Summary
We recently synthesized a series of novel makaluvamine compounds, and found that the most potent was FBA-TPQ. The effects of FBA-TPQ on human (LNCaP and PC3) and murine (TRAMP Cl) prostate cancer cells were evaluated. Potential mechanisms of action of the compound were also determined. FBA-TPQ exhibited dose-dependent cytotoxicity in the low micromolar range, inhibited proliferation, caused cell cycle arrest, and induced apoptosis in prostate cancer cell lines. The compound also decreased the expression of the androgen receptor and PSA. The results presented herein support the further development of FBA-TPQ as a novel agent for prostate cancer.
Keywords: Makaluvamine, Prostate cancer, Chemotherapy, Cell cycle, Apoptosis
Introduction
It is estimated that prostate cancer was the second most frequently diagnosed cancer in the USA in 2008, and that it caused more than 28,000 deaths [1]. Despite recent advances in the detection, diagnosis, and treatment of prostate cancer, there is a need for more effective therapies. Unfortunately, most conventional therapeutic modalities, such as androgen ablation therapy, frequently result in androgen-independent cancers. These cancers are typically more aggressive, metastatic, and resistant to chemotherapeutic agents than androgen-dependent prostate cancer [2–4]. Therefore, agents that are effective against both androgen-sensitive and androgen-independent prostate cancers, and against genotypically diverse cancers are critically needed.
In search of alternative therapeutic compounds, many scientists have turned to the subterranean marine environment. Natural products extracted from marine invertebrate animals (and synthetic derivatives of these products) have proven to be extraordinary resources for biologically active compounds [5–8]. In particular, marine sponges have proven to be an exceptionally abundant source of chemically and biologically diverse natural products [9–12].
As part of our ongoing interest in the potential therapeutic benefits of marine natural products (and in a search for less toxic analogs to conventional compounds), we recently developed a schematic for the organic synthesis of makaluvamine analogs. A number of synthetic pyrroloiminoquinone makaluvamine alkaloids with proven anticancer activities possess substitutions at the 7-position of the pyrroloiminoquinone ring. Therefore, we explored substitutions of increased steric bulk, hydrophobicity, and hydro-philicity at the 7-position of the pyrroloiminoquinone ring [13, 14]. Various synthetic makaluvamine derivatives were tested against a panel of cancer cell lines, and the 4 fluorobenzyl substituted analog (FBA-TPQ) exhibited excellent cytotoxicity in the low micromolar range against a variety of human cancer cells, including prostate cancer cells [15].
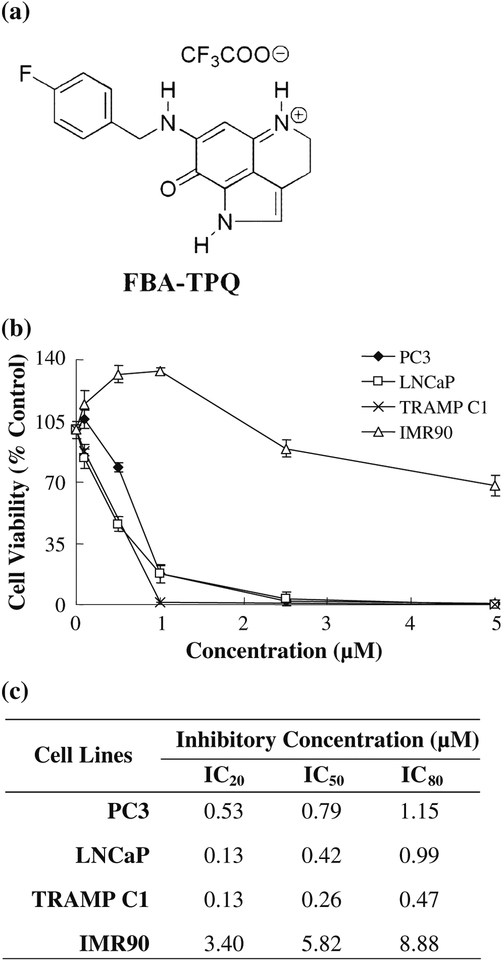
While FBA-TPQ (Fig. 1a) exhibited excellent growth inhibition against the cancer lines, the mechanisms of action responsible for these effects are largely unknown. To investigate the possible mechanisms governing the cytotoxic activity of FBA-TPQ, we employed LNCaP, PC3 and TRAMP Cl human and murine cancer cell lines as models of prostate cancer.
Fig. 1.
