D, Stoyanov JV, Gedet P, et al. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J. 2008;17:289-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadfelic M, Kalbere F, Saegesser D, et al. The course of macropscopic degeneration in the human lumbar intervertebral disc. Spine. 2006;31(12):1522-31. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro IM, Vresilovic EJ, Risbud MV. Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone. 2012;50:771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869-74. [DOI] [PubMed] [Google Scholar]
- 26.Ambard D, Cherblanc F. Mechanical behavior of annulus fibrosus: a microstructural modle of fibers reorientation. Ann Biomed Eng. 2009;37(11):2256-65. [DOI] [PubMed] [Google Scholar]
- 27.Jahnke MR, McDevitt CA. Protoeglycans of the human intervertebral disc. Electrophoretic heterogeneity of the aggregating protoegylycans of the nucleus pulposus. Biochem J. 1988;251(2):347-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nachemson A, Elfstrom G. Intravital dynamic pressure measurements in lumbar discs. A study of common movements, maneuvers and exercises. Scan J Rehabil Med Suppl. 1970;1:1-40. [PubMed] [Google Scholar]
- 29.Urban JPG, Smith S, Fairbank JCT. Nutrition of the intervertebral disc. Spine (Phila. Pa. 1976). 2004;29:2700-9. [DOI] [PubMed] [Google Scholar]
- 30.Gruhagen T, Wilde G, Soukane DM, et al. Nutrient supply and intervertebral disc metabolism. J Bone Surg Am. 2006;88(2):30-5. [DOI] [PubMed] [Google Scholar]
- 31.Scharf B, Clement CC, Yodmuang S, et al. Age-related carbonylation of fibrocartilage structural proteins drives tissue degenerative modification. Chem Biol. 2013;20:922-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes IAF, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Phila. Pa. 1976). 2004;29:2724-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila. Pa. 1976). 1990;15:1142-7. [DOI] [PubMed] [Google Scholar]
- 34.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine (Phila. Pa. 1976). 1988;13:179-87. [DOI] [PubMed] [Google Scholar]
- 35.Iatridis JC, MacLean JJ, O'Brien M, et al. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila. Pa. 1976). 2007;32:1493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams MA, McNally DS, Dolan P. Stress' distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965-72. [DOI] [PubMed] [Google Scholar]
- 37.Krismer M, Haid C, Rabl W. The contribution of annulus fibers to torque resistance. Spine (Phila. Pa. 1976). 1996;21:2551-7. [DOI] [PubMed] [Google Scholar]
- 38.Michalek AJ, Iatridis JC. Height and torsional stiffness are most sensitive to annular injury in large animal intervertebral discs. Spine J. 2012;12:425-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Fairbank JCT, Roberts S, et al. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine (Phila. Pa. 1976). 2005;30:1815-20. [DOI] [PubMed] [Google Scholar]
- 40.Benneker LM, Heini PF, Alini M, et al. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine (Phila. Pa. 1976). 2005;30:167-73. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson SJ, Steffen T. Biomechanics of the aging spine. Eur Spine J. 2003;12:S97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sairyo K, Biyani A, Goel VK, et al. Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine (Phila. Pa. 1976). 2007;32:E340-7. [DOI] [PubMed] [Google Scholar]
- 43.D'hooge R, Cagnie B, Crombez G, et al. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man Ther. 2012;17:584-8. [DOI] [PubMed] [Google Scholar]
- 44.Sowa GA, Coelho JP, Vo NV, et al. Cells from degenerative intervertebral discs demonstrate unfavorable responses to mechanical and inflammatory stimuli: a pilot study. Am J Phys Med Rehabil. 2012;91:846-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing QJ, Liang QQ, Bian Q, et al. Leg amputation accelerates senescence of rat lumbar intervertebral discs. Spine (Phila. Pa. 1976). 2012;35:E1253-61. [DOI] [PubMed] [Google Scholar]
- 46.Videman T, Battié MC, Gill K, et al. Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine (Phila. Pa. 1976). 1995;20:928-35. [DOI] [PubMed] [Google Scholar]
- 47.Videman T, Levälahti E, Battié MC. The effects of anthropometrics, lifting strength, and physical activities in disc degeneration. Spine (Phila. Pa. 1976). 2007;32:1406-13. [DOI] [PubMed] [Google Scholar]
