Abstract
The high mortality rate and lack of effective therapies make lung cancer an ideal target for novel therapeutic agents. The present study was designed to implement a novel chemical synthesis pathway and to determine the biological activities of synthetic makaluvamine analogs in human lung cancer. Seventeen compounds were synthesized and purified, and their chemical structures were elucidated on the basis of physicochemical constants and NMR spectra. Their in vitro activity was determined in human lung cancer cell lines. Based on initial screens, compound Ic was found to be the most potent, and was therefore used as a model for further studies in lung cancer cells. Ic induced both apoptosis and S-phase cell cycle arrest. Furthermore, it activated p53 and induced cleavage of PARP and caspases 8 and 9. Our preclinical data indicate that the makaluvamine analogs are potential therapeutic agents against lung cancer, providing a basis for further development of Ic (and perhaps other analogs) as a novel anti-cancer agent.
Keywords: Makaluvamine, marine alkaloid, lung cancer, apoptosis
INTRODUCTION
Cancer, particularly lung cancer, poses a major health problem worldwide. Although significant progress has been seen with early detection, treatment, and prevention of recurrence, new therapies are urgently needed in order to improve the clinical outcome for cancer patients. Natural products from marine animals and their derivatives have proven to be a rich source of novel, medicinally valuable compounds [1, 2–4]. The toxic substances produced by marine invertebrates such as sponges, bryozoans, tunicates and ascidians, assist the organisms in the deterrence of predators and the paralysis of prey [5]. As such, marine natural products exhibit potent biological activities.
Marine sponges of the genera Latrunculia, Batzella, Prianos and Zyzzya are a rich source of alkaloids bearing a pyrrolo[4,3,2-de]quinoline skeleton [6, 7]. This series of alkaloids, comprised of approximately 60 metabolites, including makaluvamines [8–13], exhibit numerous biological activities [8, 11] and exert cytotoxicity against numerous tumor cell lines [11, 14, 15]. Many also have anti-fungal [8] or antimicrobial activity [16]. A number of excellent reviews have highlighted the chemistry and bioactivity of this class of compounds [6, 17–19], prompting us to investigate the potential role of chemically synthesized derivatives of marine alkaloids as novel anti-cancer agents.
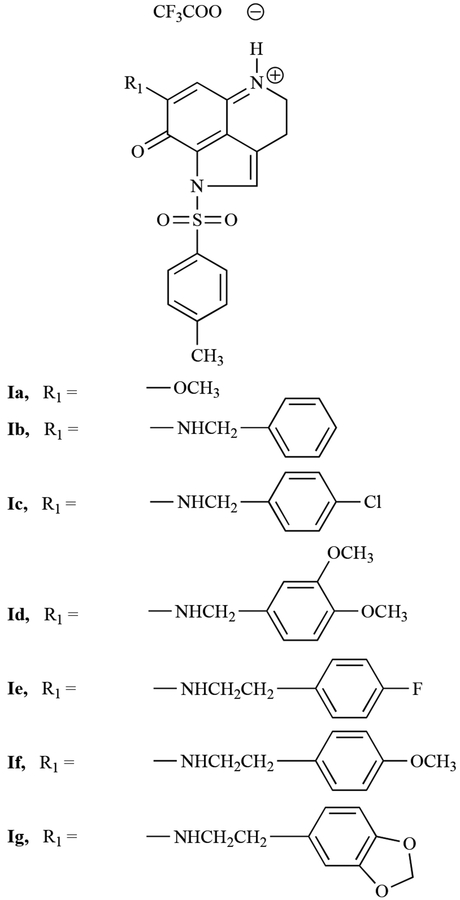
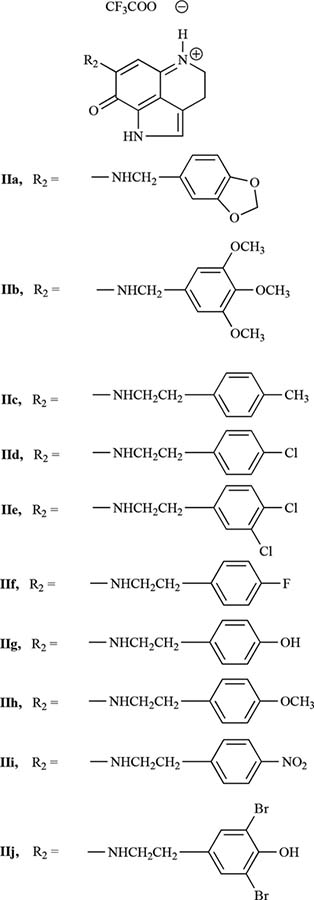
We recently synthesized a number of makaluvamine analogs and evaluated their anti-cancer activity against several human cancer cell lines [20, 21]. This manuscript describes the synthesis of two additional groups of analogs, the first group (Fig. (1)) contains a p-toluenesulfonyl (tosyl) group on the pyrrole ring N, while the second group (Fig. (2)) contains a free pyrrole N atom. Both groups contain substituents at the 6-position of the pyrroloiminoquinone ring of makaluvamines. Herein, we provide a comparison of the biological activity of these new analogs in human lung cancer cell lines.
Fig. (1).
Makaluvamine analogs containing a tosyl group on the pyrrole Nitrogen.
Fig. (2).
Makaluvamine analogs containing an un-substituted pyrrole Nitrogen. Scheme 1: A simple depiction of the procedure for synthesizing makaluvamine analogs Ib-g and IIa-j.
MATERIALS AND METHODS
Synthesis and Characterization of the Compounds
Reagents and Instrumentation
Anhydrous solvents used for reactions were purchased from Aldrich Chemical Company, and solvent evaporations were carried out in vacuo using a rotary evaporator. Thin layer chromatography (TLC) was performed on silica gel plates with a fluorescent indicator (Whatmann, silica gel, UV254, 25 μm plates). Spots were visualized by UV light (254 and 365nm) and purification by column and flash chromatography was carried out using BAKER silica gel (40μm) in the solvent systems indicated. Proton nuclear magnetic resonance 1H-NMR) and carbon nuclear magnetic resonance (13C-NMR) spectra were recorded on a Brucker DPX-300 spectrometer using tetramethylsilane (TMS) as an internal standard. Mass spectra results were recorded on the Micromass Platform LCC instrument. Chemical shift values (δ) are reported as parts per million (ppm) and coupling constants (J) as hertz (Hz).
