Abstract
The prototype 5-HT2A receptor agonist hallucinogens LSD, mescaline, and psilocybin are classified as Schedule 1 drugs of abuse by the United States Drug Enforcement Administration. Accumulating clinical evidence also suggests that acute or repeated “microdosing” with these drugs may have utility for treatment of some mental health disorders including drug abuse and depression. The goal of the present study was to evaluate LSD, mescaline, and psilocybin effects on intracranial self-stimulation (ICSS), a procedure that has been used to evaluate abuse-related effects of other classes of abused drugs. Effects of repeated LSD were also examined to evaluate potential changes in its own effects on ICSS or changes in effects produced by the abused psychostimulant methamphetamine or the prodepressant kappa opioid receptor (KOR) agonist U69,593. Male Sprague Dawley rats were implanted with microelectrodes targeting the medial forebrain bundle and trained to respond under a “frequency-rate” ICSS procedure, in which many drugs of abuse increase (or “facilitate”) ICSS. In acute dose-effect and time-course studies, evidence for abuse-related ICSS facilitation was weak and inconsistent; the predominant effect of all three drugs was dose- and time-dependent ICSS depression. Repeated LSD treatment failed to alter either its own ICSS depressant effects or the abuse-related effects of methamphetamine; however, repeated LSD did attenuate ICSS depression by U69,593. These results extend previous preclinical studies to suggest weak expression of abuse-related effects by 5-HT2A agonist hallucinogens and provide supportive evidence for therapeutic effects of repeated LSD dosing to attenuate KOR-mediated depressant effects but not abuse potential of psychostimulants.
Keywords: LSD, mescaline, psilocybin, methamphetamine, ICSS, U69, 593, microdosing
INTRODUCTION
The prototype serotonergic hallucinogens lysergic acid diethylamide (LSD), mescaline, and psilocybin have distinct molecular scaffolds but share activity as serotonin 2A (5-HT2A) receptor agonists (McKenna & Peroutka, 1989; Nichols, Frescas, Marona-Lewicka, & Kurrasch-Orbaugh, 2002; Sadzot et al., 1989), and all three compounds are categorized by the United States Drug Enforcement Administration (DEA) as Schedule 1 drugs with high abuse liability and no current clinical indications (DEA resource guide, 2017). LSD is a conformationally constrained semi-synthetic tryptamine belonging to the chemical class of compounds called ergolines (Fantegrossi, Murnane, & Reissig, 2008), whereas mescaline is a naturally occurring phenethylamine found in peyote cactus species Lophophora williamsii or Anhalonium lewini (Pikhal, 1991), and psilocybin is a tryptamine found naturally in hallucinogenic mushrooms (Tyls, Palenicek, & Horacek, 2014). There are an estimated 32 million people in the United States who have used at least one of these drugs during their lifetime (Krebs & Johansen, 2013). Although none of these compounds is currently approved for clinical use, a growing body of evidence suggests that they may have utility for treatment of some neurological or psychiatric disorders, such as obsessive-compulsive disorder (Moreno, Wiegand, Taitano, & Delgado, 2006), anxiety and depression (Carhart-Harris et al., 2016; Gasser et al., 2014; Griffiths et al., 2016; Grob et al., 2011), alcoholism (Bogenschutz et al., 2015; Krebs & Johansen, 2012), and tobacco use disorder (Johnson, Garcia-Romeu, Cosimano, & Griffiths, 2014). Moreover, there is anecdotal evidence that clinical benefit may be associated with a pattern of 5-HT2A agonist use called “microdosing,” in which subhallucinogenic doses are administered repeatedly to relieve anxiety and depression (Fadiman, 2011; Plante, 2017; Waldman, 2018; Williams, 2017).
Although LSD, mescaline, and psilocybin are categorized by the DEA as Schedule I drugs, they fail to produce robust signals in preclinical procedures used to assess abuse potential. For example, none of these drugs maintains reliable drug self-administration in laboratory animals (Fantegrossi et al., 2008; Goodwin, 2016; Yokel, 1987). Intracranial selfstimulation (ICSS) is another procedure that has been used to assess abuse liability, and in ICSS procedures, drugs of abuse often increase (or “facilitate”) responding maintained by low frequencies or intensities of brain stimulation (Carlezon & Chartoff, 2007; Negus & Miller, 2014; Wise, 1996). To our knowledge, LSD, mescaline, and psilocybin have not been previously investigated using contemporary ICSS procedures; however, acute treatment with the non-scheduled 5-HT2A agonist TCB-2 failed to produce ICSS facilitation (Katsidoni, Apazoglou, & Panagis, 2011).
The goal of the present study was to compare the effects of LSD, mescaline, and psilocybin on ICSS in rats using a “frequency-rate” procedure that has been used previously to evaluate a wide range of other drugs including monoamine transporter ligands (Bauer, Banks, Blough, & Negus, 2013a, 2013b; Bonano et al., 2015; Bonano, Glennon, De Felice, Banks, & Negus, 2014) and monoamine receptor agonists (Bauer, Banks, Blough, & Negus, 2015; Lazenka, Blough, & Negus, 2016; Lazenka, Legakis, & Negus, 2016). Initial studies characterized the potency and time course of effects produced by acute treatments with each drug. A subsequent study evaluated effects produced by repeated daily treatment with LSD for two reasons. First, as noted above, repeated microdosing has emerged as a one pattern of hallucinogen use by humans (Fadiman, 2011; Plante, 2017; Waldman, 2018; Williams, 2017). Second, repeated treatment with some other drugs (e.g. mu opioid receptor agonists) (Miller, Altarifi, & Negus, 2015) can unmask and/or enhance expression of ICSS facilitation suggestive of abuse potential, and accordingly, the present study evaluated the degree to which repeated LSD might unmask and/or increase expression of ICSS facilitation by LSD. Additionally, given evidence that acute or repeated microdosing with hallucinogens might be effective to reduce sensitivity to abused drugs or potentially depressive stimuli (Bogenschutz & Johnson, 2016; Ross et al., 2016; Whelan & Johnson, 2018), the effects of the abused monoamine releaser methamphetamine and prodepressant kappa opioid receptor agonist U69,593 were also evaluated in rats treated with repeated vehicle or LSD.
METHODS
Subjects
Studies were conducted in 52 adult male Sprague-Dawley rats (Envigo; Somerset, NJ) that weighed approximately 300 g at the time of surgery. Rats were singly housed, had ad libitum access to food and water in the home cage, and were kept under a 12-hour light/dark cycle (lights on 6AM-6PM) in a facility accredited by Association for the Assessment and Accreditation of Laboratory Animal Care. The animal use protocol (AD20002, titled “Prodepressant Depressant Effects of Pain in Rodents”) was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Council Research, 2011).
Surgery
Surgical procedures were similar to those described previously (Bonano et al., 2015). Briefly, rats were anesthetized with isoflurane (3% in oxygen; Webster Veterinary, Phoenix, AZ) for stereotaxic implantation of the cathode (0.25 mm diameter, insulated except at tip) of a stainless steel electrode (Plastics One, Roanoke, VA) into the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, and 8.8 mm ventral to the skull). Three screws were implanted into the skull, and the anode (0.125 mm diameter, uninsulated) was wrapped around one of the screws to act as a ground. Dental acrylic secured the electrode to the screws and skull. Ketoprofen (5 mg/kg) was administered intraperitoneally (IP) as a postoperative analgesic immediately and 24 hours post-surgery. Animals were allowed at least 5 recovery days before initiation of ICSS training.