Growth inhibitory activity of FBA-TPQ in prostate cancer cells, (a) Structure of FBA-TPQ. (b) Growth inhibitory activity of FBA-TPQ in prostate cancer cells and primary fibroblasts. Cells were exposed to various concentrations of FBA-TPQ for 72 hr followed by MTT assay. All assays were performed in triplicate, (c) FBA-TPQ concentrations inducing 20%, 50%, and 80% growth inhibition in prostate cancer cells, relative to the control
Materials and methods
Chemicals and reagents
All chemicals and solvents were of the highest analytical grade available. The anti-human MDM2 (SNIP14) and Bax (N-20) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-human p53 (Ab-6) antibody was from EMD Chemicals, Inc. (Gibbs-town, NJ), and easpase-3 (9662), easpase-8 (9746) and easpase-9 (9502) antibodies were from Cell Signaling Technology, Inc. (Danvers, VIA.). Cell culture media, fetal bovine serum (FBS), phosphate-buffered saline (PBS), sodium pyruvate, non-essential amino acids, penicillin-streptomycin, and other cell culture supplies were obtained from the Comprehensive Cancer Center Media Preparation Shared Facility, University of Alabama at Birmingham.
Synthesis and purification of FBA-TPQ [7-(4-fluorobenzylamino)-1,3,4,8-tetrahydropyrrolo [4,3,2-de]quinolin-8(1H)-one]
FBA-TPQ was synthesized using methods reported previously [15]. The purity of the compound was greater than 99.0%, based on 1H-NMR, 13C-NMR, and mass spectral analyses. The data for the compound is given below.
Spectral data 1H NMR (CD3OD) δ 2.95 (t, 2H, J=7.6 Hz), 3.83 (t, 2H, J=7.6 Hz), 4.57 (s, 2H), 5.40 (s, 1H), 7.01–7.16 (m, 3H) and 7.30–7.39 (m, 2H); 13C NMR (CD3OD) 5 19.4, 44.2, 47.3, 86.4, 116.5, and 116.8 (C-F coupling), 120.2, 123.6, 125.7, 127.1, 130.4 and 130.5 (C-F coupling), 133.3, 155.0 and 160.0 (C-F coupling), 165.4, 168.8, 173.2; MS (ES+) m/z 296 (M+).
Cell lines and cell culture
Human prostate cancer cell lines were obtained from the American Type Culture Collection (Rockville, MD). LNCaP (p53 wild-type, androgen-dependent) cells were cultured in RPMI 1640 media containing 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, glucose (4.5 mg/mL), and sodium bicarbonate (1.5 mg/mL). PC3 (p53 null, androgen-independent) cells were cultured in Ham’s F-12K medium containing sodium bicarbonate (1.5 mg/mL). The murine cell line, TRAMP Cl, was kindly provided by Dr. Pierre Triozzi (University of Alabama at Birmingham), and cultured in DMEM supplemented with 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 0.005 mg/mL bovine insulin and 10 nM dehydroisoandrosterone. Human IMR90 immortalized fibroblasts, a gift from Dr. S. Lee (Harvard), were cultured in DMEM. All culture media contained 10% FBS and 1% penicillin-streptomycin.
Cell survival/viability assay
The effect of FBA-TPQ on human cancer cell growth, expressed as the percentage of cell survival, was determined using the MTT assay. The cells were grown in 96-well plates at 4–5 × 103 cells per well and exposed to the test compound (0, 0.1, 0.5, 1, 2.5 and 5 μM). After incubation for 72 hr, 10 μL of the MTT solution (5 mg/mL; Sigma; St. Louis, MO) were added into each well. The plates were incubated for 2–4 hr at 37°C. The supernatant was then removed and the formazan crystals were dissolved with 100 μL of DMSO. The absorbance at 570 nm was recorded using an OPTImax microplate reader (Molecular Devices; Sunnyvale, CA). The cell survival percentages were calculated by dividing the mean OD of compound-containing wells by that of control wells.
Cell proliferation assay
Bromodeoxyuridine (BrdUrd) incorporation (Oncogene, La Jolla, CA) was employed to determine the effect of the test compound on cell proliferation. Cells were seeded in 96-well plates (8 × 103 to 1.2× 104 cells per well) and incubated with various concentrations of FBA-TPQ (0, 0.1, 0.5, 0.75, or 1.0 μM) for 24 hr. BrdUrd was added to the medium 10 hr before termination of the experiment. The extent of BrdUrd incorporation into cells was determined with an anti-BrdUrd antibody, and absorbance was measured at dual wavelengths of 450/540 nm with an OPTImax microplate reader (Molecular Devices; Sunnyvale, CA).
