Abstract
The workforce available to care for older adults has not kept pace with the need. In response to workforce limita-tions and the growing complexity of healthcare, scientists have tested new models of care that redesign clinical prac-tice. This article describes why new models of care in aging, dementia, and mental health diffuse inadequately into the healthcare systems and communities where they might benefit older adults. We review a general frame-work for the diffusion of innovations and highlight the importance of other features of innovations that deter or facilitate diffusion. Although scientists often focus on gen-erating evidence-based innovations, end-users apply their own criteria to determine an innovation’s value. In 1962, Rogers suggested six features of an innovation that facili-tate or deter diffusion suggested:relative advantage, com-patibility with the existing environment, ease or difficulty of implementation, trial-ability or ability to “test drive”,adaptability, and observed effectiveness. We describe examples of models of care in aging, dementia and mental health that enjoy a modicum of diffusion into practice and place the features of these models in the context of deter-rents and facilitators for diffusion. Developers of models of care in aging, dementia, and mental health typically fail to incorporate the complexities of health systems, the barriers to diffusion, and the role of emotion into design considerations of new models. We describe agile imple-mentation as a strategy to facilitate the speed and scale of diffusion in the setting of complex adaptive systems, social networks, and dynamic macroenvironments.
Keywords: implementation, diffusion, models of care, dementia, mental health
According to the U.S. National Academies of Sciences, Engineering, and Medicine, the workforce available to support the health and social care of older adults falls short of the growing care needs of older Americans.1–3 As the ratio of older to younger Americans increases over the next 2 decades, older adults will struggle to find family and professional providers to support their care. The National Academies reported that the workforce available to provide care for older adults was inadequate in quan-tity, skills, and distribution. The United States failed to produce an adequate number of physicians and nurses trained in the special care needs of older adults4–7,but we must also recognize that our nation failed to produce an adequate number of social workers, psychologists, thera-pists, pharmacists, direct care workers, and other types of providers fundamental to assuring excellent care for older adults2–4. Maldistribution of the workforce exacerbates the problem of inadequate numbers because many com-munities lack any providers with special training in the care of older adults. Family caregivers, the caregivers who provide the greatest percentage of day-to-day care for older adults, are declining in supply relative to demand. In addition, the physical, administrative, regulatory, and cultural environment of health care has not kept pace with the needs of older adults with multiple chronic conditions. Our nation now struggles to reengineer health care to become more age friendly.9,10 With the reemerging recog-nition of the social determinants of health, our nation also struggles to reengineer its communities to become more age friendly.11
The National Academies further highlight the work-force limitations facing older Americans with mental health needs.3–12,12 This includes older adults with lifelong severe mental illnesses such as schizophrenia;13 older adults with age-related conditions such as Alzheimer’s disease and related dementias; and older adults with con-ditions such as anxiety, depression, and insomnia. Mental health challenges of older adults also include substance use, including alcohol, marijuana and opiates.14 Nearly 1 in 5 older adults has a mental health or substance use problem.12 Older adults with mental illness have the same spectrum of common comorbid medical conditions (e.g., diabetes mellitus, hypertension, heart disease) as do older adults without mental illness.15–17 Older adults with men-tal illness die at younger ages than those without.15 Older adults with mental illness continue to face disturb-ing challenges from stigma, underrecognition, and misun-derstanding. The United States has less than 1 geriatric psychiatrist for every 10,000 older adults (vs 3–5 cardiolo-gists for every 10,000 older adults).12,18
Finally, the National Academies recently documented the workforce challenges in helping older adults live inde-pendently in the community.19 Older adults, their families, and payers all seek to reduce the use of acute and long-term care services and shift the site of care to homes. Helping people live in their homes longer requires a work-force capable of providing services in the home or the local community. Some homes require structural changes to improve mobility and safety, and some homes required upgrades in technology.
Over the past 30 years, researchers and providers have experimented with numerous new models of care seeking to achieve effective clinical practice redesign.20–25 Scientists have tested these innovative models of care in hospital, ambulatory, nursing facility, and home settings and attempted to improve transitions between these set-tings. The models of care often target persons with specific diseases, such as depression or dementia, but some also target adults with multimorbidity and social stressors. None of the tested models is a panacea,26,27 but they offer compelling prototypes and early evidence that remodeling care can overcome some of the challenges in providing excellent care to older adults.28 Despite the evidence of the models’ improvements to quality care, diffusion of these models into routine care is limited. None of these models has diffused at the level of other technological advances, such as cellular telephones, hemodialysis, or intensive care units. The goal of this article is to answer the question: “Why have evidence-based models of care in aging, dementia, or mental health not diffused widely into the healthcare system or the community?” We describe the common theoretical models of diffusion of innovation, the relative advantages of new models of care, and barriers to diffusion in the context of healthcare organizations and recommend approaches to implementation that may over-come common barriers to diffusion.
