Short abstract
Activation of the extracellular ATP ionotropic receptor P2X7 stimulates motor neuron apoptosis, whereas its inhibition in cell and animal models of amyotrophic lateral sclerosis can be protective. These observations suggest that P2X7 receptor activation is relevant to motor neuron disease and that it could be targeted for therapeutic development. Heat shock protein 90 (Hsp90) is an integral regulatory component of the P2X7 receptor complex, antagonizing ligand-induced receptor activation. Here, we show that the repressive activity of Hsp90 on P2X7 receptor activation in primary motor neurons is highly sensitive to inhibition. Primary motor neurons in culture are 100-fold more sensitive to Hsp90 inhibition by geldanamycin than other neuronal populations. Pharmacological inhibition and down-regulation of the P2X7 receptor prevented motor neuron apoptosis triggered by Hsp90 inhibition, which occurred in the absence of extracellular ATP. These observations suggest that inhibition of a seemingly motor neuron specific pool of Hsp90 leads to ligand independent activation of P2X7 receptor and motor neuron death. Downstream of Hsp90 inhibition, P2X7 receptor activated the phosphatase and tensin homolog (TPEN), which in turn suppressed the pro-survival phosphatidyl inositol 3 kinase (PI3K)/Akt pathway, leading to Fas-dependent motor neuron apoptosis. Conditions altering the interaction between P2X7 receptor and Hsp90, such as recruitment of Hsp90 to other subcellular compartments under stress conditions, or nitration following oxidative stress can induce motor neuron death. These findings may have broad implications in neurodegenerative disorders, including amyotrophic lateral sclerosis, in which activation of P2X7 receptor may be involved in both autonomous and non-autonomous motor neurons death.
Impact statement
Here we show that a motor neuron specific pool of Hsp90 that is highly sensitive to geldanamycin inhibition represses ligand-independent activation of P2X7 receptor and is critical to motor neuron survival. Activation of P2X7 receptor by Hsp90 inhibition triggers motor neuron apoptosis through the activation of PTEN, which in turn inhibits the PI3 kinase/Akt survival pathway. Thus, inhibition of Hsp90 for therapeutic applications may have the unexpected negative consequence of decreasing the activity of trophic pathways in motor neurons. The inhibition of Hsp90 as a therapeutic approach may require the identification of the Hsp90 complexes involved in pathogenic processes and the development of inhibitors selective for these complexes.
Keywords: Heat shock protein 90, motor neuron, phosphatase and tensin homolog, P2X7, apoptosis, Fas
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the loss of pyramidal neurons in the motor cortex and motor neurons in the spinal cord and brain stem. Motor neuron degeneration in ALS has two components, a motor neuron autonomous mechanism and a non-autonomous process that results from neuroinflammation mediated by microglia and astrocytes.1–5 The ionotropic purine receptor P2X7 (P2X7 receptor) is present in activated microglia in the spinal cord of ALS patients and animal models of the disease.6,7 Inhibition of the P2X7 receptor, at the time of symptom onset provides protection in transgenic mouse model of ALS, suggesting that activation of P2X7 receptor plays a role in disease progression.8–10 However, the deletion of exon 10 in the P2X7 receptor gene accelerates symptoms onset but delays progression of the disease in the same mouse model of ALS.11 Activation of P2X7 receptor in astrocytes isolated from the spinal cord of a transgenic ALS model stimulates non-autonomous motor neurons death. In agreement, inhibition of the receptor or the degradation of its endogenous ligand ATP by incubation with apyrase reverse the toxic effects of astrocytes on motor neuron survival.12 Together, these results suggest that P2X7 receptor plays an important role in ALS non-autonomous motor neurons death.
The expression of P2X7 receptor in the central nervous system is not limited to glial cells.13 Motor neurons express the P2X7 receptor both in vivo and in culture.14,15 Activation of P2X7 receptor using a high affinity agonist stimulates peroxynitrite-dependent motor neuron death in cultures.16 In contrast, motor neuron death stimulated by intracellular delivery of the oxidatively modified molecular chaperone heat shock protein 90 (Hsp90) requires P2X7 receptor activation but does not involve peroxynitrite production,15 showing that P2X7 receptor can induce cell-autonomous motor neuron death. The involvement of P2X7 receptor in both cell autonomous and non-cell autonomous motor neuron death pathways suggests this receptor is an ideal target for therapeutic intervention in ALS. While P2X7 receptor antagonists are under investigation for the treatment of a wide variety of disorders, its success in clinical trial is hampered by genetic variation within patient populations.17 Thus, an alternative approach to augment the therapeutic potential of P2X7 receptor antagonists is to target the pathways regulated by P2X7 itself.
The P2X7 receptor is an atypical member of the P2X family of purine ionotropic receptors, which activation leads to the opening of a non-selective calcium channel and prolonged stimulation leads to the opening of a high molecular pore.18–20 P2X7 receptor has an additional 200 aa c-terminal domain, which is responsible for most conductance-independent P2X7 receptor activities through the formation of cell-specific complexes that include as many as 11 distinct protein components.21–24 One of the components of the P2X7 receptor complex is Hsp90.22,23,25 Hsp90 represses P2X7 receptor by binding to the C-terminal domain, decreasing the affinity of the receptor for ATP.22–24 Hsp90 is a ubiquitous molecular chaperone that totals 1–2% of cytosolic proteins.26 Hsp90 participates in the regulation of a variety of pro-survival cellular processes through interactions with more than 300 client proteins, including numerous transcription factors and kinases such as phosphoinositide-dependent kinase-1 (PDK1) and Akt.27–37 Hsp90 is also responsible for the induction of the heat shock/stress response, which results in the expression of stress proteins such as heat shock protein 70 and 40 (Hsp70 and Hsp40).38,39 However, motor neurons fail to induce the expression of Hsp70 after Hsp90 inhibition,40,41 which results in a higher threshold for the induction of an atypical heat shock response.41,42 However, until now there was not clear understanding for this anomalous behavior. Here we report that incubation of pure primary motor neurons with low nanomolar concentrations of geldanamycin triggers cell death through ligand-independent activation of the P2X7 receptor. We also report for the first time in neurons that the influx of calcium due to activation of the P2X7 receptor stimulates the phosphatase and tensin homolog (TPEN), which inhibits the phosphatidyl inositol 3 kinase (PI3K)/Akt pathway, leading to Fas-mediated motor neuron apoptosis. Together these results provide a new understanding on specific pathways that may be responsible for the selective motor neuron degeneration in ALS.
Materials and methods
Motor neuron cultures
Motor neurons were prepared as previously described.15,43–45 Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Central Florida and Oregon State University and manipulations performed according with the National Institute of Health guidelines for the Care and Use of Laboratory Animals. Briefly, ventral spinal cords from E15 rat embryos were enzymatically disaggregated using trypsin followed by mechanical trituration. Motor neurons were recovered from the interface after centrifugation of the disaggregated spinal cord cell suspension on a 6% OptiPrep cushion (Sigma, Saint Louis, MO). Further purification of the motor neurons was achieved by immunoaffinity purification with a monoclonal antibody that recognize the extracellular domain of the p75 low-affinity neurotrophin receptor (p75NTR, Chemicon-Milliport, Billerica, MA) and magnet-assisted cell separation (Miltenyi Biotec, Auburn, CA). Motor neurons were plated on poly-DL-ornithine and laminin (Sigma) in 96 well plates (750 cells per well), 35 mm plates (20,000 cells per plate), 4-well plates (3,000 cells per well), or 8-well chamber slides (30,000 cells per well) on. The cultures were maintained in Neurobasal media with B27supplement, heat inactivated horse serum, glutamine, glutamate, 3-mercaptoethanol (all from Gibco/Invitrogen, Carlsbad, CA). When the cultures were performed in the presence of trophic factors, 1 ng/mL brain-derived neurotrophic factor (BDNF), 0.1 ng/mL glial-derived neurotrophic factor (GDNF) and 10 ng/mL cardiotrophin-1 (CT-1) were added to the media described above. The cultures were maintained at 37°C in a 5% CO2 humidified atmosphere. The culture purity was regularly confirmed by staining for Islet 1/2, choline acetyl transferase (ChAT) and Hb9. The cultures were more than 95% pure motor neurons.
Cortical neuron cultures
Cortical neurons were obtained from the cerebral cortex of fetal Sprague Dawley rats (E 17) by a papain dissociation method as described previously.46 Cultures were plated on poly-D-lysine (Sigma-Aldrich)-coated cell culture dishes and maintained in minimum essential medium (MEM) (Invitrogen, Grand Island, NY) containing 5.5 g/L glucose, 2 mM glutamine, 100 µM cystine, and supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (Invitrogen). Cultures plated at a low density (10,000 cells/mL) were maintained in MEM containing 10% horse serum (HS) (Invitrogen) instead of FBS and 10 µM BHA (Invitrogen). All experiments were initiated 24 h after plating.
NSC34 cell line culture
NSC34 motor neuron hybrid line was maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin at 37°C during the growth stage. To differentiate the cells, 10% FBS was substituted for HS and 1 µM retinoic acid was added. The cells were differentiated for a period of one week prior to treatments.
Motor neuron survival
Motor neuron survival was determined either by counting under phase contrast in four well plates (Nunc) or by calcein staining (Molecular Probes, Invitrogen) for 45 min in black 96-well plates (Greiner Bio-One, Monroe, NC) as previously described.15 Extracellular calcein was quenched with 100 mg/mL hemoglobin and the images were captured using the RUNNER (Trophos, Marseilles, France). Cell counts were obtained using the Tina® software (Trophos). The criteria for cell survival determinations by direct observation under phase contrast followed the same requirements utilized by Tina software to include only neurons with neurites longer than 3-soma diameter.15,44,45 Survival determinations by direct observation did not differ from those obtained using image analysis. Motor neuron survival is calculated as the fraction of the survival of neurons respect to the corresponding control (with or without trophic factors) after 24 h in culture.
