Abstract
Most age-related neurodegenerative diseases are associated with the misfolding and aberrant accumulation of specific proteins in the nervous system. The proteins self-assemble and spread by a prion-like process of corruptive molecular templating, whereby abnormally folded proteins induce the misfolding and aggregation of like proteins into characteristic lesions. Despite the apparent simplicity of this process at the molecular level, diseases such as Alzheimer’s, Parkinson’s, Creutzfeldt-Jakob and others display remarkable phenotypic heterogeneity, both clinically and pathologically. Evidence is growing that this variability is mediated, at least in part, by the acquisition of diverse molecular architectures by the misfolded proteins - variants referred to as proteopathic strains. The structural and functional diversity of the assemblies is influenced by genetic, epigenetic, and local contextual factors. Insights into proteopathic strains gleaned from the classical prion diseases can be profitably incorporated into research on other neurodegenerative diseases. Their potentially wide-ranging influence on disease phenotype also suggests that proteopathic strains should be considered in the design and interpretation of diagnostic and therapeutic approaches to these disorders.
Keywords: Abeta, Alzheimer’s disease, amyloid, Parkinson’s disease, prion, synuclein, tau
INTRODUCTION
As humans grow old, the likelihood of developing one or more neurodegenerative diseases steadily rises (28). These clinically and pathologically heterogeneous disorders include Alzheimer’s disease (AD), Parkinson’s disease, Lewy body dementia, amyotrophic lateral sclerosis, frontotemporal dementia, Creutzfeldt-Jakob disease (CJD), and others. Virtually all age-related neurodegenerative diseases are characterized by the aggregation of specific proteins within or among the cells of the brain (17; 44; 68; 146). This process of abnormal protein self-assembly results in the formation of distinctive lesions that help to define each disease, including intracellular inclusions and/or extracellular masses of protein (Figure 1). Strong evidence now indicates that these neurodegenerative diseases arise and progress by a molecular mechanism closely resembling the corruptive protein templating that characterizes the classical prion diseases (68; 111; 126; 146).
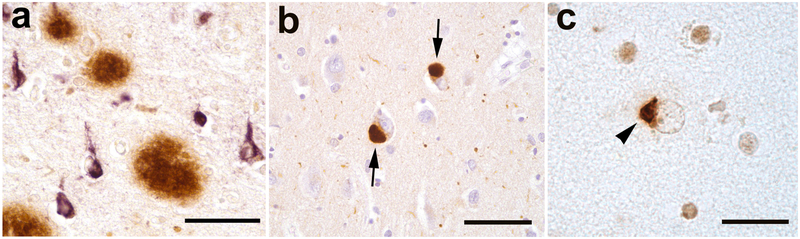
Figure 1.
Abnormal protein deposits detected immunohistochemically in three human neurodegenerative diseases. a: Extracellular Aβ (senile) plaques (brown) and intracellular neurofibrillary tangles (purple) in Alzheimer’s disease. b: Intracellular Lewy bodies (arrows) in Lewy body dementia. c: Ectopic (cytoplasmic) mass of TDP-43 (arrowhead) in amyotrophic lateral sclerosis. Nissl counterstain (blue) in b; Bars = 50μm in a and b, 20μm in c.
In many instances, the pathogenic proteins form polymeric fibrils that assemble into clumps referred to as amyloid, (17; 152) a generic term for abnormal lesions that exhibit birefringence under the light microscope after staining with the dye Congo Red (Figure 2a), and a typical cross-β X-ray fiber diffraction pattern owing to a high content of β-sheet in the constituent proteins (31; 46; 129). Using electron microscopy, amyloids are seen as rigid, generally unbranched fibrils (Figure 2b) that often are superficially similar in appearance despite deriving from any of more than 30 distinct proteins that aggregate in various organs (17; 129; 152).
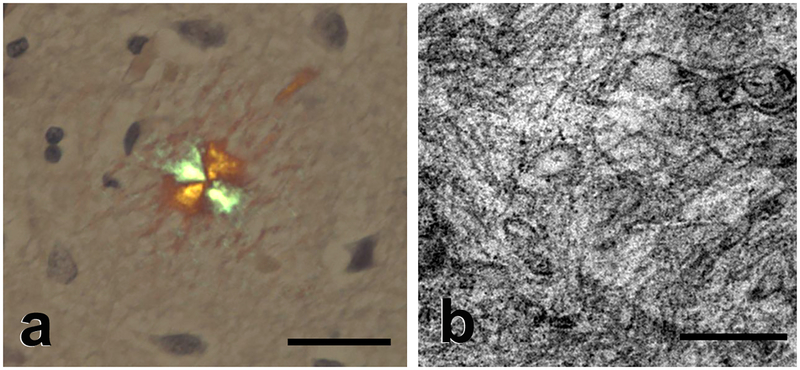
Figure 2.
Amyloid. a: Light micrograph of an amyloid plaque in the brain of an Alzheimer patient showing characteristic orange-green birefringence under illumination using crossed polarizing filters (Congo red stain, Nissl counterstain (blue); Bar = 50μm). b: Electron micrograph of massed amyloid fibrils in a plaque from an Alzheimer patient. The average width of amyloid fibrils in vivo is 7–13 nm (17). Bar = 200 nm.
Notwithstanding their general similarity in size and shape, amyloid fibrils made of a given protein can show a degree of polymorphism (101) that reflects the organization of the peptide chains within the protofilaments (17), which, in turn, is influenced by certain intrinsic and extrinsic factors (101). The omnipresence of the cross-β configuration in amyloids is consistent with an essential role of the peptide backbone in amyloidogenesis, but the amino acid side chains also influence the ultimate structure of the assemblies (17; 75). Aggregated proteins sometimes do not have the classical properties of amyloid (129), but in all known instances, ectopic deposits of protein signal the probable presence of disease. Even some well-known pathogenic proteins only inconsistently form amyloid in vivo, such as the prion protein (PrP) (26), amyloid-β (Aβ) (7), transactive response DNA binding protein-43 kDa (TDP-43) (119), huntingtin (56), and α-synuclein (120). Furthermore, amyloidogenic proteins can exist in the form of small oligomeric assemblies that lack the fibrillar structure of amyloid but that nonetheless impair organ function (32; 34; 48; 56; 84; 118). Thus, the amyloid state is indicative of a proteopathic process, and an enhanced tendency to form amyloid is common in pathogenic proteins, but amyloid per se is not always obligatory for the manifestation of disease.