THEORETICAL MODELS OF DIFFUSION OF INNOVATION
Rogers’ “Diffusion of Innovations,” originally published in 1962, is one of the earliest frameworks for understand-ing diffusion.29 The framework has been surprisingly robust across applications in myriad industries. Rogers’ framework suggests 6 features of an innovation that facili-tate or deter diffusion: relative advantage compared with current approaches, compatibility with the existing environment and resources, complexity or difficulty of implementation, “trial-ability” or ability to “test drive, adaptability to the user’s environment or needs, and observed effectiveness. Rogers also submits that end-users judge these features as a whole; weaknesses in any of these characteristics might hinder diffusion, whereas substantial strengths in any of these characteristics might overcome weaknesses in others.
Rogers’ framework specifically highlights the impor-tant role of peer-to-peer communications in facilitating the spread of new ideas. Innovators often view communication as a didactic interaction between expert and learner. Rogers stressed that: “communication is a process of con-vergence (or divergence) as two or more individuals exchange information in order to move toward each other (or apart) in the meanings that they give certain events.”29 We will return to the importance of this “exchange of information” in recommendations to improve diffusion of new models of care in aging, dementia, and mental health. Effective exchange of information often requires the inno-vator to develop strong relationships and embeddedness in the targeted organization. More recent research in behav-ioral economics also stresses the role of unconscious choices, the importance of emotions in day-to-day deci-sion-making, and the largely untapped potential of “nudge” techniques in affecting behavior change.30
In 2004, Greenhalgh published an extensive review of nearly 500 data sources reporting on the diffusion of inno-vations in service organizations with an emphasis on healthcare settings.31 Building from Rogers’ framework, the review highlighted some features of diffusion of partic-ular relevance to health care. One overarching theme was the research team’s observation that innovation can be dif-fused within organizations through three approaches: com-pletely organically or “letting it happen,” facilitating and nudging or “helping it happen,” or dictating and rule-making or “making it happen.” Greenhalgh placed consid-erable importance on relative advantage and convergent communication:
If potential users see no relative advantage in the inno-vation, they generally will not consider it further; in other words, relative advantage is a prerequisite for adoption. Nevertheless, relative advantage alone does not guarantee widespread adoption. Even so-called evidence-based innovations undergo a lengthy period of negotia-tion among potential adopters in which their meaning is discussed, contested, and reframed. Such discourse can increase or decrease the innovation’s perceived relative advantage.31
A healthcare delivery organization is a complex adaptive system, and groups of stakeholders decide whether to adopt new approaches in the context of an unpredictably changing environment. “Peer-to-peer” in this context may be more related to peer organizations, so adoption of the innovation by peer organizations may be a powerful stim-ulus for local adoption. Thus, some healthcare innovations may diffuse rapidly due to spread through patients’ social networks, whereas other innovations may spread through the social networks of healthcare organizations. Finally, the internal and external political context and personal relationships and familiarity between decision-makers and innovators often facilitates or deters local adoption.32 This notion of familiarity sometimes drives the recommenda-tion that innovators embed their teams in the environment they wish to change and, in doing so, help manage the interface between the internal and external environment.
Whether an individual or a networked group of indi-viduals judges the perception of “relative advantage,” innovators are reminded that the end-user assigns the rela-tive advantage and not the inventor. Furthermore, the end-user defines the characteristics of importance in assigning the relative advantage (e.g., outcomes of interest or of value). Because the end-user, not the inventor, judges the relative advantage (or value) of an innovation, relative advantage cannot necessarily be equated with concepts such as clinical efficacy or superiority for a specific clinical outcome. For example, the end-user may adopt the inno-vation because of perceived advantages such as time saved, fewer hassles, greater patient satisfaction, greater revenue, and better reputation or image, among many other factors. Within this broader framework of diffusion, innovators in health care can begin to see why antacids, hemodialysis, sedative-hypnotics, and antipsychotics may diffuse faster than new models of care for older adults.
