Abstract
Protein arginine deiminase (PAD) enzymes catalyze the conversion of protein- bound arginine into citrulline, an irreversible posttranslational modification with loss of a positive charge that can influence protein-protein interactions and protein structure. Protein arginine deiminase activity depends on high intracellular calcium concentrations occurring in dying cells. In this study, we demonstrate that protein citrullination is common during pyroptotic cell death in macrophages and that inhibition of PAD enzyme activity by Cl-amidine, a pan-PAD inhibitor, blocks NLRP3 inflammasome assembly and proinflammatory IL-1β release in macrophages. Genetic deficiency of either PAD2 or PAD4 alone in murine macrophages does not impair IL-1β release; however, pharmacological inhibition or small interfering RNA knockdown of PAD2 within PAD4−/− macrophages does. Our results suggest that PAD2 and 4 activity in macrophages is required for optimal inflammasome assembly and IL-1β release, a finding of importance for autoimmune diseases and inflammation.
Introduction
Citrullination is a posttranslational modification controlled by calcium-dependent enzymes, called protein arginine deiminases (PADs; peptidylarginine deiminases, EC3.5.3.15) that convert the guanidinium group of protein-bound arginine into a ureido group (1). Of the five known PAD isoforms (PAD1–4 and PAD6), only PAD2 and PAD4 are expressed in macrophages (2, 3). In murine or human macrophages, PAD enzymes are inactive, and citrullinated proteins are not present under resting conditions. However, under conditions that raise intracellular calcium (for instance, when macrophages are treated with the calcium ionophore ionomycin), several proteins, such as the type III intermediate filament vimentin, are rapidly deiminated, a process also known as cellular hypercitrullination (4, 5). Little is known about the role of PAD enzymes under physiologic conditions or during innate immune responses in macrophages. It is assumed that the intracellular calcium threshold required to activate PAD enzymes is reached during apoptosis; however, no information exists about other cell death pathways in macrophages that can activate PAD enzymes.
A large body of evidence demonstrates the importance of PAD enzyme activation in neutrophils during host defense, inflammation and autoimmunity. In neutrophils, PAD4 is required for LPS-induced histone citrullination and neutrophil extracellular trap formation (6, 7). Neutrophil extracellular traps were originally identified to avidly bind bacteria and aid in the clearance of bacterial infection (8). Later, neutrophil extracellular traps were also shown to be an important source of DNA and DNA-bound autoantigens such as histones and citrullinated vimentin in systemic lupus erythematosus and rheumatoid arthritis, respectively (9, 10).
An inflammatory form of cell death in macrophages involving caspase-1 and proinflammatory IL-1β is called pyroptosis (11) and tightly regulated at the transcriptional (12) and post-translational levels (13). Caspase-1 activity is controlled by inflammasomes, which are multiprotein signaling complexes that detect microbial-derived molecular signatures or endogenous danger signals. TLR stimulation of macrophages induces the synthesis of a biologically inactive precursor protein, pro-IL-1β as well as the NLR and AIM-2 like sensor proteins required for inflammasome assembly. Once activated, NLR or AIM2-like proteins recruit the apoptosis-associated speck-like protein containing a CARD domain (ASC) through homotypic interactions via their pyrin domains. ASC, in turn, binds procaspase-1 via its CARD domain to release active p20 caspase-1 by self-cleavage, which then matures the cytokine proforms of IL-1β and IL-18 into their biologically active counterparts (14, 15). Of the NLR proteins, the NLRP3 protein is particularly well studied and is activated by microbial toxins such as nigericin, derived from Streptomyces hygroscopicus (16), as well as danger-associated molecular patterns (DAMPs) such as extracellular ATP (17). Interestingly, high concentrations of extracellular calcium or the mobilization of calcium from ER stores also triggers NLRP3 inflammasome activation in macrophages (18–20). However, it is unknown if calcium-dependent PAD enzymes are activated in macrophages during pyroptosis.
In this study, we demonstrate that protein citrullination is induced upon inflammasome activation in murine macrophages. Furthermore, we show that PAD enzyme inhibition reduced IL-1β release as a consequence of diminished inflammasome assembly.