Amination of the Pyrroloiminoquinone Derivative, Ia
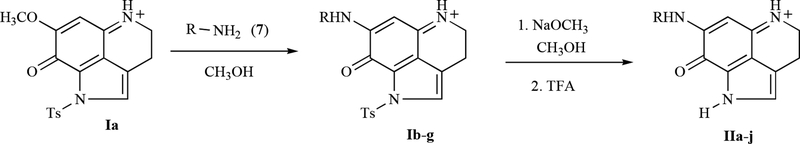
A schematic of the procedure for synthesizing the compounds appears in Fig. (3). To generate the compounds in Group I, a solution of amine (7) in anhydrous MeOH was added drop-wise to a solution of the pyrroloiminoquinone derivative, Ia, over a 15 min period. The resulting solution was stirred at room temperature for 18 hr. Upon completion of the reaction, the mixture was cooled to 0°C and quenched by addition of trifluoroacetic acid (TFA). The crude product was obtained by removal of the solvent solution under reduced pressure and purified by column chromatography over silica gel using MeOH/CHCl3.
Fig. (3).
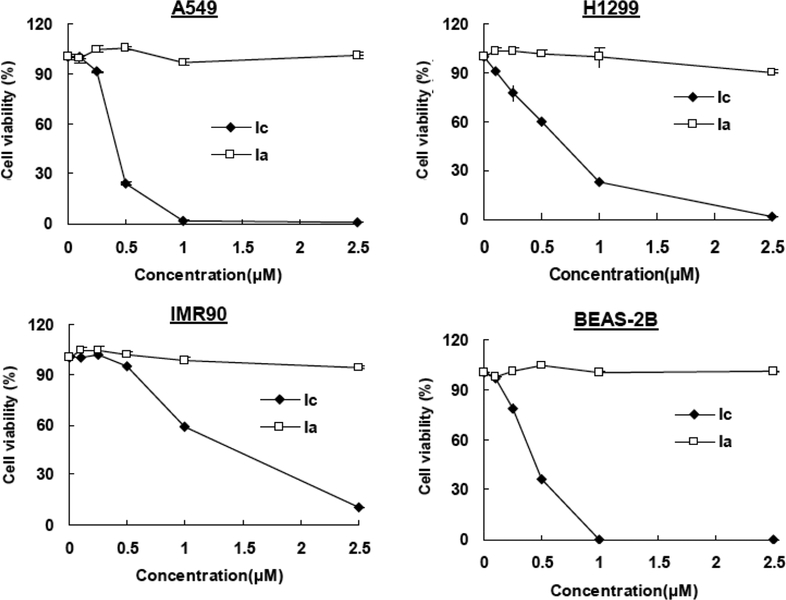
Growth inhibitory activity of makaluvamine analogs Ia and Ic in lung cancer cells. Cells were exposed to various concentrations of the compounds for 72 hr followed by MTT assay. All assays were performed in triplicate.
Detosylation
In order to generate detosylated compounds (for Group II), sodium methoxide was added to a MeOH solution of N-tosyl makaluvamine derivatives (Group I) and stirred at room temperature for 4–6 hr. The resulting solution was quenched at 0°C with TFA. The solvent was completely removed under reduced pressure and the resulting residue was co-evaporated with CHC13 to remove traces of TFA. The remaining crude compound was purified by chromatography over a column of silica gel (20×2cm) using MeOH/CHCl3.
Biological Experiments
Reagents
All chemicals and solvents used were of the highest analytical grade available. Cell culture media, phosphate-buffered saline (PBS), fetal bovine serum (FBS), sodium pyruvate, non-essential amino acids, penicillin-streptomycin, and other cell culture supplies were obtained from the Comprehensive Cancer Center Media Preparation Shared Facility, University of Alabama at Birmingham (Birmingham, AL). The antihuman p53 (Ab-6) antibody was acquired from EMD Chemicals, Inc (Gibbstown, NJ). The anti-human MDM2 (SMP14) and PARP (H-250) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and the caspase-8 (9746) and caspase-9 (9502) antibodies were purchased from Cell Signaling Technology, Inc (Danvers, MA).
Cell Culture
Human cancer cells and normal lung epithelial cells (BEAS-2B) were obtained from the American Type Culture Collection (Rockville, MD) and cultured according to their instructions. The human primary fibroblast cell line IMR90-EEA (IMR90), which was cultured in DMEM, was a gift from Dr. S. Lee (Harvard University, Boston, MA). All culture media contained 10% fetal bovine serum and 1% penicillin-streptomycin. A549 cells were grown in Ham’s F12K medium supplemented with 2 mM L-glutamine and 1.5g/L sodium bicarbonate.
Cell Survival Assay
The effects of test compounds on human cancer cell growth, expressed as the percentage of cell survival, were determined using the MTT assay [22, 23]. In brief, the cells were grown in 96-well plates at 4–5×103 cells per well and exposed to the test compounds (0, 0.1, 0.5, 1, or 2.5μM) for 72 hr. After incubation with the compounds, 10μL of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) solution (5mg/mL; Sigma; St. Louis, MO) were added to each well. The plates were incubated for 2–4 hr at 37°C. The supernatant was then removed, and the formazan crystals were dissolved in 100μL of DMSO. The absorbance at 570nm was recorded using an OPTImax microplate reader (Molecular Devices; Sunnyvale, CA). The cell survival percentages were calculated by dividing the mean OD of compound-containing wells by that of DMSO-treated control wells. Three separate experiments were accomplished to determine the IC50.
Cell Cycle Measurements
To determine the effects of the compound on the cell cycle, cells (2–3×105/well) were exposed to the test compound (0, 0.1, 0.5 or 0.75μM) and incubated for 24 hr prior to analysis. Cells were then trypsinized, washed with PBS, and fixed in 1.5mL of 95% ethanol at 4°C overnight, followed by incubation with RNAse and staining with propidium iodide (Sigma). The DNA content was determined by flow cytometry.