Immunoblotting
Cells were lysed using a NP-40 lysis buffer (1% NP-40, 40 mM Tris, pH 7.4, 0.15 M NaCl, 10% glycerol, 0.1% SDS, 0.1% deoxycholate) containing protease and phosphatase inhibitors. Cells were subjected to sonication or freeze-thaw and then centrifuged to collect supernatant. Lysate was subjected to SDS-PAGE before transfer to PVDF membrane. After blocking the membranes with Odyssey Blocking Buffer (LI-COR Biosciences), the membranes were incubated with primary antibodies overnight at 4°C, washed, incubated with secondary antibodies for 1 h at room temperature. After washing, the membranes were scanned using the LI-COR Biosciences Odyssey Infrared Imaging system. Secondary antibodies were obtained from LI-COR Biosciences (IR 680 goat anti-rabbit IgG and IR 800 goat-anti-mouse IgG or 680 donkey anti-rabbit and 700 donkey anti-goat). Primary antibodies were the following: Akt, pAkt (Ser473), pAKT (thr308), and Myc from Cell Signaling. Bands were quantified using Image Studio Licor software.
Adenoviral vectors construction
The p110-CAAX subunit of PI3K,47 A280V PDK1,48 and N-term myristolyation AKT1 (Upstate Biotechnology) were cloned separately into pAdTrack-CMV using restriction enzymes SalI and EcoRV (Genscript Corporation, Piscataway, NJ). The encoded proteins carry a C-terminus Flag-tag. The adenoviral vectors were prepared and amplified as previously described.49 Briefly, the linear vectors were electroporated in chemically competent cells containing pAdEasy1. The resulting adenoviral vector co-expressing GFP was digested with Pac1 and transfected into HEK293A cells using lipofectamine 2000. The infected cells were cultured until all cells were GFP positive, at which time cells were lifted and lysed by freeze/thaw. The viral stock was then amplified to a titer of 1 × 108 pfu.
Motor neuron transduction
Purified motor neurons (80 motor neurons/µl) were incubated with the adenoviral vectors at a multiplicity of infection of 120 for 2 h at 4°C in transduction media (1% heat inactivated horse serum, 20 µM glucose, 0.5 µg/mL insulin, 10 µM putrescine, 10 µg/mL conalbumin, and 0.3 nM sodium selenite, 20 nM progesterone). Motor neurons were then plated at a density of 3000 cells/well in a 4-well plates or 1000 cells/well in a 96-well plates and cultured for 72 h at 37°C, 5% CO2/air prior to experimentation. After 72 h and before any additional treatment, GFP positive cells per well of a 4-well plates were counted under a fluorescence microscope in two perpendicular diagonals, or using the runner for 96-well plates. The number of cells per well was recorded. The cultures were then treated with geldanamycin and the GFP-positive motor neurons were counted again after 24 h. Survival was calculated as the percent of GFP-positive cells in the well 24 h after treatment respect to the GFP-positive cells present in the same well before treatment. A similar protocol was used for the transduction of motor neurons with lentiviral vectors co-expressing GFP with control shRNA or P2X7 receptor shRNA, and PTEN shRNA (Santa Cruz Biotechnology) at a multiplicity of infection of 20.
Statistical analysis
Statistical analysis was performed and the graphs were made using Prism software (Graphpad). ANOVA (one or two way), followed by Bonferroni multiple comparison test, was used to compare multiple groups in the same experiment. When the homoscedasticity test (Brown–Forsythe and Bartlett’s tests) show differences in the standard deviation of the different groups, the Kurskal–Wallis test was used. Survival and dose response data were fit to sigmoidal curves. EC50 values were compared using the extra sum-of-squares F test. Differences between groups were considered different when P < 0.05. All experiments were performed at least in triplicate and repeated at least three times.
Results
Motor neurons are highly sensitive to inhibition of Hsp90 by geldanamycin
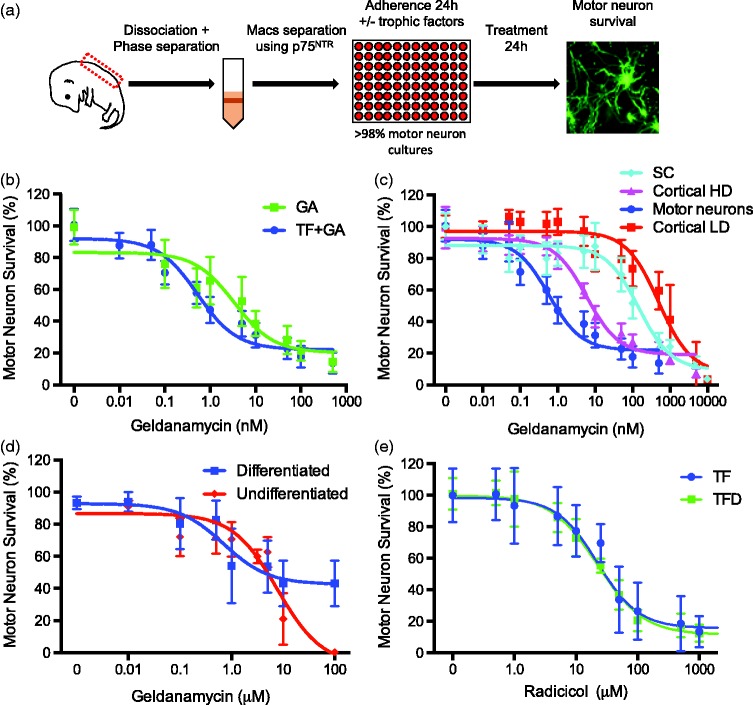
The Hsp90 inhibitor, geldanamycin, is commonly used to study interactions between Hsp90 and the P2X7 complex (Adinolfi 2003 and Migita 2006). Thus, we investigated the effects of Hsp90 inhibition by geldanamycin on the survival of rat E15 embryo motor neurons isolated by a combination of gradient centrifugation and immunopurification with antibodies to p75NTR (Figure 1(a)) as previously described.15,43,50 The cultures were over 95% positive for the motor neuron markers ChAT, Hb9, and Islet 1/2, as previously reported.15,44,45,51 Motor neurons were incubated with and without a combination of BDNF, GDNF and CT-1 (trophic factors) for 24 h prior to the addition of geldanamycin (Figure 1(a)). Trophic factor deprivation stimulated death in approximately 50% of the motor neurons (not shown).44,51–55 Incubation of 24 h old motor neuron cultures with increasing concentrations of the Hsp90 inhibitor geldanamycin stimulated concentration-dependent death independently on whether the motor neurons were cultured in the presence or absence of trophic factors (Figure 1(b)). However, motor neurons cultured in the presence of trophic factors were significantly more sensitive to Hsp90 inhibition than those that survived without trophic factors for 24 h prior to geldanamycin treatment (Figure 1(b); Table 1). In addition, the surviving motor neurons show morphological abnormalities including smaller soma size at geldanamycin concentrations as low as 0.1 nM, a concentration that do not show a significant reduction in cell viability. The half-maximal effective concentration (EC50) for Hsp90 inhibition on motor neuron survival was calculated by normalizing the survival to the corresponding control group (with or without trophic factor) incubated without geldanamycin (Figure 1(b), Table 1). These observations suggest that one or more signaling pathways regulated by trophic factors made motor neurons more dependent on Hsp90 activity. In addition, the EC50 for the induction of trophic factor-treated motor neuron death by geldanamycin was substantially lower than previously reported values in other cell types (Table 1).56–58 To confirm the higher sensitivity of motor neurons to Hsp90 inhibition in the presence of trophic factors, we investigated the effects of increasing concentrations of geldanamycin in cultures of dissociated embryo ventral spinal cord and cortical neurons at low and high culturing density (Figure 1(c); Table 1), as well as undifferentiated and differentiated cultures of the hybrid motor neuron cell line NCS34 (Fig. 1d). The EC50 for geldanamycin of purified motor neurons cultured with trophic factors was approximately 10-fold lower than the EC50 for high density cortical cultures and 1000-fold lower than low density cortical cultures (Table 1). Similarly, trophic factor-treated motor neurons were approximately 300-fold more sensitive to Hsp90 inhibition than cultures of embryo ventral spinal cord (Table 1). When differentiated, the hybrid cell line NSC34 (neuroblastoma × spinal cord) shows some of the characteristics of motor neurons and it is thus widely used as a motor neuron model.59 While differentiated NSC34 cells were over 1000-fold more sensitive to Hsp90 inhibition than undifferentiated cells, they were still 1400-fold less sensitive than motor neurons cultured with trophic factors (Figure 1(d); Table 1).
Figure 1.
Effect of Hsp90 inhibition on neuronal survival. (a) Motor neurons were prepared from 15 days old rat embryos by a combination of gradient centrifugation and immunoaffinity. Pure motor neurons were cultured for 24 h with or without a combination of BDNF, GDNF, and CT-1 previous to incubation with geldanamycin for 24 h before determine viability. Neuron survival was determined by analyzing the imagens of calcein-AM-stained neurons using the Tina software (Trophos) or by counting under phase contrast. The survival between experiments was normalized to the survival of the corresponding control group incubated without geldanamycin. (b) Motor neurons were treated with geldanamycin (0–500 nM) in the presence (TF+GA) or absence of trophic factors (GA) for 24 h. (c) Cells from whole embryo ventral spinal cords (SC), purified motor neuron with trophic factors (motor neurons), and isolated cortical neurons at high (Cortical HD) or low (Cortical LD) density were treated with geldanamycin (0–10 µM) for 24 h. (d) Undifferentiated and differentiated NSC34 cells were treated with geldanamycin (0–100 µM) for 24 h. (e) Motor neurons were cultured with (TF) and without trophic factors (TFD) and incubated with radicicol at the indicate concentrations. For all panels, values represent the mean ± SD of three to five experiments performed by quadruplicate to octuplicate (n ≥ 20 per group). The continuous lines in panels b–e represent the sigmoidal regression curves of the data using the Prism software.
Table 1.
