Abstract
Introduction
Amiodarone, a pharmaceutical extensively used to suppress atrial and ventricular tachyarrhythmias, is also known to cause many side effects on many tissues. N-acetyl-cysteine (NAC), vitamin E and vitamin C are known as antioxidants for their ability to minimize oxidative stress. In the peer-reviewed literature, there is no study reporting on the protective effects of these antioxidant agents against its hepatotoxicity.
Aim
We investigated the oxidative effects of NAC, vitamins E and C on liver tissue after amiodarone treatment.
Material and methods
Rats were randomly assigned to: control; amiodarone group; amiodarone + NAC treated group; amiodarone + Vit. E group and amiodarone + Vit. C group. Liver tissues were isolated from animals and total glutathione levels were measured.
Results
In all time intervals, the level of glutathione increased. When all time intervals were compared, the amiodarone group revealed the lowest levels. The antioxidant co-administered group was studied; the glutathione levels were statistically significantly higher than the sole amiodarone group. When vitamins E, C or N-acetyl cysteine were examined, there was no statistically significant difference among them.
Conclusions
In this study we found that hepatotoxicity capacity of amiodarone may be reduced by taking up antioxidants. In addition, the effect documented here may be reproducible and may be applied to clinical settings.
Keywords: amiodarone, toxicity, N-acetyl cysteine, vitamin E, ascorbic acid, hepatotoxicity, rats
Abstract
Wprowadzenie
Amiodaron – lek powszechnie stosowany w tachyarytmii przedsionkowej i komorowej – wywołuje wiele skutków ubocznych w różnych tkankach. N-acetylocysteina (NAC), witamina E i witamina C znane są jako przeciwutleniacze, ponieważ mają zdolność redukcji stresu oksydacyjnego. W piśmiennictwie nie ma badań opisujących ich działanie ochronne w odniesieniu do hepatotoksyczności amiodaronu.
Cel
Zbadanie wpływu oksydacyjnego NAC, witaminy E i witaminy C na tkanki wątroby po leczeniu amiodaronem.
Materiał i metody
Szczury losowo przydzielono do grupy kontrolnej, grupy otrzymującej amiodaron, grupy otrzymującej amiodaron i NAC, grupy otrzymującej amiodaron i witaminę E oraz grupy otrzymującej amiodaron i witaminę C. Od zwierząt pobrano tkanki wątroby i zbadano całkowite stężenie glutationu.
Wyniki
We wszystkich przedziałach czasowych stężenie glutationu zwiększało się. Gdy porównano wszystkie przedziały czasowe, najmniejsze jego stężenie stwierdzono w grupie leczonej amiodaronem. W grupach przyjmujących jednocześnie przeciwutleniacze stężenia glutationu były statystycznie istotnie większe niż w grupie leczonej tylko amiodaronem. Nie stwierdzono statystycznie istotnych różnic między grupami otrzymującymi witaminę E, witaminę C i NAC.
Wnioski
Zaobserwowano, że przyjmowanie przeciwutleniaczy może zmniejszać hepatotoksyczność amiodaronu. Opisany efekt jest powtarzalny, zatem może być wykorzystany w warunkach klinicznych.