Apparatus
Studies were conducted in operant conditioning chambers (29.2 cm X 30.5 cm X 24.1 cm) housed in sound-attenuating boxes and equipped with a response lever, three stimulus lights above the lever, a house light, and an ICSS stimulator (Med Associates, St Albans, VT). The intracranial electrode was connected to the stimulator via bipolar cables routed through a swivel-commutator (Model SL2C, Plastics One). Stimulus deliveries were controlled and lever-press responses were recorded with a computer and interface operated by MED-PC IV computer software (Med Associates).
Training
Procedures for training and testing were similar to those used previously to examine acute and chronic effects of other monoaminergic drugs on ICSS in rats (Bonano et al., 2015; Bonano et al., 2014; Lazenka, Blough, et al., 2016; Suyama, Banks, & Negus, 2018). Each behavioral session commenced with illumination of the house light. Rats were trained to lever press under a fixed-ratio 1 (FR 1) schedule for brain stimulation delivered via the intracranial electrode. Each stimulation consisted of a 0.5-s train of square-wave cathodal pulses (0.1 ms per pulse) at a designated frequency and amplitude and was accompanied by illumination of the three stimulus lights over the lever. During initial 60-min training sessions, the stimulation frequency and amplitude were fixed at 126 Hz and 150 μA, respectively. Amplitude was adjusted in each rat to a level sufficient to maintain response rates >30 responses/min, and terminal amplitudes ranged between 75 and 300 [jA. Frequency manipulations were then introduced during 30-min behavioral sessions consisting of three 10-min components, and each component consisted of 10 1-min frequency trials. During each component, the frequency of brain stimulation decreased across the 10 trials in 0.05 log increments from 2.2 log Hz to 1.75 log Hz. The frequency then reset to 2.2 log Hz at the start of the next component. Each trial began with an initial 10-s time out, during which the house light was off, responding had no scheduled consequences, and 5 non-contingent stimulations at the designated frequency were delivered. For the remaining 50 s of each trial, the house light was illuminated, and responding produced both brain stimulation under an FR1 schedule and illumination of the three stimulus lights as described above. ICSS training was considered complete when frequency-rate curves were not statistically different over three consecutive days of training as indicated by lack of a significant effect of ‘day’ in a two-way analysis of variance (ANOVA) with day and frequency as the main effect variables (see Data Analysis below). All training was completed within seven weeks after surgery.
Testing
Acute Dose-Effect and Time-Course Studies.
Separate groups of rats were used for initial dose-effect studies with LSD (0.0032–0.1 mg/kg; N=6), mescaline (0.32–10 mg/kg; N=6), and psilocybin (0.032–1.0 mg/kg; N=6). Test sessions were conducted once per week and consisted of three “baseline components” followed first by a 15-min time-out period and then by two “test” components. Saline vehicle or a drug dose was administered IP at the start of the time-out period. In each group, the order of saline and test-drug doses was randomized across rats using a Latin-square design. Three-component training sessions were conducted on other weekdays.
Time-course studies were also conducted in separate groups of rats with representative low and high doses of LSD (0.01 and 0.32 mg/kg; N=6), mescaline (1.0 and 10 mg/kg; N=6), and psilocybin (0.1 and 1 mg/kg; N=6). The LSD group included four rats from LSD dose-effect studies and two drug-naïve rats. The mescaline group included two rats from mescaline dose-effect studies and four drug-naïve rats. The psilocybin group included five rats from psilocybin dose-effect studies and one drug-naïve rat. Time-course test sessions were conducted weekly, and consisted of three baseline ICSS components followed first by injection of saline vehicle or a test-drug dose and then by pairs of test components that began after 10, 30, 100, 180, and 300 minutes. In each group, the order of saline and test-drug doses was randomized across rats using a Latin-square design. Three-component training sessions were conducted on other weekdays.
Repeated LSD Studies.
Twenty-seven drug-naïve rats were divided into four groups for repeated treatment with saline, 0.01, 0.1, or 0.32 mg/kg/day LSD (N=7 per group except 0.32 mg/kg/day, where N=6). Training was conducted as described above, and data from the last three days of training established the Predrug Baseline parameters of ICSS. Subsequently, the effects of repeated treatments were evaluated using a nine-day protocol. On Days 1–6, daily test sessions consisted of three baseline components followed first by a 10-min time-out period and then by two test components. The assigned treatment for each group (saline, 0.01, 0.1, or 0.32 mg/kg/day LSD) was administered IP at the start of the time-out period. On day 7, effects of cumulative LSD (0.0032–0.32 mg/kg) were evaluated in all rats. Cumulative-dosing test sessions consisted of three baseline components followed by a series of five LSD injections administered IP at 30-min intervals. Each dose increased the total, cumulative LSD dose by 0.5 log units, and a pair of ICSS test components began 10 min after each dose. On Days 8 and 9, additional cumulative-dosing test sessions were conducted with the monoamine releaser methamphetamine (0.032–0.32 mg/kg) and the kappa opioid receptor agonist U69,593 (0.056–0.56 mg/kg). The order of methamphetamine and U69,593 testing was counterbalanced across rats within an LSD repeated-dosing group, and cumulative-dosing test sessions with these drugs were identical to those with LSD with the exception that increasing doses of methamphetamine or U69,593 were administered at 30-min intervals rather than LSD. Methamphetamine and U69,593 doses were selected based on previous studies (Bauer et al., 2013b; Negus, Morrissey, Rosenberg, Cheng, & Rice, 2010).
Data analysis
The first component of each daily session was considered to be an acclimation component, and data were discarded. The primary dependent variable for the remainder of each session was the reinforcement rate in stimulations/trial during each frequency trial. To normalize these raw data, reinforcement rates from each trial were converted into the percent maximum control rate (% MCR). For dose-effect and time-course testing, the MCR was determined during the baseline components of each daily test session and was defined as the mean of the maximal stimulations observed in any frequency trial during the second and third baseline components. Thus, % MCR for each trial was calculated as (stimulations during a frequency trial/MCR)×100. Normalized data across each pair of baseline components and each pair of test components were then averaged first within each rat and then across rats to generate mean baseline and test frequency-rate curves, respectively. Frequency-rate curves were analyzed using two-way repeated measures analysis of variance (ANOVA), with drug dose or time as one factor and ICSS frequency as the other factor. For this and all other statistical analyses, a significant ANOVA was followed by a Holm–Sidak post hoc test, and the criterion for significance was set at the 95% confidence level (P < 0.05). To provide an additional summary of ICSS performance, the total number of stimulations/component delivered across all 10 frequency trials was determined for each component. The average number of total stimulations per test component was expressed as a percentage of the average number of total stimulations per component during the second and third baseline components (% baseline) in each rat and then averaged across rats. Results were analyzed by one-way ANOVA.