Material and Methods
Reagents
The following reagents were used: ultra-pure LPS, nigericin, poly(deoxyadenylic-deoxythymidylic) acid [poly(dA:dT)]/lyoVec, BAPTA-AM, calcium-free DMEM, disuccinimidyl suberate, Lipofectamine 2000 (all Invivogen), calcium chloride solution, thapsigargin, ATP (Sigma-Aldrich), CI-amidine (Millipore), BB-CI-amidine and GSK484 (Cayman Chemicals), AFM 30a (P. Thompson, University of Massachusetts Medical School, Worcester, MA) (21), iScript cDNA kit and iQ SYBR green supermix (BioRad), TRIzol (Ambion, Life technologies), fixation/permeabilization solution, mouse IL-1β ELISA kit (BD Bioscience), propidium iodide (ImmunoChemistry Technologies) and mouse TNF- alpha ELISA (eBioscience). Abs for confocal microscopy were ASC (AdipoGen), peptidyl-citrulline, clone F95 (Millipore), anti-vimentin (Abcam), goat anti-rabbit IgG AF647, goat anti-mouse IgM AF488 (Thermo Fisher Scientific), and goat anti-mouse IgM isotype control (SouthernBiotech). DAPI was from Molecular Probes.
Quantitative PCR
For quantitative PCR, cDNA was amplified with gene-specific forward and reverse primers using SYBR green mix (primers available on request). PCR was performed as follows: 5 mins at 95°C, 45 cycles (each cycle at 95°C for 30 s, 60°C for 30 s, 72°C for 30 s), and 72°C for 5 mins.
Immunoblots
Abs used for immunoblots were ASC (N-15)-R, β-actin, rabbit anti-goat IgG HRP, goat anti-mouse IgG HRP (all Santa Cruz Biotechnology), caspase-1 p20 (AdipoGen), mouse IL-1β (R&D Systems) and goat anti-rabbit IgG HRP (Biorad). The anti-citrulline detection kit was from Millipore. ASC cross-linking was performed as described (22).
Cell stimulation
Bone marrow-derived macrophages (BMDMs) were generated as described (23) from mice of either the C57BL/6 or the FVB background for wild-type (WT), PAD2−/− and PAD4−/−. BMDMs were primed with 200ng/ml LPS in RPMI 1640 for 2 h prior to all experiments, or treated with thapsigargin (300 nM) or BAPTA-AM (25 μM) for 30 mins in DMEM or Ca2+ - free DMEM media before stimulating with nigericin, as indicated. BMDMs were treated with CI-amidine, BB-CI-amidine, GSK484 or AFM 30a in doses ranging from 2 to 200 μM, 0.008 to 20 μM, 0.5 to 20 μM, 5 to 0.5 μM for 1 h. PAD2 small interfering RNA (siRNA) knockdown in BMDMs was done with mouse smart pool Accell Padi2 and scrambled siRNA (Dharmacon) according to the manufacturer’s instructions. Cells were stimulated with ATP (5 mM) for 1 h, with CaCl2 (1 mM) for 6 h, with nigericin (5 μM) for 1 h or with poly(dA:dT) (2 μg/ml) for 6 h.
ASC speck detection in human PBMCs
PBMC stimulation and ASC speck detection in monocytes via flow cytometry was performed as described previously (24). Briefly, 1 × 106 PBMCs were primed with 100 ng/ml LPS for 4 h, treated with 2, 20 or 200 μM CI-amidine, and stimulated with nigericin for 20 min.
Results
NLRP3 inflammasome activation induces citrullination in murine BMDMs.