Introduction
Amiodarone is a type 3 anti-arrhythmic agent that is widely used and has many toxic effects on different tissue functions, including hepatic toxicity, steatosis, gastrointestinal upset, hepatitis, bradycardia, liver and pancreas fibrosis. It was shown that amiodarone has cytotoxic effects on different cell types in cell culture studies. It is most extensively prescribed for the benefits as a drug used in atrial fibrillation (AF). Amiodarone can cause many adverse effects especially on the liver [1]. Many studies have concluded that amiodarone generates free radicals in vitro and in vivo which are the main causes of oxidative stress [2, 3]. Oxidative stress manifests by producing reactive oxygen species (ROS) in the cell [4]. Superoxide anion is a reactive and superoxide dismutase that converts superoxide to hydrogen peroxide, which is less reactive than oxygen anion. Glutathione peroxidase detoxifies hydrogen peroxide to water [4]. Reduced glutathione (GSH) regulates the activity of glutathione peroxidase by giving hydrogen. Therefore, GSH is considered as a detoxifying molecule. Glutathione is present mostly in reduced form (GSH) in the cell. It can be oxidized to GSSG (oxidized glutathione), which is approximately 1% of total glutathione in the cell in normal conditions [5]. GSH has an important role in detoxification and it can help regenerating some antioxidants such as vita-min C and E, which are also capable of detoxifying hydrogen peroxide [4]. In addition to them, N-acetyl cysteine (NAC) is another antioxidant molecule. It is a cys residue with acetylation and in this way it becomes a part of the cysteine pool in the cell to maintain the biosynthesis of GSH [6]. Deficiency of GSH or the ratio of GSH/GSSG could be a marker for several pathological problems [5]. As a result of AF, in myocardial tissues, high levels of superoxide and hydrogen peroxide are observed [7–10]. Also, as an oxidative stress marker, the ratio of GSSG to GSH (reduced glutathione) was found increased in blood samples of patients with AF [11]. Both AF and using amiodarone as a drug for AF can be obvious causes of increased ROS and consequently change of total concentration of GSH in cells and related tissues. Many studies have shown that using antioxidants such as NAC, vitamin C and vitamin E could be helpful to minimize the effects of oxidative stress in the cell [3]. With this information, using antioxidants could reduce oxidative stress, which is a result of amiodarone as a drug for AF.
Recently, especially amiodarone’s toxic effects on the liver have not been well studied and no clinical agents are available for prevention. Oxidative stress has been proved to be the major pathogenic mechanism of amiodarone’s tissue damage effects. From this perspective, the aim of this study is to investigate toxic effects of amiodarone and the prevention potential of NAC, vitamin E and vitamin C in a rat experimental model.
Aim
We studied the level of glutathione in rat liver tissue, following the oral administration of amiodarone and antioxidants.
Material and methods
Agents used
Amiodarone hydrochloride (Cordorone, 200 mg, Sanofi-Aventis, Istanbul, Turkey), vitamin E (Evicap, 400 I.U., İlaç ve Kimya Sanayi A.Ş., Çerkezköy, Tekirdağ, Turkey), ascorbic acid (Redoxon, 500 mg/tablet, Bayer Türk Kimya San Ltd. Şti., Istanbul, Tukey), and N-acetyl cysteine (NAC, 200 mg/tablet, Bilim İlaç San ve Tic. A.Ş., Gebze, Kocaeli, Turkey) were purchased from a pharmacy. Glutathione Assay Kit (CAS number: CS0260) was obtained from Sigma-Aldrich (St. Louis, MO).
Animals
Thirty Wistar albino male rats were used with the approval of the Animal Care and Use Committee of Hacettepe University (Permit no. 2013/5-10) and carried out in accordance with the National Guidelines for the Use and Care of Laboratory Animals. After all animals were purchased from Animal Laboratory Units of Hacettepe University, they were allowed to become adapted to the conditions of the laboratory for seven days. All animals were housed randomly selected as three animals per cages, using a 12 h light-dark cycle.
Design of the study
All rats were randomly assigned to five groups as follows: control group (untreated group, n = 6), amiodarone treated group (100 mg/kg/day, n = 6), amiodarone + Vit. E treated group (50 mg/kg/day, n = 6), amiodarone + Vit. C treated group (50 mg/kg/day, n = 6), amiodarone + NAC treated group (50 mg/kg/day, n = 6). Amiodarone and all other substance were given orally for 7 days. The amiodarone dose is higher compared to the dose used in human beings due to the fact that the metabolism of rats is higher compared to human beings [12]. All antioxidant agents were given 1 h after administration of amiodarone. All the animals were sacrificed under anesthesia on the 8th day. Liver tissues were taken from the animals, homogenized in 0.9% NaCl and 10% (w/v) homogenates were prepared. Total glutathione was determined in the liver homogenates. Experiments were carried out at the temperatures given in the procedures.