- 48.Galbusera F, Brayda-Bruno M, Wilke HJ. Is post-contrast MRI a valuable method for the study of the nutrition of the intervertebral disc? J Biomech. 2014;47:3028-34. [DOI] [PubMed] [Google Scholar]
- 49.Raty HP, Battié MC, Videman T, et al. Lumbar mobility in former elite male weightlifters, soccer players, long-distance runners, and shooters. Clin Biomech. 1997;12:325-30. [DOI] [PubMed] [Google Scholar]
- 50.Blanco JF, Graciani IF, Sanchez-Guijo FM, et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila. Pa. 1976). 2010;35:2259-65. [DOI] [PubMed] [Google Scholar]
- 51.Feng G, Yang X, Shang H, et al. Multipotential differentiation of human annulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92:675-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skelprogenitor cells in the degenerate human intervertebral disc. Spine (Phila. Pa. 1976). 2007;32:2537-44. [DOI] [PubMed] [Google Scholar]
- 53.Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson SM, Mobasheri A, Freemont AJ, et al. Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol Histopatho. 2007;22(9):1033-41. [DOI] [PubMed] [Google Scholar]
- 55.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An HS, Masuda K, Inoue N. Intervertebral disc degeneration: biological and biomechanical factors. J Orthop Sci. 2006;11(5):541-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Surg Am. 2006;88(2):76-82. [DOI] [PubMed] [Google Scholar]
- 58.Vo NV, Hartman RA, Patil PR, et al. Molecular Mechanisms of Biological Aging in Intervertebral Discs. J Orthop Res. 2016;34:1289-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumaresan S, Yoganandan N, Pintar FA, et al. Morphology of young and old cervical spine intervertebral disc tissues. Biomed Sci Instrum. 2000;36:141-6. [PubMed] [Google Scholar]
- 60.Prescher A. Anatomy and pathology of the aging spine. Eur J Radiol. 1998;27:181-95. [DOI] [PubMed] [Google Scholar]
- 61.Edelson JG, Nathan H. Stages in the natural history of the vertebral endplates. Spine (Phila. Pa. 1976).1988;13:21-6. [DOI] [PubMed] [Google Scholar]
- 62.Bibby SRS, Jones DA, Ripley RM, et al. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila. Pa. 1976). 2005;30:487-96. [DOI] [PubMed] [Google Scholar]
- 63.Gullbrand SE, Peterson J, Mastropolo R. et al. Low rate loading-induced convection enhances net transport into the intervertebral disc in vivo. Spine J. 2015;15:1028-33. [DOI] [PubMed] [Google Scholar]
- 64.Galbusera F, Mietsch A, Schmidt H, et al. Effect of intervertebral disc degeneration on cell viability: a numerical investigation. Comput Methods Biomech Biomed Engin. 2013;16:328-37. [DOI] [PubMed] [Google Scholar]
- 65.Ayotte DC, Ito K, Perren SM, et al. Direction-dependent constriction flow in a poroelastic solid: the intervertebral disc valve. J Biomech Eng. 2000;122:587-93. [DOI] [PubMed] [Google Scholar]
- 66.Niedernhofer LJ, Robbins PD. Signaling mechanisms involved in the response to genotoxic stress and regulating lifespan. Int J Biochem Cell Biol. 2008;40:176-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasty P, Campisi J, Hoeijmakers J, et al. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355-9. [DOI] [PubMed] [Google Scholar]
- 68.Guarente L. Mitochondria-a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of aging. Nature. 2000;408:239-47. [DOI] [PubMed] [Google Scholar]
- 70.Lopez-Otn C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153:1194-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila. Pa. 1976). 2004;29:2691-9. [DOI] [PubMed] [Google Scholar]
- 72.Tengblad A, Pearce RH, Grimmer BJ. Demonstration of link protein in proteoglycan aggregates from human intervertebral disc. Biochem J. 1984;222:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scott JE, Bosworth TR, Cribb AM, et al. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 1994;184:73-82. [PMC free article] [PubMed] [Google Scholar]
- 74.Sztrolovics R, Grover J, Cs-Szabo G, et al. The characterization of versican and its message in human articular cartilage and intervertebral disc. J Orthop Res. 2002;20:257-66. [DOI] [PubMed] [Google Scholar]
- 75.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila. Pa. 1976). 1995;20:1307-14. [DOI] [PubMed] [Google Scholar]
- 76.Adams P, Eyre DR, Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehabil. 1997;16:22-9. [DOI] [PubMed] [Google Scholar]