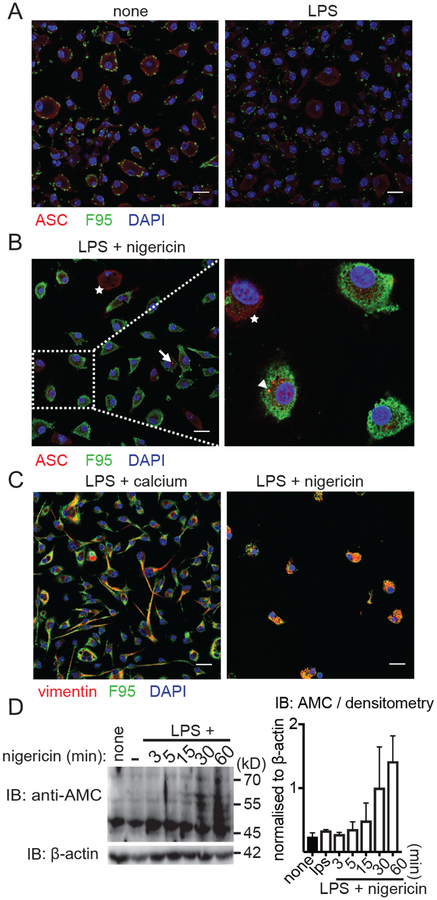
To test if proteins are citrullinated in murine BMDMs following stimulation with LPS, we compared cells either left untreated or stimulated by LPS using a citrulline-specific Ab (F95) and imaged BMDMs by confocal microscopy. Untreated BMDMs showed very little citrullination, which did not increase in LPS- treated cells (Fig. 1A), but was slightly higher in intensity compared with the isotype control (Supplemental Figure 1A). In contrast, BMDMs primed with LPS and subsequently stimulated with an NLRP3 inflammasome activator, nigericin, were strongly citrullinated. Citrullination was observed at an early time point after nigericin addition, when the inflammasome adaptor ASC was beginning to redistribute into ASC specks. Interestingly, in some instances, we also noticed a colocalization of citrullinated proteins with ASC specks (Fig. 1B, arrowhead). However, not all ASC specks were citrullinated (Fig. 1B, triangle). Unactivated cells with an even cytoplasmic distrubution of ASC were also not citrullinated (Fig. 1B, star). As a control, we used a known substrate for PAD enzymes during apoptosis, the intermediate filament vimentin (5). Interestingly, vimentin was strongly citrullinated in macrophages during inflammasome activation with either extracellular calcium or nigericin (Fig. 1C). To confirm the microscopically observed inflammasome-induced protein citrullination by another citrulline- specific detection method, we again stimulated BMDMs with LPS and nigericin and used a chemical citrulline-specific modification of the immunoblotted cellular lysates (with 2,3-butanedione and antipyrine in an acidic environment), that was then detected by an anti-modified citrulline Ab (25). In agreement with the microscopy data, we observed a rapid and pronounced protein citrullination in LPS-primed and nigericin-activated BMDMs (Fig. 1D). Together, these data indicate that protein citrullination is widespread following NLRP3 inflammasome activation in macrophages.
Figure 1. Inflammasome activation induces protein citrullination in murine macrophages.
(A) Confocal images of BMDMs stained with anti-ASC Ab (red), F95 (green), and DAPI (nuclei, blue) either left untreated (left panel) or primed with LPS for 2h (right panel). (B) BMDMs were stimulated with LPS and nigericin for 15 min and prepared for confocal imaging as in (A). Citrullinated and non-citrullinated ASC specks are indicated by an arrowhead or triangle, respectively, and nonactivated cells with evenly distributed cytoplasmic ASC are marked by stars. (C) Confocal images of BMDMs stained with anti-vimentin Ab (red) and F95 (green) stimulated for 6h (Ca2+) and 60 min (nigericin) as indicated. (D) Anti-modified citrulline (AMC) immunoblot of cell lysates from LPS-primed and nigericin-stimulated BMDMs at the indicated time points (one representative immunoblot of three independent experiments and quantification by band densitometry; mean ± SEM). Images were analyzed by Image J. Scale bar, 20 μm.
PAD enzyme inhibition impairs inflammasome activation and IL-1β release in macrophages.
We next sought to identify which PAD enzymes are responsible for the protein citrullination in macrophages during pyroptosis. There are five homologs of PAD enzymes (PAD1–4 and PAD6) (26), of which only PAD2 and PAD4 are expressed in human monocytes and macrophages (2). Because no data on PAD expression in murine macrophages exist, we performed quantitative RT-PCR for PAD enzyme expression in murine C57BL/6 BMDMs (Supplemental Figure 1B). Comparable to human macrophages, we found only PAD2 and PAD4 expressed in murine BMDMs.