Glutathione assay
The level of total glutathione (GSH and GSSG) in the cell was measured using the Glutathione Assay Kit (Sigma-Aldrich, USA) according to the manufacturer’s instructions. Liver samples was firstly deproteinized with 5% 5-sulfosalicylic acid solution and then centrifuged to remove the precipitated protein. Supernatants of the samples were used for the determination of the glutathione according to the manufacturer’s protocol. Samples were read with kinetically at 1 min intervals for 5 min at 412 nm using an ELISA reader (EZ Read 400 Micro-plate Reader, Biochrom).
Statistical analysis
Data are presented as mean ± SD in Table I and mean ± SEM in Figure 1. Differences between groups were analyzed using analysis of variance (ANOVA) for triplicate measurements. The level of significance was taken as p < 0.05 in all instances. GraphPad Prism 5.01 for Windows (GraphPad Software) was used for statistical analysis. In cases of significance, Bonferroni adjusted Mann-Whitney U test was used to determine the efficient group.
Table I.
Total glutathione data of all groups (μM)
| t [min] | Control | Amiodarone | P-value** | Amiodarone + Vit. C | Amiodarone + NAC | Amiodarone + Vit. E |
|---|---|---|---|---|---|---|
| 1 | 584.2 ±91.2 | 150.5 ±87.1 | < 0.0001 | 321.4 ±126.1 p*** < 0.0001 |
332.8 ±118.7 p*** < 0.0001 |
372.7 ±92.0 p*** < 0.0001 |
| 2 | 607.7 ±91.4 | 162.2 ±96.3 | < 0.0001 | 334.3 ±134.1 p*** < 0.0001 |
345.7 ±125.6 p*** < 0.0001 |
386.8 ±95.2 p*** < 0.0001 |
| 3 | 627.0 ±88.2 | 176.3 ±104.7 | < 0.0001 | 347.7 ±142.7 p*** < 0.0001 |
360.1 ±132.8 p*** < 0.0001 |
402.3 ±99.2 p*** < 0.0001 |
| 4 | 644.3 ±83.6 | 188.8 ±113.8 | < 0.0001 | 363.6 ±151.5 p*** < 0.0001 |
375.5 ±140.0 p*** < 0.0001 |
419.7 ±104.4 p*** < 0.0001 |
| 5 | 659.4 ±77.4 | 205.4 ±122.5 | < 0.0001 | 380.0 ±157.7 p*** < 0.0001 |
392.8 ±147.4 p*** < 0.0001 |
440.6 ±109.3 p*** < 0.0001 |
| P-value* | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
2-way ANOVA
comparison between control vs. amiodarone groups/Bonferroni adjusted Mann-Whitney U
comparison between groups vs. amiodarone group/Bonferroni adjusted Mann-Whitney U.
All data are presented as mean ± SD. The level of significance was taken as p < 0.05. t – time, Vit. C – vitamin C, NAC – N-acetyl cysteine, Vit. E – vitamin E.