RELATIVE ADVANTAGE OF NEW MODELS OF CARE
Given the primacy of “relative advantage,” it is important to examine the evidence that these new models improve the care of older adults. In support of the National Aca-demies’ recommendations for improving the workforce for the care of older adults, researchers sought to identify models of comprehensive care that demonstrated better quality, outcomes, and efficiency of care.25 Fifteen models were identified that improved at least one aspect of care. These models included interdisciplinary primary care, models that supplement primary care, transitional care, models of acute care in people’s homes, nurse and physi-cian teams for nursing home residents, and models of comprehensive care in hospitals. The reviewers concluded that “many comprehensive models of chronic care for older adults have been shown to be capable of improving important outcomes, but the nation’s ability to benefit from these advances will depend on the models’ inherent diffusability, on additional rigorous research, on the inten-sity of the consumer demand for such models including from patients, family caregivers, policy makers, and health insurers and, finally, on public and private insurers’ ability and willingness to reimburse providers adequately for the costs of operating these models.”25
Other reviews have reached similar conclusions that there is high-quality evidence of the advantage of these models over usual care, but the relative advantage may be difficult to achieve in real-world healthcare settings.20,33,34 Although new models improve some outcomes, it is less clear that these models improve efficiency and cost of care or other aspects of end-users’ perceptions of relative advantage.26,27 If end-users define relative advantage as better quality and lower cost and no need for practice redesign, then the evidence base for these models is much weaker. A review of the effectiveness of and economic case for collaborative care models found that “the weight of evidence fails to match the enthusiasm for a solution to the major clinical challenge of the decade. Previous reviews have shown how subjective conclusions about effectiveness of comprehensive care can be.”27 Because similar reviews have reported uncertain findings from comprehensive care models, decision makers could reason-ably conclude that relative advantage is still subjective.35 We and others have argued that these models have shown proof of concept and that diffusion requires more oppor-tunities to develop the models in real-world settings.28,36,37 In other words, we may have an evidence base for some models of care that demonstrate efficacy, but not effective-ness, whereas some models may be effective but are viewed as having poor relative advantage. Some models of care thrive in the greenhouse of a clinical trial but not in a hurricane in the real world of complex adaptive systems. Some models of care thrive in the isolated systems con-structed in clinical trials but not in the larger healthcare system.
BARRIERS TO DIFFUSION IN THE CONTEXT OF HEALTH CARE ORGANIZATIONS
Given that more than 79 clinical trials of collaborative care demonstrated improvement in depression and anxiety outcomes in primary care, one might ask, “Why are there not more collaborative care programs available nationwide?”34 Of evaluated models, the Improving Mood—Promoting Access to Collaborative Treatment for Late Life Depression (IMPACT) model of collaborative care for late-life depression may be the most rigorously evaluated and the most widely disseminated.38–42 With regard to the quadruple aim, this model of care has been shown to improve patient and provider satisfaction, improve outcomes, and reduce the cost of care.38,43 The model was tested in multiple healthcare settings and has been replicated in numerous sites across the United States but remains unavailable to the majority of older adults with depression. Thus, there are clearly barriers to the widespread diffusion of this model of care (and other models of collaborative care for aging, dementia, and mental health). We can examine these potential barriers in the context of the 6 features described in Rogers’ framework.
The IMPACT model includes a team-based approach to primary care and a care manager working with a primary care provider and consultant psychiatrist to provide protocol-driven care to a group of individuals with depres-sion whose outcomes are carefully tracked on critical clini-cal outcomes. Thus, the model requires practice redesign, including redefining professional roles, new training and potentially new personnel, and regular ambulatory monitor-ing of a defined population. Setting aside relative advantage, with regard to Roger’s criteria for compatibility” and “trial-ability,” collaborative care may face barriers such as high start-up costs, an inadequate workforce, and inadequate third-party payment. For example, in our past experience in implementing an integrated behavioral health program into the primary care practices of a large healthcare system, the implementation failed because there were a limited number of behavioral health providers and because of financial non-sustainability. This initial diffusion attempt predates more recent changes in Medicare reimbursement that promise better financial support for collaborative care. In this case, system leadership judges the relative advantage of the pro-gram (and financial sustainability) rather than an individual patient or provider.
We more recently attempted to implement an adapted version of the IMPACT model because the local condi-tions to support diffusion had changed. These changed conditions included favorable healthcare system priorities, leadership buy-in based on patient needs, available resour-ces in terms of the workforce and intramural funding, and institutional pressures to transition from a fee-for-service model to a population health-based model. These pres-sures from new payment models led to greater perceived value of behavioral health services by healthcare system leadership. We highlight this renewed attempt at diffusion of the IMPACT model because health care represents a dynamic complex adaptive system, and changes such as new financial models, leadership, regulations, technical barriers, competing products, and patient demands may facilitate or block diffusion of the same model. For exam-ple, although the potential for Medicare reimbursement for some components of collaborative care now offers the opportunity to defray the cost of delivering this service, our targeted practice decided against billing for these activities because of perceived administrative barriers in the required documentation and billing. Thus, new reve-nue streams are assessed for relative advantage.44
Even with a favorable environment for financial sup-port and workforce, other criteria in the Roger’s frame-work could lower the likelihood of diffusion of the IMPACT model. For example, in examining the IMPACT model’s “complexity or difficulty to implement,” one rec-ognizes the barrier of requiring provider training not only in the model, but also in how to work as a team, assign accountability, and develop information technology to monitor outcomes. “Trial-ability” in the Roger’s frame-work refers to the ease with which a provider or patient could try the intervention and see if it works and do so with low cost, low hassle, and little chance of adverse effects. Because the IMPACT model for most practices would require hiring and training a care manager and developing the information technology to monitor out-comes, end-users could perceive the trial-ability of the IMPACT model as poor. With regard to Roger’s criteria for “adaptability to the user’s environment or needs,” many collaborative care programs, including IMPACT, distinguish themselves in terms of adaptability to the macro-environment and adaptability or customizing to individual patients. Finally, purveyors of these models often struggle with identifying the end-user: is it the patient, the family, the provider, the healthcare system, insurers, or policy makers?