Cell cycle measurements
To determine the effects of the test compound on the cell cycle, cells (2–3 × 105) were exposed to FBA-TPQ (0, 0.1, 0.5 and 1 μM) and incubated for 24 hr prior to analysis. Cells were trypsinized, washed with PBS, and fixed in 1.5 mL of 95% ethanol at 4°C overnight, followed by incubation with RNAse and staining with propidium iodide (Sigma, MO). The DNA content was determined by flow cytometry.
Detection of apoptosis
Cells in early and late stages of apoptosis were detected using an Annexin V-FITC kit from BioVision (Mountain View, CA). In this procedure, 2–3 × 105 cells were exposed to the test compound (0, 0.1, 0.5 and 1 μM) for 24 hr prior to analysis. Cells were collected and washed with serum-free media. Cells were then resuspended in 500 μL of Annexin V binding buffer, followed by addition of 5 μL of Annexin V-FITC and 5 μL of propidium iodide (PI). The samples were incubated in the dark for 5 min at room temperature and analyzed with a Becton Dickinson FACSCalibur instrument (Ex=488 nm; Em=530 nm). Cells positive for Annexin V-FITC alone (early apoptosis) and Annexin V-FITC and PI (late apoptosis) were counted.
Western blot analysis
Cultured cells were exposed to various concentrations of the test compound for 24 hr. Cell lysates were fractionated with identical amounts of protein by SDS-PAGE and transferred to Bio-Rad trans-Blot nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The nitrocellulose membrane was incubated in blocking buffer (Tris-buffered saline containing 0.1% Tween 20 and 5% nonfat milk) for 1 hr at room temperature, then with the appropriate primary antibody overnight at 4°C or 2 hr at room temperature with gentle shaking. Finally, the membrane was washed three times with washing buffer (Tris-buffered saline containing 0.1% Tween 20) for 15 min, incubated with goat anti-mouse/rabbit IgG-horseradish peroxidase-conjugated antibody (Bio-Rad) for 1 hr at room temperature, and washed again (in triplicate). The proteins of interest were detected by enhanced chemiluminescence reagents from Perkin Elmer LAS, Inc (Boston, MA).
AR and prostate-specific antigen (PSA) expression and PSA promoter-luciferase assay
LNCaP cells were seeded on 6-well plates for 48 hr, then exposed to either vehicle, decursin (30 μM), or FBA-TPQ (3 μM) in complete medium containing 10% FBS for 24 hr. Decursin, a natural product known to inhibit the expression of the AR [16], was used as a reference for comparison. Cell lysates were then examined for AR and PSA expression by Western blotting.
To determine PSA promoter activity, LNCaP cells stably transfected with a luciferase reporter plasmid driven by a 6-kb PSA promoter were seeded into 6-well plates in regular medium. When the cells grew to 40–50% confluence, they were incubated in phenol red-free RPMI 1640 media with 10% charcoal-stripped serum for 24 hr. The cells were treated with 0.5 nM mibolerone combined with FBA-TPQ (0, 0.3, 1.0, or 3.0 μM) for another 24 hr. Conditioned medium was used for the secreted PSA assay as previously described [17]. Cell lysates were processed according to the Promega Luciferase System protocol (Promega Corp., Madison, WI) and analyzed using a luminometer.
Data and statistical analysis
The data were expressed as means and standard deviations, and the significance of differences was analyzed by ANOVA and Student’s t-test as appropriate.
Results
FBA-TPQ decreases the growth of prostate cancer cells, but not fibroblasts
FBA-TPQ was evaluated for its effects on prostate growth inhibition in vitro. At a concentration of 1 μM, FBA-TPQ potently reduced the survival of LNCaP cells (p53 wt), PC3 cells (p53 null), and TRAMP Cl cells (inactived p53) (Fig. 1b). Normal IRM90 fetal lung fibroblasts were less sensitive to the inhibitory effects of FBA-TPQ compared to prostate cancer cells. The synthetic marine alkaloid exhibited IC50 values in the low micromolar range in all tested prostate cancer cell lines (Fig. 1c).