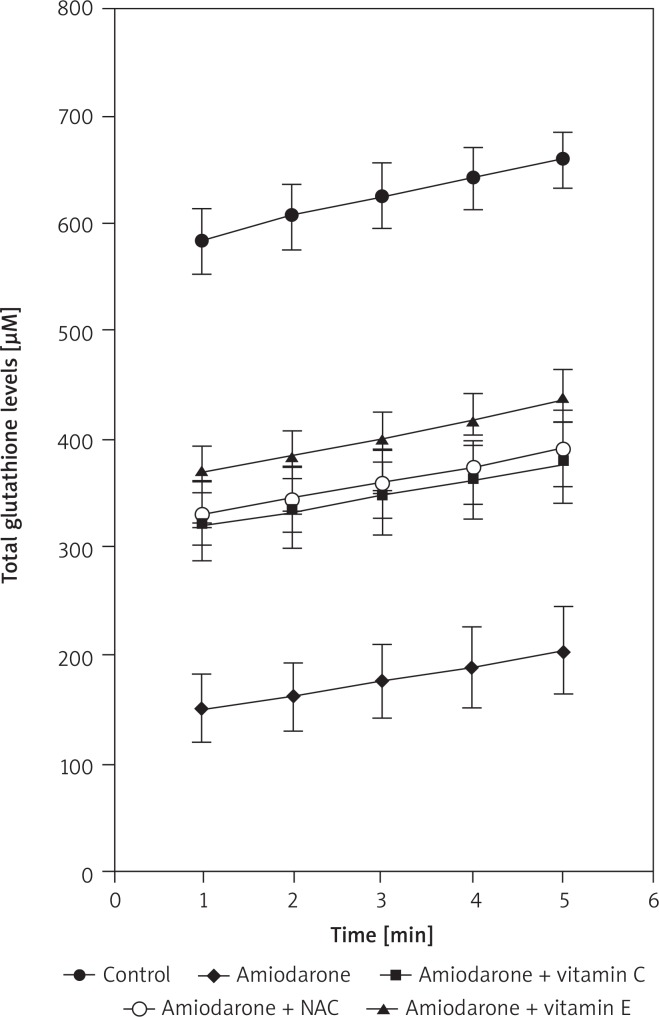
Fig. 1.
Results of total glutathione levels (μM) in rat liver tissues with kinetic read at 1-minute intervals for 5 min
Results
Total glutathione amount of the liver samples of rats was measured spectrophotometrically with kinetic read at 1-minute intervals for 5 minutes. When the groups were examined individually there was a significant increase in glutathione levels with time (p < 0.0001) (Table I). When separate time intervals were compared, the amiodarone treated group had the lowest levels compared to control and each antioxidant co-administered group in all time intervals (p < 0.0001), which may demonstrate the protective potential of antioxidants (Fig. 1). When the antioxidants were compared, there was no difference between amiodarone + NAC, amiodarone + Vit. C and amiodarone + Vit. E (p > 0.05 for each).
Discussion
Hepatic injury is a common manifestation of drug toxicity and may be reflected as different clinical features [13]. Mitochondrial dysfunction is one of these features and clinical studies scrutinizing mitochondrial dysfunction came a long way for decades [14]. Mitochondrial dysfunction manifests itself in different ways such as membrane permeabilization, oxidative phosphorylation impairment, inhibition of fatty acid oxidation and mitochondrial DNA depletion [14]. Within the cell, mitochondria are the source of ROS production through the mitochondrial respiratory chain. In this respiratory chain, electrons enter the system and directly react with oxygen to generate superoxide anion radical. Then this radical is converted to hydrogen peroxide (H2O2) by superoxide dismutase. Hydrogen peroxide is detoxified to water by glutathione peroxidase which uses reduced glutathione (GSH). In healthy tissues, all reactive oxygen species are detoxified properly by mitochondrial systems. However, in different pathophysiological situations, GSH depletion was seen in liver mitochondria, which reduce the capacity of H2O2 detoxification. Hydrogen peroxide accumulation causes mitochondrial dysfunction. Amiodarone can also induce hepatotoxicity due to the mitochondrial dysfunction [15–18]. Also, many studies have documented that it has long-term effects on different tissues. After discontinuation of the drug, the effects of amiodarone were still detected in patients’ different tissues including liver, lung and adipose tissue [19]. In our study, we measured total glutathione level (total GSSG and GSH in μM) of rat livers which were administered with amiodarone. Compared to the control group (which has high levels of glutathione), the amiodarone administered group showed dramatically reduced glutathione levels at each time interval, which shows the reduced detoxification capacity of mitochondria. On the other hand, it was shown that treatment with antioxidants such as vitamin C, vitamin E and NAC has preventive effects on reduced detoxification capacity of mitochondria [20–23]. Both cell culture studies and animal model experiments on amiodarone in relation to several antioxidants show similar beneficial effects of antioxidants to reduce toxicity of amiodarone [24–27]. These antioxidants have similar mechanisms in antioxidant action via scavenging ROS [28, 29]. Also, NAC has importance for production of glutathione in the cell because its cysteine part together with glutamate and glycine is a precursor of glutathione [30]. Glutathione is already an antioxidant in the cell; therefore, NAC administration has a kind of backup. To analyze the antioxidant effects of vitamin C, vitamin E and NAC on amiodarone toxicity, we treated rats with both amiodarone and these antioxidants separately as described above. All antioxidants that we used showed increased glutathione levels when they were coadministered with amiodarone compared to the reduced glutathione level of the amiodarone group. They could not produce glutathione like in the control group but when compared to the effect of amiodarone only, we can say that antioxidant usage is successful in producing glutathione in the cell. The group receiving vitamin E coadministered with amiodarone had the highest level of glutathione, followed by the NAC + amiodarone group and Vit. C + amiodarone group. It is already known that separate treatments of NAC, vitamin C and vitamin E on cultured cells have antioxidant effects [3].