Antipsychotic prescribing practices for persons with dementia are a prime example of practice diffusion at a point where geriatrics, dementia care, and mental health care intersect. The use of these medications rapidly diffused through peer-to-peer communication, as well as marketing, trial-ability, ease of use, and perceptions of rel-ative advantage. Perceptions of relative advantage are not limited to research evidence of better patient outcomes. In 2005, it was reported that the use of atypical antipsychotic medications to treat behavioral and psychological symp-toms associated with dementia resulted in higher mortality but scant evidence of improvement in targeted symp-toms.45 This information led the Food and Drug Adminis-tration to affix a black box warning that advised of potential serious harms in the use of atypical antipsy-chotics in persons with dementia. Despite this warning, high rates of antipsychotic use in long-term care persisted. Practitioners and caregivers apparently viewed these medi-cations as offering an advantage over other alternatives. Regulatory pressures from the Centers for Medicare and Medicaid Services, including greater emphasis on enforce-ment of gradual dose regulations during individual facility site surveys and open online publication of long-term care facility antipsychotic use rates, have had some effect on decreasing antipsychotic prescribing.46 This example again demonstrates the primacy of the end-user’s assessment of relative advantage.
EXPLORING NEW APPROACHES TO FACILITATING DIFFUSION
Most humans live and work within organized, connected networks of other individuals. We often make unconscious choices, and emotions we associate with our choices typi-cally influence those choices. Assuming that human net-works operate as complex adaptive systems, plans to facilitate diffusion must account for social networks, emo-tions, systems within systems, and shared decision-making. In the more common approach of academia, researchers simply move directly to prescribing a new model of care to health systems because their proposed solution meets the researchers’ criteria for relative advantage. This approach bypasses the required and complicated step of matching the end-user’s needs and the end-user’s definition of relative advantage. Greenhalgh’s summary suggested that innovation can be diffused within organizations through three approaches: completely organically or “letting it happen,” facilitating and nudging or “helping it happen,” or dictating and rule-making or “making it hap-pen”.31 Health services researchers may find great appeal in regulatory actions that “make it happen” because such an approach has the least level of variation between differ-ent end-users. This centralized approach is easier to imple-ment by focusing on the average user without taking into account the personalized perception of relative advantage and emotions of each end-user. Examples of such “rule-making” include changes in antipsychotic and opioid pre-scribing, minimum criteria for organizations to participate in Accountable Care Organizations, and the health insur-ance requirement of prior authorization for certain high-priced healthcare services. The two main disadvantages of “rule-making” are the high levels of relative advantage and the time needed to convince national policy makers to adapt such a centralized regulation and the failure of such a centralized approach in improving health outcomes for certain medical conditions that require changing the health behaviors of highly engaged end-users.
To facilitate faster implementation of healthcare innovation in a complex adaptive healthcare delivery organization, our group at Indiana University and others over the past decade started exploring new “helping it happen” diffusion strategies. In particular, we began an elusive search for scalability and a better understanding of how end-users assign relative advantage.47–55 These emerging meth-ods are often referred to as Implementation Science.56 There are two relatively different extant definitions of implementation science. The first defines implementation from the perspective of an observer: the scientific method of identifying barriers to and facilitators of implementing evidence-based healthcare innovations \in the real world.57 The second is from the perspective of an engineer: the method of developing tools, processes, and strategies to select evidence-based innovations and rapidly implement-ing such innovations in the local environment.57 Using this second perspective, researchers at the Indiana University Center for Innovation and Implementation Science devel-oped a process called agile implementation to actively select, implement, evaluate, expand, and sustain evidence-based healthcare innovations in a local, complex, adaptive healthcare delivery organization. We view agile implemen-tation as a cluster of 8 hierarchical steps as shown in Table 1 and displayed operationally in Figure 1. In many respects, agile implementation reorders and reprioritizes the implementation steps so that the organization is pull-ing the solution forward rather than the innovator pushing it forward. Although the agile implementation process lies squarely within the Greenhalgh concept of “helping it happen,” it seeks reach agreement on relative advantage from the perspective of potential customers, use the poten-tial of nudge techniques, and proactively address other facilitators in Roger’s framework, as illustrated in Table 2. For example, we have trained more than 40 change agents in the agile implementation process. These agents are embedded in the quality improvement departments of two healthcare systems affiliated with Indiana University. The main goal of such agents is to facilitate behavioral changes in the various semiautonomous members of healthcare delivery organizations, including patients, family caregiv-ers, clinicians, administrators, and other members who may move in and out of the system and intersect with multiple other systems. We are evaluating the agile imple-mentation process within the Great Lakes Practice Trans-formation Network. In 2015, we launched this network through funding support from the Centers for Medicare and Medicaid Services to provide technical assistance in practice transformation for clinicians across the Midwest. The Great Lakes Practice Transformation Network includes 2,100 primary and specialty practices across 7 Midwestern states (www.glptn.org). The network has trained more than 60 quality improvement advisors in the agile implementation process, embedded these agents in network-affiliated practices, and provided them with regu-lar online and face-to-face “time and space” for the devel-opment and sustainability of a peer-to-peer group–based problem-solving social network platform. Over the past 24 months, the quality improvement advisors have been able to use the agile implementation process and the social network platform to facilitate improvement in vari-ous care processes such as depression screening and man-agement in primary care, quality of diabetes and hypertension care, and inappropriate imaging testing for chronic back pain. These advisors have also been able to facilitate implementation of new models of care such as behavioral health integration models, population health management, and transitional care.