- 77.Binch ALA, Shapiro IM, Risbud MV. Syndecan-4 in intervertebral discs and cartilage: Saint or sinner? Matrix Biol. 2016;52:355-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu k, Wang X, Zhang Q, et al. Sp1 downregulates proinflammatory cytokine-induced catabolic gene expression in nucleus pulposus cells. Mol Med Rep. 2016;14(4):3961-8. [DOI] [PubMed] [Google Scholar]
- 79.Yang H, Lui H, Li X, et al. TNF-α and TGF-β1 regulate Syndecan-4 expression in nucleus pulposus cells: role of the mitogen-activated protein kinase and NF-kB pathways. Connect Tissue Res. 2015;56:281-7. [DOI] [PubMed] [Google Scholar]
- 80.Sztrolovics R, Alini M, Mort JS, et al. Age-related changes in fibromodulin and lumican in human intervertebral discs. Spine (Phila. Pa. 1976). 1999;24:1765-71. [DOI] [PubMed] [Google Scholar]
- 81.Hollander AP, Heathfield TF, Liu JJ, et al. Enhanced denaturation of the alpha (II) chains of type-II collagen in normal adult human intervertebral discs compared with femoral articular cartilage. J Orthop Res. 1996;14:61-6. [DOI] [PubMed] [Google Scholar]
- 82.Pokharna HK, Phillips FM. Collagen crosslinks in human lumbar intervertebral disc aging. Spine (Phila. Pa. 1976). 1998;23:1645-8. [DOI] [PubMed] [Google Scholar]
- 83.Sivan SS, Tsitron E, Wachtel E, et al. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J. 2006;399:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verzijl N, DeGroot J, Ben ZC, et al. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: candidate amino acids involved in Advanced Glycation End-products. Matrix Biol. 2014;34:89-95. [DOI] [PubMed] [Google Scholar]
- 85.Verzijl N, DeGroot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114-23. [DOI] [PubMed] [Google Scholar]
- 86.Rostogi A, Kim H, Twomey JD, et al. MMP-2 mediates local degradation and remodeling of collagen by annulus fibrosus cells of the intervertrbral discs. Arthritis Res. Ther. 2013;15(2):R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wuetz K, Haglund L. Inflammatory mediators in intervertebral disc degeneration and discogenic pain. Global Spine J. 2013;3:175-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moon HJ, Yurube T, Lozito TP, et al. Effects of secreted factors in culture medium of annulus fibrosus cells on microvascular endothelial cells: elucidating the possible pathomechanisms of matrix degradation and nerve in-growth in disc degeneration. Osteoarthr Cartil. 2014;22:344-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le Maitre CL, Pockert A, Buttle DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652-5. [DOI] [PubMed] [Google Scholar]
- 90.Feng H, Danfelter M, Stromqvist B, et al. Extracellular matrix in disc degeneration. J Bone Joint Surg. 2006;88:25-9. [DOI] [PubMed] [Google Scholar]
- 91.Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10:161-7. [DOI] [PubMed] [Google Scholar]
- 92.Rannou F, Lee TS, Shou RH, et al. Intervertebral disc degeneration: the role of mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen SQ, Lin JP, Zheng QK, et al. Protective effects of paeoniflorin agaist FasL-induced apoptosis of intervertebral dics annulus fibrosus cells via Fas-FasL signaling pathway. Exp Ther. 2015;10(6):2351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montane J, Klimek-Abercrombie A, Potter KJ, et al. Metabolic stress, IAPP and islet amyloid. Diabetes Obes Metab. 2012;14:68-77. [DOI] [PubMed] [Google Scholar]
- 95.Zhang M, Hu R, Liang G, et all. Structural and energetic insight into the cross-seeding amyloid assemblies of human IAP and rat IAPP. J Phys Chem. 2014;B118:7026-36. [DOI] [PubMed] [Google Scholar]
- 96.Venkatanarayan A, Raulji P, Chakravarti ND, et al. IAPP-driven metabolic reprogramming induces regression of p53-deficient tumors in vivo. Nature. 2015;517:626-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lutz TA. The role of amylin in the control of energy and homeostasis. Am J Physiol Regul Integr Comp Physiol. 2010;298:1475-84. [DOI] [PubMed] [Google Scholar]
- 98.Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta 2 diabetes. Nat Immunol. 2010;11:897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu X, Wang K, Hau W, et al. Down-regulation of islet amyloid polypetide expression induces death of human annulus fibrosus cells via mitochondrial and death receptor pathways. Biochim Biophys Acta, Mol Basis Dis. 2017;1863(6):1479-91. [DOI] [PubMed] [Google Scholar]
- 100.Liang T, Zhang LL, Xia W, et al. Individual collagen fibril thickening and stiffening of annulus fibrosus in degenerative intervertebral disc. Spine (Phila. Pa. 1976). 2017;42(19):E1104-11. [DOI] [PubMed] [Google Scholar]