EC50 of different cell types treated with geldanamycin.
| Cell Type | EC50 (95% CI) | Ratio to Motor Neurons + TF | R2 |
|---|---|---|---|
| Motor neuron plus TF | 0.5 nM (0.4 nM to 0.6 nM) | 1 | 0.89 |
| Motor neuron minus TF | 3.4 nM (2.4 nM to 4.8 nM) | 7 | 0.74 |
| Embryo ventral spinal cord | 146 nM (118.3 nM to 180.3 nM) | 292 | 0.84 |
| High density cortical neurons | 5.9 nM (4.95 nM to 7.1 nM) | 12 | 0.94 |
| Low density cortical neurons | 497.6 nM (404.8 nM to 611.7 nM) | 995 | 0.80 |
| NSC34 undifferentiated | 7.75 µM (6.1 µM to 9.8 µM) | 15500 | 0.82 |
| NSC34 differentiated | 0.70 µM (0.4 µM to 1.2 µM) | 1400 | 0.65 |
Note: Cells were treated with geldanamycin (0.01 nM to 100 µM) for 24 h. Cell survival was measured using calcein-AM staining as described in material and methods. Values represent the mean and 95% CI of at least three independent experiments. Data were fit to a sigmoidal curve using Prism software (Graphpad). The EC50 values were compared using the extra sum-of-squares F test. EC50 different for each group compared with motor neuron plus trophic factors P < 0.0001.
Surprisingly, motor neurons did not show the same response to inhibition of Hsp90 by radicicol, another Hsp90 inhibitor with a different scaffold and distinct interaction with the Hsp90 ATP binding site.60 The EC50 for radicicol toxicity was the same whether motor neurons were incubated with or without trophic factors (Figure 1(e)), which suggest there is a pool of Hsp90 inhibited by low geldanamycin concentrations that is responsible for the prevention of motor neurons death. The high sensitivity to geldanamycin was not due to variations in overall content of Hsp90 protein in motor neurons, as no difference was observed in protein levels between spinal cord cultures and motor neurons (Supplemental Figure 1). In summary, these results reveal that a pool of Hsp90 that is highly sensitive to inhibition by geldanamycin has a specific motor neuron regulatory function not present in other cell types, and that this pool is involved in signaling pathways regulated by trophic factors.
Hsp90 inhibition activates P2X7 receptor
Hsp90 associates and represses the ligand-dependent activation of the P2X7 ionotropic ATP-gated receptor,22,25 which activation stimulates motor neuron death.15,16 Thus, we investigated whether motor neuron death stimulated by Hsp90 inhibition with low geldanamycin concentrations was dependent on the activation of the P2X7 receptor. Motor neurons were incubated with a 0.5 nM concentration of geldanamycin, which stimulated 50% motor neuron death in the presence of trophic factors but had no effect on the survival of trophic factor-deprived motor neurons. The inhibition of P2X7 receptor using brilliant blue G (BBG), KN-62, and the irreversible inhibitor oxidized ATP (oATP)61 prevented motor neuron death stimulated by incubation with 0.5 nM geldanamycin in the presence of trophic factors (Figure 2(a)). Geldanamycin-induced motor neuron death was also prevented by transduction of motor neurons with a lentiviral vector expressing a shRNA targeting P2X7 receptor (Santa Cruz Biotechnology, (Figure 2(b)), which reduced the expression of the receptor by ∼50%, as previously described.15 In contrast, delivery of scrambled control shRNA did not affect motor neuron survival. Furthermore, incubation with the ATP degrading enzyme apyrase had no effect on motor neuron death stimulated by Hsp90 inhibition (Figure 2(a)), suggesting that the activation of the receptor by Hsp90 inhibition is ligand independent. P2X7 receptor is a preferential calcium channel and some of its effects are mediated by the calcium influx.18,62 The intracellular calcium chelator BAPTA-AM prevented motor neuron death stimulated by low geldanamycin concentrations (Figure 2(a)), suggesting that it is the influx of calcium that induces motor neuron death rather than the formation of a pore. Together, our results reveal that inhibition of Hsp90 is sufficient to activate the P2X7 receptor in the absence of ligand, increasing intracellular calcium, which in turn stimulates motor neuron death.
Figure 2.
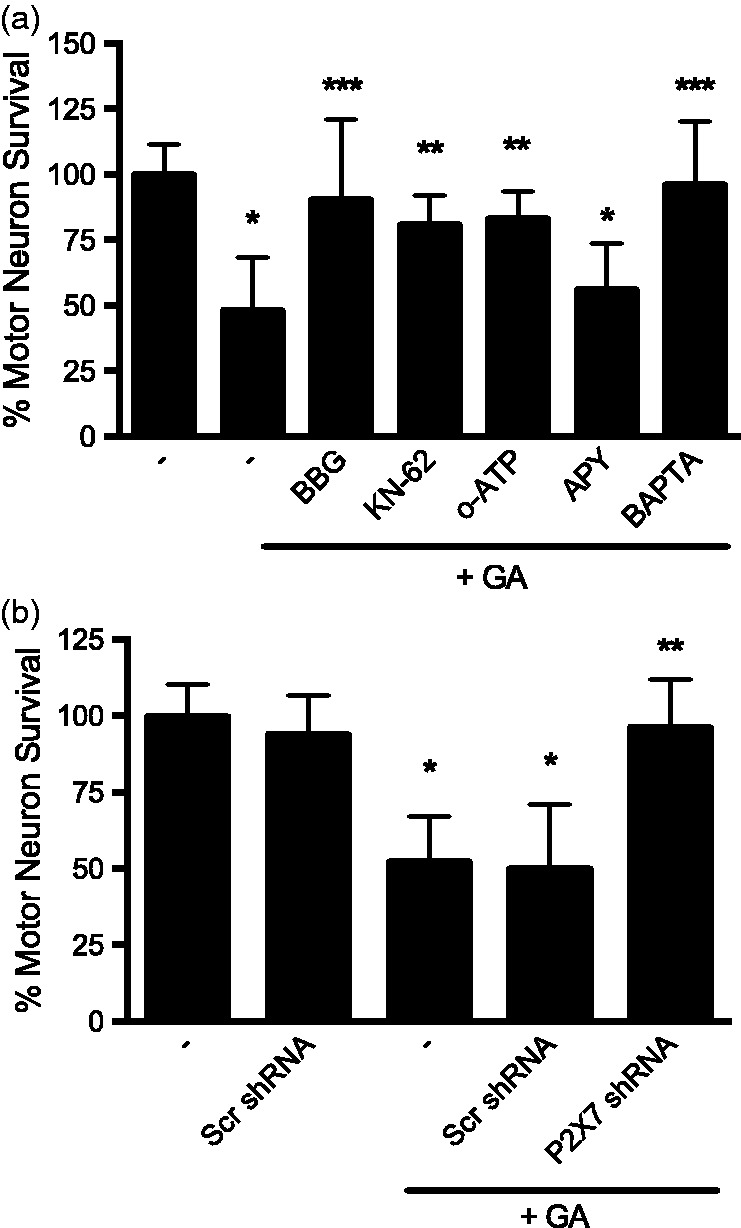
Hsp90 inhibition activates the P2X7 receptor. (a) Motor neurons were incubated with trophic factors and 0.5 nM geldanamycin in the absence or presence of the P2X7 receptor inhibitors Brilliant Blue G (BBG, 10 µM), KN-62 (1µM), and the irreversible inhibitor oxidized ATP (oATP, 1 µM), the ATP degrading enzyme apyrase (APY, 1 U/mL) or the calcium chelator BAPTA-AM (BAPTA, 1 µM) for 24 h. (b) Motor neurons were transduced with P2X7 receptor or scrambled shRNA lentiviral particles co-expressing GFP for 48 h and then cultured in the presence of 0.5 nM geldanamycin (GA) for additional 24 h. Survival was assessed by counting GFP-positive motor neurons for the transfection experiments and normalized to the number of GFP-positive neurons before the addition of geldanamycin. All other treatments were quantified using calcein-AM staining and normalized to control as described in materials and methods. *P < 0.05 versus control; **P < 0.05 versus GA, ***p<0.01 versus GA. Differences were determined by one-way ANOVA followed by Bonferroni post hoc test. Columns represent the mean ± SD of at least three independent experiments performed by quadruplicate to octuplicate (n ≥ 16 per group).