Because citrullination was common during pyroptosis, we consequently assessed the effect of PAD enzyme inhibition on inflammasome activation. In LPS-primed and then pan-PAD inhibitor (Cl-amidine)-treated BMDMs, we observed a marked reduction of IL-1β following NLRP3 activation with Ca2+, ATP, or nigericin (Fig. 2A). Of note, there was no increase in cytoxicity due to PAD inhibitor treatment in BMDMs (Supplemental Fig 1C). TNF-αproduction is commonly used as a readout to demonstrate specificity of a given inhibitor toward the inflammasome. TNF-a production was not diminished by addition of Cl-amidine to LPS-treated BMDMs as compared to LPS-treated and Ca2+ -, ATP - or nigericin-activated BMDMs. This indicates the inflammasome specific effect of the PAD inhibitor (Fig. 2B). Furthermore, treatment with Cl-amidine led to a reduction of the p20 active caspase-1 subunit in the supernatant of BMDMs stimulated with the NLRP3 agonists Ca2+, ATP or nigericin (Fig. 2C) and consistently also to a reduction in overall protein citrullination (Fig. 2D, 2E). Subsequently, we determined whether PAD enzyme blockade inhibited caspase-1 directly or at an earlier step and at the level of NLRP3 inflammasome assembly. We speculated that Cl-amidine treatment of NLRP3 inflammasome- activated BMDMs would block ASC multimerization. Therefore, we treated LPS -primed BMDMs with ATP in the presence of the pan-PAD inhibitor and found a marked reduction in ASC oligo- / multimers (Fig. 2F), indicating that PAD enzymes have a previously unknown role in the assembly of the NLRP3 inflammasome. In addition, we determined whether PAD inhibitor treatment blocks ASC speck assembly in human monocytes. Therefore, we employed a FACS-based method to detect ASC speck formation in human peripheral blood monocytes (24) and found that Cl-amidine blocked nigericin-induced ASC speck formation (Supplemental Fig. 1D, 1E). In conclusion, these data indicate that PAD enzyme blockade impairs NLRP3 inflammasome assembly and IL-1β secretion in macrophages.
Figure 2. PAD enzyme inhibition blocks IL-1β release in NLRP3-activated macrophages.
ELISA for IL-1β (A) and TNF-α (B) of supernatants (SNs) from NLRP3-activated BMDMs stimulated as indicated and treated with increasing concentrations of the pan-PAD inhibitor Cl-amidine (n=4, mean ± SEM for duplicate samples). (C) Immunoblot for IL-1β, caspase-1 and β-actin of SNs and cell lysate (CL) from BMDMs stimulated as in (A) (one representative out of four independent experiments is shown). (D) AMC immunoblot of CL from BMDMs treated with LPS plus ATP or (E) LPS plus nigericin and increasing concentrations of Cl-amindine (one representative of two independent experiments). (F) Immunoblot analysis of DSS cross-linked ASC of LPS- and ATP-stimulated BMDMs treated with increasing concentrations of Cl-amidine (one representative of two independent experiments and quantification by band densiometry). dA:dT, poly (dA:dT).
PAD enzyme inhibition partially impairs AIM2 inflammasome activation.
We next assessed whether the effect of PAD inhibition would extend onto a non-NLR inflammasome such as the AIM2 inflammasome, which assembles upon binding of cytoplasmic dsDNA. LPS-primed BMDMs were, therefore treated with CI-amidine and then transfected with poly(dA:dT). Again, a dose dependent inhibition of IL-1b release by CI-amidine was observed; however, the inhibition was only roughly 50%, whereas we observed a reduction of ~ 85% in CI-amidine- treated and then NLRP3 inflammasome-activated BMDMs (Supplemental Fig. 2A, 2B). TNF-α secretion was not attenuated in the presence of the PAD inhibitor (Supplemental Fig. 2C). Hence, these data demonstrate that PAD enzymes may be more relevant for NLRP3 than for AIM2 inflammasome assembly and activation.
PAD enzyme inhibition does not significantly impair TLR4 induced pro-IL-1β protein synthesis.
In the context of inflammasome signaling, upregulation of NLR proteins as well as pro-IL-1β in response to TLR4 priming is an essential component of inflammasome activation and proinflammatory IL-1β production (12). PAD4 has recently been shown to interact with the E2F-1 transcription factor in HL60 granulocytic cells (27). Moreover, E2F-1 is also regulated by TLR4 signaling (28). In HL60 cells, the E2F-1 transcription factor is recruited to inflammatory gene promoters in response to TLR4 stimulation to upregulate IL-1β and TNF-α gene expression, and Cl-amidine treatment hindered binding of E2F-1 to the IL-1β promoter (27). Therefore, we addressed whether PAD inhibition in macrophages exerted an inhibitory effect already at the level of pro-IL-1β protein synthesis. We thus treated LPS-primed BMDMs with Cl-amidine in the exact order of ‘inhibitor to LPS’ that all prior experiments had been performed with. In this experimental setup, we did not observe a relevant reduction in pro-IL-1β protein synthesis. However, when BMDMs were incubated with the PAD inhibitor prior to LPS stimulation, there was a minor reduction in pro-IL-1β synthesis (Supplemental Fig. 1F, 1G), although not enough to explain the extent of IL-1β inhibition at the level of cytokine maturation (Fig. 2A, 2C). Because the assay conditions employed throughout the study encompassed treatment with the PAD inhibitor after LPS priming, we conclude that the major mechanism of IL-1β reduction by PAD enzyme inhibition is not at the level of pro-IL-1β synthesis.