ALZHEIMER’S DISEASE AND THE AGGREGATION OF Aβ AND TAU
Alzheimer’s disease is the most prevalent age-associated neurodegenerative disorder (114) and the most frequent form of amyloidosis in humans (41). The principal clinical attribute of AD is a progressive deterioration of cognitive function, usually over a period of 7–10 years (57). Analysis of biomarkers for AD indicates that the disease process begins in the brain two decades or more before the onset of demonstrable cognitive impairment (64). A definitive diagnosis of AD in a patient with dementia requires the presence of two canonical histopathologic lesions in the brain: Aβ (senile) plaques (heterogeneous structures consisting of extracellular deposits of multimeric Aβ peptide and a variable array of reactive cells), and neurofibrillary tangles (intracellular, fibrillar polymers of hyperphosphorylated tau protein) (Figure 1a). In addition to plaques, Aβ accumulates, albeit inconsistently, in the walls of brain blood vessels as cerebral amyloid angiopathy (CAA) (117).
Aβ is a cleavage product of the Aβ-precursor protein (APP), a 695–770 amino acid, single membrane-spanning protein that is expressed in cells throughout the body and is particularly abundant in the nervous system (47; 127). APP is sequentially cleaved by an extra-membranous enzyme called β-secretase (β-amyloid cleaving enzyme [BACE]) and an intramembranous enzyme complex called gamma-secretase to yield Aβ fragments that are usually 40 or 42 amino acids long (Aβ40 and Aβ42, respectively). In addition, smaller populations of C-terminally and N-terminally truncated and/or modified Aβ fragments are present (107; 108). Although Aβ40 is the predominant isoform that is generated by brain cells, Aβ42 has two additional hydrophobic residues at the C-terminal end that greatly enhance its tendency to aggregate; for this reason, Aβ42 is thought to be the most culpable player in the early development of AD (57).
Tau is a microtubule-binding protein that, in its normal state, is believed to stabilize cellular microtubules (132). In the tauopathies, tau misfolds and becomes hyperphosphorylated; in this state the molecules aggregate to form soluble oligomers and long, β-sheet-rich polymers that bundle together as neurofibrillary tangles. Tauopathy can afflict both neurons and glial cells (76), and occurs in association with numerous brain disorders other than AD. In the primary tauopathies, cognitive deterioration is linked to neurofibrillary tangles that generally exist in the absence of Aβ plaques (23; 76; 132). In many instances however, including AD, tauopathy appears to be secondary to various types of injury or stress to the brain (95).
For years the relative importance of Aβ and tau in the pathogenesis of AD has been the subject of debate (15; 79). The supposed antithesis of Aβ and tau in the AD scheme is, of course, a false dichotomy; abnormalities of both proteins are essential for the full clinicopathologic expression of AD (128). Although the number of tangles generally correlates more strongly with the degree of dementia than does the number of plaques (10; 25), genetic and biochemical evidence implicates the misfolding and multimerization of Aβ as the initial and indispensable occurrence in the ontogeny of AD (52; 57). This concept has been embodied in the amyloid (Aβ) cascade hypothesis, first formulated as such by John Hardy and colleagues (52; 53). The amyloid cascade hypothesis holds that Aβ aggregation is causative in AD, and other manifestations, including tauopathy, are downstream (more on this below).
Misfolded Aβ disrupts brain function in multiple ways. Studies in animal models show that Aβ plaques themselves have cytotoxic properties (94; 105), but, as in the case of many amyloidogenic proteins, Aβ also forms small, soluble, oligomeric assemblies that contribute to neuronal dysfunction and death (34; 48; 84). Hydrophobic amino acids are exposed on Aβ oligomers, facilitating toxic interactions with lipid membranes; as oligomers self-assemble into amyloid fibrils, their reactive surface area diminishes, rendering fibrils at least partially protective (128). With the proliferation and maturation of plaques, the equilibrium between insoluble fibrils and soluble oligomers is thought to shift in favor of generating oligomers, which then are free to impair the function of cells (128). A protective role of Aβ fibrils, however, by no means negates the centrality of Aβ aggregation in AD, as discussed below in a comparison with PrP prion disease. Additional evidence for a primary role of Aβ in AD has come from genetics.
AMYLOID, GENETICS, AND THE ORIGINS OF THE Aβ CASCADE HYPOTHESIS
In his description of amyloid in the early 1850’s, Rudolf Virchow initially believed that amyloid (meaning “starch-like”) resembled cellulose, but Friedreich and Kekule’s 1859 report that amyloid consists mainly of protein profoundly re-oriented inquiry into the nature of the substance [see (112)]. The proteins in some systemic amyloidoses were eventually identified in the early 1970s (13; 130), and the Aβ that forms plaques and CAA was sequenced in the mid-1980’s by Glenner and Wong (42) and Masters and colleagues (88). This knowledge quickly led to the localization of the gene for APP on human chromosome 21 (45; 72; 140), which, despite some early missteps (51), propelled genetic investigations of AD forward. In 1990, a missense mutation in the APP gene was linked to an autosomal dominant form of CAA (81; 143), and in 1991, for the first time a specific mutation (in the APP gene) was found to segregate with a familial form of Alzheimer’s disease (43).
It is hard to overstate the significance of these discoveries for defining the subsequent course of research on AD. In furnishing the first indisputable evidence for a specific molecular abnormality as a causative feature of AD, they helped to clarify research objectives, establish a plausible framework for the development of disease-modifying therapies, and lay the groundwork for the creation of genetically modified rodent models of AD. In so doing, the genetic findings also kindled the formulation of the Aβ cascade hypothesis as the dominant mechanistic framework for understanding the causation and course of AD.
The Aβ cascade hypothesis was further solidified by the discovery of autosomal dominant transmission of AD due to mutations in the genes for presenilin-1 and presenilin-2. The presenilins are related components of gamma-secretase, the intramembranous enzyme complex that, in series with extramembranous β-secretase, liberates Aβ from APP (47; 127). Presenilin-1 is encoded on chromosome 14, and presenilin-2 is encoded on chromosome 1. The majority of autosomal dominant mutations associated with AD occur in the genes that code for the presenilins, especially presenilin-1 (51; 57). Indeed, all known AD-linked mutations affect the production, removal, trafficking, and/or tendency to aggregate of Aβ (52).
Most of the AD mutations – specifically those in the presenilins and the APP regions flanking Aβ - influence the processing of APP, but mutations within the Aβ segment often modify its aggregation potential and tissue specificity (47). Remarkably, a rare genetic variant in the APP gene that causes an amino acid substitution at position 2 of Aβ (A673T, according to APP770 numbering) reduces the production (67) and aggregation propensity (8) of Aβ, and also lowers the risk of developing AD (67). In contrast, substitution of valine for alanine at this position (A673V) increases the production and aggregability of Aβ, and in homozygous carriers causes an autosomal recessive form of AD (27). These genetic data leave little doubt that Aβ plays a critical role in hereditary forms of AD.