Hsp90 inhibition/P2X7 receptor activation in the presence of trophic factors triggers motor neuron death via PTEN-mediated suppression of the PI3K/Akt pathway
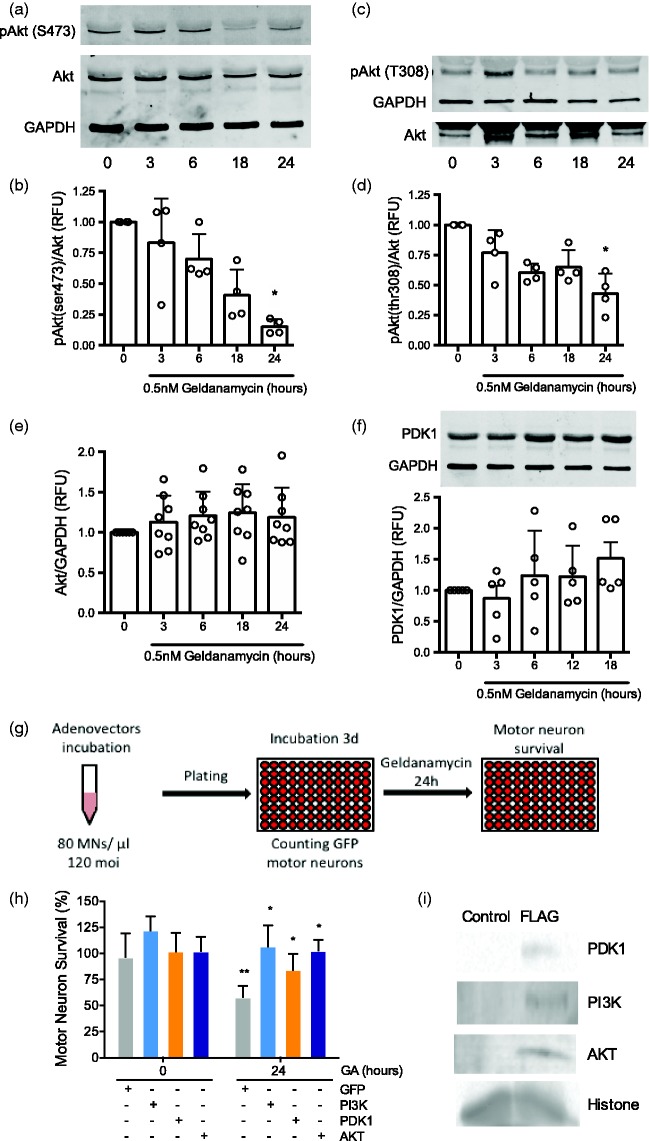
In the presence of trophic factors, motor neurons depend on the activation of the PI3K/AKT pathway for survival; these pathways are inactive in conditions of trophic factor deprivation.43,63–67 Thus, we hypothesized that the selective sensitivity of motor neurons to geldanamycin treatment in the presence of trophic factors is triggered by inhibition of the phosphatidylinositol 3 kinase (PI3K)/Akt pathway downstream of P2X7 receptor activation. We investigated this hypothesis by assessing the progression of AKT phosphorylation after Hsp90 inhibition. Incubation of motor neurons with 0.5 nM geldanamycin causes a significative reduction in Akt phosphorylation at Ser 473 (Figure 3(a) and (b)) and Thr 308 (Figure 3(c) and (d)) after 24 h without altering total Akt levels (Figure 3(a), (b), and (e)). In addition, inhibition of Hsp90 did not affect the levels of the phospholipid-dependent kinase 1 (PDK1) (Figure 3(f)), which activates Akt due to the production of phosphatidyl inositol 3,4,5 triphosphate (PIP3) by the phosphatidyl inositol 3 kinase (PI3K).68 To determine which component of the PI3K/Akt pathway was hindered by Hsp90 inhibition, motor neurons were transduced with adenoviral vectors that co-expressed GFP and constitutively active Myc tagged Akt1, PDK1 or PI3K. Three days later, GFP-positive motor neurons were counted prior to Hsp90 inhibition and surviving motor neurons were counted 24 h after incubation 0.5 nM geldanamycin (Figure 3(g)). Overexpression of constitutively active Akt1, PDK1 and PI3K prevented motor neuron death stimulated by Hsp90 inhibition (Figure 3(h) and (i)), suggesting that inactivation of the PI3K/Akt pathway occurs at the level of PI3K signaling or upstream. In cellular cancer models, the phosphatase and tensin homolog (PTEN), a well-established inhibitor of the PI3K/Akt pathway, is activated downstream of P2X7 receptor.69–71 PTEN opposes the PI3K activity through dephosphorylation of PIP3 to phosphatidylinositol-3,4-diphosphate (PIP2), which prevents the activation of PDK1.68,72 Motor neuron death in the presence of low geldanamycin concentrations was prevented by incubation with the PTEN inhibitor VO-OH(pic) or PTEN downregulation by shRNA (Santa Cruz Biotechnology, Figure 4(a)). The expression of shRNA decreased PTEN levels by ∼50%, determined by infrared Western blot (Figure 4(b)). In contrast, the expression of scrambled shRNA prior to the incubation with geldanamycin did not affect motor neuron survival. These results suggest that inhibition of Hsp90 disabled the PI3K/Akt pro-survival pathway by activation of PTEN.
Figure 3.
Hsp90 inhibition inactivates the PI3K/AKT pathway. Motor neurons cultured with trophic factors were incubated with 0.5 nM geldanamycin and protein harvested at the indicated times as described in material and methods. (a) Representative infrared Western blots for phosphoserine 473 Akt. (b) Quantification of four Western blots showing phospho Akt/Akt fluorescent intensities, the ratios were standardized between experiments using the values of time 0. *P < 0.01 vs. time 0. (c) Representative infrared Western blot for phosphothreonine 308 Akt. (d) Quantification of four Western blots showing phospho Akt/Akt fluorescent intensities as indicated in (b). *P < 0.02 vs. time 0. (e) Quantification of eight Western blots of total Akt fluorescence respect to GAPDH fluorescence. Calculations were performed as in (b). (f) Representative infrared Western blot for total PDK1 (upper panel) and quantification of five Western blots of PDK1 respect to GAPDH fluorescence (lower panel). Calculations were performed as indicated in (b). For all panels, columns represent the mean ± SD of at least of the indicated number of independent experiments for each graphic, while the circles represent the individual values used to calculate the mean and SD. Statistical analysis was performed using the Kruskal–Wallis test followed by the Dunn’s multiple comparisons test. (g) Purified motor neurons were incubated with adenoviral vectors expressing green fluorescent protein (GFP) alone or in combination with constitutive active PI3K, PDK1 or myristoylated Akt as described in materials and methods before plating and incubated for three days. GFP-positive motor neurons were counted before addition of 0.5 nM geldanamycin for 24 h. Motor neuron survival was determined as the proportion of GFP-positive motor neurons present after incubation with geldanamycin respect to the number of GFP-positive motor neurons before the incubation with geldanamycin. (h) Expression of constitutive active PI3K, PDK1, or myristoylated Akt prevented motor neuron death stimulated by 0.5 nM geldanamycin in the presence of trophic factors. Values are the mean ± SD of at least four independent experiments performed by quadruplicate (n ≥ 16 per group). Results were analyzed by two-way ANOVA followed by Bonferroni post hoc test. *P < 0.001 versus GA-treated GFP, **P < 0.05 versus GFP before the incubation with geldanamycin. (i) Representative infrared Western blot for Myc tag showing the expression of the recombinant proteins PI3K, PDK1, and Akt.
Figure 4.
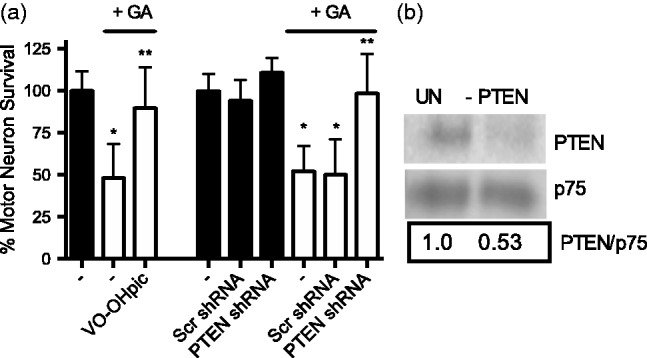
Inhibition of PTEN prevents geldanamycin-induced motor neuron death. (a) Motor neurons were incubated with 0.5 nM geldanamycin for 24 h in the absence or presence of the PTEN inhibitor, VO-OH(pic) (VOOH, 2 µM) or 48 h after transducing with PTEN or scrambled shRNA lentiviral particles. Cells were counted and normalized to control. Columns represent the mean ± SD of at least three independent experiments performed by quadruplicate to octuplicate (n ≥ 16 per group). *P < 0.05 versus respective control, **P < 0.05 versus respective control + GA by one-way ANOVA followed by Bonferroni post hoc test. (b) Downregulation of PTEN expression by PTEN shRNA was confirmed by infrared western blot 48 h after motor neuron transduction and normalized to p75 receptor.
Hsp90 inhibition leads to stimulation of Fas ligand transcription by FOXO3a and Fas receptor activation
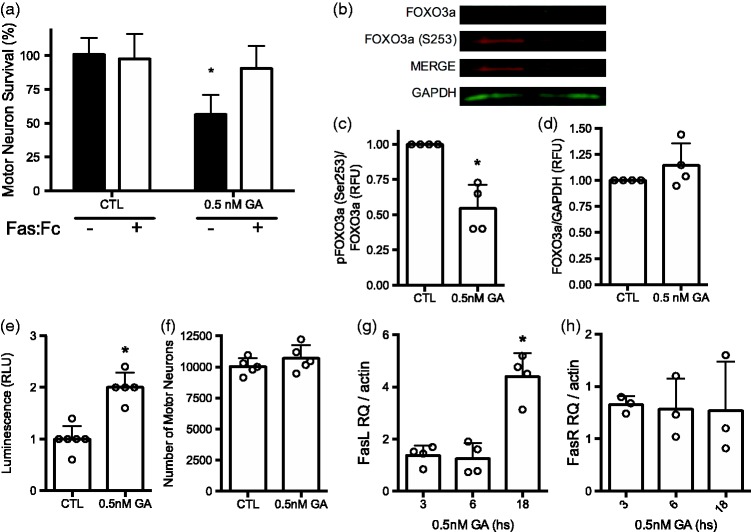
In conditions of trophic factor deprivation, the inactivation of the PI3K/Akt pathway leads to motor neuron death by activation of the Fas receptor,43,51 suggesting that Hsp90 inhibition with low geldanamycin concentrations may activate the same cell death pathway. Indeed, incubation Fas:Fc, a decoy that prevents Fas activation, blocked motor neuron death stimulated by inhibition of Hsp90 with low geldanamycin concentrations in the presence of trophic factors (Figure 5(a)).
Figure 5.