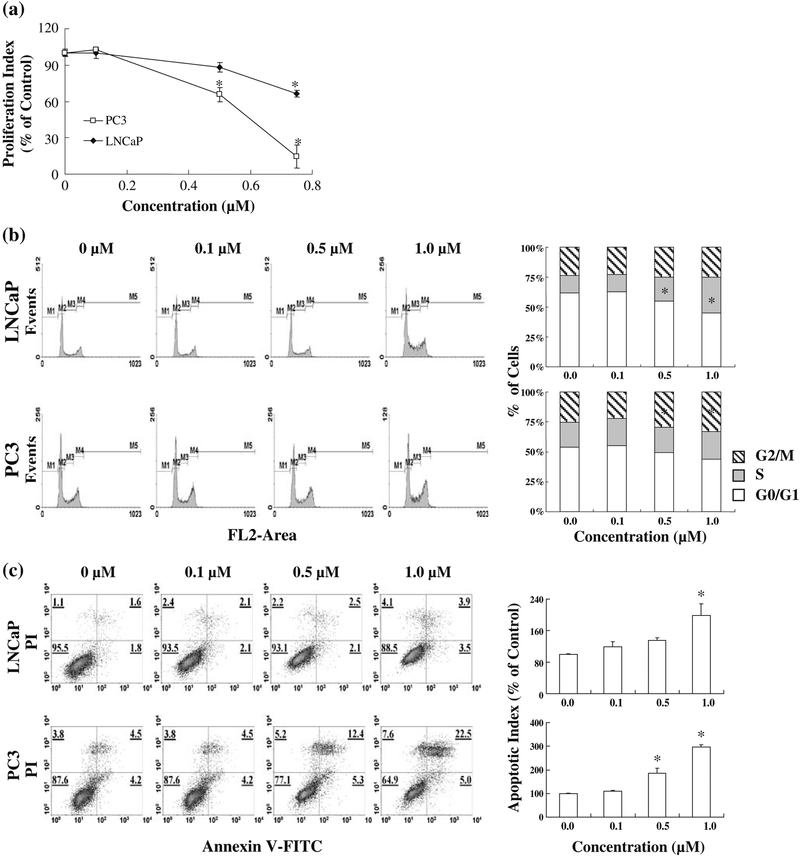
FBA-TPQ inhibits prostate cancer cell proliferation and induces cell cycle arrest and apoptosis
As the cell growth inhibitory effects of FBA-TPQ observed using the MTT assay provided a general view of cell survival, but did not reflect distinct mechanisms of action, we investigated the effects of FBA-TPQ on cell proliferation, cell cycle progression, and apoptosis. FBA-TPQ inhibited the proliferation of human prostate cancer cells in a dose-dependent manner (Fig. 2a). PC3 cells were more sensitive than the LNCaP cells, with a significant decrease in proliferation beginning at 0.5μM (p<0.05), and the PC3 cell proliferation index being decreased by approximately 85% after exposure to 0.75 μM of the compound. The LNCaP proliferation index was also significantly decreased (by ~30%, p<0.05) at this concentration.
Fig. 2.
Effects of FBA-TPQ on the proliferation (a), cell cycle followed by assessment of proliferation by BrdUrd incorporation, cell progression (b), and apoptosis (c) of human prostate cancer cells. cycle by flow cytometry, and apoptosis by Annex in V/PI flow Cells were exposed to various concentrations of FBA-TPQ for 24 hr cytometry assays. All assays were performed in triplicate.
*p<0.05
As shown in Fig. 2b, the synthetic compound induced significant S phase cell cycle arrest in LNCaP cells and G2/M arrest in PC3 cells. We also observed that FBA-TPQ induced apoptosis in prostate cancer cells in a concentration-dependent manner (Fig. 2c). In both human prostate cancer cell lines, a 1.0 μM concentration of FBA-TPQ elicited a significant (p<0.05) increase in apoptosis compared to the control, although the PC3 cells were again more sensitive than the LNCaP cells.
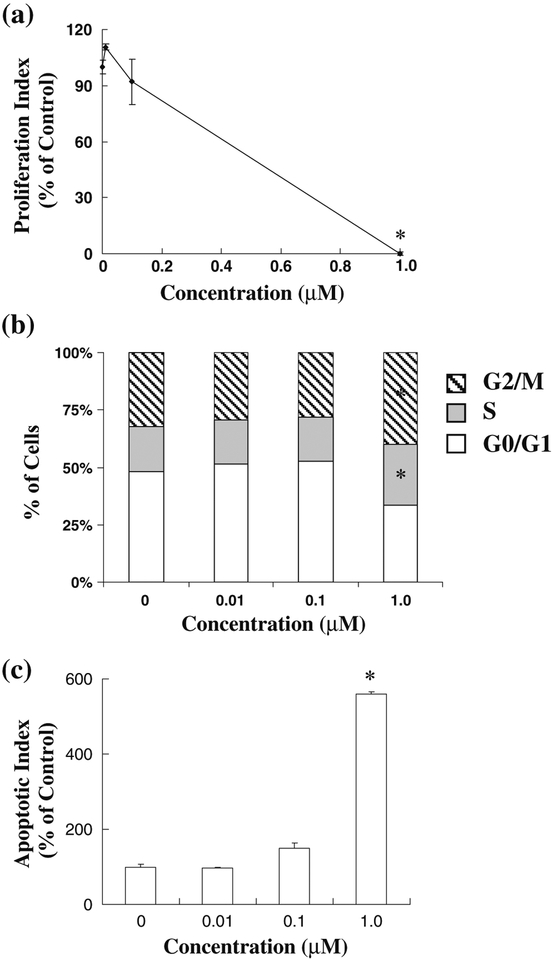
To confirm these effects, the murine TRAMP Cl prostate cancer cell line was also exposed to FBA-TPQ and examined for its effects on proliferation, cell cycle progression and apoptosis. Similar to the human PC3 cells, exposure to 1.0 μM of the compound significantly decreased proliferation, leading to an almost complete inhibition of proliferation (Fig. 3a, p<0.05). Interestingly, FBA-TPQ induced cell cycle arrest in both the S and G2/M phases of the cell cycle (Fig. 3b). The murine cells were also highly sensitive to the compound in terms of apoptosis, with the 1 μM concentration increasing apoptosis by more than five-fold (Fig. 3c, p<0.05).