But autosomal dominant and recessive causes are operative in less than 1% of all AD cases (57). Is it possible that idiopathic AD arises by a different mechanism, i.e., one in which the aggregation of misfolded Aβ is not a pivotal feature? This seems unlikely. Genetic variations are estimated to contribute as risk factors in ~70–80% of all AD cases (95; 139; 155). Most of these myriad genetic variants individually have a small impact on overall risk (58), but several together may have additive or emergent/interactive impact. By far the most salient genetic risk factor for AD is the E4 variant of apolipoprotein E (ApoE4) (158).
Apolipoprotein E (ApoE) is involved in lipid transport throughout the body, and it is the main apolipoprotein in the brain (18). ApoE has three major isoforms in humans, designated ApoE2, ApoE3 and ApoE4. The APOE gene is on chromosome 19, and the APOE4 allele dose-dependently increases the risk of AD (18). Worldwide, the most common isoform of the protein is ApoE3 (~78%), followed by ApoE4 (~14%) and ApoE2 (~8%) (83). Although the mechanism by which ApoE4 predisposes to AD is probably multifactorial (109; 158), bearers of APOE4 begin to accumulate Aβ in the brain at least a decade earlier in life than do non-bearers (116; 147; 148). In this sense, APOE4 appears to confer risk by expediting the Aβ cascade.
Whether hereditary or idiopathic, all AD involves the same core clinical and pathological features: progressive dementia in the context of Aβ plaques and neurofibrillary tangles. Notably, though, the characteristics of the lesions can vary within and among AD cases (see Proteopathic Aβ strains in Alzheimer’s disease, below). The Aβ cascade hypothesis identifies Aβ as the prime mover in the pathogenesis of AD, but the means by which the protein actually causes disease has been uncertain. Here, parallels with the molecular attributes of PrP prions have established a compelling model for understanding how the transformation of Aβ leads to the dementia of AD.
THE PRION PARADIGM OF NEURODEGENERATIVE DISEASE
Prototypical prions are multimeric assemblies of misfolded PrP that impel the misfolding and aggregation of other PrP molecules by a process of corruptive molecular templating (110; 111). PrP, encoded by a gene on chromosome 20, is expressed in nervous and non-nervous tissues and can undergo a variety of post-translational modifications (98). PrP prions cause progressive, fatal neurodegenerative disorders that include CJD, kuru, Gerstmann-Sträussler-Scheinker syndrome (GSS), fatal insomnias, and variably protease-sensitive prionopathy in humans; in nonhuman species they include scrapie in sheep and goats, bovine spongiform encephalopathy (BSE) in cattle, chronic wasting disease in cervids, and several others (1; 60; 61; 90). The prion diseases are defined histopathologically by spongiform change, loss of neurons, astrocytosis, and the accumulation of PrP (Figure 3). Notably, however, these properties vary considerably among the prion diseases, as does the presence of accompanying anomalies such as tauopathy (26).
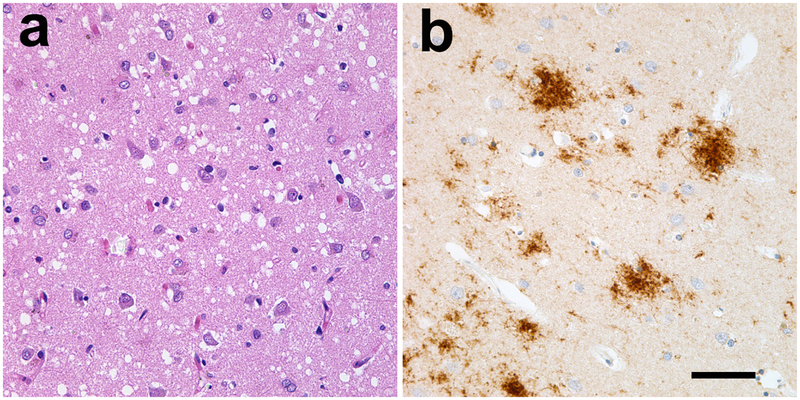
Figure 3.
The pathology of Creutzfeldt-Jakob disease. Common features of CJD are spongiform degeneration (seen as holes in a; hematoxylin and eosin stain) and the variable accumulation of prion protein (b; PrP immunostain, brown, Nissl counterstain, blue). Both types of lesion vary in type and extent among patients, and amyloid per se is rare in most cases of human prion disease. Bar = 50μm for both panels.
Prion diseases have long intrigued researchers because some of them – notably scrapie and kuru - are infectious with a very long incubation period. A frank immune response to infection is absent, and the infectious agent is unusually durable and resistant to harsh treatments such as radiation, heat and formaldehyde (110). Kuru is the prototypical infectious prion disease in humans. It is a rapidly progressive (usually 3–12 months) encephalopathy that was largely confined to the Fore linguistic/cultural group of Papua New Guinea in the 20th century. In early investigations, D. Carleton Gajdusek and Vincent Zigas noted that kuru had a strong tendency to run in families; hence, their leading hypothesis initially was that kuru was an atypical genetic (“heredofamilial”) disorder [see (33)]. Several incongruities, however, gave them pause – adult females were much more frequently affected than were males, but among children boys became ill nearly as often as girls. In addition, Gajdusek’s exploration of the fringes of kuru territory disclosed occasional Fore females who developed kuru when married into neighboring (kuru-free) tribes, as would be expected for a genetic disorder, but they also made the contradictory observation that females from neighboring tribes sometimes manifested kuru after moving into Fore territory (33).
The experimental transmission of kuru to chimpanzees (38) ultimately substantiated the longstanding suspicion that endocannibalism – usually of deceased family members – caused the preferential spread of the kuru agent within families. Females and children were more likely than adult males to participate in the mortuary feasts (a.k.a. transumption) (153). Following the cessation of endocannibalism in the 1950’s, the incidence of kuru began a long, slow decline; rare cases continued to appear into the 21st century (2), with incubation periods of some patients thought to exceed 50 years (22). Remarkably, the striking loss of Fore women and children during the kuru epidemic resulted in the selection of a polymorphism in the gene for PrP that protects against kuru, possibly the strongest example of genetic selection yet discovered in a human population (4).
The prion puzzle was complicated by the recognition that, in addition to infection, prion diseases also can be idiopathic or genetic in origin (110; 111). The infectivity of PrP prions is made possible by an unprecedented mechanism by which a pathogenic molecular conformation of PrP (PrP Scrapie [PrPSc]) structurally corrupts normal, endogenous PrP molecules (PrP Cellular [PrPC]) by templated structural conversion (1; 110). Efficient transmission of prion disease is critically dependent on the compatibility of the host and the agent (110; 145; 150), and the host can influence the characteristics of the aggregates independent of PrP sequence differences (24). Functionally significant variations in prion traits are associated with differences in PrP amino acid sequence, protease sensitivity, resistance to denaturants, and glycosylation patterns (89; 150), but a crucial factor governing infectivity and phenotype is the molecular conformation of PrPSc (39; 103; 137). Such structural/functional variants of PrP prions are commonly referred to as prion strains, the heterogeneity of which is linked to heterogeneity in the presentation of the disease (21; 124). As is discussed below, other disease-related proteins share with PrP prions the capacity to form variant strains (17; 31; 137) (Figure 4).