Hsp90 inhibition induces FOXO3a- and Fas-dependent motor neuron apoptosis. (a) Motor neurons were cultured with trophic factors and incubated with or without 0.5 nM geldanamycin alone or in combination with 1 µg/mL Fas:Fc (+) for 24 h before determine survival as described in Materials and Methods. The data were analyzed using two-way ANOVA followed by Sidak’s multiple comparisons test. *P < 0.05 versus CTL. (b) Representative infrared Western blot for total FOXO3a and FOXO3a phosphorylated at serine 253 [FOXO3a (S253)] in homogenates of motor neurons treated with 0.5 nM geldanamycin (GA) for 16 h. (c) Quantification of four western blots showing FOXO3a-S253 fluorescent intensities respect to total FOXO3a, the ratios were standardized between experiments using the values of control. *P < 0.001 vs. control. (d) Total relative expression of FOXO3a respect to GAPDH was calculated and standardized as described in (c). For (c) and (d) for all panels, columns represent the mean ± SD of the indicated number of independent experiments for each graphic, while the circles represent the individual values used to calculate the mean and SD. (e) Motor neurons were transduced with adenoviral particles expressing luciferase under the control of fork head response element (FHRE) and incubated for 24 h before treating the cultures with 0.5 nM geldanamycin for additional 16 h, at which time the luminescence was quantified. Bars represent the mean ± SD of five experiments performed in octuplicate. Circles are the mean of the replicates of each one of the independent experiments. *P < 0.01 vs. control. (f) Motor neuron cultures used in (e) were stained with calcein. AM and the number of motor neurons were determined as described in material and methods. Bars represent the mean ± SD of five experiments performed in octuplicate. Circles are the mean of the replicates from each one of the independent experiments. Statistical analysis in (c), (d), (e) and (f) was performed using the unpaired t test. (g) Quantitative RT-PCR for Fas ligand normalized to β-actin. Motor neurons were cultured with and without geldanamycin for the indicate times before total RNA was extracted and processed as described in materials and methods. Values are standardized to the ratio Fas ligand to β-actin in motor neurons cultured without geldanamycin. Bars represent the mean ± SD of four experiments performed in triplicate. Circles are the mean of the independent experiments. *P < 0.001 vs. untreated, 3 and 6 h. By Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (h) Quantitative RT-PCR for Fas receptor normalized to β-actin. Cultures and treatments were performed as described in (g).
When the PI3K/Akt pathways is inactivated in motor neurons, the dephosphorylation of forkhead box 03 (FOXO3a) transcription factor on Ser 253 leads to FasL expression and Fas receptor activation.73–76 Incubation of motor neurons with low geldanamycin concentrations reduced FOXO3a phosphorylation (Figure 5(b) and (c)), with no change in total levels of the transcription factor (Figure 5(b) and (d)). The activity of FOXO3a was measured by transducing motor neurons with a reporter construct carrying luciferase under the control of the forkhead responsive element (FHRE).74 There was a two-fold increase in the luciferase activity in motor neurons incubated with geldanamycin for 16 h compared with untreated controls (Figure 5(e)) with no changes in cell number (Figure 5(f)). In addition, while inhibition of Hsp90 stimulated the expression of Fas ligand (Figure 5(g)), it did not affect the expression of Fas receptor, as determined by qRT-PCR (Figure 5(h)). Together, these results reveal that at low levels of geldanamycin, the activation of the P2X7 receptor leads to PTEN activation and subsequent inhibition of the PI3K/Akt pathway, which is sufficient to stimulate Fas ligand expression and activate the Fas death pathway.
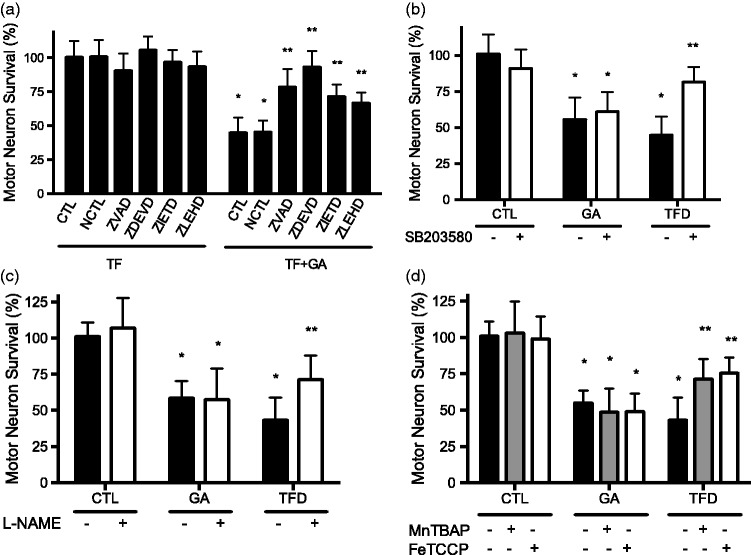
In motor neurons, Fas receptor activates the Fas-Associated protein with Death Domain (FADD), which in turn activates the caspase cascade starting with caspase 8 leading to activation of the effector caspase 3 and motor neuron apoptosis.43,51 In addition, Fas also activates an apparently specific motor neuron pathway through interaction with the Death domain-Associated protein (DAXX), that leads to activation of p38-MAP kinase, expression of neuronal nitric oxide synthase (nNOS), production of peroxynitrite, tyrosine nitration, and caspase activation, which triggers motor neuron apoptosis.51 Motor neuron death stimulated by Hsp90 inhibition with low geldanamycin concentrations was prevented by incubation with the pan caspase inhibitor z-VAD and selective inhibitors for caspases 3, 8 and 9 (Figure 6(a)). However, neither inhibition of p38 MAP kinase (Figure 6(b)) nor of nitric oxide production (Figure 6(c)) prevented motor neuron apoptosis induced by incubation with low geldanamycin concentrations. Similar results were obtained using the superoxide and peroxynitrite scavengers MnTBAP and FeTCCP (Figure 6(d)). In contrast, inhibition of p38, prevention of nitric oxide production, and incubation with MnTBAP and FeTCCP prevented trophic factor deprivation-induced motor neuron death (Figure 6(b) to (d)). These results reveal that inhibition of Hsp90 stimulates motor neuron apoptosis through a FADD-dependent but DAXX-independent Fas death pathway.
Figure 6.
Inhibition of Hsp90 stimulates motor neuron apoptosis independently of p38 activation and the production of peroxynitrite. (a) Geldanamycin stimulates caspase-dependent motor neuron death. Purified motor neurons were cultured in the presence of trophic factors and were treated without (TF) or with 0.5 nM geldanamycin (TF+GA) and 50 µM of the following selective caspase inhibitors: NCTL (negative control), Z-VAD (pan caspase inhibitor), Z-DEVD (caspase 3), Z-IETD (caspase 8), and Z-LEHD (caspase 9). Motor neuron survival was assessed by calcein-AM staining and normalized to motor neurons cultured in the presence of trophic factors without treatment (TF-CTL). Statistical analysis was performed using two-way ANOVA followed by Bonferroni post hoc test, *P < 0.001 versus CTL, **P < 0.01 versus TF+GA CTL. (b) Geldanamycin stimulates p38-independent motor neuron apoptosis. Motor neurons were culture in the presence (CTL) and absence of trophic factors (TFD) or in the presence of trophic factors or with trophic factor and 0.5 nM geldanamycin (GA). Each treatment was combined with inhibition of p38 MAPK using the selective inhibitor SB203580 (5 µM). (c) Inhibition of nitric oxide production did not affect geldanamycin-stimulated motor neuron death. Motor neurons were cultured as described in (b) in combination with the nitric oxide synthase inhibitor L-NAME (100µM). (d) Motor neuron death stimulated by geldanamycin was not affected by scavenging of superoxide and peroxynitrite. Motor neurons were cultured as described in (b) in combination with superoxide and peroxynitrite scavengers MnTBAP (50µM) or FeTCPP (50 µM). Trophic factor deprivation was included as a positive control for the treatments. Statistical analysis was performed using two-way ANOVA followed by Bonferroni post hoc test, *P < 0.05 versus control, **P < 0.001 versus TFD. Columns represent the mean ± SD of at least three independent experiments. In all cases, motor neurons were incubated for 24 h with the treatments before survival was assessed by calcein-AM staining and normalized to control.
Hsp90 inhibition with higher geldanamycin concentrations also triggered the death of motor neurons that survive for 24 h in the absence of trophic factors (Figure 1(b)). We next investigated whether prevention of Fas receptor activation using Fas:Fc could prevent geldanamycin-induced cell death in motor neurons cultured with and without trophic factor. Motor neurons were cultured with increasing concentrations of geldanamycin and in the presence and absence of trophic factors and Fas:FC. After blocking the Fas pathway, there were no differences in the EC50 for geldanamycin induction of motor neuron death in the presence and absence of trophic factors (Table 2). In fact, prevention of Fas activation shifted the EC50 of geldanamycin toxicity for motor neurons cultured with trophic factors to the same value observed in conditions of trophic factor deprivation, which did not change in the presence and absence of Fas:Fc (Table 2). In agreement with these results, blocking the Fas pathways had no effect on the EC50 for the induction of motor neuron death by radicicol (Table 2). There results indicate that in the presence of trophic factors, inhibition of a seemingly motor neuron specific pool of Hsp90 with low concentrations of geldanamycin activates Fas-dependent motor neuron death. While low geldanamycin concentrations stimulate Fas-dependent motor neuron death, radicicol or higher concentrations of geldanamycin trigger Fas-independent motor neuron death both in the presence and absence of trophic factors.
Table 2.
EC50 of motor neurons treated with Fas:Fc.
| Treatment | EC50 (95% CI) | EC50 different from Motor Neuron plus TF | R2 |
|---|---|---|---|
| Motor neuron plus TF + GA | 0.6 nM (0.4 nM to 0.7 nM) | 0.89 | |
| Motor neuron plus TF + GA + Fas:Fc | 3.9 nM (2.8 nM to 5.4 nM) | P<0.0001 | 0.77 |
| Motor neuron minus TF + GA | 3.2 nM (2.2 nM to 4.5 nM) | P<0.0001 | 0.78 |
| Motor neuron minus TF + GA + Fas:Fc | 3.3 nM (2.2 nM to 4.9 nM) | P<0.0001 | 0.83 |
| Motor neuron plus TF + radicicol | 25 µM (19 µM to 31 µM) | 0.80 | |
| Motor neuron plus TF + radicicol + Fas:Fc | 21 µM (18 µM to 24 µM) | 0.93 | |
| Motor neuron minus TF + radicicol | 21 µM (18 µM to 34 µM) | 0.73 | |
| Motor neuron minus TF + radicicol + Fas:Fc | 22 µM (18 µM to 25 µM) | 0.82 |
Note: Cells were treated with geldanamycin (0 to 100 µM) with or without Fas:Fc (1 µg/mL) for 24 h. Similar conditions were used to incubate motor neurons radiciol (0 to 10 mM). Cell survival was measured using calcein-AM staining as described in material and methods. Values represent the mean and 95% CI of at least three independent experiments. Data were fit to a sigmoidal curve using Prism software (Graphpad). The EC50 values were compared using the extra sum-of-squares F test.