Data from the study of repeated LSD were analyzed using a similar approach, with the exception that baseline MCR and total stimulations were calculated from the three-day Predrug Baseline components conducted before any LSD administration (six total Predrug Baseline components). There were four different groups treated with different doses of repeated LSD, and analyses were conducted both within group and across groups. For within-group comparisons, the Predrug Baseline frequency-rate curve was compared to the Day 7 frequency-rate curve to evaluate the degree to which repeated LSD administration altered baseline ICSS. Analyses were conducted using two-way repeated measures ANOVA with treatment day and frequency as the two factors. Additionally, frequency-rate curves determined during cumulative dosing with each test drug were examined by two-way repeated measures ANOVA with dose and frequency as the two factors. For between-group analyses, frequency-rate curves for a given dose of a given test drug were compared across LSD-treatment groups by two-way ANOVA with frequency as a within-subject factor and treatment group as a between-subjects factor. Additionally, dose-effect curves relating dose of each drug to the total number of stimulations per component were compared across LSD-treatment groups using two-way ANOVA with dose as a within-subject factor and treatment group as a between-subjects factor.
Drugs
(+) Lysergic acid diethylamide (+) tartrate, mescaline HCl, psilocybin, and U69,593 HCl were provided by the National Institute on Drug Abuse drug supply program (Bethesda, MD). (+)-Methamphetamine HCl was purchased from a commercial supplier (Sigma Aldrich, St. Louis, MO). All drugs were dissolved in sterile water and doses are expressed as the salt forms listed above.
RESULTS
Dose-effect and time-course studies with acute LSD, mescaline and psilocybin dosing
For the 25 rats used in dose-effect and time-course studies, the mean±SEM MCR was 55±6 stimulations per trial, and the mean±SEM number of stimulations per component was 253±17. In general, LSD, mescaline, and psilocybin produce weak and inconsistent evidence of ICSS facilitation at lower doses and more consistent ICSS depression at higher doses.
Figure 1 shows acute effects of LSD (0.0032–0.32 mg/kg; N=6), mescaline (0.32–10 mg/kg; N=6), and psilocybin (0.032–1 mg/kg; N=6) on full ICSS frequency-rate curves (Panels A, C, E) and the summary measure of total stimulations per component (Panels B, D, F). Statistical results for this and all subsequent figures are in the figure legends. LSD produced biphasic effects with low doses tending to increase ICSS while higher doses decreased ICSS. Specifically, ICSS facilitation was observed at one brain-stimulation frequency (1.95 log Hz) after 0.0032 mg/kg LSD and at two frequencies (2.0 and 2.05 log Hz) after 0.01 mg/kg LSD, and 0.01 mg/kg LSD also significantly increased the summary measure of total stimulations per component summed across all frequencies. Conversely, 0.032 mg/kg LSD did not significantly alter ICSS, and a higher dose of 0.1 mg/kg LSD significantly decreased ICSS across a broad range of frequencies (2.0–2.15 log Hz) and also significantly decreased the number of stimulations per component. A higher dose of 0.32 mg/kg LSD was also tested in a subset of three rats; results were not included in statistical analysis, but this dose generally produced a further decrease in ICSS (also, see time-course data below). In contrast to LSD, mescaline only decreased ICSS across a broad range of frequencies (2.0–2.2 log Hz) at the highest dose (10 mg/kg), and effects on the summary measure of total stimulations per component approached significance (P=0.052). Similarly, psilocybin only decreased ICSS across a broad range of frequencies (2.0–2.2 log Hz) at the highest dose (1.0 mg/kg), and this high dose also significantly decreased the number of stimulations per component.
Figure 1.
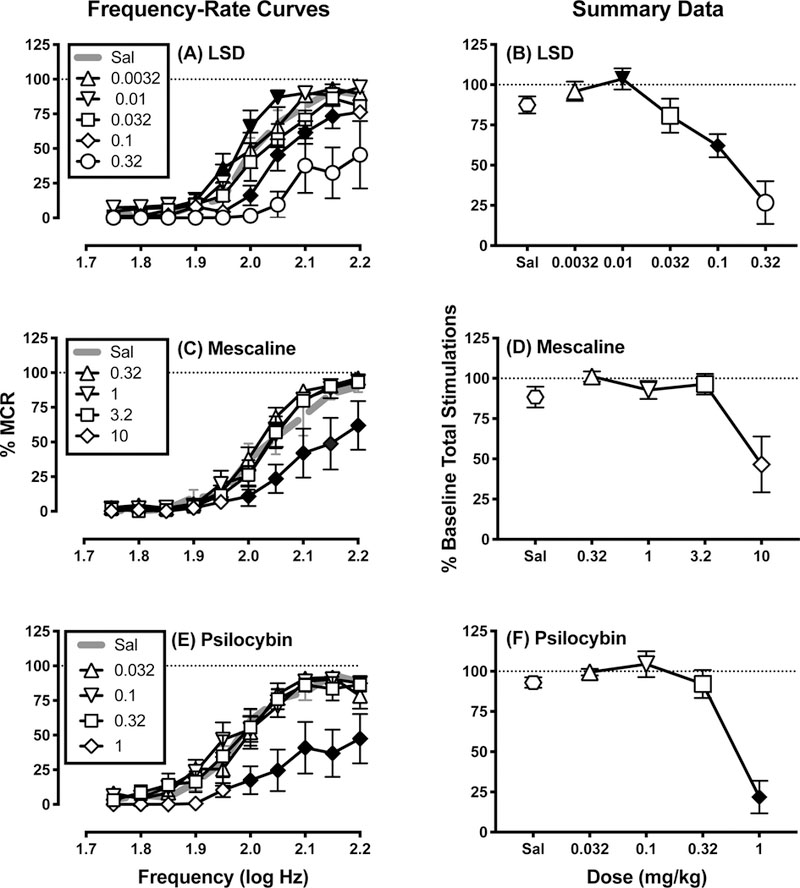
Effects of acute LSD (A,B), mescaline (C,D), and psilocybin (E,F). Left panels (A,C,E) show full frequency-rate curves. Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: reinforcement rate expressed as percentage of maximum control reinforcement rate (% MCR). Filled symbols represent frequencies at which ICSS rates after drug administration were statistically different from rates after saline administration as determined by two-way ANOVA followed by Holm-Sidak post hoc test, p < 0.05. Right panels (B,D,F) show the number of stimulations per component collapsed across all frequencies. Abscissae: drug dose in mg kg−1. Ordinates: percentage of baseline number of stimulations per component. Filled points indicate doses at which ICSS was significantly increased or decreased in at least one trial of the frequency-rate curves in the left panels. All points show mean ± SEM for N=6 rats. Statistical results were as follows (only significant main effects of dose and significant frequency × dose interaction results are shown for two-way ANOVAs in Panels A,C,E; the main effect of frequency was always significant). (A) significant main effect of LSD dose [F(4, 20) = 35.81, p<0.001] and frequency × dose interaction [F(36, 180) = 2.817, p<0.001]; (B) significant effect of dose F(1.783, 8.917) = 30.17, p<0.001; (C) significant main effect of mescaline dose [F(4, 20) = 4.578, p=0.009] and frequency × dose interaction [F(36, 180) = 1.794, p=0.007]; (D) effect of dose approached significance F(1.229, 6.145) = 5.504, p=0.052; (E) significant main effect of psilocybin dose [F(4, 20) = 15.51, p<0.001] and frequency × dose interaction [F(36, 180) = 1.86, p=0.004]; (G) significant effect of dose F(2.301, 11.51) = 24.72, p<0.001.