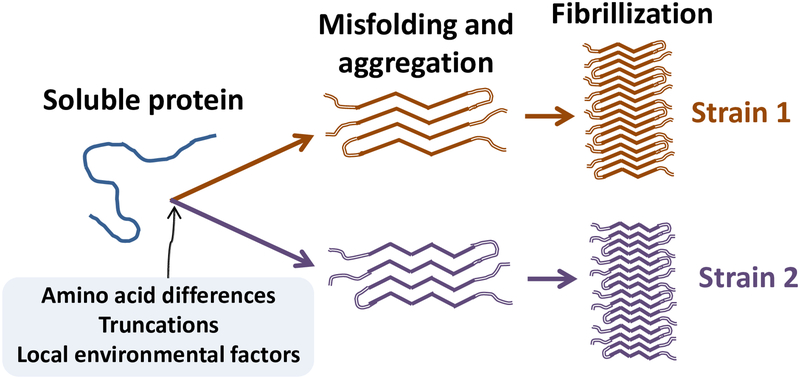
Figure 4.
Schematic diagram of proteopathic strain formation. Pathogenic proteins are most likely to misfold from an unfolded or partially folded state. The path to a given strain is influenced by such factors as sequence differences, truncation of the protein, post-translational modifications, and the milieu in which the seeds form and propagate. As the molecules acquire excess β-sheet, they bind to one another and multimerize into small oligomers, protofibrils, and/or amyloid fibrils. In addition to their influence on disease presentation, different molecular strains are sometimes reflected in the presence, distribution, and/or morphology of resultant lesions in the brain.
PRION STRAINS
In 1961, Pattison and Millson noted that the disease phenotype caused by intracerebral inoculation of goats with scrapie-affected brain homogenates faithfully recapitulated the disease that had been seen in the donor (100). They surmised that the infectious agent – then supposed by most to be an unusual virus – existed in the form of variant, genetically defined viral strains that produced clinically distinct signs. In the same year, Chandler reported the first successful transmission of scrapie to mice (16). He made the important observation that the nature of the resulting disease – called either “scratching” or “drowsy” in goats – is governed by both the strain of inoculum and the type of host. Despite strong evidence even then that the infective agent was highly unorthodox (99), the existence of disease strains was invoked for years afterwards as evidence that, in order to replicate in its alternative forms, the causative agent must be a microbe or virus. The emerging idea that prion strains are enciphered solely in the protein itself was met with incredulity by many researchers, as the complexity of strain characteristics was thought to require a nucleic acid genome (110). Although the maintenance of specific features through multiple passages is considered to be characteristic of microbial strains, an important attribute of prion strains is that they can be post-translationally modified and/or selected for by a different type of host (14; 151).
Prion strains in mammals have been classified as PrP variants that elicit typical pathogenic and phenotypic traits in the host (21; 39; 125). For instance, PrPSc is potentially amyloidogenic, but amyloid plaques per se are uncommon in most human and nonhuman prion diseases (26). Two exceptions are variant CJD (vCJD), which is transmitted to humans by exposure to the agent of BSE (154), and GSS, in which disease-associated amino acid substitutions facilitate terminal truncations of PrP that render the protein highly amyloidogenic (26). Interestingly, although tauopathy is inconsistent in occurrence and form in human prion diseases, the GSS cases are notable in that they manifest a type of tauopathy that is reminiscent of that in AD (26).
Genetic factors play a role in the pathobiology of prion strains by determining the primary sequence of PrP as well as the compatibility of the host and agent; for example, an amino acid polymorphism (M or V) at position 129 of PrP distinctly influences disease phenotype (39). Just as a rare variant in the primary structure of APP (A673T) can protect against AD (67), a G127V substitution in PrP is able to prevent prion disease (and therefore was strongly selected for by the kuru epidemic among the Fore people) (4). Amino acid sequence is one of several factors that influence a crucial trait that determines prion functionality: the multidimensional architecture of the molecules (3; 39). Recently developed thiophene-based probes can distinguish tertiary and/or quaternary molecular features of prion strains based on dissimilar fluorescence emission profiles when bound to aggregated PrPSc (87). Remarkably, prion strains can mutate and undergo preferential amplification under selection pressure (9; 21; 39; 40; 82). The notion that PrP prions and other proteopathic seeds exist in various states with distinct biological properties has considerable explanatory power for the protean nature of protein-based molecular information transfer (19; 20; 80; 92; 102; 111; 131).
PROTEOPATHIC Aβ STRAINS IN ALZHEIMER’S DISEASE
The deposition of Aβ in the brain can be stimulated in experimental animals by the introduction of seeds of aggregated Aβ (68). These seeds bear obvious structural and functional similarities to PrP prions. Specifically, Aβ seeds, like PrP prions, consist solely of a particular protein (Aβ rather than PrP); they are resistant to high temperature or formaldehyde (36; 93), they can spread within the brain and to the brain from the periphery (29; 30; 157), and they are extremely long-lived in the brain (156). These commonalities support the inclusion of Aβ seeds within the expanding prion family (111; 146). They also suggest that Aβ seeds might share with PrP prions the ability to aggregate into variant structural/functional strains. Although research on Aβ strains lags somewhat behind that of PrP prions, evidence is mounting that not all aggregates of Aβ are alike. Indeed, the potential to generate conformationally distinct protein strains is considered to be a common property of aggregation-prone proteins (17; 31; 137).
A given protein can form amyloid fibrils of varying morphologies under the influence of different environmental conditions such as pH, temperature, ionic strength, and protein concentration, and the resulting fibrillar shapes are associated with differences in molecular arrangement (101). The molecular architecture of misfolded Aβ, like that of other amyloidogenic proteins, can be conveyed to newly formed fibrils by a process of templated structural conversion, or seeding, in a strain-specific fashion (85; 133; 149). Similar to PrP prions (above), strains of other pathogenic proteins are subject to conformational selection in which the morphotype best suited to a given environment prevails (101).
It is important to note that AD and other neurodegenerative diseases, unlike prion diseases, have not been demonstrated to be infectious under everyday circumstances. Recent reports have found evidence for the seeded induction of Aβ deposition in persons treated with cadaver-derived pituitary hormones (65) or who had received transplants of cadaver-derived dura mater (37; 50) – clearly extraordinary (and now discontinued) circumstances. The patients who were analyzed in these reports had died of iatrogenic CJD due to the presence of PrP prions in the biomaterials, and it appears likely that some of the materials also contained Aβ seeds (69; 77). However, the Aβ deposition in the recipients was not accompanied by tauopathy, and it cannot be known whether they eventually would have developed AD had they not succumbed to CJD. A study of human growth hormone recipients in the United States found no evidence of increased AD among surviving growth hormone recipients, at least as of 2008 (63). It will be instructive to monitor these patients over the coming years to determine if AD or other neurodegenerative diseases become disproportionately frequent with longer incubation periods.