Detection of Apoptosis
Cells in early and late stages of apoptosis were detected using an Annexin V-FITC apoptosis detection kit from Bio-Vision (Mountain View, CA) [22, 23]. In apoptosis experiments, 2–3×105 cells were exposed to the test compound (0, 0.1, 0.5 or 0.75μM) and incubated for 24 hr prior to analysis. Cells were collected and washed with serum-free media, then re-suspended in 500μL, of Annexin V binding buffer followed by addition of 5μL, of Annexin V-FITC and 5μL, of propidium iodide (PI). The samples were incubated in the dark for 5 min at room temperature and analyzed with a Becton Dickinson FACSCalibur instrument (Ex=488 nm; Em=530 nm). Cells that were positive for Annexin V-FITC alone (early apoptosis) and Annexin V-FITC and PI (late apoptosis) were counted.
Western Blot Analysis
The protein expression levels were assessed following exposure of cells to various concentrations of the compounds for 24 hr [22, 23]. Whole cell lysates were fractionated with identical amounts of protein by SDS-PAGE and transferred to Bio-Rad trans-Blot nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The nitrocellulose membrane was incubated in blocking buffer (Tris-buffered saline containing 0.1% Tween 20 and 5% nonfat milk) for 1 hr at room temperature. Then the membrane was incubated with the appropriate primary antibody overnight at 4°C or 2 hr at room temperature with gentle shaking. The membrane was washed three times with rinsing buffer (Tris-buffered saline containing 0.1% Tween 20) for 15 min, then incubated with goat anti-mouse/rabbit IgG-horseradish peroxidase-conjugated antibody (Bio-Rad) for 1 hr at room temperature. After repeating the washes in triplicate, the protein of interest was detected by enhanced chemiluminescence reagents from PerkinElmer LAS, Inc (Boston, MA).
RESULTS
Synthesis and Characterization of the Compounds
All of the test compounds were synthesized in two steps starting from the tricyclic pyrroloiminoquinone compound, Ia (see Fig. (3)). This compound was prepared following the 4,6,7-trimethoxyindole approach described previously [24]. Treatment of Ia with a solution of amine (7) in anhydrous methanol at room temperature provided the tosylated members of the first group of analogs, Ib-g (Fig. (1)) in 30–51% yields. Compounds IIa-j (Fig. (2)) were prepared by treatment of appropriately substituted group I compounds with NaOMe in MeOH, which resulted in 48–62% yields of the detosylated compounds. All final products were completely characterized, and purity was confirmed by 1H-NMR, 13C-NMR, and MS spectroscopy (Table 1).
Table 1.
1H-NMR, 13C-NMR, and MS Spectral Data of 1,3,4,8-Tetrahydropyrrolo[4,3,2-de]quinolin-8(1H)-one Alkaloid Analogs
| Compd. | 1H-NMR δ Values (ppm)a | 13C-NMR δ Values (ppm)a | MS (ES+) b, m/z (M+) |
|---|---|---|---|
| Ib | (CDCl3) δ 2.45 (s, 3H), 2.98 (t, 2H, J = 7.2 Hz), 3.96 (t, 2H, J = 7.0 Hz), 4.45 (d, 2H, J = 5.7 Hz), 6.01 (s, 1H), 7.33–7.43 (m, 7H), 7.69 (s, 1H) and 8.01 (d, 2H, J = 10.5 Hz). | (CDCl3) δ 18.0, 21.8, 42.6, 48.3, 87.8, 118.0, 123.2, 127.7, 128.2, 128.3, 128.8, 129.0, 129.3, 130.2, 133.0, 134.2, 147.3, 150.3, 156.9 and 166.6 | 432 |
| Ic | 1H NMR (CDCl3) δ 2.46 (s, 3H), 2.93–3.04 (m, 2H), 3.92–4.04 (m, 2H), 4.45 (bs, 2H), 6.14 (bs, 1H), 7.07 (bs, 1H), 7.20–7.27 (m, 2H), 7.35–7.40 (m, 4H), 7.67 (s, 1H), 8.02 (d, 2H, J = 8.1 Hz). | (CDCl3) δ 18.0, 21.8, 42.7, 47.5, 88.0, 117.9, 123.1, 127.6, 128.2, 129.0, 129.5, 129.7, 130.2, 132.7, 132.9, 134.9, 147.4, 150.3, 157.1 and 166.5. | 466 |