Fig. 3.
Effects of FBA-TPQ on the proliferation (a), cell cycle progression (b) and apoptosis (c) of murine TRAMP C1 prostate cancer cells. Cells were exposed to various concentrations of FBATPQ for 24 hr, followed by assessment using the BrdUrd incorporation assay, flow cytometry or Annexin V/flow cytometry.
*p<0.05
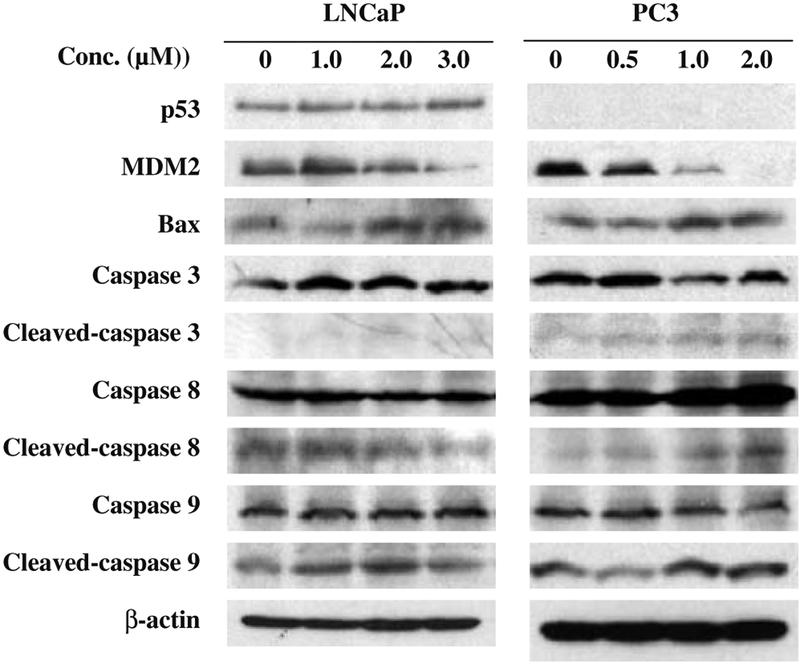
FBA-TPQ modulates the expression of apoptosis-related proteins
As our data suggested that the induction of apoptosis is one major mechanism by which FBA-TPQ exerts its anticancer effects, we assessed the expression of several apoptosis-related proteins following FBA-TPQ exposure. Consistent with the Annexin V/flow cytometry experiments, FBA-TPQ activated p53 (in LNCaP cells), down-regulated MDM2, increased Bax, and induced the cleavage of caspases 3, 8, and 9 (Fig. 4)
Fig. 4.