Phenotypic variation in Aβ deposits
In the PrP prionoses, different strains of PrPSc often produce distinctive patterns of lesion configuration and distribution in the brain (26; 103). A key sign that vCJD is caused by a novel PrP prion strain, possibly originating from cows with BSE, was the discovery of unusual lesions called florid plaques in affected humans (62). As in the case of PrP prions, evidence is growing that Aβ can form strain-like variants both in vitro (91; 96; 97; 104; 142) and in vivo (54; 85; 93; 121; 123; 134; 149). Histologically, cerebral Aβ deposits in AD patients manifest considerable morphologic variation (Figure 5) that may be indicative of intra-individual strain-like differences in molecular structure (80).
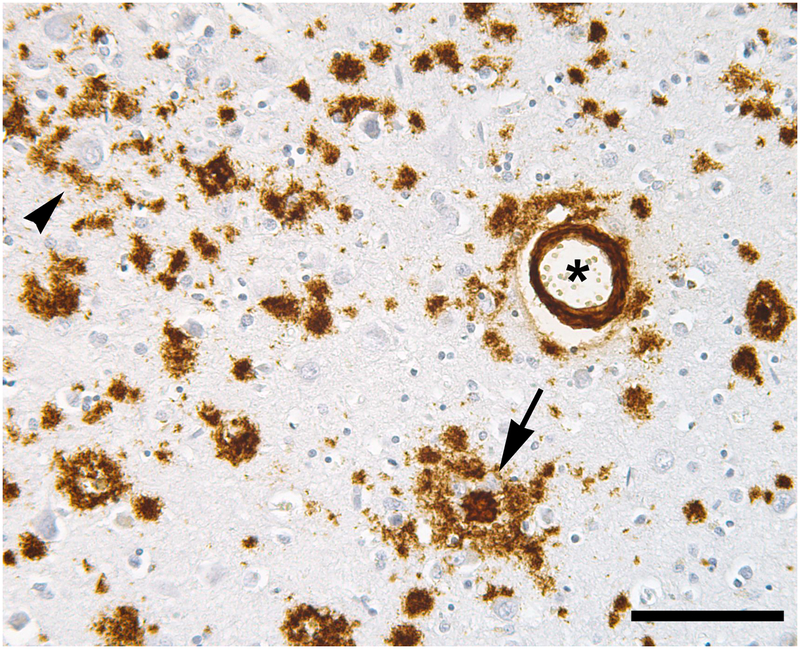
Figure 5.
Aβ deposits in AD are heterogeneous. A dense-core (amyloid) plaque is marked by an arrow, a cluster of diffuse Aβ deposits by an arrowhead, and a blood vessel with mural and perivascular Aβ by an asterisk. Immunostain for Aβ (brown); Nissl counterstain (blue). Bar = 100μm.
Genetic Determinants of Structurally Variant Aβ Assemblies
Genetic mutations that substitute amino acids within Aβ can engender distinct disease phenotypes. The first evidence that a mutation in the gene for APP can cause disease arose from investigation of a rare familial form of cerebral amyloid angiopathy in the Netherlands (81; 143). A G->C nucleotide change (the “Dutch” mutation) alters amino acid 22 of Aβ (APP: E693Q), causing Aβ deposition in the cerebral vasculature (81; 143), which leads to catastrophic cerebral hemorrhage by approximately 50 years of age (86). In contrast, the “Arctic” mutation at the same locus (yielding E693G) results in clinical AD but with profuse, atypical Aβ plaques lacking the classical dense amyloid core (70). As mentioned earlier, different amino acid substitutions at position 2 of Aβ either cause dementia (27) or protect against it (67); these findings collectively confirm that not all multimeric Aβ is the same at the molecular or histologic level, and that molecular differences can influence the nature of the disease. The amino acid sequence is a strong determinant of folded protein structure, but, as in the case of PrP prions, the amino acid sequence of Aβ need not be altered in order for the aggregated protein to manifest different structural and functional properties (141). In addition to germline mutations and polymorphisms, the role of somatic mosaicism (12; 35) and/or epigenetic modifications (78) in the ontogeny and heterogeneity of AD and other neurodegenerative disorders is a nascent and promising topic for further investigation.
Aβ Strains in Idiopathic Alzheimer’s Disease
The hypothesis that Aβ is able to aggregate into diverse strains is supported by the differential affinity of amyloid-binding agents for Aβ lesions. Pittsburgh compound B (PiB) (N‐methyl‐[11C]2‐(4′‐methylaminophenyl)‐6‐hydroxybenzothiazole) is a radiolabeled diagnostic imaging agent derived from the histochemical dye Thioflavin-T. PiB crosses the blood-brain barrier and, at low-nanomolar concentrations, selectively binds with high affinity and stoichiometry to Aβ plaques (73) and CAA (66) in humans. Unexpectedly, deposits of human-sequence Aβ in APP-transgenic mice are deficient in high-affinity binding of PiB (74). Similar observations were made in species that naturally accumulate human-type sequence Aβ with age. For example, nonhuman primates deposit high levels of Aβ in plaques and CAA as they grow old (55; 123), yet the lesions lack significant high-affinity PiB binding, suggestive of post-translational differences in misfolded Aβ among species (123). Although monkeys show some cognitive decline with age (6), they do not manifest the full behavioral and pathologic phenotype of AD, most notably the presence of widespread cerebral tauopathy. These findings together suggest that the degree of high-affinity PiB binding reflects post-translational characteristics of misfolded Aβ, and thus could furnish clues to the uniquely human vulnerability to AD (80; 122; 123).
The affinity of PiB binding to Aβ appears also to vary among humans with AD (59; 121). In one extraordinary occurrence, a patient who had died of AD was discovered to have very high levels of Aβ in the brain, yet virtually no high-affinity binding of PiB (121). Though histopathologically unexceptional, biochemically this patient had an especially high ratio of Aβ40:Aβ42; indeed, the amounts of various Aβ fragments could be an important determinant of the strain-like character and pathogenicity of aggregated Aβ (108). While the existence of this PiB-negative case would seem to argue against high-affinity PiB binding as a marker of pathogenic Aβ, it is worth noting that the manifestation of clinical disease in this case occurred in the context of Aβ levels that were an order of magnitude greater than in other AD cases (121).
Several other experimental approaches have added to the evidence for Aβ strains in humans. Using a biophysical method developed for the analysis of PrP prions, Safar and colleagues reported that a rapidly progressive form of AD is associated with specific structural properties of the aggregated Aβ, especially Aβ42 (20). Lu, Tycko and colleagues used Aβ derived from two clinically distinct AD cases to seed the formation of daughter fibrils, which consisted of synthetic Aβ40, that were remarkably distinct when analyzed by solid-state nuclear magnetic resonance and electron microscopy (85). These studies, along with those of PiB and conformation-sensitive dyes, substantiate the conclusion that not all Aβ aggregates are alike, and that strain-like variations of amyloidogenic proteins can markedly influence the bioactivity of the assemblies (142).