| Id | (CDCI3) δ 2.45 (s, 3H), 2.97 (bt, 2H, J = 6.6 Hz), 3.88 (s, 6H), 3.98 (bt, 2H, J = 4.5 Hz), 4.39 (d, 2H, J = 5.1 Hz), 6.80–6.89 (m, 3H), 7.06 (bs, 1H), 7.38 (d, 2H, J = 8.4 Hz), 7.67 (s, 1H) and 8.02 (d, 2H, J=8.4Hz). | (CDCI3) δ 18.0, 21.8, 29.7, 42.5, 48.2, 56.0, 56.1, 87.8, 111.6, 111.7, 118.0, 121.0, 123.2, 126.6, 127.8, 128.1, 129.0, 130.2, 133.0, 147.3, 149.5, 149.5, 150.1, 156.9 and 166.7. | 492 |
| Ie | 1H NMR (CDCI3) δ 2.46 (s, 3H), 2.93–2.99 (m, 4H), 3.54–3.62 (m, 2H), 3.98 (bt, 2H, J = 6.9 Hz), 6.20 (bs, 1H), 6.84 (bs, 1H), 7.02 (t, 2H, J = 8.5 Hz), 7.19 (dd, 2H, J1 = 8.7, J2 = 5.7 Hz), 7.39 (d, 2H, J = 8.4 Hz), 7.67 (s, 1H) and 8.02 (d, 2H, J = 8.4 Hz). | (CDCI3) δ 18.0, 21.8, 29.7, 33.3, 42.5, 45.0, 87.5, 115.8, 116.0, 118.0, 123.1, 127.8, 128.2, 129.0, 130.1, 130.2 (2C), 132.4, 132.5, 133.0, 147.3, 150.6, 156.8, 160.4 and 166.4. | 464 |
| If | (CDCI3) δ 2.46 (s, 3H), 2.89–2.96 (m, 4H), 3.52–3.58 (m, 2H), 3.81 (s, 3H), 3.97 (bt, 2H, J = 6.9 Hz), 6.19 (bs, 1H), 6.87 (d, 2H, J = 8.4 Hz), 7.13 (d, 2H, J = 8.4 Hz), 7.38 (d, 2H, J = 8.4 Hz), 7.65 (s, 1H) and 8.02 (d, 2H, J = 8.4 Hz). | (CDCI3) δ 18.0, 21.8, 33.2, 42.4, 45.2, 55.3, 87.5, 114.5, 118.0, 123.2, 127.9, 128.1, 128.7, 128.9, 129.7, 130.2, 133.1, 147.2, 150.6, 156.7, 158.8 and 166.5. | 476 |
| Ig | (CDCI3) δ 2.45 (s, 3H), 2.88 (t, 2H, J = 6.6 Hz), 2.97 (t, 2H, J = 6.6 Hz), 3.57–3.49 (m, 2H), 3.96 (t, 2H, J = 7.2 Hz), 5.95 (s, 2H), 6.06 (bs, 1H), 6.64–6.67 (m, 2H), 6.75 (d, 1H, J = 7.8 Hz), 6.91 (bs, 1H), 7.38 (d, 2H, J = 8.4 Hz), 7.66 (s, 1H) and 8.01 (d, 2H, J = 8.4 Hz). | (CDCI3) δ 18.0, 21.8, 29.7, 33.7, 42.5, 45.1, 87.3, 101.1, 108.7, 108.8, 118.0, 121.7, 123.1, 127.3, 127.8, 128.2, 128.9, 130.3, 133.0, 146.8, 147.3, 148.2, 150.8, 156.8 and 166.3. | 490 |
| IIg | (CD3OD) δ 2.87 (t, 2H, J = 7.5 Hz), 2.94 (t, 2H, J = 7.8 Hz), 3.54 (t, 2H, J = 6.9 Hz), 3.83 (t, 2H, J = 7.5 Hz), 5.38 (s, 1H), 6.71 (d, 2H, J = 8.4 Hz), 7.06 (d, 2H, J = 8.4 Hz) and 7.14 (s, 1H). | (CD3OD) δ 19.5, 34.4, 44.2, 46.5, 85.2, 116.5 (2C), 120.2, 124.0, 127.0, 127.2, 130.0, 130.9 (2C), 155.0, 157.4, 159.8 and 168.5. | 307 |
| IIi | (CD3OD) δ 2.95 (t, 2H, J = 7.8 Hz), 3.12 (t, 2H, J = 7.2 Hz), 3.68 (t, 2H, J = 6.9 Hz), 3.84 (t, 2H, J = 7.8 Hz), 5.42 (s, 1H), 7.15 (s, 1H), 7.51 (d, 2H, J = 9.0 Hz) and 8.17 (d, 2H, J = 9.0 Hz). | (CD3OD) δ 19.5, 34.8, 44.2, 45.3, 85.4, 120.2, 123.8, 124.8 (2C), 127.2, 129.8, 131.1 (2C), 147.5, 148.4, 155.1, 159.9 and 168.5. | 337 |
| IIj | (CD3OD) δ 2.86 (t, 2H, J = 6.8 Hz), 2.95 (t, 2H, J = 7.6 Hz), 3.55 (t, 2H, J = 7.6 Hz), 3.85 (t, 2H, J = 7.6 Hz), 5.38 (s, 1H), 7.15 (s, 1H) and 7.39 (s, 2H). | (CD3OD) δ 19.5, 33.6, 44.2, 45.9, 85.4, 112.3, 120.2, 123.9, 125.5, 127.2 (2C), 133.5, 133.8 (2C), 151.3, 155.1, 159.7 and 168.5. | 466 |
1H-NMR and 13C-NMR) spectra were recorded on a Brucker DPX-300 spectrometer. The chemical shift values are reported as parts per million (ppm) relative to tetramethylsilane as internal standard.
Mass spectra were recorded on a Micromass Platform LCC Electrospray instrument.
Initial Screening for Growth Inhibition in Cancer Cells
Seventeen compounds were tested for their in vitro anticancer activity using the MTT assay. Three human lung cancer cell lines were cultured with test compounds Ia-g and IIa-j at concentrations ranging from 0.01μM to 2.5μM for 72 hr, then cell viability was determined. The inhibitory effects of these compounds on cell growth are illustrated in Table 2. Most of the analogs in both series showed impressive activity against all three of the tested cell lines. However, Ia, which was used as a control for the core structure of these compounds, did not show any major activity. This finding indicates that the amino N atom at the 6-position of the pyrroloiminoquinone ring enhances the activity of the makaluvamine analogs.
Table 2.