PAD2 and 4 synergistically regulate IL-1β release in NLRP3 inflammasome- activated BMDMs.
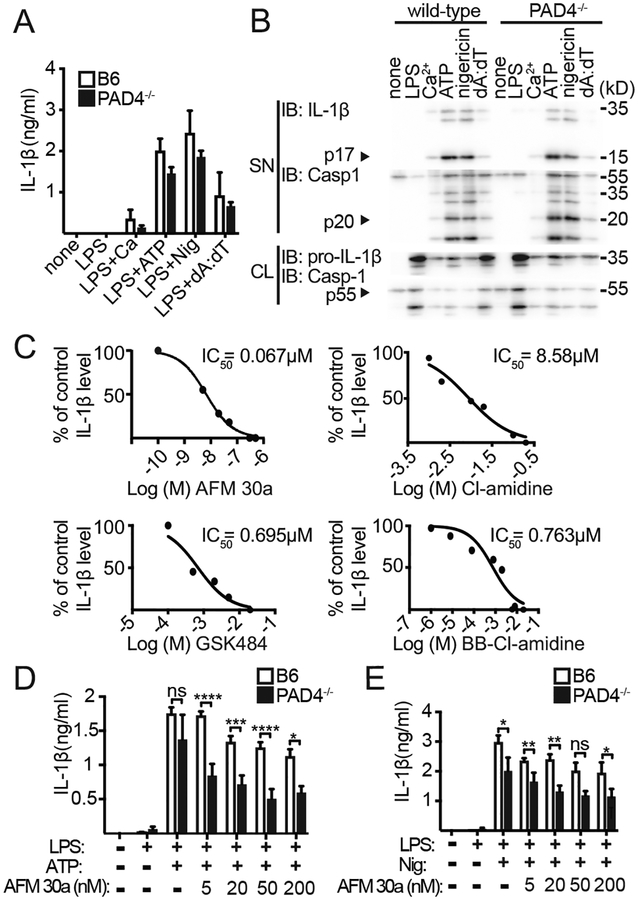
We next sought to establish which of the PAD enzymes expressed in macrophages, PAD2 and/or PAD4, was required for IL-1β release and inflammasome assembly. Comparing BMDMs from WT to those from PAD2−/− and PAD4−/− single-deficient mice, we observed only a minor influence of PAD enzymes on Ca2+-, ATP- or nigericin-induced IL-1β maturation (Fig. 3A, 3B, Supplemental Fig 2D). Because PAD2/PAD4 double-deficient mice are not available (because of the close proximity of these two genes in the murine genome), we used siRNA to knock down PAD2 within PAD4−/− and WT macrophages. Knockdown of PAD2 within PAD4−/− macrophages resulted in some reduction of IL-1β release (Supplemental Fig. 2E); however, because primary macrophages are difficult to transfect with siRNA, we sought additional evidence by testing a PAD2-specific inhibitor (AFM 30a) and a PAD4-specific inhibitor (GSK484) as well as another pan-PAD inhibitor, BB-CI-amidine. All of these efficiently suppressed IL-1β release from LPS- and nigericin-treated BMDMs, and the calculated IC50 values were lower than with Cl-amidine (Fig. 3C). However, higher concentrations of either the PAD2-specific or the PAD4- specific inhibitor were also sufficient to block IL-1β production in WT BMDMs. Therefore, to demonstrate a synergistic role for both PAD enzymes on IL-1β production, we next tested whether the PAD2-specific inhibitor AFM 30a would help to further reduce IL-1β production within PAD4−/− BMDMs when used at lower inhibitor concentrations. Indeed, the addition of the PAD2 inhibitor to PAD4−/− BMDMs significantly adds to the suppression of IL-1β release in this assay (Fig. 3D, 3E). Mechanistically, PAD2/4 enzyme activity was dependent on intracellular and/or extracellular Ca2+, as global protein citrullination was suppressed in BAPTA-AM-treated BMDMs and/or Ca2+- free medium-exposed BMDMs alongside with a reduction in mature p17 IL-1β (Supplemental Fig 2F). Together, these data suggest that the activity of PAD2 and 4 enzymes is important for efficient IL-1β maturation in NLRP3 inflammasome-activated macrophages.