Table 1.
Indiana University Agile Implementation Framework [related Roger’s criteria are shown in brackets]
| 1. Conduct proactive surveillance and proactive confirmation of clinical opportunities to assure that a demand to solve a specific clinical problem emanates from the end-users. |
| 2. Identify an evidence based, scalable, and sustainable solution capable of solving the local healthcare delivery system’s specific problem. [compatibility and complexity] |
| 3. Determine the criteria and the process by which to turn off a failed solution within predetermined time window. [trial-ability and observed effectiveness]] |
| 4. Localize the content, the processes, and the desired outcomes of the selected solution into the local complex adaptive healthcare delivery system. [adaptability] |
| 5. Develop a timely and actionable feedback loop to monitor the implementation process and adjust the process dynamically. [trial-ability and observed effectiveness] |
| 6. Create a process to detect the impact of the selected solution on the overall performance of the entire complex adaptive health. Innovations may have unintended consequences in the form of improving narrowly defined outcomes but worsening overall patient or system outcomes. [relative advantage and observed effectiveness] |
| 7. Create a process to detect emergent behaviors that may facilitate or deter the accomplishment of the desired outcomes of the selected evidence-based solution. This step recognizes the ever-changing landscape of a complex adaptive system. [relative advantage] |
| 8. Develop and annually update a minimally (not maximally) standardized operational manual for implementing the evidence-based solution that is robust across organizations and offer others the ability to adapt the innovation. [adaptability, complexity, compatibility] |
Figure 1.
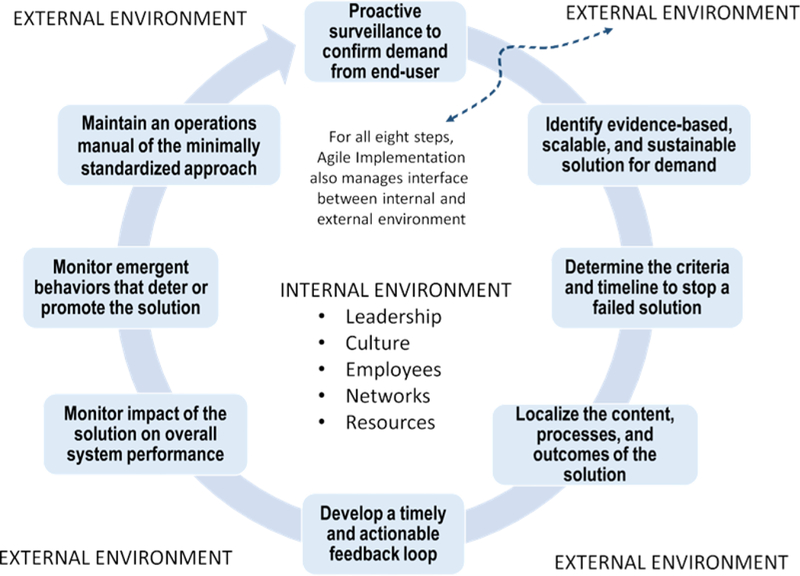
Agile implementation framework.
Table 2.