Discussion
Our results demonstrate that motor neuron survival in the presence of trophic factors is dependent on the prevention of ligand-independent activation of the P2X7 receptor by a pool of Hsp90 with high affinity for geldanamycin. The presence of the P2X7 receptor in neurons is controversial.13,77 Although some reports fail to find the receptor in spinal cord neurons, the presence of P2X7 receptor in brain stem and spinal motor neuron was demonstrated by in situ hybridization.14,78 Several different methods have been used for the detection of the receptor, with the most recent using a fusion protein, which allows the detection of most P2X7 receptor isoforms.78 However, the receptor has a number of splice variants making nearly impossible to account for all variants with a single method.13 The presence of the P2X7 receptor has been demonstrated in cultured motor neurons by RT-PCR, Western Blot and in functional studies.15,16 Our results show that three different inhibitors and the down regulation of the P2X7 receptor protect motor neurons from apoptosis stimulated by inhibition of Hsp90, providing further support for the expression of the receptor in motor neurons.
The main difference between motor neurons that survive in the presence and absence of trophic factors is a partial resistance to apoptosis mediated by the expression of the FLICE-inhibitor protein (FLIP) in surviving motor neurons.43 The 7-fold difference in the EC50 for the stimulation of motor neuron apoptosis by geldanamycin in the presence and absence of trophic factors was abolished by treatment of the cultures with the Fas-ligand decoy Fas:Fc, revealing the activation of two distinct cell death mechanisms by geldanamycin. One mechanism, activated by low concentrations of geldanamycin, depends as a pre-requisite on the presence of active trophic factor signaling pathways. In these conditions, the inhibition of the PI3K/Akt pathway leads to activation of the Fas pathway and motor neuron apoptosis. The second mechanism, at higher geldanamycin concentrations, is independent of both trophic factor signaling and the Fas pathway. In addition, these results suggest that the Hsp90 pool with high affinity for geldanamycin is motor neuron specific. This may indicate that P2X7 receptor and Hsp90 form a protein complex that confers Hsp90 higher affinity for geldanamycin, and that this complex is exclusive to motor neurons, because induction of cell death in every other cell type tested required at least an order of magnitude higher concentration of the inhibitor. An alternative explanation is that the pathway activated by inhibition of Hsp90 by low geldanamycin concentrations is specific to motor neurons. The composition of the P2X7 receptor complex seems to be cell type dependent activating different signaling pathways in different cell types.18,20,24 Our results showing the presence of a P2X7 receptor complex in motor neurons with a form of Hsp90 with high affinity for geldanamycin are in agreement with previous reports suggesting that the P2X7 receptor forms a complex with Hsp90 and at least other 11 other proteins, which all evidence indicates is cell specific.18,20,23–25
Phosphorylated Hsp90 modulates the activation of the P2X7 receptor by its ligand ATP.22,25 The affinity of geldanamycin and ATP for Hsp90 depends on post-translational modifications and the interaction with client proteins and co-chaperones.79–82Furthermore, post-translational modifications and co-chaperons affect also the affinity of Hsp90 for client proteins.29,37,82,83 The combination of post-translational modifications and protein interactions of Hsp90 in the P2X7 receptor complex in motor neurons may provide the conditions that make the chaperone exceptionally sensitive to geldanamycin inhibition. In agreement with these observations, Hsp90 associated to the P2X7 receptor complex seems to be particularly sensitive to inhibition by geldanamycin but not by radicicol, another inhibitor of the ATPase activity of Hsp90 that differs in its interaction with the ATP binding site.60
One of the most important functions of Hsp90 as a molecular chaperone is conferring cell protection during conditions of stress. Interestingly, motor neurons have an atypical heat shock response.42,84 In normal conditions, heat shock factor 1 (HSF1) is kept in the cytoplasm by a complex that includes Hsp90 and Hsp70.38,39 As a consequence of protein unfolding that leads to a higher demand for Hsp90, the complex dissociates releasing HSF1, which migrates to the nucleus and activates the expression of proteins associate with the stress response38,39 Motor neurons have a high threshold for the activation of HSF1, which leads to a deficient stress response due to differential regulation of multichaperone complexes.39,41,42,85 We found that concentrations of geldanamycin in the picomolar range were sufficient to stimulate the death of purified motor neurons in culture. In contrast, activation of the heat shock response in motor neurons requires 20 to 200-fold higher geldanamycin concentrations.41,85 The requirement of Hsp90 in the P2X7 receptor complex may explain motor neurons high threshold and atypical stress response. Because Hsp90 is needed to prevent the activation of P2X7 receptor, dependence of the stress response on removal of the chaperone from protein complexes could result in motor neuron death. In fact, this interpretation may also provide an explanation for how unfolded proteins can stimulate motor neuron death by promoting an aberrant interaction with the Hsp90 pool highly sensitive to geldanamycin inhibition, thus removing the chaperone from the P2X7 receptor complex. Such mechanism might be relevant in ALS where Hsp90 is found in protein aggregates.86 This interpretation is further supported by the reported protection provided by down-regulation of PTEN in motor neurons from transgenic mice carrying ALS-liked mutant SOD.87
Motor neurons are highly dependent on the supply of trophic factors for survival both in culture and in vivo.52,88,89 The protection provided by trophic factors is mediated by activation of the PI3K/Akt pathway. Indeed, motor neurons are highly dependent on the activity of the PI3K/Akt pathway and inhibition of this pathway stimulates death by apoptosis.43,63–67 PDK1 and Akt are known clients of Hsp90. Hsp90 has the double function of preventing the degradation of the activated kinase, and through the binding of co-chaperons participates in directing the kinases to their targets.90,91 Inhibition of Hsp90 in motor neurons down-regulated the PI3K/Akt pathway as determined by decreased Akt phosphorylation. Consistent with this interpretation, overexpression of constitutive active PDK1 and Akt prevented motor neuron death induced by inhibition of Hsp90. However, overexpression of the p110 constitutive active catalytic subunit of the PI3K also prevented motor neuron apoptosis induced by inhibition of Hsp90. To the best of our knowledge, PI3K is not a client of Hsp90, suggesting that Hsp90 inhibition is acting upstream of this kinase. Inhibition of PTEN, a well-known down regulator of the PI3K/Akt pathway has recently been shown to improve motor neuron survival and function in cell culture and in vivo models of motor neuron degeneration.87,92,93 PTEN inhibits PDK1 activation by catalyzing the dephosphorylation of phosphatidylinositol 3,4,5 triphostate (PIP3) to phosphatidylinositol 3,4 biphosphate (PIP2). Low levels of PIP3 prevent the activation of PDK1, inhibiting the activation of Akt and its down-stream survival signaling.68 PTEN is also activated downstream of the P2X7 receptor in other cellular models.69–71 Our results show for the first time the regulation of PTEN downstream of P2X7 receptor in neurons. In addition, this is the first demonstration that the P2X7 receptor stimulates cell death by inhibiting trophic factor-activated survival pathways. The over-activation of PI3K by transduction with a constitutively active enzyme probably overwhelms the capacity of PTEN to decrease the levels of PIP3, keeping the pathway active and the motor neurons alive.
Trophic factor deprivation triggers Fas-dependent motor neuron apoptosis through the activation of two different pathways downstream of Fas receptor, a DAXX-dependent and a FADD-dependent pathway.43,51 We have recently identified Hsp90 as a target for nitration by peroxynitrite. Nitrated Hsp90 triggers motor neuron apoptosis through the activation of the FADD component of the Fas pathway that is independent of peroxynitrite formation.15 Activation of the DAXX component of the pathway leads to peroxynitrite formation, nitration of Hsp90, and subsequent activation of the FADD-dependent pathway, inducing motor neuron apoptosis.15 The results showed here support the notion that both nitrated Hsp90 and inhibition of Hsp90 act downstream of peroxynitrite formation in the motor neuron death pathway. Both pathways are mediated by P2X7 receptor and Fas activation. However, there are significant differences between the mechanisms activated by inhibition of Hsp90 and nitrated Hsp90. One very important difference between the two conditions is that nitrated Hsp90 stimulates cell death by a gain-of-function, while induction of motor neuron death by geldanamycin is due to the loss-of-function of a specific pool of Hsp90. In spite of the differences, the requirement for P2X7 receptor activation in both conditions suggests that the inhibition of the purine receptor by Hsp90 is key for motor neuron survival. This inhibition seems to be easily perturbed by post-translational modifications or inhibition of the chaperone, suggesting that nonspecific inhibition of Hsp90 would be detrimental in therapeutic treatments of neurodegenerative disease. Therefore, Hsp90 inhibitors that specifically target the pool of Hsp90 with a gain-of-function leading to cell death, such as nitrated Hsp90, while avoiding pro-survival complexes of Hsp90 are critical for therapeutic success in neurodegenerative diseases.
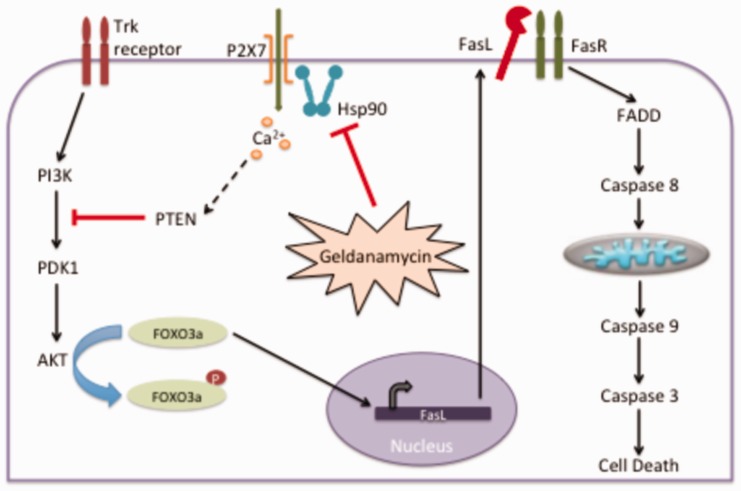
In summary, the results reveal that Hsp90 repression of P2X7 receptor is critical to prevent motor neuron apoptosis induced by activation of PTEN followed by inactivation of Akt, dephosphorylation of FOXO3a, and expression of Fas ligand (Figure 7). The requirement of Hsp90 in the P2X7 receptor complex may be the cause for the motor neuron high threshold and atypical stress response. This atypical stress response can be the cause of motor neuron predisposition to degeneration in diseases such as spinal and muscular atrophy and ALS.