Conclusions
Our results are important in terms of understanding the toxic effects of amiodarone and its prevention via use of antioxidants. In our previous study, we demonstrated the decrease of amiodarone cytotoxicity using antioxidants in cell culture [3]. Now, we showed the toxicity reducing capacity of antioxidants in animal experiments. To make a comprehensive conclusion, further analysis such as separate measurement of GSSG and GSH amounts, glutathione peroxidase enzyme activity and measurement of lipid peroxidation products could be supportive. In addition, we believe that this study is reproducible, and clinical studies may be performed based on these findings.
Disclosure
The authors report no conflict of interest.
Biography

References
- 1.Mason JW. Amiodarone. N Engl J Med. 1987;316:455–466. doi: 10.1056/NEJM198702193160807. [DOI] [PubMed] [Google Scholar]
- 2.Vereckei A, Blazovics A, Gyorgy I, Feher E, Toth M, Szenasi G, Zsinka A, Foldiak G, Feher J. The role of free radicals in the pathogenesis of amiodarone toxicity. J Cardiovasc Electrophysiol. 1993;4:161–177. doi: 10.1111/j.1540-8167.1993.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 3.Durukan AB, Erdem B, Durukan E, Sevim H, Karaduman T, Gurbuz HA, Gurpinar A, Yorgancioglu C. May toxicity of amiodarone be prevented by antioxidants? A cell culture study. J Cardiothor Surg. 2012;7:61. doi: 10.1186/1749-8090-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25:287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camera E, Picardo M. Analytical methods to investigate glutathione and related compounds in biological and pathological processes. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:181–206. doi: 10.1016/s1570-0232(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 6.Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cycteine supplements and exercise-induced oxidaive stress. J Int Soc Sports Nutr. 2005;2:38–44. doi: 10.1186/1550-2783-2-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JP, Chen MC, Liu WH, Yang CH, Chen CJ, Chen YL, Pan KL, Tsai TH, Chang HW. Atrial myocardial nox2 containing NADPH oxidase activity contribution to oxidative stress in mitral regurgitation: potential mechanism for atrial remodeling. Cardiovasc Pathol. 2011;20:99–106. doi: 10.1016/j.carpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 9.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Youn JY, Kim AY, Ramirez RJ, Gao L, Ngo D, Chen P, Scovotti J, Mahajan A, Cai H. NOX4-dependent hydrogen peroxide overproduction in human atrial fibrillation and hl-1 atrial cells: relationship to hypertension. Front Physiol. 2012;3:140. doi: 10.3389/fphys.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC., Jr Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larrey D. Drug-induced liver diseases. J Hepatol. 2000;32:77–88. doi: 10.1016/s0168-8278(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 14.Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335–353. doi: 10.1111/j.1472-8206.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 15.Berson A, Descatoire V, Sutton A, Fau D, Maulny B, Vadrot N, Feldmann G, Berthon B, Tordjmann T, Pessayre D. Toxicity of alpidem, a perpheral benzodiazepine receptor ligand, nut not zolpidem, in rat hepatocytes: role of mitochondrial permeability transition and metabolic activation. J Pharmacol Exp Ther. 2001;299:793–800. [PubMed] [Google Scholar]