Summary Table of Recommendations to Improve Diffusion
| 1. Promote bidirectional communication between implementation scientists and healthcare decision-makers by embedding these scientists in the targeted healthcare ecosystem. |
| 2. Support a network of nationally representative health care systems which are funded to serve as the surveillance system, early adopters, practical adapters, and pragmatic assessors of evidence-based models of care. |
| 3. Increase the cross-collaboration between implementation scientists focused in health care systems with scientists in other major industries such as engineering, safety, manufacturing, and distribution. |
| 4. Facilitate input from patients, citizen scientists, and social networks to allow for truly innovative ideas from end-users to reach other scientists more efficiently. |
| 5. Demand solutions that begin with scalability as a key requirement of the original product design. |
When attempting to facilitate behavior change in indi-viduals or in organizations, we have referred to the impor-tance of social networks, but exploiting social networks is itself part of a larger framework of “nudge” approaches that use findings from behavioral economics. 58 These approaches emphasize the unconscious and emotional aspects of decision-making rather than focusing on only the conscious and cognitive aspects.30 The more we are able to make our innovations consistent with the default option, with local norms, and with positive emotions that make us feel better about ourselves and our choices, the more likely we are to see diffusion of our innovations.59
CONCLUSIONS
Many would argue that some features of innovation and diffusion of innovation within health care differ from other service industries, although many would agree that features important to these other industries are also impor-tant in health care. Healthcare innovators learn perhaps too little from lessons from other industries. Such lessons would include the value of simple checklists used to improve safety in air travel, simulation modeling used to improve prototyping in engineering, user experience design in consumer electronics, and the behavioral economics principles used to improve uptake of consumer products. Proof of concept and early evidence of relative advantage are very early prerequisites for diffusion. Health services researchers often stop at the point of generating new knowledge, hoping for spontaneous diffusion or that a third party will accept the burden of facilitating diffusion. We find little evidence that this hidden distribution net-work exists in the realm of new models of care for older adults, particularly those focused on aging, dementia, and mental health. Development of distribution networks is needed to expedite introduction of evidence-based prac-tices into standard clinical practice. This could be accom-plished through a number of methods, including application of the successful network development strat-egies used in the pharmaceutical, technology, and service industries. Other alternatives include creation of small business startups whose mission is to distribute new evidence-based practices or models of care. It should also not be ignored that companies in other industries that have had some of the greatest successes in innovation (e.g., Apple, Google, Amazon) place a high premium on innovation and often view innovation as a job belonging to everyone in the organization. The Great Lakes Practice Transformation Network provides another example of developing a network to support the diffusion of innovation into the healthcare system.
Finally, the frameworks that we discuss (complex adaptive system theory, diffusion theory) too often focus on the supply portion of the diffusion cycle. Innovators will not eliminate implementation gaps without working hand in hand with the demand side by creating pulling forces from the potential true end-users of the innovation, such as patients and family caregivers, as well as health system leaders. The first step in the agile implementation process is proactive surveillance of the local healthcare system to identify and confirm demand. The process of mastering the process of diffusion and the skills needed to do so are important skill gaps of health services research-ers, implementation scientists, and policy makers. Embeddedness and engagement for the purposes of facili-tating diffusion also tend to draw scientists away from the competing demand of developing new knowledge. For those frustrated with slow diffusion, implementation science and its collaborations with engineers and behavioral scientists, among others, offers a new avenue to generate new knowledge.
ACKNOWLEDGMENTS
Financial Disclosure: This work was supported by the Agency for Healthcare Research and Policy Patient Safety Learning Laboratories Program (P30HS024384).
Sponsor’s Role: This work was supported in part by the Agency for Healthcare Research and Policy Patient Safety Learning Laboratories Program (P30HS024384). The 2017 NHCGNE Leadership Conference, where a version of this paper was first presented, was funded in part by the National Institute on Aging [R13AG056140 and R13AG056140– 01A1]. The sponsors had no role in the design, conduct, writ-ing, or decision to publish this manuscript. The content is solely the responsibility of the authors and does not necessar-ily represent the official views of the NIH.
Footnotes
Conflict of Interest: None.
REFERENCES
- 1.Institute of Medicine, Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [Google Scholar]