Figure 2 shows the time course of effects produced by acute treatment with low and high doses of LSD (0.01 and 0.32 mg/kg; Panels A-C; N=6), mescaline (1 and 10 mg/kg; Panels D-F; N=6), and psilocybin (0.1 and 1 mg/kg, Panels G-I; N=6). In these time-course studies, the low dose of 0.01 mg/kg LSD failed to alter ICSS at any time, and the high dose of 0.32 mg/kg LSD depressed ICSS across a broad range of brain-stimulation frequencies after 10 and 30 min. The low dose of 1.0 mg/kg mescaline facilitated ICSS at one brain-stimulation frequency (2.05 log Hz) after 10 min, and the high dose of 10 mg/kg mescaline depressed ICSS across a range of frequencies at 10 min. The low dose of 0.1 mg/kg psilocybin facilitated ICSS at one frequency (2.05 log Hz) after 30 min, and the high dose of 1.0 mg/kg depressed ICSS across a range of frequencies at 10 and 30 min. Figure 2 shows that all drug effects had dissipated by 100 min, and similarly, no dose of any drug produced significant effects on ICSS after 180 or 300 min (data not shown).
Figure 2.

Time course of effects produced by selected doses of LSD (A-C), mescaline (D-F), and psilocybin (G-I). Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: reinforcement rate expressed as percentage of maximum control reinforcement rate (% MCR). Filled symbols represent frequencies at which ICSS rates after drug administration were statistically different from rates after saline administration as determined by two-way ANOVA followed by Holm-Sidak post hoc test, p < 0.05. All points show mean ± SEM for N=6 rats. Statistical results were as follows (only significant main effects of time and significant frequency × time interaction results are shown; the main effect of frequency was always significant). (A) significant main effect of time [F(2, 150) = 184.7, p<0.001] and frequency × time interaction [F(18, 150) = 18, p<0.001]; (B) significant main effect of time [F(2, 10) = 6.23, p=0.018] and frequency × time interaction [F(18, 90) = 4.499, p<0.001]; (C) no significant main effect of time or frequency × time interaction; (D) significant main effect of time [F(2, 10) = 4.706, p=0.036] and frequency × time interaction [F(18, 90) = 2.29, p=0.006]; (E) no significant main effect of time or frequency × time interaction; (F) no significant main effect of time or frequency × time interaction; (G) significant main effect of time [F(2, 10) = 56.41, p<0.001] and frequency × time interaction [F(18, 90) = 1.811, p=0.036]; (H) significant main effect of time [F(2, 10) = 17.95, p<0.001] and frequency × time interaction [F(18, 90) = 2.841, p=0.001]; (I) no significant main effect of time or frequency × time interaction.
Effects 7-day repeated LSD treatment
For the 27 rats used in repeated LSD studies, the mean±SEM MCR was 56±1 stimulations per trial, and the mean±SEM number of stimulations per component was 232±16. Predrug baseline frequency-rate curves were not different from the Day 7 baseline frequency-rate curves determined in any group, indicating that baseline ICSS was not altered by repeated treatment with saline or any LSD dose (data not shown; P>0.05 for main effect of “Day” and “Frequency x Day” interaction in all groups). Figure 3 shows within-group analyses of effects produced by cumulative dosing with LSD (Panels A-D), methamphetamine (Panels E-H), and U69,593 (Panels I-L) in each LSD-dosing group (N=7 in all groups except 0.32 mg/kg/day LSD, where N=6). Cumulative LSD (0.0032–0.32 mg/kg) and U69,593 (0.056–056 mg/kg) produced only dose-dependent ICSS depression in all groups, whereas cumulative methamphetamine (0.032–0.32 mg/kg) produced dose-dependent ICSS facilitation in all groups. Figure 4 shows the same data graphed for between-group analyses. The left panels of Figure 4 compare ICSS frequency-rate curves after the highest cumulative doses of LSD (0.32 mg/kg, Panel A), methamphetamine (0.32 mg/kg Panel C), and U69,593 (0.56 mg/kg, Panel E) in each LSD-treatment group. There was a tendency for LSD treatment to attenuate effects of all three drugs; however, the only significant changes were that 0.1 and 0.32 mg/kg/day LSD attenuated ICSS depression by U69,593 (Panel E). The right panels of Figure 4 compare dose-effect curves for LSD (Panel B), methamphetamine (Panel D), and U69,593 (Panel F) on the summary measure of total stimulations per component. This analysis again showed a tendency for repeated LSD to modestly alter effects of all three drugs, and in particular to attenuate effects of the highest dose of each drug; however, none of these effects met criteria for statistical significance.
Figure 3.
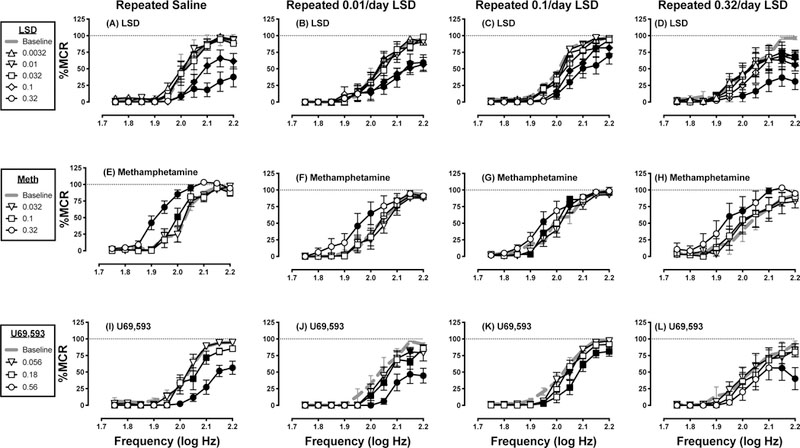
Effects on LSD (A-D), methamphetamine (E-H), and U69,593 after repeated treatment for 7 consecutive days with saline (A,E,I), 0.01 mg/kg/day LSD (B,F,J), 0.1 mg/kg/day LSD (C,G,K), or 0.32 mg/kg/day LSD (D,H,L). Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: reinforcement rate expressed as percentage of maximum control reinforcement rate (% MCR). Filled symbols represent frequencies at which ICSS rates after drug administration were statistically different from test-day baseline rates as determined by two-way ANOVA followed by Holm-Sidak post hoc test, p < 0.05. All data are mean ± SEM for N=7 for all groups except 0.32 mg/kg/day LSD, where N=6. Statistical results were as follows (only significant main effects of dose and significant frequency × dose interaction results are shown; the main effect of frequency was always significant). (A) significant main effect of LSD dose [F(5, 30) = 26.01, p<0.001] and frequency × dose interaction [F(45, 270) = 5.811, p<0.001]; (B) significant main effect of LSD dose [F(5, 30) = 7.431, p<0.001] and frequency × dose interaction [F(45, 270) = 3.7, p<0.001]; (C) significant main effect of LSD dose [F(5, 30) = 9.556, p<0.001] and frequency × dose interaction [F(45, 270) = 2.857, p<0.001]; (D) significant main effect of LSD dose [F(5, 25) = 8.094, p<0.001] and frequency × dose interaction [F(45, 225) = 2.638, p<0.001]; (E) significant main effect of methamphetamine dose [F(3, 18) = 45.26, p<0.001] and frequency × dose interaction [F(27, 162) = 6.239, p<0.001]; (F) significant main effect of methamphetamine dose [F(3, 18) = 5.355, p=0.008]; (G) significant frequency × dose interaction [F(27, 162) = 2.499, p<0.001]; (H) significant main effect of methamphetamine dose [F(3, 15) = 15.58, p<0.001]; (I) significant main effect of U69,593 dose [F(3, 18) = 28.85, p<0.001] and frequency x dose interaction [F(27, 162) = 5.841, p<0.001]; (J) significant main effect of U69,593 dose [F(3, 18) = 5.355, p<0.001]; (K) significant main effect of U69,593 dose [F(3, 18) = 8.991, p<0.001] and frequency × dose interaction [F(27, 162) = 2.585, p<0.001]; (L) significant main effect of U69,593 dose [F(3, 15) = 7.989, p=0.002] and frequency × dose interaction [F(27, 135) = 1.622, p=0.039].