- 101.Nasto LA, Wang D, Robinson AR, et al. Genotoxic stress accelerates age-associated degenerative changes in intervertebral discs. Mech Ageing Dev. 2013;134(1-2):35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang D, Nasto LA, Roughley P, et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthr Cartil. 2012;20:896-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mellor FE, Breen AC. Ionizing radiation exposure and the development of intervertebral disc degeneration- no case to answer. Spine J. 2013;13:224-6. [DOI] [PubMed] [Google Scholar]
- 104.Vo N, Niedernhofer LJ, Nasto LA, et al. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res. 2013;31:831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mavrogonatou E, Kletsas D. High osmolality activates the G1 and G2 cell cycle checkpoints and affects the DNA integrity of nucleus pulposus intervertebral disc cells triggering an enhanced DNA repair response. DNA Repair (Amst). 2009;8:930-43. [DOI] [PubMed] [Google Scholar]
- 106.Urban JP. The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans. 2002;30:858-64. [DOI] [PubMed] [Google Scholar]
- 107.Holm S, Maroudas A, Urban JP, et al. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101-19. [DOI] [PubMed] [Google Scholar]
- 108.Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disc: Effect of fluid flow on solute transport. Clin Orthop Relat Res. 1982;170:296-302. [PubMed] [Google Scholar]
- 109.Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113-30. [PMC free article] [PubMed] [Google Scholar]
- 110.Ohshima H, Urban JPG. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine (Phila. Pa. 1976). 1992;17:1079-82. [DOI] [PubMed] [Google Scholar]
- 111.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829-35. [DOI] [PubMed] [Google Scholar]
- 112.Gu W, Zhu Q, Gao X, et al. Simulation of the progression of intervertebral disc degeneration due to decreased nutritional supply. Spine (Phila. Pa. 1976). 2014;39:E1411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shirazi-Adl A, Taheri M, Urban JPG. Analysis of cell viability in intervertebral disc: effect of endplate permeability on cell population. J Biomech. 2010;43:1330-6. [DOI] [PubMed] [Google Scholar]
- 114.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614-36. [DOI] [PubMed] [Google Scholar]
- 115.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Acosta JC, O'Loghlen A, Banito A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006-18. [DOI] [PubMed] [Google Scholar]
- 117.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513-22. [DOI] [PubMed] [Google Scholar]
- 119.Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature; 2011;479:232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9(3):R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roberts S, Evans EH, Kletsas D, et al. Senescence in human intervertebral discs. Eur Spine J. 2006;15:S312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gruber HE, Ingram JA, Davis DE, et al. Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J. 2009;9:210-5. [DOI] [PubMed] [Google Scholar]
- 124.Kim KW, Chung HN, Ha KY, et al. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J. 2009;9:658-66. [DOI] [PubMed] [Google Scholar]
- 125.Heathfield SK, Le Maitre CL, Hoyland JA. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res Ther. 2008;10:R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dimozi A, Mavrogonatou E, Sklirou A, et al. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater. 2015;30:89-103. [DOI] [PubMed] [Google Scholar]
- 127.Phillips KL, Cullen K, Chiverton N, et al. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthr Cartil. 2015;77:1165. [DOI] [PubMed] [Google Scholar]
- 128.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nerlich AG, Bachmeier BE, Schleicher E, et al. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann NY Acad Sci. 2007;1096:239-48. [DOI] [PubMed] [Google Scholar]
- 130.Bank RA, Bayliss MT, Lafeber FP, et al. Aging and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330:345-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ali R, Le Maitre CL, Richardson SM, et al. 2008. Connective tissue growth factor expression in human intervertebral disc: implications for angiogenesis in intervertebral disc degeneration. Biotech Histochem. 2008;83:239-45. [DOI] [PubMed] [Google Scholar]