Growth Inhibitory Activity of Makaluvamine Analogs in Human Lung Cancer Cells. Cells were Exposed to Various Concentrations of the Compounds for 72 hr Followed by MTT Assay. All Assays were Performed in Triplicate
| A549 | H838 | H1299 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IC20b | IC50 | IC80 | IC20 | IC50 | IC80 | IC20 | IC50 | IC80 | |
| Ia | >5.00 | >5.00 | >5.00 | >5.00 | >5.00 | >5.00 | >5.00 | >5.00 | >5.009 |
| Ib | 1.11 | 1.39 | 1.76 | 1.60 | 2.05 | 2.69 | 0.39 | 0.88 | 1.72 |
| Ic | 0.30 | 0.39 | 0.53 | 0.50 | 1.41 | 3.07 | 0.29 | 0.58 | 1.16 |
| Id | 1.08 | 1.55 | 2.14 | 1.72 | 2.55 | 3.75 | 0.22 | 0.77 | 2.03 |
| Ie | 2.30 | 2.71 | 3.18 | 2.53 | 2.96 | 3.54 | 1.64 | 2.15 | 3.00 |
| If | 2.50 | 3.10 | 3.72 | 2.56 | 2.93 | 3.44 | 2.14 | 2.81 | 3.87 |
| Ig | 0.29 | 3.01 | 5.53 | 1.74 | 2.61 | 3.92 | 0.84 | 1.69 | 3.26 |
| IIa | 1.32 | 2.21 | 3.37 | 0.39 | 1.05 | 2.27 | 0.35 | 0.67 | 1.19 |
| IIb | 1.08 | 2.10 | 3.48 | 0.64 | 0.93 | 1.35 | 0.37 | 0.91 | 1.87 |
| IIc | 0.85 | 1.95 | 3.63 | 0.56 | 1.51 | 3.25 | 0.40 | 0.81 | 1.48 |
| IId | 1.09 | 3.33 | 5.56 | 0.79 | 1.29 | 2.04 | 0.88 | 1.84 | 3.06 |
| IIe | 0.73 | 3.01 | 5.81 | 0.84 | 1.80 | 3.35 | 0.35 | 1.22 | 2.93 |
| IIf | 1.18 | 2.84 | 5.20 | 0.69 | 1.71 | 3.46 | 0.51 | 1.16 | 2.26 |
| IIg | 3.16 | >10.00 | >10.00 | 4.00 | 7.94 | >10.00 | 4.07 | >10.00 | >10.00 |
| IIh | 0.77 | 1.99 | 3.57 | 0.43 | 1.23 | 2.76 | 0.36 | 0.69 | 1.20 |
| Iii | 0.18 | 0.56 | 1.29 | 0.42 | 0.59 | 0.83 | 0.20 | 0.42 | 0.78 |
| IIj | 3.22 | 8.82 | >10.00 | 7.47 | 9.71 | 10.85 | 7.70 | >10.00 | >10.00 |
ND: Not determined;
IC, inhibitory concentration. IC20, IC50, and IC80 are the concentrations of drug that inhibit growth by 20%, 50%, and 80%, respectively, relative to the control.
The analog that showed the greatest activity was the N-tosyl chlorobenzyl analog, Ic (Mean IC50 0.72μM). Among the compounds that contained a N atom at the 6-position of the ring, the least active analogs were the hydroxyphenethyl analog, IIg, and the structurally similar 2,6-dibromohydroxy-phenethyl analog, IIj (Mean IC50> 10μM). Similar effects of the compounds were also observed in human MCF-7 breast cancer cells (data not shown). The presence of the N-tosyl group did not seem to affect the activity of the compounds because analogs from both Groups I (Ib-g) and II (IIa-j) were active against the tested cell lines. Since Ic consistently exhibited potent activity against the lung cancer cells, we compared the effects of Ic and Ia on the survival of lung cancer (A549 and HI299) cell lines and normal (non-malignant) (IMR90 and BEAS-2B) cells, and found that there was a dose-dependent decrease in cell growth following Ic exposure in all of the cells, while Ia had minimal effects (Fig. (3)).
Examination of the Effects of the Compounds on the Cell Cycle
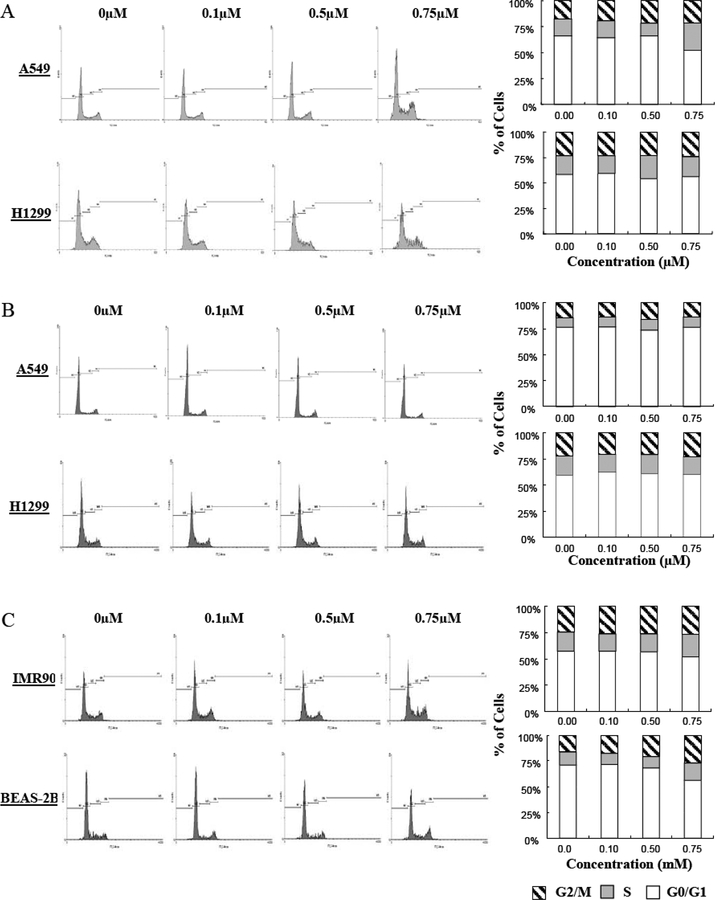
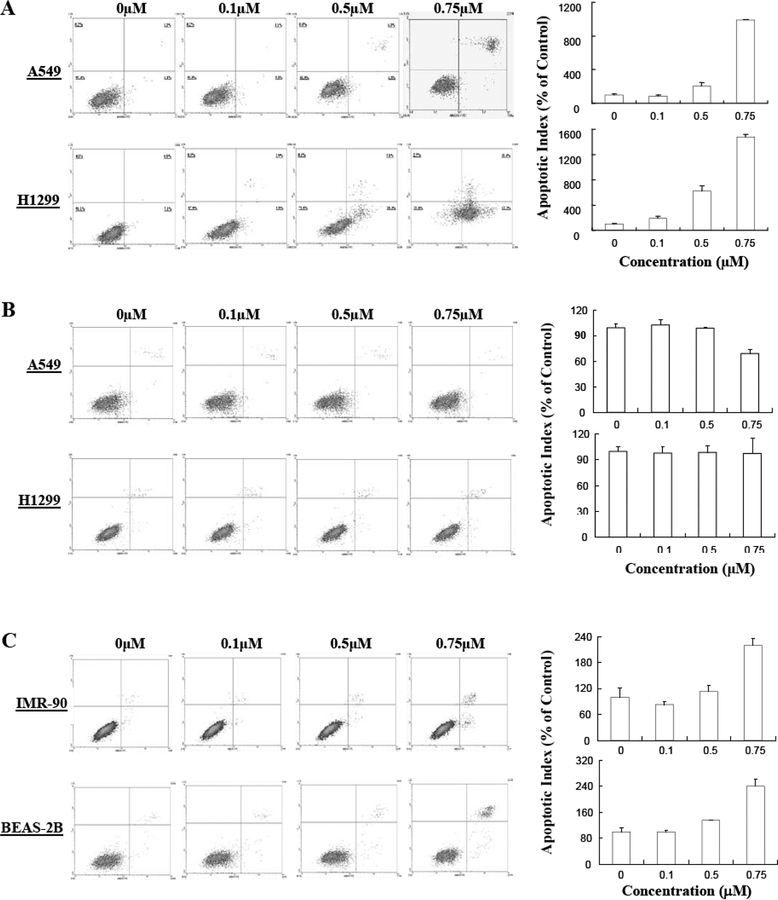