Figure 4.
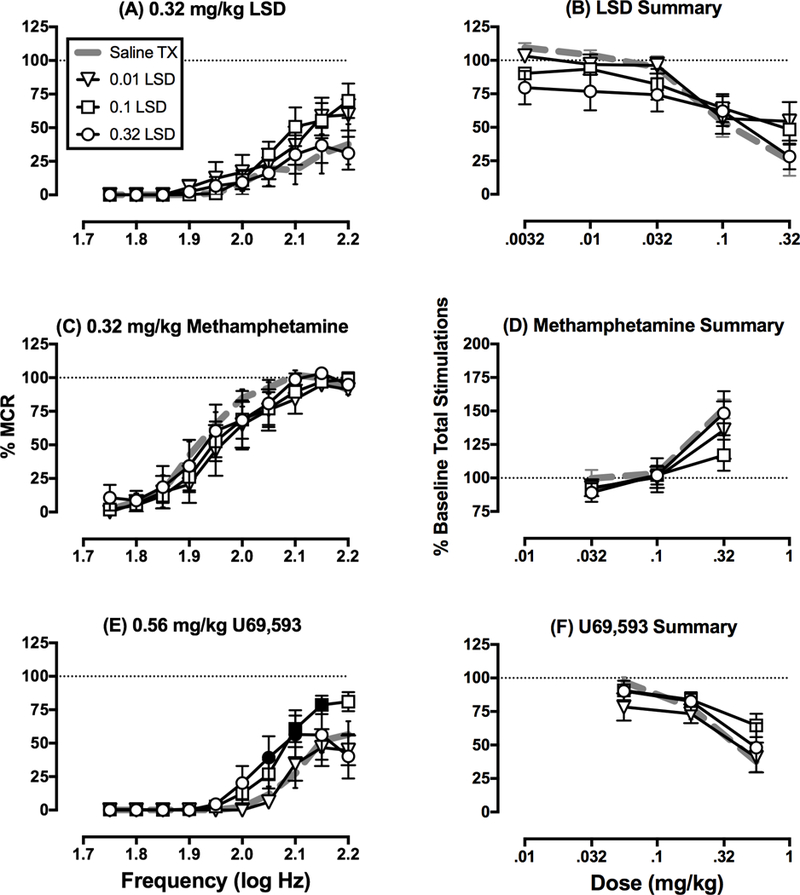
Comparison of effects produced by LSD (A,B), methamphetamine (C,D), and U69,593 (E,F) after repeated treatment with saline or LSD. Data are the same as in Figure 3 but grouped here for analysis of drug effects across LSD treatment groups. Left panels (A,C,E) show full frequency-rate curves determined after the highest cumulative dose of drug. Abscissae: frequency of electrical brain stimulation in log Hz. Ordinates: reinforcement rate expressed as percentage of maximum control reinforcement rate (% MCR). Filled symbols in Panel C show frequencies at which LSD treatment attenuated ICSS depression by 0.56 mg/kg U69,593 as determined by two-way ANOVA followed by Holm-Sidak post hoc test, p < 0.05. In all three panels, there was a significant main effect of frequency; however, there was not a significant main effects of LSD treatment group for any drug, and there was a significant frequency × treatment group interaction only for U69,593 in Panel C [F(27,207)=1.966, p=0.0045]. Right panels (B,D,F) show the number of stimulations per component collapsed across all frequencies. Abscissae: drug dose in mg kg−1. Ordinates: percentage of baseline number of stimulations per component. There was a significant main effect of drug dose for all drugs, but there was not a significant main effect of LSD treatment group or a significant drug dose x treatment group interaction for any drug. All data are mean ± SEM for N=7 for all groups except 0.32 mg/kg/day LSD, where N=6.
DISCUSSION
This study evaluated effects of the prototype hallucinogens LSD, mescaline, and psilocybin in an ICSS procedure that has been used to evaluate abuse potential of drugs from other pharmacological classes. There were three main findings. First, in acute dose-effect and time-course studies, lower doses of these compounds produced weak and inconsistent evidence for abuse-related ICSS facilitation, but higher doses produced reliable and robust ICSS depression. Second, the rate-decreasing effects of LSD were not altered after a regimen of repeated daily dosing. In particular, there was no evidence for tolerance to ICSS depression and increased expression of ICSS facilitation. Lastly, repeated LSD treatment also did not alter either baseline ICSS or methamphetamine-induced ICSS facilitation, but repeated LSD did significantly attenuate U69,593-induced ICSS depression.
LSD, mescaline and psilocybin produced primarily ICSS depression in initial dose-effect and time-course studies. Although statistically significant ICSS facilitation was occasionally observed, this facilitation was weak relative to effects of many other classes of abused drugs (e.g. in comparison to methamphetamine in this study) and inconsistent across repeated determinations of similar conditions (e.g. 0.01 mg/kg LSD produced significant ICSS facilitation in the initial dose-effect study, but it failed to produce ICSS facilitation in either the time-course or cumulative-dosing studies). Thus, the present results are consistent with the finding that the non-scheduled 5-HT2A agonist TCB-2 also produced only ICSS depression in a frequency-rate ICSS procedure in rats (Katsidoni et al., 2011). A relatively high range of LSD doses (0.05–0.2 mg/kg) was evaluated in rats using an early type of procedure, in which ICSS was maintained in rats by a single frequency and amplitude of brain stimulation, and ICSS rates were monitored continuously for 100 min after LSD administration (Olds & Olds, 1963). As in the present study, LSD usually depressed ICSS during the first hour after its administration, although both baseline ICSS rates and LSD effects were variable across rats. Taken together the present and previous studies agree in finding that acute dosing with 5-HT2A agonist hallucinogens fails to produce reliable evidence of abuse-related facilitation in ICSS procedures.