Figure 3. PAD2 and PAD4 are required for NLRP3-dependent IL-1β release.
(A) IL-1β ELISA of supernatant (SN) from WT compared to PAD4-/- BMDMs. BMDMs were primed with LPS and stimulated with Ca2+, ATP, nigericin and poly(dA:dT) (WT n=3; PAD4-/- , n=3; mean ± SEM for duplicate samples). (B) Immunoblot for IL-1β and caspase-1 from SN and cell lysates (CL) of WT and PAD4−/− BMDMs stimulated as indicated. (C) Production of IL-1β from WT BMDMs stimulated with LPS and nigericin and treated with AFM 30a (n=3), CI-amidine (n=4), GSK484 (n=4), or BB-CI-amidine (n=4), as measured by ELISA. Cytokine levels are normalized to uninhibited control cells. Nonlinear regression analysis was performed, and log (M) of the indicated inhibitors versus the normalized response (variable slope) is presented. (D) IL-1β ELISA of SN from WT and PAD4−/− BMDMs primed with LPS and stimulated with ATP, or nigericin (E) and treated with increasing concentrations of AFM 30a (n=3, mean ± SEM for duplicate samples). *p<0.05; **p<0.01; ***p<0.001, ****p<0.0001 by two-way ANOVA. dA:dT, poly(dA:dT).
Discussion
We found that inflammasome activation in macrophages induces prominent protein citrullination. Different Ab-based methods exist for the identification of citrullinated amino acids, and we used the mAb F95 raised against 10 citrullinated residues (Fig. 1 A–C) together with an alternative approach, a chemical modification with 2,3-butanedione and antipyrine together with an anti-modified citrulline Ab (Fig. 1D, 2D, 2E), to confirm our results by two independent methods.
To date, and perhaps because the important role of PAD4 in the process of NETosis in neutrophils, citrullination has been studied extensively within these cells. However, there are clearly cell type-specific requirements for the activation of PAD enzymes, as stimulation of TLR4 alone is sufficient to induce citrullination in neutrophils (7), but not in macrophages (Fig 1A, 1D). Only a few earlier studies have reported citrullination secondary to extracellular calcium or ionomycin treatment within macrophages (5) (2). Interestingly, extracellular calcium is also known to induce pyroptotic cell death in macrophages (18–20), and the bacterial pore-forming toxin nigericin triggers the release of calcium from intracellular stores (29), which suggests that the cellular milieu favors PAD enzyme activation during pyroptotic cell death.
By using a pan-PAD inhibitor, Cl-amidine, we show that peptidylarginine enzyme activity is required for ASC multimerization and IL-1β maturation in murine macrophages stimulated by the NLRP3 agonists ATP, Ca2+ and nigericin (Fig. 2A, 2C, 2F). Because pan-PAD inhibitor treatment of PBMCs also leads to a reduction of ASC speck formation in human monocytes, these findings are relevant beyond murine macrophages (Supplemental Fig 1D, 1E). Importantly, PAD2 and PAD4 single-deficient BMDMs were not impaired in IL-1β maturation (Fig. 3A, 3B, Supplemental Fig 2D). Only when PAD2 was inhibited by a specific PAD2 inhibitor, AFM 30a, or siRNA-mediated transcriptional gene inactivation within BMDMs of PAD4−/− mice did we obtain results that recapitulated the effect of pan-PAD inhibition (Fig. 3D, 3E, Supplemental Fig. 2E). In addition to the currently most effective inflammasome inhibitor, MCC950, which has a reported IC50 of 7.5nM (30), we have now identified several PAD inhibitors as potent inhibitors of NLRP3 inflammasome activation and IL-1β production in macrophages (Figure 3C). Since the single-enzyme selectivity for GSK484 and AFM 30a is rhoughly 15-fold and 47-fold, respectively (31) (21), we assume that at higher concentrations these inhibitors may also block their respective isozyme counterparts (Fig. 3C). Our results further show that PAD inhibition does not have a major impact on IL-1β and TNF-α protein expression in BMDMs, a finding that is of interest because recent studies demonstrated that several cytokine promoters are regulated by PAD enzymes in myeloid granulocytic HL60 cells (27, 28). In BMDMs, however, PAD inhibition did not influence TNF-α (Fig. 2B) or pro-IL-1β synthesis (Supplemental Fig. 1F, 1G) to any degree that could explain the observed effect of PAD inhibiton on IL-1β maturation (i.e., pro-IL-1β cleavage into mature IL-1β) (Fig. 2A, 2C).