- 132.Feng C, Yang M, Lan M, et al. ROS: Crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxid Med Cell Longev. 2017;2017:5601593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim KW, Chung HN, Ha KY, et al. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J. 2009;9(8):658-66. [DOI] [PubMed] [Google Scholar]
- 134.Gruber HE, Chow Y, Hoelscher GL, et al. Micromass culture of human anulus cells: morphology and extracellular matrix production. Spine. 2010;35(10):1033-8. [DOI] [PubMed] [Google Scholar]
- 135.Gruber HE, Watts JA, Riley FE, et al. Mitochondrial bioenergetics, mass, and morphology are altered in cells of the degenerating human annulus. J. Orthop Res. 2013;31(8):1270-5. [DOI] [PubMed] [Google Scholar]
- 136.Ding F, Shao ZW, Yang SH, et al. Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells. Apoptosis. 2012;17(6):579-90. [DOI] [PubMed] [Google Scholar]
- 137.Nasto LA, Robinson AR, Ngo K, et al. Mitochondrial-derived reactive oxygen species play a causal role in aging-related intervertebral disc degeneration. J Orthop. Res. 2013;31(7):1150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park EY, Park JB. High glucose-induced oxidative stress promotes autophagy through mitochondrial damage in rat notochordal cells. Int Orthop. 2013;37(12):2507-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park JS, Park JB, Park IJ, et al. Accelerated premature stress-induced senescence of young annulus fibrosus cells of rats by high glucose-induced oxidative stress. Int Orthop. 2014;38:1311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hou G, Lu H, Chen M, et al. Oxidative stress participates in age-related changes in rat lumbar intervertebral discs. Arch Gerontol Geriatr. 2014;59(3):665-9. [DOI] [PubMed] [Google Scholar]
- 141.Suzuki S, Fujita N, Hosogane N, et al. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther. 2015;17:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang X, Jin L, Yao L, et al. Antioxidative nanofullerol prevents intervertebral disk degeneration. Int J Nanomed. 2014;9(1):2419-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei A, Brisby H, Chung SA, et al. Bone morphogenetic protein-7 protects human intervertebral disc cells in vitro from apoptosis. Spine J. 2008;8(3):466-74. [DOI] [PubMed] [Google Scholar]
- 144.Yang L, Rong Z, Zeng M, et al. Pyrroloquinoline quinone protects nucleus pulposus cells from hydrogen peroxide-induced apoptosis by inhibiting the mitochondria-mediated pathway. Eur Spine J. 2015;24(8):1702-10. [DOI] [PubMed] [Google Scholar]
- 145.Yang D, Wang D, Shimer A, et al. Glutathione protects human nucleus pulposus cells from cell apoptosis and inhibition of matrix synthesis. Connect Tissue Res. 2014;55(2):132-9. [DOI] [PubMed] [Google Scholar]
- 146.Mavrogonatou E, Angelopoulou MT, Kletsas D. The catabolic effect of TNFa on bovine nucleus pulposus intervertebral disc cells and the restraining role of glucosamine sulfate in the TNFa-mediated up-regulation of MMP-3. J Orthop Res. 2014;32:1701-7. [DOI] [PubMed] [Google Scholar]
- 147.Poveda L, Hottiger M, Boos N, et al. Peroxynitrite induces gene expression in intervertebral disc cells. Spine. 2009;34(11):1127-33. [DOI] [PubMed] [Google Scholar]
- 148.Chen JW, Ni BB, Li B, et al. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cell Physiol Biochem. 2014;34(4):1175-89. [DOI] [PubMed] [Google Scholar]
- 149.Cai XY, Xia Y, Yang SH, et al. Ropivacaine- and bupivacaine-induced death of rabbit annulus fibrosus cells in vitro: involvement of the mitochondrial apoptotic pathway. Osteoarthr Cartil. 2015;23(10):1763-75. [DOI] [PubMed] [Google Scholar]
- 150.Chen JW, Ni BB, Zheng XF, et al. Hypoxia facilitates the survival of nucleus pulposus cells in serum deprivation by down-regulating excessive autophagy through restricting ROS generation. Int J Biochem Cell Biol. 2015;59:1-10. [DOI] [PubMed] [Google Scholar]
- 151.Pearce RH, Mathieson JM, Mort JS, et al. Effect of age on the abundance and fragmentation of link protein of the human intervertebral disc. J Orthop Res. 1989;7:861-7. [DOI] [PubMed] [Google Scholar]
- 152.Sztrolovics R, Alini M, Roughley PJ, et al. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326:235-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine (Phila. Pa. 1976). 1997;22:2781-95. [DOI] [PubMed] [Google Scholar]