The present study extended these findings by also evaluating effects of repeated daily LSD treatment. These repeated-dosing studies were conducted for two reasons. First, repeated dosing can increase expression of abuse-related effects of some drugs in ICSS procedures. For example, morphine (Miller et al., 2015) and nicotine (Freitas, Carroll, & Negus, 2016) both produce primarily ICSS depression in drug-naïve rats, but repeated daily administration of doses that initially produce ICSS depression results in tolerance to ICSS-depressant effects and increased expression of abuse-related ICSS facilitation. In the present study, repeated daily treatment with either low or high LSD doses failed to produce either significant tolerance to LSD-induced ICSS depression or emergence of any ICSS facilitation. Thus, as with acute-dosing, these repeated-dosing studies also failed to provide evidence for an abuse-related effect of LSD. A second reason for evaluating effects of repeated LSD administration was that a pattern of drug use called “microdosing” has emerged as one mode of consumption for 5-HT2A agonist hallucinogens (Fadiman, 2011; Plante, 2017; Waldman, 2018; Williams, 2017). Microdosing is not a precisely defined dosing regimen, but it generally involves repeated administration of several relatively low, subhallucinogenic drug doses at intervals of a day or two, and it is reputed to produce sustained anxiolytic and/or antidepressant effects. Research on effects of hallucinogen microdosing is in its infancy. In the only published study to date, a microdosing regimen of psilocin administration (3 doses of 0.05 or 0.075 mg/kg administered on alternate days) produced weak evidence for enhancement rather than relief of anxiety-like behavior in rats tested with an elevated-plus maze procedure (Horsley, Palenicek, Kolin, & Vales, 2018). In the present study, repeated dosing with the relatively low dose of 0.01 mg/kg LSD was also ineffective to unmask abuse-related ICSS facilitation by LSD. Taken together, the weak and unreliable ICSS facilitation by 5-HT2A agonist hallucinogens after acute or repeated treatment in ICSS procedures agrees with the finding of unreliable self-administration by rhesus monkeys of 5-HT2A agonists in drug self-administration procedures (Fantegrossi, Woods, & Winger, 2004; Goodwin, 2016).
The weak evidence for ICSS facilitation by 5-HT2A agonist hallucinogens is superficially at odds with the legal status of these compounds as DEA Schedule 1 drugs of abuse. However, ICSS in rats is most predictive for abuse potential of drugs that maintain sustained patterns of frequent drug use in humans (e.g. amphetamine-like psychostimulants, morphine-like mu opioid receptor agonists, nicotine-like nicotinic acetylcholine receptor agonists, diazepam-like GABAa receptor positive allosteric modulators) (Negus & Miller, 2014). ICSS is less effective to detect abuse potential of drugs that produce dissociative effects and typically maintain patterns of transient and infrequent use (e.g. ketamine, salvinorin) (Hillhouse, Porter, & Negus, 2014) Thus, the failure of LSD, psilocybin, and mescaline to produce reliable ICSS facilitation in this study is consistent with the clinical observation that these drugs do not maintain patterns of sustained and frequent use, but rather are consumed with relatively intermittent frequency during relatively transient periods of a user’s lifespan (Chilcoat & Schutz, 1996; Coney, Maier, Ferris, Winstock, & Barratt, 2017; Johnson, Griffiths, Hendricks, & Henningfield, 2018).
The absence of significant tolerance to ICSS-depressant effects after a week of repeated daily LSD treatment contrasts with evidence for tolerance to subjective and some physiological effects of LSD in humans (Isbell, Wolbach, Wikler, & Miner, 1961). Tolerance has also been reported to some LSD effects in rats, but resistance to tolerance is also often observed. For example, LSD doses of 0.04–0.39 mg/kg produced a dose-dependent decrease in food-maintained responding in rats, and consistent with the present study, doses of 0.13 and 0.195 mg/kg produced similar decreases in operant responding to approximately 40% of control rates. Intriguingly, though, complete tolerance developed to the rate-decreasing effects of 0.13 mg/kg LSD when that dose was administered daily for eight days, but daily treatment with the slightly higher dose of 0.195 mg/kg for up to 10 days failed to produce tolerance (Freedman, Appel, Hartman, & Molliver, 1964). In another example, daily administration of 0.1 mg/kg LSD for 21 days failed to produce tolerance to LSD-induced decreases in food-maintained responding in rats, but tolerance did develop when the frequency of injections was increased to three doses per day (Kovacic & Domino, 1976).
Treatment with hallucinogens has been proposed to produce therapeutic effects for some mental health disorders including drug abuse and depression (Bogenschutz et al., 2015; Ross et al., 2016; Whelan & Johnson, 2018). To address this issue, the present study also evaluated effects of repeated LSD on abuse-related ICSS facilitation produced by the monoamine releaser methamphetamine (Bauer et al. 2013b) and anhedonia-related ICSS depression produced by the kappa opioid receptor agonist U69,593 (Todtenkopf, Marcus, Portoghese, & Carlezon, 2004). There was a tendency for LSD treatment to attenuate effects produced by the highest doses of both drugs; however, this attenuation was significant only for U69,593 and only using the frequency-rate curve analysis (see Figure 4E). Consequently, these results do not support the use of LSD treatment to treat abuse of psychostimulants like methamphetamine, but they do provide qualified support for the potential of LSD to alleviate symptoms of depression that might be mediated by activation of endogenous kappa-opioid receptor signaling (Knoll & Carlezon, 2010). Further research will be required to clarify both the extent to which repeated treatment with LSD or other 5-HT2A hallucinogens might alleviate mental health disorders and the underlying mechanisms of those effects.
Two limitations of the present study warrant mention. First, this study examined hallucinogen effects only in males, and effects in females may differ. For example, we have previously reported subtle but significant sex differences in the potency and duration of ICSS effects produced by other serotonergic drugs including flibanserin (a mixed 5-HT1A agonist and 5-HT2A antagonist marketed for hypoactive sexual arousal disorder in women) and 3,4 methylenedioxymethamphetamine (MDMA, an indirect monoamine receptor agonist that promotes presynaptic release of serotonin, dopamine and norepinephrine) (Lazenka, Blough, et al., 2016; Lazenka, Suyama, Bauer, Banks, & Negus, 2017).