Because activation of the NLRP3 inflammasome and subsequent maturation of the proinflammatory cytokine IL-1β is dependent on protein-protein interaction between NLRP3 and vimentin (32), it is tempting to speculate that PAD enzyme activity may be required for vimentin citrullination and subsequent PAD-dependent vimentin disassembly (33) to generate vimentin fragments that could serve as a scaffolding platform for the assembly of the NLRP3 inflammasome protein complex. In summary, we demonstrate an important role for PAD2 and 4 in inflammasome formation and the release of proinflammatory IL-1β. We believe that the identification of pyroptotic macrophages as a source of citrullinated proteins could be of great relevance to inflammation and autoimmune diseases.
Supplementary Material
Key Points:
Citrullination in macrophages is induced during pyroptosis
The NLRP3 inflammasome is regulated by Ca2+ dependent PAD enzymes
Acknowledgements:
We thank Prof. C. Becker and Dr. M. Leppkes, University Hospital of Erlangen, for providing the PAD4−/− mice. We thank Prof. E. Latz for advice and discussion and U. Strube for organizational support (both University of Bonn).
This study was supported by the German Research Foundation DFG BO 4325/1–1 (LB) and HiLF of the MHH (LB). This work was supported in part by NIH grant R35 GM118112 (P.R.T.)
Footnotes
Disclosures: P.R.T. is a consultant for Celgene and Disarm Therapeutics, founded Padlock Therapeutics, and has received fees from Bristol Myers Squibb. The other authors have no financial conflicts of interest.
References:
- 1.Fujisaki M, and Sugawara K. 1981. Properties of peptidylarginine deiminase from the epidermis of newborn rats. J Biochem 89: 257–263. [DOI] [PubMed] [Google Scholar]
- 2.Vossenaar ER, Radstake TR, van der Heijden A, van Mansum MA, Dieteren C, de Rooij DJ, Barrera P, Zendman AJ, and van Venrooij WJ. 2004. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis 63: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Mechin MC, Vincent C, Nachat R, Yamada M, Takahara H, Simon M, Guerrin M, and Serre G. 2007. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum 56: 3541–3553. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, Di Pucchio T, Sims GP, Mittereder N, and Mustelin T. 2015. Characterization of the Hypercitrullination Reaction in Human Neutrophils and Other Leukocytes. Mediators Inflamm 2015: 236451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asaga H, Yamada M, and Senshu T. 1998. Selective deimination of vimentin in calcium ionophore-induced apoptosis of mouse peritoneal macrophages. Biochem Biophys Res Commun 243: 641–646. [DOI] [PubMed] [Google Scholar]
- 6.Li PX, Li M, Lindberg MR, Kennett MJ, Xiong N, and Wang YM. 2010. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 207: 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neeli I, Khan SN, and Radic M. 2008. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180: 1895–1902. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, and Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 9.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, and Gilliet M. 2011. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA-Peptide Complexes in Systemic Lupus Erythematosus. Sci Transl Med 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen PJ, Fox DA, Pennathur S, and Kaplan MJ. 2013. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci Transl Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latz E, Xiao TS, and Stutz A. 2013. Activation and regulation of the inflammasomes. Nat Rev Immunol 13: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu JH, Monks BG, Fitzgerald KA, Hornung V, and Latz E. 2009. Cutting Edge: NF-kappa B Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J Immunol 183: 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Liu ZH, and Xiao TS. 2017. Post-translational regulation of inflammasomes. Cell Mol Immunol 14: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, and Fitzgerald KA. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–U516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathinam VAK, Jiang ZZ, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, and Fitzgerald KA. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, and Nunez G. 2013. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 38: 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelegrin P, and Surprenant A. 2007. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1 beta release through a dye uptake-independent pathway. J Biol Chem 282: 2386–2394. [DOI] [PubMed] [Google Scholar]