Figure 7.
Inhibition of Hsp90 induces motor neuron cell death via P2X7/PTEN dependent pathway.
Supplemental Material
Supplemental Material for Ligand-independent activation of the P2X7 receptor by Hsp90 inhibition stimulates motor neuron apoptosis by Amy L Strayer, Cassandra N Dennys-Rivers, Karina C Ricart, Narae Bae, Joseph S Beckman, Maria Clara Franco and Alvaro G Estevez in Experimental Biology and Medicine
ACKNOWLEDGEMENTS
The authors thank Dr. Chris Henderson for his support and useful discussions during the development of the research and Brian Kaspar, Nicolas Wein, Kathrin Meyer, and Carlos Miranda for their critical comments on the manuscript. We thank Dr. Jian Zhong for providing the construct with the p110-CAAX subunit of PI3K, and Dr. Neil Cashman for the NSC34 cells.
Authors’ contributions
ALS and CND contributed equally to the paper. ALS, CND, KCR, NB, JSB, MCF and AGE designed and conducted experiments, and interpreted results. MCF and AGE contribute equally to the intellectual design of the experiments and the interpretation of the results. ALS, CND, JSB, MCF and AGE wrote the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH/NINDS [grant R01NS36761 to AGE and R01NS102479 to MCF] and the ALS Association [to JSB].
References
- 1.Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci 2008;28:2075–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 2006;312:1389–92 [DOI] [PubMed] [Google Scholar]
- 3.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 2007;10:615–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz-Amarilla P, Olivera-Bravo S, Trias E, Cragnolini A, Martinez-Palma L, Cassina P, Beckman J, Barbeito L. Phenotypically aberrant astrocytes that promote motoneuron damage in a model of inherited amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 2011;108:18126–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathis S, Couratier P, Julian A, Corcia P, Le Masson G. Current view and perspectives in amyotrophic lateral sclerosis. Neural Regen Res 2017;12:181–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sluyter R, Bartlett R, Ly D, Yerbury JJ. P2X7 receptor antagonism in amyotrophic lateral sclerosis. Neural Regen Res 2017;12:749–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volonte C, Apolloni S, Parisi C, Amadio S. Purinergic contribution to amyotrophic lateral sclerosis. Neuropharmacology 2016;104:180–93 [DOI] [PubMed] [Google Scholar]
- 8.Cervetto C, Frattaroli D, Maura G, Marcoli M. Motor neuron dysfunction in a mouse model of ALS: gender-dependent effect of P2X7 antagonism. Toxicology 2013;311:69–77 [DOI] [PubMed] [Google Scholar]
- 9.Apolloni S, Amadio S, Parisi C, Matteucci A, Potenza RL, Armida M, Popoli P, D'Ambrosi N, Volonte C. Spinal cord pathology is ameliorated by P2X7 antagonism in a SOD1-mutant mouse model of amyotrophic lateral sclerosis. Dis Models Mech 2014;7:1101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett R, Sluyter V, Watson D, Sluyter R, Yerbury JJ. P2X7 antagonism using Brilliant Blue G reduces body weight loss and prolongs survival in female SOD1(G93A) amyotrophic lateral sclerosis mice. PeerJ 2017; 5:e3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apolloni S, Amadio S, Montilli C, Volonte C, D'Ambrosi N. Ablation of P2X7 receptor exacerbates gliosis and motoneuron death in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Hum Mol Genet 2013;22:4102–16 [DOI] [PubMed] [Google Scholar]
- 12.Gandelman M, Peluffo H, Beckman JS, Cassina P, Barbeito L. Extracellular ATP and the P2X7 receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis. J Neuroinflammation 2010;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miras-Portugal MT, Sebastian-Serrano A, de Diego Garcia L, Diaz-Hernandez M. Neuronal P2X7 receptor: involvement in neuronal physiology and pathology. J Neurosci 2017;37:7063–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, Umemura A, Mase M, Yamada K, Shimada S. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res 2008;1194:45–55 [DOI] [PubMed] [Google Scholar]
- 15.Franco MC, Ye Y, Refakis CA, Feldman JL, Stokes AL, Basso M, Melero Fernandez de Mera RM, Sparrow NA, Calingasan NY, Kiaei M, Rhoads TW, Ma TC, Grumet M, Barnes S, Beal MF, Beckman JS, Mehl R, Estevez AG. Nitration of Hsp90 induces cell death. Proc Natl Acad Sci Usa U S A 2013;110:E1102–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandelman M, Levy M, Cassina P, Barbeito L, Beckman JS. P2X7 receptor-induced death of motor neurons by a peroxynitrite/FAS-dependent pathway. J Neurochem 2013;126:382–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHugh SM, Roman S, Davis B, Koch A, Pickett AM, Richardson JC, Miller SR, Wetten S, Cox CJ, Karpe F, Todd JA, Bullmore ET. Effects of genetic variation in the P2RX7 gene on pharmacodynamics of a P2X(7) receptor antagonist: a prospective genotyping approach. Br J Clin Pharmacol 2012;74:376–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volonte C, Apolloni S, Skaper SD, Burnstock G. P2X7 receptors: channels, pores and more. CNS Neurol Disord Drug Targets 2012;11:705–21 [DOI] [PubMed] [Google Scholar]
- 19.North RA. Molecular physiology of P2X receptors. Physiol Rev 2002;82:1013–67 [DOI] [PubMed] [Google Scholar]
- 20.Costa-Junior HM, Sarmento Vieira F, Coutinho-Silva R. C terminus of the P2X7 receptor: treasure hunting. Purinerg Signall 2011;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M, Spelta V, Sim J, North RA, Surprenant A. Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J Biol Chem 2001;276:23262–7 [DOI] [PubMed] [Google Scholar]
- 22.Adinolfi E, Kim M, Young MT, Di Virgilio F, Surprenant A. Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J Biol Chem 2003;278:37344–51 [DOI] [PubMed] [Google Scholar]
- 23.Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J 2001;20:6347–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS. Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: this dissociation regulates P2X(7) pore formation. Am J Physiol Cell Physiol 2009;297:C430–9 [DOI] [PubMed] [Google Scholar]
- 25.Migita K, Ozaki T, Shimoyama S, Yamada J, Nikaido Y, Furukawa T, Shiba Y, Egan TM, Ueno S. HSP90 regulation of P2X7 receptor function requires an intact cytoplasmic C-terminus. Mol Pharmacol 2016;90:116–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Didelot C, Schmitt E, Brunet M, Maingret L, Parcellier A, Garrido C. Heat shock proteins: endogenous modulators of apoptotic cell death. In: Gaestel M (ed) Molecular chaperones in health and disease Berlin: Springer, 2006, pp.171–98 [DOI] [PubMed]
- 27.Gaestel M. Molecular chaperones in signal transduction. Handbook Exp Pharmacol 2006;172:93–109 [DOI] [PubMed] [Google Scholar]
- 28.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005;5:761–72 [DOI] [PubMed] [Google Scholar]
- 29.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–94 [DOI] [PubMed] [Google Scholar]
- 30.Pearl LH, Prodromou C. Structure and in vivo function of Hsp90. Curr Opin Struct Biol 2000;10:46–51 [DOI] [PubMed] [Google Scholar]
- 31.Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol 2001;188:281–90 [DOI] [PubMed] [Google Scholar]
- 32.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 2003;228:111–33 [DOI] [PubMed] [Google Scholar]
- 33.Zhao R, Davey M, Hsu Y-C, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, Boone C, Emili A, Houry WA. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the Hsp90 chaperone. Cell 2005;120:715–27 [DOI] [PubMed] [Google Scholar]
- 34.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci 2002;59:1640–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pratt WB, Morishima Y, Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J Biol Chem 2008;283:22885–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim Biophys Acta 2012;1823:648–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Buchner J. Structure, function and regulation of the hsp90 machinery. Biomed J 2013;36:106–17 [DOI] [PubMed] [Google Scholar]
- 38.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell 2010;40:253–66 [DOI] [PubMed] [Google Scholar]
- 39.Robinson MB, Gifondorwa DJ, E MC. Mechanisms of the motoneuron stress response and its relevance in neurodegeneration. In: McNeill AS (ed) Neurodegeneration: theory, disorders and treatments New York: Nova Science Publishers, Inc., 2010
- 40.Newbern J, Taylor A, Robinson M, Li L, Milligan CE. Decreases in phosphoinositide-3-kinase/Akt and extracellular signal-regulated kinase 1/2 signaling activate components of spinal motoneuron death. J Neurochem 2005;94:1652–65 [DOI] [PubMed] [Google Scholar]
- 41.Batulan Z, Taylor DM, Aarons RJ, Minotti S, Doroudchi MM, Nalbantoglu J, Durham HD. Induction of multiple heat shock proteins and neuroprotection in a primary culture model of familial amyotrophic lateral sclerosis. Neurobiol Dis 2006;24:213–25 [DOI] [PubMed] [Google Scholar]
- 42.Batulan Z, Shinder GA, Minotti S, He BP, Doroudchi MM, Nalbantoglu J, Strong MJ, Durham HD. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci 2003;23:5789–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raoul C, Henderson CE, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol 1999;147:1049–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estevez AG, Spear N, Manuel SM, Radi R, Henderson CE, Barbeito L, Beckman JS. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci 1998;18:923–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahawneh MA, Ricart KC, Roberts BR, Bomben VC, Basso M, Ye Y, Sahawneh J, Franco MC, Beckman JS, Estevez AG. Cu,Zn-superoxide dismutase increases toxicity of mutant and zinc-deficient superoxide dismutase by enhancing protein stability. J Biol Chem 2010;285:33885–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Zulueta M, Ensz LM, Mukhina G, Lebovitz RM, Zwacka RM, Engelhardt JF, Oberley LW, Dawson VL, Dawson TM. Manganese superoxide dismutase protects nNOS neurons from NMDA and nitric oxide-mediated neurotoxicity. J Neurosci 1998;18:2040–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markus A, Zhong J, Snider WD. Raf and Akt mediate distinct aspects of sensory axon growth. Neuron 2002;35:65–76 [DOI] [PubMed] [Google Scholar]