To determine whether the decrease in growth was due to changes in the cell cycle, we investigated whether Ic could induce cell cycle arrest. As shown in Fig. (4A), Ic induced considerable S-phase arrest in A549 cells, but exerted a lesser effect on H1299 cells. As expected, Ia did not exert any appreciable effect on cell cycle progression (Fig. (4B)). To determine whether the effect of Ic was specific for cancer cells, we also examined whether the compound altered cell cycle progression in the “normal” cells. As shown in Fig. (4C), Ic induced modest cell cycle arrest in both cells lines, with IMR90 exhibiting a slight increase in S phase cells, and the BEAS-2B cells showing an increase in cells in G2/M. Because A549, IMR90 and BEAS-2B cells express wild-type p53, while H1299 cells do not express the protein [25], this indicates that p53 may play a role in Ic-induced cell cycle arrest.
Fig. (4).
Effects of the makaluvamine analogs on cell cycle progression. Effects of Ic (A) and Ia (B) on human lung cancer cells. (C) Effects of Ic on human non-malignant lung epithelial cells (BEAS-2B) and fibroblasts (IMR90). Cells were exposed to various concentrations of Ic or Ia for 24 hr, followed by determination of cell cycle distribution. All assays were performed in triplicate.
Induction of Apoptosis in Human Cancer Cells
Because the growth inhibition of H1299 cells appeared to be independent of the cell cycle, we examined whether Ic also could induce apoptosis. As illustrated in Fig. (5A), Ic induced apoptosis in a dose-dependent manner in both A549 (p53 wild-type) and HI299 (p53 null) cell lines, indicating that Ic can induce apoptosis, regardless of the p53 status of the cells. On the other hand, Ia did not increase apoptosis in either cell line (Fig. (5B)). The normal cells also exhibited an increase in apoptosis following exposure to Ic, although to a lesser extent than the lung cancer cells (Fig. (5C)).
Fig. (5).
Induction of apoptosis in by makaluvamine analogs. (A) Effects of Ic (A) and Ia (B) on human lung cancer cells. (C) Effects of Ic on human non-malignant lung epithelial cells (BEAS-2B) and fibroblasts (IMR90). The cells were exposed to various concentrations of Ic or Ia for 24 hr, followed by assessment of apoptosis. All assays were performed in triplicate.
Alterations in the Expression of Proteins Related to Apoptosis
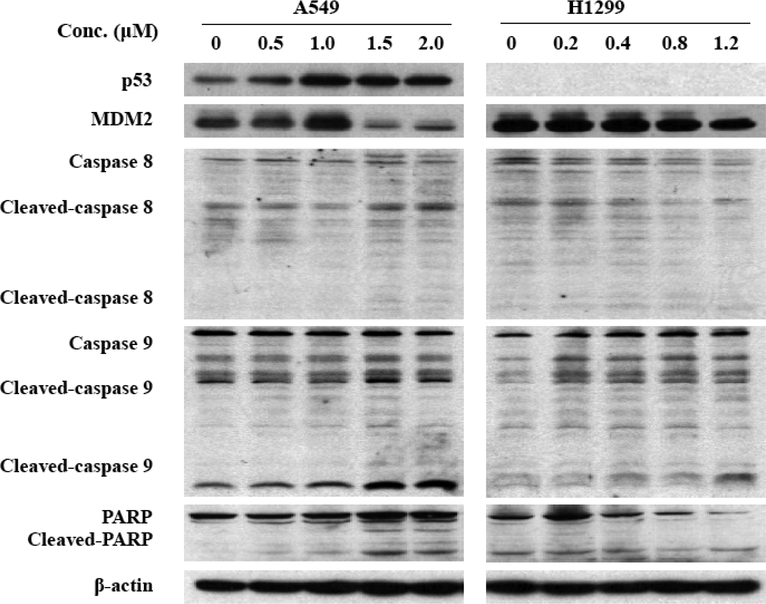
We next investigated the possible mechanism(s) responsible for the pro-apoptotic activity of Ic by evaluating their effects on the expression level of various proteins involved in apoptosis. Ic activated p53 (in A549 cells), down-regulated MDM2, and increased cleavage of PARP and caspases 8 and 9 (Fig. (6)).
Fig. (6).
Effects of the makaluvamine analog Ic on the expression of various apoptosis-related proteins in human lung cancer cells. Cells were exposed to various concentrations of Ic for 24 hr, and the target proteins were detected by immunoblotting with specific antibodies.