Experimental Induction of Aβ Strains
Aβ deposition can be seeded in the brain by a prion-like process when brain extracts containing aggregated Aβ are injected intracerebrally into APP-transgenic mice expressing human-sequence Aβ (71; 93). In the absence of seeding, APP-transgenic mouse models normally begin to manifest cerebral Aβ deposits at a predictable age. In the seeding paradigm, however, injection of a small amount of Aβ-rich brain extract into the brains of host APP-transgenic mice induces accelerated Aβ deposition that then spreads from the site of injection (49; 157). As in humans with AD, APP-transgenic mice exhibit molecular structural differences in Aβ; these strain-like characteristics are preserved when the Aβ is seeded into suitable transgenic host mice (36; 54; 93; 134; 149).
Two different lines of transgenic mice used in the Aβ-seeding studies (APP23 mice (135) and APP-PS1 mice expressing human APP and human presenilin-1 (113)) develop Aβ plaques that differ morphologically under the light microscope (54). When a small amount of Aβ-laden brain extract from one of these models is injected into the brain of the other model, the seeded lesion morphologies and the molecular architecture of the Aβ as assessed by thiophene-based amyloid probes reflect characteristics of both the seeding extract and the host mice (54). Interestingly, the exogenous seeds also modified the relative amounts of Aβ40 and Aβ42 in the Aβ deposits that were induced in the host mice (54). Brain extracts from AD patients carrying either the Swedish or Arctic mutation were shown to seed distinct Aβ pathologies that could be serially propagated and preserved after multiple passages (149). These observations, along with evidence that soluble Aβ aggregates differ in normal aging and Alzheimer’s disease (106) and can form assemblies that retain their properties after repeated passage in vitro (104), support the concept that Aβ aggregates resemble PrP prions in their ability to form and propagate functionally variant strains. Furthermore, tauopathy can be cross-seeded by pre-aggregated Aβ seeds (144); it will be informative to determine whether distinct Aβ strains differentially seed tauopathy in animal models. Here again, a systematic comparison to PrP prion diseases, in which tauopathy is inconsistent and heterogeneous (26), could provide clues to the molecular relationship between Aβ deposition and neurofibrillary tangles in the Aβ cascade.
CONCLUSIONS: THE WIDENING SPECTRUM OF PROTEOPATHIC STRAINS
The heterogeneity of age-related neurodegenerative diseases has defied a straightforward explanation. In Alzheimer’s disease, multiple factors probably contribute to phenotypic variability. These include genetic, epigenetic, and environmental risk factors, as well as the location of the primary protein aggregation event(s) in the brain, and, especially in very old patients, the co-existence of separate pathogenic processes. Research increasingly supports the likelihood that the molecular architecture of the aggregated Aβ protein also plays a part in disease heterogeneity.
In addition to Aβ and PrP, burgeoning evidence indicates that other disease-associated proteins exhibit strain-like heterogeneity, including tau (11; 138), α-synuclein (102), superoxide dismutase-1 (5), and amyloidogenic proteins outside the nervous system, such as insulin (136) and possibly AA amyloid (152). A key, open question is the mechanism by which variant strains are linked to variant disease phenotypes, but high-resolution structural definition of PrP prions and other seeds remains a challenge. Emerging and evolving methods for interrogating protein structure, such as spectroscopy, surface reactivity, cryo-electron microscopy and others should help to define conformational elements and intermolecular contact points that contribute to the pathogenicity of the proteins (115). In this regard, it is important to investigate amyloid generated both in vitro and in vivo, as the latter includes auxiliary substances that can influence the form and function of the material (152). From a broader perspective, it will be profitable to establish the variety and prevalence of proteopathic strains in the general population, as the conformation of the protein assemblies could influence the course of disease and the efficacy of therapeutic interventions.
For decades, the prion diseases have presented a compelling model for the concept that a single protein, through the assumption of myriad forms, can yield diverse disease phenotypes. Integration of the prion concept into our thinking about other neurodegenerative diseases is likely to spawn needed insights into their origin, clinicopathologic course, and idiosyncrasies. This more cohesive research effort could accelerate the discovery of unified therapeutic strategies that can be applied to the many diseases of protein aggregation that currently lack effective treatment.
ACKNOWLEDGMENTS
I gratefully acknowledge helpful discussions with Mathias Jucker, David Lynn, Yury Chernoff, Anil Mehta, Harry LeVine III, Thomas Wingo, and Silke Vogelgesang, as well as the expert technical assistance of Jeromy Dooyema. This work was made possible by grants from the NIH (AG025688, AG040589, RR000165, OD11132), the CART Foundation, the Humboldt Foundation, and the MetLife Foundation.