- 48.Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem 2000;275:40400–6 [DOI] [PubMed] [Google Scholar]
- 49.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 1998;95:2509–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science 1999;286:2498–500 [DOI] [PubMed] [Google Scholar]
- 51.Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron 2002;35:1067–83 [DOI] [PubMed] [Google Scholar]
- 52.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, Berkemeler L, Phillips HS, Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature 1993;363:266–70 [DOI] [PubMed] [Google Scholar]
- 53.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson Lc Moffet B, Vandlen RA, Koliastos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science 1994;266:1062–4 [DOI] [PubMed] [Google Scholar]
- 54.Estevez AG, Sampson JB, Zhuang YX, Spear N, Richardson GJ, Crow JP, Tarpey MM, Barbeito L, Beckman JS. Liposome-delivered superoxide dismutase prevents nitric oxide-dependent motor neuron death induced by trophic factor withdrawal. Free Radic Biol Med 2000;28:437–46 [DOI] [PubMed] [Google Scholar]
- 55.Ye Y, Quijano C, Robinson KM, Ricart KC, Strayer AL, Sahawneh MA, Shacka JJ, Kirk M, Barnes S, Accavitti-Loper MA, Radi R, Beckman JS, Estevez AG. Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J Biol Chem 2007;282:6324–37 [DOI] [PubMed] [Google Scholar]
- 56.Drysdale MJ, Brough PA. Medicinal chemistry of Hsp90 inhibitors. Curr Top Med Chem 2008;8:859–68 [DOI] [PubMed] [Google Scholar]
- 57.Gorska M, Popowska U, Sielicka-Dudzin A, Kuban-Jankowska A, Sawczuk W, Knap N, Cicero G, Wozniak F. Geldanamycin and its derivatives as Hsp90 inhibitors. Front Biosci 2012;17:2269–77 [DOI] [PubMed] [Google Scholar]
- 58.Alcazar A, Cid C. High cytotoxic sensitivity of the oligodendrocyte precursor cells to HSP90 inhibitors in cell cultures. Exp Neurol 2009;216:511–4 [DOI] [PubMed] [Google Scholar]
- 59.Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn 1992;194:209–21 [DOI] [PubMed] [Google Scholar]
- 60.Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 1999;42:260–6 [DOI] [PubMed] [Google Scholar]
- 61.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem 1993;268:8199–203 [PubMed] [Google Scholar]
- 62.Di Virgilio F, Schmalzing G, Markwardt F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018;28:392–404 29439897 10.1016/j.tcb.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 63.Dolcet X, Egea J, Soler RM, Martin-Zanca D, Comella JX. Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor-induced motoneuron survival. J Neurochem 2002;73:521–31 [DOI] [PubMed] [Google Scholar]
- 64.Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. J Neurosci 1999;19:9160–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, deLapeyriere O. GFRalpha 1 is required for development of distinct subpopulations of motoneuron. J Neurosci 2000;20:4992–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milligan CE, Prevette D, Yaginuma H, Homma S, Cardwell C, Fritz LC, Tomaselli KJ, Oppenheim RW, Schwartz LM. Peptide inhibitors of the ICE protease family arrest programmed cell death of motoneurons in vivo and in vitro. Neuron 1995;15:385–93 [DOI] [PubMed] [Google Scholar]
- 67.Li L, Prevette D, Oppenheim RW, Milligan CE. Involvement of specific caspases in motoneuron cell death in vivo and in vitro following trophic factor deprivation. Mol Cell Neurosci 1998;12:157–67 [DOI] [PubMed] [Google Scholar]
- 68.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta 2008;1784:150–8 [DOI] [PubMed] [Google Scholar]
- 69.Ghalali A, Wiklund F, Zheng H, Stenius U, Hogberg J. Atorvastatin prevents ATP-driven invasiveness via P2X7 and EHBP1 signaling in PTEN-expressing prostate cancer cells. Carcinogenesis 2014;35:1547–55 [DOI] [PubMed] [Google Scholar]
- 70.Miraglia E, Hogberg J, Stenius U. Statins exhibit anticancer effects through modifications of the pAkt signaling pathway. Int J Oncol 2012;40:867–75 [DOI] [PubMed] [Google Scholar]
- 71.Mistafa O, Ghalali A, Kadekar S, Hogberg J, Stenius U. Purinergic receptor-mediated rapid depletion of nuclear phosphorylated Akt depends on pleckstrin homology domain leucine-rich repeat phosphatase, calcineurin, protein phosphatase 2A, and PTEN phosphatases. J Biol Chem 2010;285:27900–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weng L, Brown J, Eng C. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum Mol Genet 2001;10:237–42 [DOI] [PubMed] [Google Scholar]
- 73.Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci 2004;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999;96:857–68 [DOI] [PubMed] [Google Scholar]
- 75.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem 1999;274:16741–6 [DOI] [PubMed] [Google Scholar]
- 76.Santo EE, Stroeken P, Sluis PV, Koster J, Versteeg R, Westerhout EM. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res 2013;73:2189–98 [DOI] [PubMed] [Google Scholar]
- 77.Illes P, Khan TM, Rubini P. Neuronal P2X7 receptors revisited: do they really exist? J Neurosci 2017;37:7049–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaczmarek-Hajek K, Zhang J, Kopp R, Grosche A, Rissiek B, Saul A, Bruzzone S, Engel T, Jooss T, Krautloher A, Schuster S, Magnus T, Stadelmann C, Sirko S, Koch-Nolte F, Eulenburg V, Nicke A. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. Elife 2018;7:e36217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao R, Fiskus W, Yang Y, Lee P, Joshi R, Fernandez P, Mandawat A, Atadja P, Bradner JE, Bhalla K. HDAC6 inhibition enhances 17-AAG-mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood 2008;112:1886–93 [DOI] [PubMed] [Google Scholar]
- 80.Fiskus W, Rao R, Fernandez P, Herger B, Yang Y, Chen J, Kolhe R, Mandawat A, Wang Y, Joshi R, Eaton K, Lee P, Atadja P, Peiper S, Bhalla K. Molecular and biologic characterization and drug sensitivity of pan-histone deacetylase inhibitor-resistant acute myeloid leukemia cells. Blood 2008;112:2896–905 [DOI] [PubMed] [Google Scholar]
- 81.Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ. Targeting HSP90 for cancer therapy. Br J Cancer 2009;100:1523–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walton-Diaz A, Khan S, Bourboulia D, Trepel JB, Neckers L, Mollapour M. Contributions of co-chaperones and post-translational modifications towards Hsp90 drug sensitivity. Future Med Chem 2013;5:1059–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mollapour M, Tsutsumi S, Donnelly AC, Beebe K, Tokita MJ, Lee MJ, Lee S, Morra G, Bourboulia D, Scroggins BT, Colombo G, Blagg BS, Panaretou B, Stetler-Stevenson WG, Trepel JB, Piper PW, Prodromou C, Pearl LH, Neckers L. Swe1Wee1-dependent tyrosine phosphorylation of Hsp90 regulates distinct facets of chaperone function. Mol Cell 2010;37:333–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci 2005;25:9735–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor DM, Tradewell ML, Minotti S, Durham HD. Characterizing the role of Hsp90 in production of heat shock proteins in motor neurons reveals a suppressive effect of wild-type Hsf1. Cell Stress Chaper 2007;12:151–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Basso M, Samengo G, Nardo G, Massignan T, D'Alessandro G, Tartari S, Cantoni L, Marino M, Cheroni C, De Biasi S, Giordana MT, Strong MJ, Estevez AG, Salmona M, Bendotti C, Bonetto V. Characterization of detergent-insoluble proteins in ALS indicates a causal link between nitrative stress and aggregation in pathogenesis. PLoS One 2009;4:e8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirby J, Ning K, Ferraiuolo L, Heath PR, Ismail A, Kuo SW, Valori CF, Cox L, Sharrack B, Wharton SB, Ince PG, Shaw PJ, Azzouz M. Phosphatase and tensin homologue/protein kinase B pathway linked to motor neuron survival in human superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain 2011;134:506–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oppenheim RW, Yin QW, Prevette D, Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature 1992;360:755–7 [DOI] [PubMed] [Google Scholar]
- 89.Sendtner M, Arakawa Y, Stockli KA, Kreutzberg GW, Thoenen H. Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival. J Cell Sci Suppl 1991;15:103–9 [DOI] [PubMed] [Google Scholar]
- 90.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem 2002;277:39858–66 [DOI] [PubMed] [Google Scholar]
- 91.Fujita N, Sato S, Ishida A, Tsuruo T. Involvement of Hsp90 in Signaling and Stability of 3-Phosphoinositide-dependent Kinase-1. J Biol Chem 2002;277:10346–53 [DOI] [PubMed] [Google Scholar]
- 92.Yang DJ, Wang XL, Ismail A, Ashman CJ, Valori CF, Wang G, Gao S, Higginbottom A, Ince PG, Azzouz M, Xu J, Shaw PJ, Ning K. PTEN regulates AMPA receptor-mediated cell viability in iPS-derived motor neurons. Cell Death Dis 2014;5:e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ning K, Drepper C, Valori CF, Ahsan M, Wyles M, Higginbottom A, Herrmann T, Shaw P, Azzouz M, Sendtner M. PTEN depletion rescues axonal growth defect and improves survival in SMN-deficient motor neurons. Hum Mol Genet 2010;19:3159–68 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Ligand-independent activation of the P2X7 receptor by Hsp90 inhibition stimulates motor neuron apoptosis by Amy L Strayer, Cassandra N Dennys-Rivers, Karina C Ricart, Narae Bae, Joseph S Beckman, Maria Clara Franco and Alvaro G Estevez in Experimental Biology and Medicine