FBA-TPQ alters the expression of various apoptosis-related proteins in human prostate cancer cells. Cells were exposed to several concentrations of the test compound for 24 hr. The target proteins were detected by immunoblotting with specific antibodies
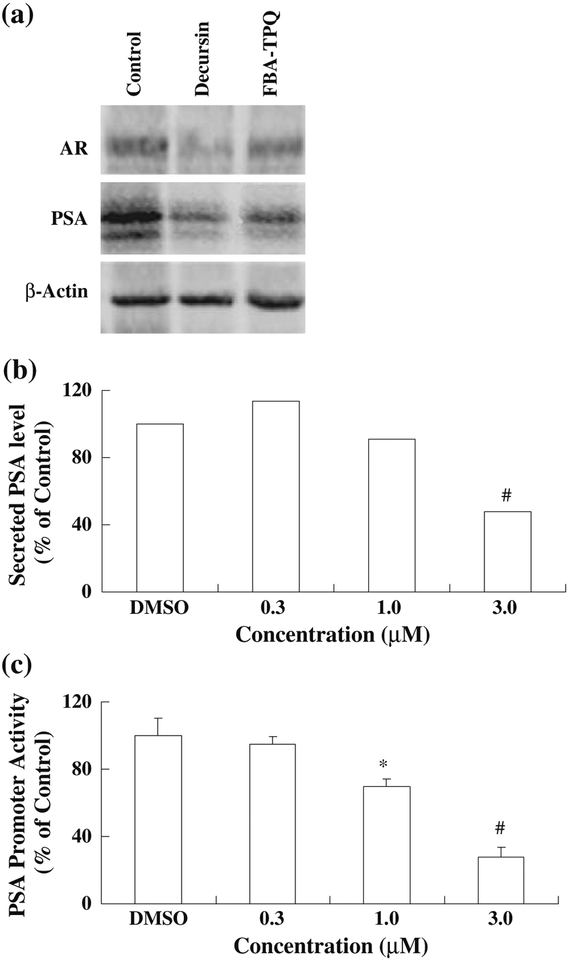
FBA-TPQ decreases expression of the AR and PSA, secreted PSA levels, and PSA promoter activity
Given the differences in the responses of the cells to the compound, and the different levels of androgen sensitivity of the cell lines, we next determined the effect of FBA-TPQ on the expression of the androgen receptor (AR) and PSA in LNCaP cells following exposure to 3 μM FBA-TPQ. We found that this concentration of the compound led to dramatic decreases in both the AR and PSA comparable to those induced by decursin (Fig. 5a). Additionally, FBA-TPQ significantly decreased the secretion of PSA into the cell culture media (Fig. 5b), and decreased PSA promoter activity (Fig. 5c) (p<0.01).
Fig. 5.
Effects of FBA-TPQ on the expression of the androgen receptor and PSA (a), secreted PSA (b), and PSA promoter activity (c) in LNCaP cells. Cells were exposed to either 3 μM FBA-TPQ or decursin (AR and PSA western blots) or various concentrations (0–3 μM) of FBA-TPQ for 24 hr. Target proteins were detected by western blotting (a), ELISA (b), or the Promega Luciferase System assay (c).
*p<0.05, #p<0.01
Discussion
Cancer initiation, progression, and metastasis are often the result of myriad genetic mutations. Thus, the genetic and phenotypic heterogeneity of various cancers presents a constant challenge for researchers and physicians. Marine-derived compounds have proven to be a rich source of medicinally valuable compounds that are effective against a variety of cancers [10, 18]. However, the limited quantities of these products have often precluded their use in biological investigations. Fortunately, recent technological and methodological advances have improved the organic synthesis of marine natural products and, over the past decade, a number of marine natural products (i.e., bryostatin-1, kahalalide-F, ecteinascidin-743, discodermo-lide) have entered various phases of advanced pre-clinical and clinical evaluation as potential anti-cancer agents [19, 20].
We recently synthesized several analogs of makaluvamines, a sub-class of marine alkaloids, in an attempt to generate more potent and less toxic compounds. FBA-TPQ, one of these analogs, showed excellent anti-tumor activity in various types of human cancer [15], prompting the present study. We have made four major observations: 1) FBA-TPQ strongly inhibits the growth of prostate cancer cells, but not normal cells, 2) FBA-TPQ affects cell proliferation and cell cycle progression, 3) the induction of apoptosis appears to be a major mechanism by which the marine alkaloid exerts its anti-cancer effects, however 4) the makaluvamine analog also decreases AR and PSA levels.
We have also demonstrated that FBA-TPQ inhibits the growth of both p53 wild-type (LNCaP) and non-functional (PC3, TRAMP Cl) cancer cells, as well as androgen-dependent (LNCaP, TRAMP Cl) and -independent (PC3) prostate cancer cells. These findings are important because prostate cancers, particularly advanced cancers, are often androgen refractory and have non-functional p53 [21]. Given the activity of FBA-TPQ against the various models examined here, we believe that the compound will prove to be effective across a broad spectrum of cancers, including advanced prostate cancers.
The regulation of the cell cycle is governed by a complex interaction among numerous proteins, including p53, MDM2, E2F1 and p21 [22–24]. It is possible that the observed cell cycle arrest is due in part to decreased MDM2 expression. Previous studies have demonstrated that MDM2 inhibition results in increased p53 protein expression and cell cycle arrest, in addition to decreased E2F1 and increased p21 protein expression [25, 26]. These reports are consistent with our findings and provide insight into the molecular targets of this compound and subsequent effects on proliferation and cell cycle progression.