- 2.Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 3.Institute of Medicine. The Mental Health and Substance Use Workforce for Older Adults: In Whose Hands? Washington, DC: National Academies Press; 2012. [PubMed] [Google Scholar]
- 4.Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: Can the U.S. health care workforce do the job? Health Aff 2009;28:64–74. [DOI] [PubMed] [Google Scholar]
- 5.Warshaw GA, Bragg EJ, Brewer DE, Meganathan K, Ho M. The develop-ment of academic geriatric medicine: Progress toward preparing the nation’s physicians to care for an aging population. J Am Geriatr Soc 2007;55:2075–2082. [DOI] [PubMed] [Google Scholar]
- 6.Besdine R, Boult C, Brangman S et al. Caring for older Americans: The future of geriatric medicine. J Am Geriatr Soc 2005;53(6 Suppl): S245–S256. [DOI] [PubMed] [Google Scholar]
- 7.Kirwin P Canary in a coal mine: Geriatric psychiatry in crisis. Am J Geriatr Psychiatry 2016;24:690–692. [DOI] [PubMed] [Google Scholar]
- 8.Gitlin LN, Schulz R. Family caregiving of older adults In: Prohask T, Anderson L, Binstock R, eds. Public Health for an Aging Society. Baltimore, MD: Johns Hopkins University Press; 2012:181–204. [Google Scholar]
- 9.Lin SY, Lewis FM. Dementia friendly, dementia capable, and dementia positive: Concepts to prepare for the future. Gerontologist 2015;55: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeste DV, Blazer DG II, Buckwalter KC et al. Age-friendly communities ini-tiative: Public health approach to promoting successful aging. Am J Geriatr Psychiatry 2016;24:1158–1170. [DOI] [PubMed] [Google Scholar]
- 11.Golant SM. The origins, programs, and benefits of age-friendly commun-ities. Gerontologist 2016;56:597–598. [Google Scholar]
- 12.Bartels SJ, Naslund JA. The underside of the silver tsunami—older adults and mental health care. N Engl J Med 2013;368:493–496. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim F, Cohen CI, Ramirez PM. Successful aging in older adults with schizophrenia: Prevalence and associated factors. Am J Geriatr Psychiatry 2010;18:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to Medicare beneficiaries. JAMA Intern Med 2016;176:990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrie HC, Tu W, Tabbey R, Purnell CE, Ambuehl RJ, Callahan CM. Health outcomes and cost of care among older adults with schizophrenia: A 10-year study using medical records across the continuum of care. Am J Geriatr Psychiatry 2014;22:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schubert CC, Boustani M, Callahan CM et al. Comorbidity profile of dementia patients in primary care: Are they sicker? J Am Geriatr Soc 2006; 54:104–109. [DOI] [PubMed] [Google Scholar]
- 17.Bartels SJ. Caring for the whole person: Integrated health care for older adults with severe mental illness and medical comorbidity. J Am Geriatr Soc 2004;52(12 Suppl):S249–S257. [DOI] [PubMed] [Google Scholar]
- 18.Aneja S, Ross JS, Wang Y et al. US cardiologist workforce from 1995 to 2007: Modest growth, lasting geographic maldistribution especially in rural areas. Health Aff 2011;30:2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Academies of Sciences, Engineering, and Medicine. Strengthening the Workforce to Support Community Living and Participation for Older Adults and Individuals with Disabilities: Proceedings of a Workshop. Washington, DC: The National Academies Press, 2017. [PubMed] [Google Scholar]
- 20.Huntley AL, Thomas R, Mann M et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta-analysis. Fam Pract 2013;30:266–275. [DOI] [PubMed] [Google Scholar]
- 21.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: A cumulative meta-analysis and review of longer-term out-comes. Arch Intern Med 27 2006;166:2314–2321. [DOI] [PubMed] [Google Scholar]
- 22.Reilly S, Miranda-Castillo C, Malouf R et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev 2015;1:CD008345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes J, Panagioti M, Alam R, Checkland K, Cheraghi-Sohi S, Bower P. Effectiveness of case management for ‘at risk’ patients in primary care: A systematic review and meta-analysis. PloS One 2015;10:e0132340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaMantia MA, Scheunemann LP, Viera AJ, Busby-Whitehead J, Hanson LC. Interventions to improve transitional care between nursing homes and hospitals: A systematic review. J Am Geriatr Soc 2010;58:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boult C, Green AF, Boult LB, Pacala JT, Snyder C, Leff B. Successful models of comprehensive care for older adults with chronic conditions: Evidence for the Institute of Medicine’s “Retooling for an Aging America” report. J Am Geriatr Soc 2009;57:2328–2337. [DOI] [PubMed] [Google Scholar]
- 26.Kane RL. What can improve chronic disease care? J Am Geriatr Soc 2009; 57:2338–2345. [DOI] [PubMed] [Google Scholar]
- 27.Kane RL. The enthusiasm:evidence ratio for comprehensive chronic disease care? J Am Geriatr Soc 2015;63:1940–1943. [DOI] [PubMed] [Google Scholar]
- 28.Callahan CM. Controversies regarding comprehensive chronic care: Coordinated care: The drug-free wonder drug. J Am Geriatr Soc 2015;63: 1938–1940. [DOI] [PubMed] [Google Scholar]
- 29.Rogers EM. Diffusion of Innovations. New York: Free Press of Glencoe; 1962. [Google Scholar]
- 30.Marteau TM, Hollands GJ, Fletcher PC. Changing human behavior to pre-vent disease: The importance of targeting automatic processes. Science 2012; 337:1492–1495. [DOI] [PubMed] [Google Scholar]