DISCUSSION
Marine natural products have attracted the attention of scientists from many disciplines, including organic chemistry, medicinal chemistry, pharmacology, and biology. As a result of improvements in deep-sea sample collection and large-scale drug production through aquaculture, the number of biologically active alkaloids isolated from marine sources has increased significantly [26]. There are approximately a dozen marine alkaloids currently undergoing various phases of human clinical trials for the treatment of multiple cancers [27,28].
The majority of bioactive marine alkaloids have been isolated from marine sponges [29] and exhibit numerous therapeutic properties including anti-inflammatory, antitumor, immunosuppressive, antiviral, anti-malarial and antibiotic characteristics [8, 11, 16]. Unfortunately, a number of these alkaloids are only available in minute quantities, thus precluding their use in biological investigations. Despite improvements in aquaculture, laboratory synthesis of these compounds and their analogs is the only practical way to obtain these compounds in larger quantities.
We recently developed novel methods that allowed us to synthesize several analogs of makaluvamines, a subclass of marine alkaloids. In addition to developing the compounds, we also accomplished initial evaluations of their activity against several human cancer cell lines [20, 21]. Further studies with the most active of these compounds are currently underway. However, during our studies, we observed that the pyrroloiminoquinone moiety of makaluvamines is crucial for their activity; therefore, no major changes can be made to the tricyclic ring system. Since further modifications may be needed to enhance the efficacy or “drug-like” properties of these compounds, we thought it necessary to generate compounds that could undergo more extensive modifications. Two areas of the makaluvamine molecule are amenable to modifications: 1) the 6-position of the pyrroloiminoquinone ring, and 2) the pyrrole N atom. Therefore, as a continuing effort to generate new potential therapeutics, we synthesized two classes of compounds by modifying each of these areas. As many pyrroloiminoquinone alkaloids with proven anti-cancer activities have substitutions at the 6-position of the ring, we decided to explore simple substitutions with increased steric bulk, hydrophobicity, and hydrophilicity at this position on the pyrroloiminoquinone ring. To our knowledge, the anti-cancer activities of N-tosylpyrroloiminoquinones have never been explored, thus making this report the first of its kind. Moreover, this study represents the first attempt to evaluate the anti-cancer activities of these synthetic makaluvamine analogs, and the first demonstration that their biological activities are related to specific modifications of the core structure.
The results of our study indicate that the presence of a N atom at the 6-position of pyrroloiminoquinone is crucial for the anti-cancer activity of the makaluvamine analogs. Although the most active analog identified from these studies was a N-tosyl analog (Ic), in most cases, N-tosyl substitution did not significantly alter compound activity. Analogs with OH substituents on the side chain phenyl ring were the least active against lung cancer.
The present investigations demonstrated at least four major points: 1) The anti-cancer activity of the makaluvamine derivatives is dose–, structure–, and cell type–dependent; 2) Induction of apoptosis is the major mechanism by which Ic exerts its cytotoxicity, although cell cycle arrest and effects on proliferation may also be involved; 3) Ic regulates the expression of cell cycle- and apoptosis-related proteins; and 4) The anti-cancer activity of compound Ic appears to occur via both p53–dependent and –independent pathways, as the viability of both A549 (p53 wt) and H1299 (p53 null) cells were affected by the compound.
It is generally believed that p53 activation is one of the major mechanisms of action for many DNA damaging agents, including irradiation and numerous chemotherapeutic agents. However, approximately 50% of all human malignancies lack functional p53 [30], rendering them un-responsive to these DNA-damaging therapies. As all of the human cell lines tested in the present study were responsive to the synthetic marine alkaloids, and were particularly sensitive to Ic, we hypothesize that these compounds may prove to be efficacious against a broad spectrum of human cancers, including those lacking functional p53. Moreover, we observed that there was a decrease in MDM2 in both cell lines. Since decreasing MDM2 has been shown to result in anticancer effects (decreased proliferation, increased apoptosis and decreased cell viability) independent of the p53 status of the cells or tumors [31], it is possible that Ic exerts its effects at least partially through MDM2. Additional molecular and pharmacological investigations are warranted to further elucidate the underlying mechanisms of action and to further establish Ic, and potentially other analogs, as novel anticancer agents.
Scheme 1.
Synthesis of makaluvamine analogs Ib-g and IIa-j.
ACKNOWLEDGEMENTS
This work was supported by NIH grants R01 CA112029 and R01 CA121211. E. Rayburn was supported by a DoD grant W81XWH-06-1-0063 and a T32 fellowship from the NIH/UAB Gene Therapy Center (CA075930). S. E. Velu was supported by a UAB Breast Spore pilot grant and a translational research grant from the UAB Council of University-Wide Interdisciplinary Research Centers.