Footnotes
DISCLOSURE STATEMENT
Dr. Walker is on the scientific advisory board of proMIS Neurosciences. The author is not aware of any other affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Aguzzi A, Polymenidou M. 2004. Mammalian prion biology: one century of evolving concepts. Cell 116:313–27 [DOI] [PubMed] [Google Scholar]
- 2.Alpers MP. 2008. Review. The epidemiology of kuru: monitoring the epidemic from its peak to its end. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 363:3707–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angers R, Christiansen J, Nalls AV, Kang HE, Hunter N, et al. 2014. Structural effects of PrP polymorphisms on intra- and interspecies prion transmission. Proc Natl Acad Sci U S A 111:11169–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asante EA, Smidak M, Grimshaw A, Houghton R, Tomlinson A, et al. 2015. A naturally occurring variant of the human prion protein completely prevents prion disease. Nature 522:478–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayers JI, Fromholt S, Koch M, DeBosier A, McMahon B, et al. 2014. Experimental transmissibility of mutant SOD1 motor neuron disease. Acta Neuropathol 128:791–803 [DOI] [PubMed] [Google Scholar]
- 6.Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, et al. 1991. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging 12:99–111 [DOI] [PubMed] [Google Scholar]
- 7.Basun H, Bogdanovic N, Ingelsson M, Almkvist O, Naslund J, et al. 2008. Clinical and neuropathological features of the arctic APP gene mutation causing early-onset Alzheimer disease. Arch Neurol 65:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benilova I, Gallardo R, Ungureanu AA, Castillo Cano V, Snellinx A, et al. 2014. The Alzheimer disease protective mutation A2T modulates kinetic and thermodynamic properties of amyloid-beta (Abeta) aggregation. J Biol Chem 289:30977–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry DB, Lu D, Geva M, Watts JC, Bhardwaj S, et al. 2013. Drug resistance confounding prion therapeutics. Proc Natl Acad Sci U S A 110:E4160–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, et al. 1995. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol 52:81–8 [DOI] [PubMed] [Google Scholar]
- 11.Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ. 2015. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 129:221–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bushman DM, Kaeser GE, Siddoway B, Westra JW, Rivera RR, et al. 2015. Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer’s disease brains. Elife 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buxbaum JN, Linke RP. 2012. A molecular history of the amyloidoses. J Mol Biol 421:142–59 [DOI] [PubMed] [Google Scholar]
- 14.Cancellotti E, Mahal SP, Somerville R, Diack A, Brown D, et al. 2013. Post-translational changes to PrP alter transmissible spongiform encephalopathy strain properties. The EMBO Journal 32:756–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellani RJ, Smith MA. 2011. Compounding artefacts with uncertainty, and an amyloid cascade hypothesis that is ‘too big to fail’. J Pathol 224:147–52 [DOI] [PubMed] [Google Scholar]
- 16.Chandler RL. 1961. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet 1:1378–9 [DOI] [PubMed] [Google Scholar]
- 17.Chiti F, Dobson CM. 2006. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–66 [DOI] [PubMed] [Google Scholar]
- 18.Chouraki V, Seshadri S. 2014. Genetics of Alzheimer’s disease. Adv Genet 87:245–94 [DOI] [PubMed] [Google Scholar]
- 19.Clavaguera F, Hench J, Goedert M, Tolnay M. 2015. Invited review: Prion-like transmission and spreading of tau pathology. Neuropathol Appl Neurobiol 41:47–58 [DOI] [PubMed] [Google Scholar]
- 20.Cohen ML, Kim C, Haldiman T, ElHag M, Mehndiratta P, et al. 2015. Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-beta. Brain 138:1009–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collinge J, Clarke AR. 2007. A general model of prion strains and their pathogenicity. Science 318:930–6 [DOI] [PubMed] [Google Scholar]
- 22.Collinge J, Whitfield J, McKintosh E, Frosh A, Mead S, et al. 2008. A clinical study of kuru patients with long incubation periods at the end of the epidemic in Papua New Guinea. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences 363:3725–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, et al. 2014. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowell J, Hughson A, Caughey B, Bessen RA. 2015. Host determinants of prion strain diversity independent of prion protein genotype. J Virol 89:10427–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crystal H, Dickson D, Fuld P, Masur D, Scott R, et al. 1988. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 38:1682–7 [DOI] [PubMed] [Google Scholar]
- 26.DeArmond SJ, Ironside JW, Bouzamondo-Bernstein E, Peretz D, Fraser JR. 2004. Neuropathology of Prion Diseases In Prion Biology and Diseases, ed. Prusiner SB:777–856. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- 27.Di Fede G, Catania M, Morbin M, Rossi G, Suardi S, et al. 2009. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science 323:1473–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan GW. 2011. The aging brain and neurodegenerative diseases. Clin Geriatr Med 27:629–44 [DOI] [PubMed] [Google Scholar]
- 29.Eisele YS, Fritschi SK, Hamaguchi T, Obermuller U, Fuger P, et al. 2014. Multiple factors contribute to the peripheral induction of cerebral beta-amyloidosis. J Neurosci 34:10264–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, et al. 2010. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330:980–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg D, Jucker M. 2012. The amyloid state of proteins in human diseases. Cell 148:1188–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang YS, Tsai KJ, Chang YJ, Kao P, Woods R, et al. 2014. Full-length TDP-43 forms toxic amyloid oligomers that are present in frontotemporal lobar dementia-TDP patients. Nat Commun 5:4824. [DOI] [PubMed] [Google Scholar]
- 33.Farquhar J, Gajdusek DC. 1981. Kuru: Early Letters and Field Notes from the Collection of D. Gajdusek Carleton. New York: Raven Press [Google Scholar]
- 34.Ferreira ST, Lourenco MV, Oliveira MM, De Felice FG. 2015. Soluble amyloid-beta oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front Cell Neurosci 9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank SA. 2014. Somatic mosaicism and disease. Curr Biol 24:R577–81 [DOI] [PubMed] [Google Scholar]
- 36.Fritschi SK, Cintron A, Ye L, Mahler J, Buhler A, et al. 2014. Abeta seeds resist inactivation by formaldehyde. Acta Neuropathol 128:477–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frontzek K, Lutz MI, Aguzzi A, Kovacs GG, Budka H. 2016. Amyloid-beta pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt-Jakob disease after dural grafting. Swiss Med Wkly 146:w14287. [DOI] [PubMed] [Google Scholar]
- 38.Gajdusek DC, Gibbs CJ, Alpers M. 1966. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature 209:794–6 [DOI] [PubMed] [Google Scholar]
- 39.Gambetti P, Cali I, Notari S, Kong Q, Zou WQ, Surewicz WK. 2011. Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol 121:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaemmaghami S, Watts JC, Nguyen HO, Hayashi S, DeArmond SJ, Prusiner SB. 2011. Conformational transformation and selection of synthetic prion strains. J Mol Biol 413:527–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghiso J, Frangione B. 2002. Amyloidosis and Alzheimer’s disease. Adv Drug Deliv Rev 54:1539–51 [DOI] [PubMed] [Google Scholar]
- 42.Glenner GG, Wong CW. 1984. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–90 [DOI] [PubMed] [Google Scholar]
- 43.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, et al. 1991. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349:704–6 [DOI] [PubMed] [Google Scholar]
- 44.Goedert M 2015. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science 349:1255555. [DOI] [PubMed] [Google Scholar]
- 45.Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. 1987. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science 235:877–80 [DOI] [PubMed] [Google Scholar]
- 46.Greenwald J, Riek R. 2010. Biology of amyloid: structure, function, and regulation. Structure 18:1244–60 [DOI] [PubMed] [Google Scholar]
- 47.Haass C, Kaether C, Thinakaran G, Sisodia S. 2012. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2:a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haass C, Selkoe DJ. 2007. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8:101–12 [DOI] [PubMed] [Google Scholar]