- 31.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: Systematic review and recommen-dations. Milbank Q 2004;82:581–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Best A, Greenhalgh T, Lewis S, Saul JE, Carroll S, Bitz J. Large-system trans-formation in health care: A realist review. Milbank Q 2012;90:421–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: A systematic review and meta-analysis. Int J Geriatr Psychiatry 2013;28: 889–902. [DOI] [PubMed] [Google Scholar]
- 34.Archer J, Bower P, Gilbody S et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reuben DB. Organizational interventions to improve health outcomes of older persons. Med Care 2002;40:416–428. [DOI] [PubMed] [Google Scholar]
- 36.Callahan CM, Sachs GA, Lamantia MA, Unroe KT, Arling G, Boustani MA. Redesigning systems of care for older adults with Alzheimer’s disease. Health Aff 2014;33:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuben DB. Better ways to care for older persons: Is anybody listening? J Am Geriatr Soc 2009;57:2348–2349. [DOI] [PubMed] [Google Scholar]
- 38.Unutzer J Clinical practice. Late-life depression. N Engl J Med 2007;357: 2269–2276. [DOI] [PubMed] [Google Scholar]
- 39.Grypma L, Haverkamp R, Little S, Unutzer J. Taking an evidence-based model of depression care from research to practice: making lemonade out of depression. Gen Hosp Psychiatry 2006;28:101–107. [DOI] [PubMed] [Google Scholar]
- 40.Katon WJ, Schoenbaum M, Fan MY et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry 2005; 62:1313–1320. [DOI] [PubMed] [Google Scholar]
- 41.Unutzer J, Powers D, Katon W, Langston C. From establishing an evidence-based practice to implementation in real-world settings: IMPACT as a case study. Psychiatr Clin North Am 2005;28:1079–1092. [DOI] [PubMed] [Google Scholar]
- 42.Unutzer J, Katon W, Callahan CM et al. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA 2002;288:2836–2845. [DOI] [PubMed] [Google Scholar]
- 43.Katon WJ, Seelig M. Population-based care of depression: Team care approaches to improving outcomes. J Occup Environ Med 2008;50:459–467. [DOI] [PubMed] [Google Scholar]
- 44.Borson S, Chodosh J, Cordell C et al. Innovation in care for individuals with cognitive impairment: Can reimbursement policy spread best prac-tices? Alzheimers Dement 2017;13:1168–1173. [DOI] [PubMed] [Google Scholar]
- 45.Schneider LS, Tariot PN, Dagerman KS et al. Effectiveness of atypical anti-psychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538. [DOI] [PubMed] [Google Scholar]
- 46.Mort JR, Sailor R, Hintz L. Partnership to decrease antipsychotic medication use in nursing homes: Impact at the state level. S D Med 2014;67:67–69. [PubMed] [Google Scholar]
- 47.Alder CA, Callahan CM, Boustani MA, Hendrie HC, Austrom MG. Pro-viding care to the caregiver: Implementing the PREVENT Model in a real world memory care clinic In: Thyrian JR, HoffMann W, eds. Dementia Care Research: Scientific Evidence, Current Issues and Future Perspectives. Miami, FL: Pabst Science Publishers; 2012:34–42. [Google Scholar]
- 48.Boustani MA, Sachs GA, Alder CA et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health 2011;15:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callahan CM, Boustani MA, Weiner M et al. Implementing dementia care models in primary care settings: The Aging Brain Care Medical Home. Aging Ment Health 2011;15:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boustani MA, Munger S, Gulati R, Vogel M, Beck RA, Callahan CM. Selecting a change and evaluating its impact on the performance of a complex adaptive health care delivery system. Clin Interv Aging 2010;5: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boustani MA, Frame A, Munger S et al. Connecting research discovery with care delivery in dementia: The development of the Indianapolis Discovery Network for Dementia. Clin Interv Aging 2012;7:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stetler CB, McQueen L, Demakis J, Mittman BS. An organizational frame-work and strategic implementation for system-level change to enhance research-based practice: QUERI Series. Implement Sci 2008;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagedorn H, Hogan M, Smith JL et al. Lessons learned about implement-ing research evidence into clinical practice. Experiences from VA QUERI. J Gen Intern Med 2006;21 Suppl 2:S21–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crabtree BF, Nutting PA, Miller WL et al. Primary care practice transfor-mation is hard work: Insights from a 15-year developmental program of research. Med Care 2011;49 Suppl:S28–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanter MH, Lindsay G, Bellows J, Chase A. Complete care at Kaiser Permanente: Transforming chronic and preventive care. Jt Comm J Qual Patient Saf 2013;39:484–494. [DOI] [PubMed] [Google Scholar]
- 56.Rubenstein LV, Pugh J. Strategies for promoting organizational and practice change by advancing implementation research. J Gen Intern Med 2006;21 Suppl 2:S58–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azar J AN, Boustani M. The Indiana University Center for Health Innovation and Implementation Science: Bridging healthcare research and delivery to build a learning healthcare system. Z Evid Fortbild Qual Gesundhwes 2015;109:138–143. [DOI] [PubMed] [Google Scholar]
- 58.Kahneman D Thinking Fast, Thinking Slow. New York: Farrar, Straus & Giroux; 2013. [Google Scholar]
- 59.King D GF, Vlaev I, Darzi A. Approaches based on behavioral economics could help nudge patients and providers toward lower health spending growth. Health Aff 2013;32:661–668. [DOI] [PubMed] [Google Scholar]