- 154.Xu H, Mei Q, Xu B, et al. Expression of matrix metalloproteinases is positively related to the severity of disc degeneration and growing age in the East Asian lumbar disc herniation patients. Cell Biochem Biophys. 2014;70:1219-25. [DOI] [PubMed] [Google Scholar]
- 155.Zhao CQ, Zhang YH, Jiang SD, et al. ADAMTS-5 and intervertebral disc degeneration: the results of tissue immunohistochemistry and in vitro cell culture. J Orthop Res. 2011;29:718-25. [DOI] [PubMed] [Google Scholar]
- 156.Bachmeier BE, Nerlich AG, Weiler C, et al. Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors, and the activating TNF-alpha-converting enzyme suggests activation of the TNF-alpha system in the aging intervertebral disc. Ann NY Acad Sci. 2007;1096:44-54. [DOI] [PubMed] [Google Scholar]
- 157.Zhao CQ, Wang LM, Jiang LS, et al. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247-61. [DOI] [PubMed] [Google Scholar]
- 158.Zhu Q, Gao X, Gu W. Temporal changes of mechanical signals and extracellular composition in human intervertebral disc during degenerative progression. J Biomech. 2014;47:3734-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141-79. [DOI] [PubMed] [Google Scholar]
- 160.Wuertz K, Vo N, Kletsas D, et al. Inflammatory and catabolic signaling in intervertebral discs: the roles of NF-kB and MAP kinases. Eur Cell Mater. 1012;23:103-20. [DOI] [PubMed] [Google Scholar]
- 161.Nasto LA, Seo HY, Robinson AR, et al. ISSLS prize winner: inhibition of NF-kB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine (Phila. Pa. 1976). 2012;37:1819-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Le Maitre C, Hoyland J, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1b and TNFa expression profile. Arthritis Res Ther. 2007;9:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang P, Han J, Hui L. MAPK signaling in inflammation-associated cancer development. Protein Cell. 2010;1:218-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689-737. [DOI] [PubMed] [Google Scholar]
- 166.Gruber HE, Hoelscher GL, Ingram JA, et al. Senescent vs. non-senescent cells in the human annulus in vivo: cell harvest with laser capture microdissection and gene expression studies with microarray analysis. BMC Biotech. 2010;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang X, Wang H, Yang H, et al. Tumor necrosis factor-a and interleukin-1b-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-kB axis: implications in inflammatory disc disease. Am J Pathol. 2014;184:2560-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tian Y, Yuan W, Fujita N, et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kB. Am J Pathol. 2013;182:2310-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang W, Yu XH, Wang C, et al. Interleukin-1b in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262-72. [DOI] [PubMed] [Google Scholar]
- 170.Studer RK, Gilbertson LG, Georgescu H, et al. P38 MAPK inhibition modulates rabbit nucleus pulposus cell response to IL-1. J Orthop Res. 2008;26:991-8. [DOI] [PubMed] [Google Scholar]
- 171.Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197-207. [DOI] [PubMed] [Google Scholar]
- 172.Pratsinis H, Kletsas D. 2015. Growth factors in intervertebral disc homeostasis. Connect Tissue Res. 2015;49:273-6. [DOI] [PubMed] [Google Scholar]
- 173.Pratsinis H, Constantinou V, Pavlakis K, et al. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012;30:958-64. [DOI] [PubMed] [Google Scholar]
- 174.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007;16:1858-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martin JT, Kim DH, Milby AH, et al. In vivo performance of an acellular disc-like angle ply structure (DAPS) for total disc replacement in a small animal model. J Orthop Res. 2016;35:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maidhof R, Rafiuddin A, Chowdhury F, et al. Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair. J Orthop Res. 2016;35:32-40. [DOI] [PubMed] [Google Scholar]
- 177.Gawri R, Shiba T, Pilliar R, et al. Inorganic polyphosphates enhances nucleus pulposus tissue formation in vitro. J Orthop Res. 2016;35:41-50. [DOI] [PubMed] [Google Scholar]
- 178.Li Z, Lang G, Karfeld-Sulzer LS, et al. Heterodimeric BMP-2/7 for nucleus pulposus regeneration-in vitro and ex vivo studies. J Orthop Res. 2016;35:51-6 [DOI] [PubMed] [Google Scholar]
- 179.Daniels J, Binch AA, Le Maitre CL. Inhibiting IL-1 signaling pathways to inhibit catabolic processes in disc degeneration. J Orthop Res. 2016;35:74-85. [DOI] [PubMed] [Google Scholar]