Additionally, since MDM2 inhibition also induces apoptosis, the decrease in expression of this oncogene may also be partly responsible for the pro-apoptotic effects observed in our studies. Consistent with our data, MDM2 inhibition has been shown to result in the upregulation of Bax in LNCaP cells [27]. Moreover, these effects can occur independently of the p53 status of cells [27].
Our data also indicate that FBA-TPQ decreases secreted PSA levels in a dose-dependent manner. MDM2 was shown to interact with the PSA promoter [28]. We speculate that decreased PSA promoter activity following FBA-TPQ exposure may inhibit the recruitment of MDM2 to the promoter, subsequently decreasing PSA transcription and ultimately the levels of secreted PSA. Thus, it is possible that the effects of FBA-TPQ may be, at least partially, mediated by inhibition of MDM2.
In conclusion, we have demonstrated that FBA-TPQ has potent biological activity against prostate cancers of diverse backgrounds. Although we have identified the induction of apoptosis as a major mechanism of action of the compound, we also observed anti-proliferative and cell-cycle inhibitory effects, as well as a decrease in the AR and PSA. Thus, more studies investigating the translational capacity of this compound in various sub-types of cancer are warranted.
Acknowledgements
This work was supported by NEH grants R01 CAI 12029 and ROI CA121211. E. Rayburn was supported by a DoD grant (W81XWH-06-1-0063) and a T32 fellowship from the NEH/UAB Gene Therapy Center (CA075930). The apoptosis analyses were performed by the Flow Cytometry Core of the Arthritis and Musculoskeletal Center, which is supported in part by an NIH grant (P60 AR20614). S. E. Velu was supported by a translational research grant from the UAB Council of University-Wide Interdisciplinary Research Centers.
Contributor Information
Yong Zhang, Hormel Institute, University of Minnesota, Austin, MN 55912, USA.
Wei Wang, Division of Clinical Pharmacology, Department of Pharmacology and Toxicology, University of Alabama at Birmingham, 1670 University Blvd, 113 Volker Hall, Birmingham, AL 35294, USA.
Elizabeth R. Rayburn, Division of Clinical Pharmacology, Department of Pharmacology and Toxicology, University of Alabama at Birmingham, 1670 University Blvd, 113 Volker Hall, Birmingham, AL 35294, USA Gene Therapy Center, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
Ruiwen Zhang, Division of Clinical Pharmacology, Department of Pharmacology and Toxicology, University of Alabama at Birmingham, 1670 University Blvd, 113 Volker Hall, Birmingham, AL 35294, USA; Gene Therapy Center, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
References
- 1.American Cancer Society, Inc. Leading Sites of New Cancer Cases and Deaths-2007 Estimates. Surveillance Research. 2007. Available online. http://www.cancer.org/downloads/STT/CAFF2Q07PWSecured.pdf. Accessed 9 December 2008 [Google Scholar]
- 2.Quinn DI, Henshall SM, Sutherland RL (2005) Molecular markers of prostate cancer outcome. Eur J Cancer 41:858–887 doi: 10.1016/j.ejca.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP (2005) The current role of chemotherapy in metastatic hormone-refractory prostate cancer. Urology 65:3–7 doi: 10.1016/j.urology.2005.03.053 [DOI] [PubMed] [Google Scholar]
- 4.Tammela T (2004) Endocrine treatment of prostate cancer. J Steroid Biochem Mol Biol 92:287–295 doi: 10.1016/j.jsbmb.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 5.Haefner B (2003) Drags from the deep: marine natural products as drag candidates. Drag Discov Today 8:536–544 doi: 10.1016/SI359-6446(03)02713-2 [DOI] [PubMed] [Google Scholar]
- 6.Blunt JW, Copp BR, Munro MH et al. (2006) Marine natural compounds. Nat Prod Rep 23:26–78 doi: 10.1039/b502792f [DOI] [PubMed] [Google Scholar]
- 7.Marris E (2006) Marine natural products: drags from the deep. Nature 443:904–905 doi: 10.1038/443904a [DOI] [PubMed] [Google Scholar]
- 8.Schwartsmann G, Brondani da Rocha A, Berlinck RG, Jimeno J (2001) Marine organisms as a source of new anticancer agents. Lancet Oncol 2:221–225 doi: 10.1016/S1470-2045(00)00292-8 [DOI] [PubMed] [Google Scholar]