Second, ICSS has been less extensively validated for its utility to predict drug effectiveness to treat drug abuse or depression than to predict abuse potential (Carlezon & Chartoff, 2007; Negus & Miller, 2014). Consequently, the implications of the present results for clinical utility of repeated LSD to treat psychostimulant abuse or depression should be regarded with appropriate caution, especially insofar as clinical practice would likely use hallucinogens in conjunction with other treatment modalities (e.g. hypnotherapy or psychotherapy) rather than as a stand-alone treatment (Bogenschutz & Ross, 2018). Additionally, it may be necessary to modify experimental-design parameters for optimal detection of therapeutic effects produced by repeated LSD or other hallucinogens. For example, the sample size in this study was consistent with our previous studies of repeated treatment with other drugs (Altarifi, Rice, & Negus, 2013; Sakloth & Negus, 2018; Suyama et al., 2018), and it was sufficient to detect effects of repeated LSD on subsequent effects of LSD itself and of U69,593; however, it may have been underpowered to detect effects on methamphetamine. Specifically, a post hoc power analysis was conducted on results after repeated LSD shown in Figure 4A,C,E to determine the power of each experiment (Faul, Erdfelder, Lang, & Buchner, 2007). Assuming alpha=0.05, calculated power to detect a statistically significant interaction between frequency and LSD treatment was >0.99 for data with LSD (Figure 4A) and U69,593 (Figure 4C), but for data with methamphetamine (Figure 4C), calculated power was 0.51. An increase in power to 0.8 would require an increase in sample size from 6–7 per group to 11 per group. As one other example, the regimens of repeated dosing used in this study might not adequately mimic the microdosing regimens being touted for therapeutic purposes (Fadiman, 2011; Plante, 2017; Waldman, 2018; Williams, 2017). Microdosing involves the intermittent use of doses approximately one tenth of the hallucinogenic dose, and the lowest dose of repeated LSD in this study (0.01 mg/kg/day) was selected in part because it is approximately one tenth of the doses commonly used in drug discrimination procedures that model subjective effects of hallucinogens in rats (0.08–0.1 mg/kg) (Appel, West, & Buggy, 2004; Eshleman et al., 2014; Killinger, Peet, & Baker, 2010). However, if this 0.01 mg/kg/day dose of repeated LSD is adjusted by allometric scaling to account for species differences in general metabolic rates (human equivalent dose ÷ rat dose 6.2 = 0.0016 mg/kg/day; (Nair & Jacob, 2016)), it is still above the recommended dose for LSD microdosing in humans (~0.01 mg, or 0.0002 mg/kg in a 50 kg human). Thus, it may be of interest in the future to test effects of repeated dosing with even lower LSD doses at other dosing intervals. It should be noted, though, that attenuation of U69,593-induced ICSS depression was produced only by the higher doses of repeated LSD (0.1 and 0.32 mg/kg/day), but not by the lowest LSD dose of 0.01 mg/kg/day. Consequently, these results do not support effectiveness of microdosing to alleviate depression symptoms that might be mediated by activation of endogenous kappa-opioid receptor signaling (Knoll & Carlezon, 2010).
Public significance statement-.
Hallucinogens are drugs of abuse and may also have therapeutic potential for some mental health disorders. The current study uses an intracranial self-stimulation procedure in rats to examine abuse-related effects and potential therapeutic effects of acute and repeated treatment with prototype hallucinogens.
ACKNOWLEDGEMENTS
Funded by grants R01DA033930 and T32DA007027 from the National Institute on Drug Abuse of the National Institutes of Health.
References
- Altarifi AA, Rice KC, & Negus SS (2013). Abuse-related effects of micro-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behavioural Pharmacology, 24(5–6), 459–470. doi: 10.1097/FBP.0b013e328364c0bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel JB, West WB, & Buggy J (2004). LSD, 5-HT (serotonin), and the evolution of a behavioral assay. Neuroscience & Biobehavioral Reviews, 27(8), 693–701. doi: 10.1016/j.neubiorev.2003.11.012 [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, & Negus SS (2013a). Rate-dependent effects of monoamine releasers on intracranial self-stimulation in rats: implications for abuse liability assessment. Behavioural Pharmacology, 24(5–6), 448–458. doi: 10.1097/FBP.0b013e328363d1a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, & Negus SS (2013b). Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. British Jornal of Pharmacology, 168(4), 850–862. doi: 10.1111/j.1476-5381.2012.02214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, & Negus SS (2015). Role of 5-HT(2)C receptors in effects of monoamine releasers on intracranial self-stimulation in rats. Psychopharmacology, 232(17), 3249–3258. doi: 10.1007/s00213-015-3982-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, & Strassman RJ (2015). Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol, 29(3), 289–299. doi: 10.1177/0269881114565144 [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, & Johnson MW (2016). Classic hallucinogens in the treatment of addictions. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 250–258. doi: 10.1016/j.pnpbp.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, & Ross S (2018). Therapeutic Applications of Classic Hallucinogens. Current Topics in Behavioral Neurosciences, 36, 361–391. doi: 10.1007/7854_2016_464 [DOI] [PubMed] [Google Scholar]
- Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, … Negus SS (2015). Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. British Jornal of Pharmacology, 172(10), 2433–2444. doi: 10.1111/bph.13030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, & Negus SS (2014). Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology, 231(1), 199–207. doi: 10.1007/s00213-013-3223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, … Nutt DJ (2016). Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry, 3(7), 619–627. doi: 10.1016/S2215-0366(16)30065-7 [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr., & Chartoff EH (2007). Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nature Protocols, 2(11), 2987–2995. doi: 10.1038/nprot.2007.441 [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, & Schutz CG (1996). Age-specific patterns of hallucinogen use in the US population: an analysis using generalized additive models. Drug and Alcohol Dependence, 43(3), 143–153. [DOI] [PubMed] [Google Scholar]
- Coney LD, Maier LJ, Ferris JA, Winstock AR, & Barratt MJ (2017). Genie in a blotter: A comparative study of LSD and LSD analogues’ effects and user profile. Hum Psychopharmacol, 32(3). doi: 10.1002/hup.2599 [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, & Gatch MB (2014). Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology, 231(5), 875–888. doi: 10.1007/s00213-013-3303-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiman J (2011). The Psychedelic Explorer’s Guide: Safe, Therapeutic, and Sacred Journeys: park street press. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, & Reissig CJ (2008). The behavioral pharmacology of hallucinogens. Biochemical Pharmacology, 75(1), 17–33. doi: 10.1016/j.bcp.2007.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Woods JH, & Winger G (2004). Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behavioural Pharmacology, 15(2), 149–157. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Freedman DX, Appel JB, Hartman FR, & Molliver ME (1964). Tolerance to Behavioral Effects of Lsd-25 in Rat. Journal of Pharmacology and Experimental Therapeutics, 143, 309–318. [PubMed] [Google Scholar]