- 16.Berson A, Fau D, Fornacciari R, Degove-Goddard P, Sutton A, Descatoire V, Haouzi D, Lettéron P, Moreau A, Feldmann G, Pessayre D. Mechanisms for experimental buprenorphine hepatotoxicity: major role of mitochondrial dysfunction versus metabolic activation. J Hepatol. 2001;34:261–269. doi: 10.1016/s0168-8278(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 17.Larosche I, Letteron P, Fromenty B, Vadrot N, Abbey-Toby A, Feldmann G, Pessayre D, Mansouri A. Tamoxifen inhibits topoisomerases, depletes mitochondrial DNA, and triggers steatosis in mouse liver. J Pharmacol Exp Ther. 2007;321:526–535. doi: 10.1124/jpet.106.114546. [DOI] [PubMed] [Google Scholar]
- 18.Waldhauser KM, Torok M, Ha HR, Thomet U, Konrad D, Brecht K, Follath F, Krähenbühl S. Hepatocellular toxicity and pharmacological effect of amiodarone and amiodarone derivatives. J Pharmacol Exp Ther. 2006;319:1413–1423. doi: 10.1124/jpet.106.108993. [DOI] [PubMed] [Google Scholar]
- 19.Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol. 2011;54:773–794. doi: 10.1016/j.jhep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 21.Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, Ozguner F, Dogan A, Ibrisim E. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. Eur Heart J. 2008;29:625–631. doi: 10.1093/eurheartj/ehn011. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigo R, Vinay J, Castillo R, Cereceda M, Asenjo R, Zamorano J, Araya J, Castillo-Koch R, Espinoza J, Larraín E. Use of vitamins C and E as a prophylactic therapy to prevent postoperative atrial fibrillation. Int J Cardiol. 2010;138:221–228. doi: 10.1016/j.ijcard.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 23.Korantzopoulos P, Kolettis TM, Kountouris E, Dimitroula V, Karanikis P, Pappa E, Siogas K, Goudevenos JA. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol. 2005;102:321–326. doi: 10.1016/j.ijcard.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 24.Golli-Bennour EE, Bouslimi A, Zouaoui O, Nouira S, Achour A, Bacha H. Cytotoxicity effects of amiodarone on cultured cells. Exp Toxicol Pathol. 2012;64:425–430. doi: 10.1016/j.etp.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Bolt MW, Racz WJ, Brien JF, Massey TE. Effects of vitamin E on cytotoxicity of amiodarone and N-desethylamiodarone in isolated hamster lung cells. Toxicology. 2001;166:109–118. doi: 10.1016/s0300-483x(01)00451-6. [DOI] [PubMed] [Google Scholar]
- 26.Card JW, Racz WJ, Brien JF, Massey TE. Attenuation of amiodarone-induced pulmonary fibrosis by vitamin E is associated with suppression of transforming growth factor-beta1 gene expression but not prevention of mitochondrial dysfunction. J Pharmacol Exp Ther. 2003;304:277–283. doi: 10.1124/jpet.102.043208. [DOI] [PubMed] [Google Scholar]
- 27.Ágoston M, Örsi F, Fehér E, Hagymási K, Orosz Z, Blázovics A, Fehér J, Vereckei A. Silymarin and vitamin E reduce amiodarone-induced lysosomal phospholipidosis in rats. Toxicology. 2003;190:231–241. doi: 10.1016/s0300-483x(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 28.Viswanatha Swamy AH, Wangikar U, Koti BC, Thippeswamy AH, Ronad PM, Manjula DV. Cardioprotective effect of ascorbic acid on doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol. 2011;43:507–511. doi: 10.4103/0253-7613.84952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyewole AO, Birch-Machin MA. Mitochondria-targeted antioxidants. FASEB J. 2015;29:4766–4771. doi: 10.1096/fj.15-275404. [DOI] [PubMed] [Google Scholar]
- 30.Sansone RA, Sansone LA. Getting a knack for NAC: N-acetyl-cysteine. Innov Clin Neurosci. 2011;8:10–14. [PMC free article] [PubMed] [Google Scholar]