- 18.Murakami T, Ockinger J, Yu JJ, Byles V, McColl A, Hofer AM, and Horng T. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. P Natl Acad Sci USA 109: 11282–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schoneberg T, Schaefer M, Krugel U, Smajilovic S, Brauner-Osborne H, Baerwald C, and Wagner U. 2012. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, and Chae JJ. 2012. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492: 123–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muth A, Subramanian V, Beaumont E, Nagar M, Kerry P, McEwan P, Srinath H, Clancy K, Parelkar S, and Thompson PR. 2017. Development of a Selective Inhibitor of Protein Arginine Deiminase 2. J Med Chem 60: 3198–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi HX, Wang Y, Li XH, Zhan XM, Tang M, Fina M, Su LJ, Pratt D, Bu CH, Hildebrand S, Lyon S, Scott L, Quan JX, Sun QH, Russell J, Arnett S, Jurek P, Chen D, Kravchenko VV, Mathison JC, Moresco EMY, Monson NL, Ulevitch RJ, and Beutler B. 2016. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol 17: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt-Lauber C, Bossaller L, Abujudeh HH, Vladimer GI, Christ A, Fitzgerald KA, Latz E, Gravallese EM, Marshak-Rothstein A, and Kay J. 2015. Gadolinium-based compounds induce NLRP3-dependent IL-1beta production and peritoneal inflammation. Ann Rheum Dis 74: 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad F, Mishra N, Ahrenstorf G, Franklin BS, Latz E, Schmidt RE, and Bossaller L. 2018. Evidence of inflammasome activation and formation of monocyte-derived ASC specks in HIV-1 positive patients. AIDS 32: 299–307. [DOI] [PubMed] [Google Scholar]
- 25.Senshu T, Sato T, Inoue T, Akiyama K, and Asaga H. 1992. Detection of citrulline residues in deiminated proteins on polyvinylidene difluoride membrane. Anal Biochem 203: 94–100. [DOI] [PubMed] [Google Scholar]
- 26.Vossenaar ER, Zendman AJ, van Venrooij WJ, and Pruijn GJ. 2003. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 25: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 27.Ghari F, Quirke AM, Munro S, Kawalkowska J, Picaud S, McGouran J, Subramanian V, Muth A, Williams R, Kessler B, Thompson PR, Fillipakopoulos P, Knapp S, Venables PJ, and La Thangue NB. 2016. Citrullination-acetylation interplay guides E2F-1 activity during the inflammatory response. Sci Adv 2: e1501257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim CA, Yao F, Wong JJY, George J, Xu H, Chiu KP, Sung WK, Lipovich L, Vega VB, Chen J, Shahab A, Zhao XD, Hibberd M, Wei CL, Lim B, Ng HH, Ruan YJ, and Chin KC. 2007. Genome-wide mapping of RELA(p65) binding identifies E2F1 as a transcriptional activator recruited by NF-kappa B upon TLR4 activation. Mol Cell 27: 622–635. [DOI] [PubMed] [Google Scholar]
- 29.Katsnelson MA, Rucker LG, Russo HM, and Dubyak GR. 2015. K+ Efflux Agonists Induce NLRP3 Inflammasome Activation Independently of Ca2+ Signaling. J Immunol 194: 3937–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coll RC, Robertson AAB, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KHG, Masters SL, Schroder K, Cooper MA, and O’Neill LAJ. 2015. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21: 248–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, Bicker KL, Bingham RP, Campbell M, Chen YH, Chung CW, Craggs PD, Davis RP, Eberhard D, Joberty G, Lind KE, Locke K, Maller C, Martinod K, Patten C, Polyakova O, Rise CE, Rudiger M, Sheppard RJ, Slade DJ, Thomas P, Thorpe J, Yao G, Drewes G, Wagner DD, Thompson PR, Prinjha RK, and Wilson DM. 2015. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol 11: 189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, Sennello JA, Kutuzov MA, Sitikov A, Davis JM, Lam AP, Cheresh P, Kamp D, Shumaker DK, Budinger GR, and Ridge KM. 2015. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun 6: 6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inagaki M, Takahara H, Nishi Y, Sugawara K, and Sato C. 1989. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J Biol Chem 264: 18119–18127. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.