REFERENCES
- [1].Haefner B Drugs from the deep: marine natural products as drug candidates. Drug Discov. Today, 2003, 8, 536–44. [DOI] [PubMed] [Google Scholar]
- [2].Blunt JW; Copp BR; Munro MH; Northcote PT; Prinsep MR Marine natural products. Nat. Prod. Rep, 2006, 23, 26–78. [DOI] [PubMed] [Google Scholar]
- [3].Marris E Marine natural products: drugs from the deep. Nature, 2006, 443, 904–905. [DOI] [PubMed] [Google Scholar]
- [4].Schwartsmann G; Brondani da Rocha A; Berlinck RG; Jimeno J Marine organisms as a source of new anticancer agents. Lancet Oncol, 2001, 2, 221–5. [DOI] [PubMed] [Google Scholar]
- [5].Williams DH; Stone MJ; Hauck PR; Rahman SK Why are secondary metabolites (natural products) biosynthesized? J. Nat. Prod, 1989, 52, 1189–1208. [DOI] [PubMed] [Google Scholar]
- [6].Antunes EM; Copp BR; Davies-Coleman MT; Samaai T Pyrroloiminoquinone and related metabolites from marine sponges. Nat. Prod. Rep, 2005, 22, 62–72. [DOI] [PubMed] [Google Scholar]
- [7].Faulkner DJ Marine natural products. Nat. Prod. Rep, 2002, 19, 1–48. [DOI] [PubMed] [Google Scholar]
- [8].Carney JR; Scheuer PJ; Kelly-Borges M A new bastadin from the sponge Psammaplysilla purpurea. Tetrahedron, 1993, 49, 8483–6. [DOI] [PubMed] [Google Scholar]
- [9].Casapullo A; Cutignano A; Bruno I; Bifulco G; Debitus C; Gomez-Paloma L; Riccio R Makaluvamine P, a new cytotoxic pyrroloiminoquinone from Zyzzya cf. fuliginosa. J. Nat. Prod, 2001, 64, 1354–6. [DOI] [PubMed] [Google Scholar]
- [10].Hu JF; Schetz JA; Kelly M; Peng JN; Ang KK; Flotow H; Leong CY; Ng SB; Buss AD; Wilkins SP; Hamann MT New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J. Nat. Prod, 2002, 65, 476–80. [DOI] [PubMed] [Google Scholar]
- [11].Radisky DC; Radisky ES; Barrows LR; Copp BR; Kramer RA; Ireland CM Novel cytotoxic topoisomerase II inhibiting pyrroloiminoquinones from Fijian sponges of the genus Zyzzya. J. Am. Chem. Soc, 1993,115, 1632–1638. [Google Scholar]
- [12].Schmidt EW; Harper MK; Faulkner DJ Makaluvamines H-M and damirone C from the pohnpeian sponge Zyzzya fuliginosa. J. Nat. Prod, 1995, 58, 1861–7. [DOI] [PubMed] [Google Scholar]
- [13].Venables DA; Concepcion GP; Matsumoto SS; Barrows LR; Ireland CM Makaluvamine N: a new pyrroloiminoquinone from Zyzzya fuliginosa. J. Nat. Prod, 1997, 60, 408–10. [DOI] [PubMed] [Google Scholar]
- [14].Gunasekera SP; Zuleta IA; Longley RE; Wright AE; Pomponi SA Discorhabdins S, T, and U, new cytotoxic pyrroloiminoquinones from a deep-water Caribbean sponge of the genus Batzella. J. Nat. Prod 2003, 66, 1615–7. [DOI] [PubMed] [Google Scholar]
- [15].Dijoux MG; Schnabel PC; Hailock YF; Boswell JL; Johnson TR; Wilson JA; Ireland CM; van Soest R; Boyd MR; Barrows LR; Cardellina JH 2nd. Antitumor activity and distribution of pyrroloiminoquinones in the sponge genus Zyzzya. Bioorg. Med. Chem 2005,13, 6035–44. [DOI] [PubMed] [Google Scholar]
- [16].Perry NB; Blunt JW; Munro MHG Cytotoxic pigments from new Zealand sponges of the genus latrunculia : discorhabdins a, b and c. Tetrahedron, 1988, 44, 1727–34. [Google Scholar]
- [17].Ding Q; Chichak K; Lown JW Pyrroloquinoline and pyridoacridine alkaloids from marine sources. Curr. Med. Chem, 1999, 6, 1–27. [PubMed] [Google Scholar]
- [18].Urban S; Hickford SJH; Blunt JW; Munro MHG Bioactive marine alkaloids. Curr. Org. Chem, 2000, 4, 778–821. [Google Scholar]
- [19].Harayama Y; Kita Y Pyrroloiminoquinone alkaloids: discorhabdins and makaluvamines. Curr. Org. Chem, 2005, 9, 1567–88. [Google Scholar]
- [20].Shinkre BA; Raisch KP; Fan L; Velu SE Analogs of the marine alkaloid makaluvamines: synthesis, topoisomerase II inhibition, and anticancer activity. Bioorg. Med. Chem. Lett, 2007, 17, 2890–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shinkre BA; Raisch KP; Fan L; Velu SE Synthesis and antiproliferative activity of benzyl and phenethyl analogs of makaluvamines. Bioorg. Med. Chem 2008, 16, 2541–2549. [DOI] [PubMed] [Google Scholar]
- [22].Li M; Zhang Z; Hill D; Chen X; Wang H; Zhang R Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res, 2005, 65, 8200–8. [DOI] [PubMed] [Google Scholar]
- [23].Wang W; Rayburn ER; Hao M; Zhao Y; Hill DL; Zhang R; Wang H Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides. Prostate, 2008, 68, 809–19. [DOI] [PubMed] [Google Scholar]
- [24].Velu SE; Pillai SK; Lakshmikantham MV; Billimoria AD; Culpepper JS; Cava MP Efficient Syntheses of the Marine Alkaloids Makaluvamine D and Discorhabdin C: The 4,6,7-Trimethoxyindole Approach. J. Org. Chem, 1995, 60, 1800–1805. [Google Scholar]
- [25].Mitsudomi T,; Steinberg SM; Nau MM; Carbone D; D’Amico D; Bodner S; Oie HK; Linnoila RI; Mulshine JL; Minna JD; et al. p53 gene mutations in non-small-cell lung cancer cell lines and their correlation with the presence of ras mutations and clinical features. Oncogene, 1992, 7, 171–180. [PubMed] [Google Scholar]
- [26].Pomponi SA The bioprocess-technological potential of the sea. Journal of Biotechnology, 1999, 70, 5–13. [Google Scholar]
- [27].Newman DJ; Cragg GM Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod, 2004, 67, 1216–38. [DOI] [PubMed] [Google Scholar]
- [28].Simmons TL; Andrianasolo E; McPhail K; Flatt P; Gerwick WH Marine natural products as anticancer drugs. Mol. Cancer Ther, 2005, 4, 333–42. [PubMed] [Google Scholar]
- [29].Sipkema D; Franssen MC; Osinga R; Tramper J; Wijffels RH Marine sponges as pharmacy. Mar. Biotechnol. (NY), 2005, 7,142–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lain S; Lane D Improving cancer therapy by non-genotoxic activation of p53. Eur. J. Cancer, 2003, 39, 1053–60. [DOI] [PubMed] [Google Scholar]
- [31].Zhang Z; Li M; Wang H; Agrawal S; Zhang R Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc. Natl. Acad. Sei. USA, 2003,100, 11636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]