- 9.Dijoux MG, Gamble WR, Hallock YF, Cardellina JH, van Soest R, Boyd MR (1999) A new discorhabdin from two sponge genera. J Nat Prod 62:636–637 doi: 10.1021/np980465r [DOI] [PubMed] [Google Scholar]
- 10.Carney JR, Scheuer PJ, Kelly-Borges M (1993) A new bastadin from the sponge Psammaplysilla purpurea. J Nat Prod 56:153–157 doi: 10.1021/np50091a025 [DOI] [PubMed] [Google Scholar]
- 11.Casapullo A, Cutignano A, Bruno I et al. (2001) Makaluvamine P, a new cytotoxic pyrroloiminoquinone from Zyzzya cf. fuliginosa. J Nat Prod 64:1354–1356 doi: 10.1021/npO10053+ [DOI] [PubMed] [Google Scholar]
- 12.Sipkema D, Franssen MC, Osinga R, Tramper J, Wijffels RH (2005) Marine sponges as pharmacy. Mar Biotechnol 7:142–162 doi: 10.1007/s10126-004-0405-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinkre BA, Raisch KP, Fan L, Velu SE (2007) Analogs of the marine alkaloid makaluvamines: synthesis, topoisomerase II inhibition, and anticancer activity. Bioorg Med Chem Lett 17:2890–2893 doi: 10.1016/j.bmcl.2007.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velu SE, Pillai SK, Lakshmikantham MV, Billimoria AD, Culpepper JS, Cava MP (1995) Efficient syntheses of the marine alkaloids makaluvamine D and discorhabdin C: the 4,6,7-trimethoxyindole approach. J Org Chem 60:1800–1805 doi: 10.1021/jo00111a043 [DOI] [Google Scholar]
- 15.Shinkre BA, Raisch KP, Fan L, Velu SE (2008) Synthesis and antiproliferative activity of benzyl and phenethyl analogs of makaluvamines. Bioorg Med Chem 16:2541–2549 [DOI] [PubMed] [Google Scholar]
- 16.Jiang C, Lee HJ, Li GX et al. (2006) Potent antiandrogen and androgen receptor activities of an Angelica gigas-containing herbal formulation: identification of decursin as a novel and active compound with implications for prevention and treatment of prostate cancer. Cancer Res 66:453–463 doi: 10.1158/0008-5472.CAN-05-1865 [DOI] [PubMed] [Google Scholar]
- 17.Cho SD, Jiang C, Malewicz B et al. (2004) Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol Cancer Ther 3:605–611 [PubMed] [Google Scholar]
- 18.Barrows LR, Radisky DC, Copp BR et al. (1993) Makaluvamines, marine natural products, are active anti-cancer agents and DNA topo II inhibitors. Anticancer Drag Des 8:333–347 [PubMed] [Google Scholar]
- 19.Newman DJ, Cragg GM (2004) Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod 67:1216–1238 doi: 10.1021/np040031y [DOI] [PubMed] [Google Scholar]
- 20.Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gervvick WH (2005) Marine natural products as anticancer drugs. Mol Cancer Ther 4:333–342 [PubMed] [Google Scholar]
- 21.Schlomm T, Iwers L, Kirstein P et al. (2008) Clinical significance of p53 alterations in surgically treated prostate cancers. Mod Pathol 21:1371–1378 doi: 10.1038/modpathol.2008.10 [DOI] [PubMed] [Google Scholar]
- 22.Schafer KA (1998) The cell cycle: a review. Vet Pathol 35:461–478 [DOI] [PubMed] [Google Scholar]
- 23.Seville LL, Shah N, Westwell AD, Chan WC (2005) Modulation of pRB/E2F functions in the regulation of cell cycle and in cancer. Curr Cancer Drug Targets 5:159–170 doi: 10.2174/1568009053765816 [DOI] [PubMed] [Google Scholar]
- 24.Zinkel S, Gross A, Yang E (2006) BCL2 family in DNA damage and cell cycle control. Cell Death Differ 13:1351–1359 doi: 10.1038/sj.cdd.4401987 [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Oliver P, Zhang Z, Agrawal S, Zhang R (2003) Chemosensitization and radiosensitization of human cancer by antisense anti-MDM2 oligonucleotides: in vitro and in vivo activities and mechanisms. Ann N Y Acad Sci 1002:217–235 doi: 10.1196/annals.1281.025 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Wang H, Li M, Rayburn ER Agrawal S, Zhang R (2005) Stabilization of E2F1 protein by MDM2 through the E2F1 ubiquitination pathway. Oncogene 24:7238–7247 doi: 10.1038/sj.one.1208814 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Zhang R (2005) p53-independent activities of MDM2 and their relevance to cancer therapy. Curr Cancer Drag Targets 5:9–20 doi: 10.2174/1568009053332618 [DOI] [PubMed] [Google Scholar]
- 28.Gaughan L, Logan IR Neal DE, Robson CN (2005) Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res 33:13–26 doi: 10.1093/nar/gkil41 [DOI] [PMC free article] [PubMed] [Google Scholar]