- Freitas K, Carroll FI, & Negus SS (2016). Comparison of effects produced by nicotine and the alpha4beta2-selective agonist 5-I-A-85380 on intracranial self-stimulation in rats. Experimental and Clinical Psychopharmacology, 24(1), 65–75. doi: 10.1037/pha0000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, & Brenneisen R (2014). Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. Journal of Nervous and Mental Disease, 202(7), 513–520. doi: 10.1097/NMD.0000000000000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AK (2016). An intravenous self-administration procedure for assessing the reinforcing effects of hallucinogens in nonhuman primates. Journal of Pharmacological and Toxicological Methods, 82, 31–36. doi: 10.1016/j.vascn.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, … Klinedinst MA (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol, 30(12), 1181–1197. doi: 10.1177/0269881116675513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, & Greer GR (2011). Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of General Psychiatry, 68(1), 71–78. doi: 10.1001/archgenpsychiatry.2010.116 [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH, & Negus SS (2014). Dissociable effects of the noncompetitive NMDA receptor antagonists ketamine and MK-801 on intracranial self-stimulation in rats. Psychopharmacology, 231(13), 2705–2716. doi: 10.1007/s00213-014-3451-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley RR, Palenicek T, Kolin J, & Vales K (2018). Psilocin and ketamine microdosing: effects of subchronic intermittent microdoses in the elevated plus-maze in male Wistar rats. Behavioural Pharmacology. doi: 10.1097/FBP.0000000000000394 [DOI] [PubMed] [Google Scholar]
- Isbell H, Wolbach AB, Wikler A, & Miner EJ (1961). Cross tolerance between LSD and psilocybin. Psychopharmacologia, 2, 147–159. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, & Griffiths RR (2014). Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol, 28(11), 983–992. doi: 10.1177/0269881114548296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, Hendricks PS, & Henningfield JE (2018). The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. doi: 10.1016/j.neuropharm.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsidoni V, Apazoglou K, & Panagis G (2011). Role of serotonin 5-HT2A and 5-HT2C receptors on brain stimulation reward and the reward-facilitating effect of cocaine. Psychopharmacology, 213(2–3), 337–354. doi: 10.1007/s00213-010-1887-7 [DOI] [PubMed] [Google Scholar]
- Killinger BA, Peet MM, & Baker LE (2010). Salvinorin A fails to substitute for the discriminative stimulus effects of LSD or ketamine in Sprague-Dawley rats. Pharmacology Biochemistry and Behavior, 96(3), 260–265. doi: 10.1016/j.pbb.2010.05.014 [DOI] [PubMed] [Google Scholar]
- Knoll AT, & Carlezon WA Jr. (2010). Dynorphin, stress, and depression. Brain Research, 1314, 56–73. doi: 10.1016/j.brainres.2009.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic B, & Domino EF (1976). Tolerance and limited cross-tolerance to the effects of N, N-dimethyltryptamine (DMT) and lysergic acid diethylamide-25 (LSD) on food-rewarded bar pressing in the rat. Journal of Pharmacology and Experimental Therapeutics, 197(3), 495–502. [PubMed] [Google Scholar]
- Krebs TS, & Johansen PO (2012). Lysergic acid diethylamide (LSD) for alcoholism: metaanalysis of randomized controlled trials. J Psychopharmacol, 26(7), 994–1002. doi: 10.1177/0269881112439253 [DOI] [PubMed] [Google Scholar]
- Krebs TS, & Johansen PO (2013). Over 30 million psychedelic users in the United States. F1000Res, 2, 98. doi: 10.12688/f1000research.2-98.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Blough BE, & Negus SS (2016). Preclinical Abuse Potential Assessment of Flibanserin: Effects on Intracranial Self-Stimulation in Female and Male Rats. Journal of Sexual Medicine, 13(3), 338–349. doi: 10.1016/j.jsxm.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Legakis LP, & Negus SS (2016). Opposing effects of dopamine D1- and D2-like agonists on intracranial self-stimulation in male rats. Experimental and Clinical Psychopharmacology, 24(3), 193–205. doi: 10.1037/pha0000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Suyama JA, Bauer CT, Banks ML, & Negus SS (2017). Sex differences in abuse-related neurochemical and behavioral effects of 3,4-methylenedioxymethamphetamine (MDMA) in rats. Pharmacology Biochemistry and Behavior, 152, 52–60. doi: 10.1016/j.pbb.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna DJ, & Peroutka SJ (1989). Differentiation of 5-hydroxytryptamine2 receptor subtypes using 125I-R-(−)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin. Journal of Neuroscience, 9(10), 3482–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, & Negus SS (2015). Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Experimental and Clinical Psychopharmacology, 23(5), 405–414. doi: 10.1037/pha0000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, & Delgado PL (2006). Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. Journal of Clinical Psychiatry, 67(11), 1735–1740. [DOI] [PubMed] [Google Scholar]
- Nair AB, & Jacob S (2016). A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm, 7(2), 27–31. doi: 10.4103/0976-0105.177703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. (2011). Guide for the care and use of laboratory animals (8th ed). Washington, DC: National Academy Press. [Google Scholar]
- Negus SS, & Miller LL (2014). Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacological Reviews, 66(3), 869–917. doi: 10.1124/pr.112.007419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, & Rice KC (2010). Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology, 210(2), 149–159. doi: 10.1007/s00213-009-1770-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, & Kurrasch-Orbaugh DM (2002). Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD). Journal of Medicinal Chemistry, 45(19), 4344–4349. [DOI] [PubMed] [Google Scholar]
- Olds ME, & Olds J (1963). Pharmacological Patterns in Subcortical Reinforcement Behavior. International Journal of Neuropharmacology, 2, 309–325. [DOI] [PubMed] [Google Scholar]
- Plante SG (2017). LSD microdoses make people feel sharper, and scientists want to know how. Retrieved from https://www.theverge.com/2017/4/24/15403644/microdosing-lsd-acid-productivity-benefits-brain-studies
- Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, … Schmidt BL (2016). Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol, 30(12), 1165–1180. doi: 10.1177/0269881116675512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, & Titeler M (1989). Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology, 98(4), 495–499. [DOI] [PubMed] [Google Scholar]
- Sakloth F, & Negus SS (2018). Naltrexone maintenance fails to alter amphetamine effects on intracranial self-stimulation in rats. Experimental and Clinical Psychopharmacology, 26(2), 195–204. doi: 10.1037/pha0000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama JA, Banks ML, & Negus SS (2018). Effects of repeated treatment with methcathinone, mephedrone, and fenfluramine on intracranial self-stimulation in rats. Psychopharmacology. doi: 10.1007/s00213-018-5029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, & Carlezon WA Jr. (2004). Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology, 172(4), 463–470. doi: 10.1007/s00213-003-1680-y [DOI] [PubMed] [Google Scholar]
- Tyls F, Palenicek T, & Horacek J (2014). Psilocybin--summary of knowledge and new perspectives. European Neuropsychopharmacology, 24(3), 342–356. doi: 10.1016/j.euroneuro.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Waldman A (2018). A Really Good Day: How Microdosing Made a Mega Difference in My Mood, My Marriage, and My Life: Anchor books. [Google Scholar]
- Whelan A, & Johnson MI (2018). Lysergic acid diethylamide and psilocybin for the management of patients with persistent pain: a potential role? Pain Manag. doi: 10.2217/pmt-2017-0068 [DOI] [PubMed] [Google Scholar]
- Williams A (2017). How LSD Saved One Woman’s Marriage. Retrieved from https://www.nytimes.com/2017/01/07/style/microdosing-lsd-ayelet-waldman-michael-chabon-marriage.html
- Wise RA (1996). Addictive drugs and brain stimulation reward. Annual Review of Neuroscience, 19, 319–340. doi: 10.1146/annurev.ne.19.030196.001535 [DOI] [PubMed] [Google Scholar]
- Yokel RA (1987). Intravenous self-adminstration: response rates, the effects of pharmacological challenges, and drug preference In Bozarth MA (Ed.), Methods of assesing the reinforcering properties of abused drugs (pp. 1–34). New York: Springer-Verlag. [Google Scholar]