- 49.Hamaguchi T, Eisele YS, Varvel NH, Lamb BT, Walker LC, Jucker M. 2012. The presence of Abeta seeds, and not age per se, is critical to the initiation of Abeta deposition in the brain. Acta Neuropathol 123:31–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamaguchi T, Taniguchi Y, Sakai K, Kitamoto T, Takao M, et al. 2016. Significant association of cadaveric dura mater grafting with subpial Abeta deposition and meningeal amyloid angiopathy. Acta Neuropathol [DOI] [PubMed] [Google Scholar]
- 51.Hardy J 2006. A hundred years of Alzheimer’s disease research. Neuron 52:3–13 [DOI] [PubMed] [Google Scholar]
- 52.Hardy J, Selkoe DJ. 2002. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–6 [DOI] [PubMed] [Google Scholar]
- 53.Hardy JA, Higgins GA. 1992. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–5 [DOI] [PubMed] [Google Scholar]
- 54.Heilbronner G, Eisele YS, Langer F, Kaeser SA, Novotny R, et al. 2013. Seeded strain-like transmission of beta-amyloid morphotypes in APP transgenic mice. EMBO reports 14:1017–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heuer E, Rosen RF, Cintron A, Walker LC. 2012. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Current pharmaceutical design 18:1159–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffner G, Djian P. 2014. Polyglutamine Aggregation in Huntington Disease: Does Structure Determine Toxicity? Mol Neurobiol [DOI] [PubMed] [Google Scholar]
- 57.Holtzman DM, Morris JC, Goate AM. 2011. Alzheimer’s disease: the challenge of the second century. Science translational medicine 3:77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humphries C, Kohli MA. 2014. Rare Variants and Transcriptomics in Alzheimer disease. Curr Genet Med Rep 2:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikonomovic MD, Abrahamson EE, Price JC, Hamilton RL, Mathis CA, et al. 2012. Early AD pathology in a [C-11]PiB-negative case: a PiB-amyloid imaging, biochemical, and immunohistochemical study. Acta Neuropathol 123:433–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imran M, Mahmood S. 2011. An overview of animal prion diseases. Virology Journal 8:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imran M, Mahmood S. 2011. An overview of human prion diseases. Virology Journal 8:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ironside JW, Head MW, Bell JE, McCardle L, Will RG. 2000. Laboratory diagnosis of variant Creutzfeldt-Jakob disease. Histopathology 37:1–9 [DOI] [PubMed] [Google Scholar]
- 63.Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, et al. 2013. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol 70:462–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jack CR, Holtzman DM. Jr 2013. Biomarker modeling of Alzheimer’s disease. Neuron 80:1347–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaunmuktane Z, Mead S, Ellis M, Wadsworth JD, Nicoll AJ, et al. 2015. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature 525:247–50 [DOI] [PubMed] [Google Scholar]
- 66.Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, et al. 2007. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 62:229–34 [DOI] [PubMed] [Google Scholar]
- 67.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, et al. 2012. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488:96–9 [DOI] [PubMed] [Google Scholar]
- 68.Jucker M, Walker LC. 2013. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jucker M, Walker LC. 2015. Neurodegeneration: Amyloid-beta pathology induced in humans. Nature 525:193–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalimo H, Lalowski M, Bogdanovic N, Philipson O, Bird TD, et al. 2013. The Arctic AbetaPP mutation leads to Alzheimer’s disease pathology with highly variable topographic deposition of differentially truncated Abeta. Acta Neuropathol Commun 1:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, et al. 2000. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci 20:3606–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, et al. 1987. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325:733–6 [DOI] [PubMed] [Google Scholar]
- 73.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, et al. 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55:306–19. [DOI] [PubMed] [Google Scholar]
- 74.Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, et al. 2005. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer’s disease brain but not in transgenic mouse brain. J Neurosci 25:10598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knowles TP, Vendruscolo M, Dobson CM. 2014. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol 15:384–96 [DOI] [PubMed] [Google Scholar]
- 76.Kovacs GG. 2015. Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 41:3–23 [DOI] [PubMed] [Google Scholar]
- 77.Kovacs GG, Lutz MI, Ricken G, Strobel T, Hoftberger R, et al. 2016. Dura mater is a potential source of Abeta seeds. Acta Neuropathol 131:911–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, et al. 2015. The epigenetics of aging and neurodegeneration. Prog Neurobiol 131:21–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee VM. 2001. Biomedicine. Tauists and beta-aptists united--well almost! Science 293:1446–7 [DOI] [PubMed] [Google Scholar]
- 80.Levine H 3rd, Walker LC 2010. Molecular polymorphism of Abeta in Alzheimer’s disease. Neurobiol Aging 31:542–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, et al. 1990. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248:1124–6 [DOI] [PubMed] [Google Scholar]
- 82.Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C. 2010. Darwinian evolution of prions in cell culture. Science 327:869–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu CC, Kanekiyo T, Xu H, Bu G. 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9:106–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu P, Reed MN, Kotilinek LA, Grant MK, Forster CL, et al. 2015. Quaternary structure defines a large class of Amyloid-beta oligomers neutralized by sequestration. Cell Rep 11:1760–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. 2013. Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154:1257–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maat-Schieman M, Roos R, van Duinen S. 2005. Hereditary cerebral hemorrhage with amyloidosis-Dutch type. Neuropathology: Official Journal of the Japanese Society of Neuropathology 25:288–97 [DOI] [PubMed] [Google Scholar]
- 87.Magnusson K, Simon R, Sjolander D, Sigurdson CJ, Hammarstrom P, Nilsson KP. 2014. Multimodal fluorescence microscopy of prion strain specific PrP deposits stained by thiophene-based amyloid ligands. Prion 8:319–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. 1985. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 82:4245–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McKintosh E, Tabrizi SJ, Collinge J. 2003. Prion diseases. J Neurovirol 9:183–93 [DOI] [PubMed] [Google Scholar]
- 90.Mead S, Reilly MM. 2015. A new prion disease: relationship with central and peripheral amyloidoses. Nat Rev Neurol 11:90–7 [DOI] [PubMed] [Google Scholar]
- 91.Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fandrich M. 2009. Abeta(1–40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol 386:869–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melki R 2015. Role of Different Alpha-Synuclein Strains in Synucleinopathies, Similarities with other Neurodegenerative Diseases. J Parkinsons Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, et al. 2006. Exogenous induction of cerebral {beta}-amyloidogenesis is governed by agent and host. Science 313:1781–4 [DOI] [PubMed] [Google Scholar]
- 94.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, et al. 2008. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 451:720–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, et al. 2012. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71:362–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nilsson KP, Aslund A, Berg I, Nystrom S, Konradsson P, et al. 2007. Imaging distinct conformational states of amyloid-beta fibrils in Alzheimer’s disease using novel luminescent probes. ACS Chem Biol 2:553–60 [DOI] [PubMed] [Google Scholar]
- 97.Paravastu AK, Leapman RD, Yau WM, Tycko R. 2008. Molecular structural basis for polymorphism in Alzheimer’s beta-amyloid fibrils. Proc Natl Acad Sci U S A 105:18349–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parchi P, Saverioni D. 2012. Molecular pathology, classification, and diagnosis of sporadic human prion disease variants. Folia Neuropathol 50:20–45 [PubMed] [Google Scholar]
- 99.Pattison IH. 1972. Scrapie--a personal view. J Clin Pathol Suppl (R Coll Pathol) 6:110–4 [PMC free article] [PubMed] [Google Scholar]
- 100.Pattison IH, Millson GC. 1961. Scrapie produced experimentally in goats with special reference to the clinical syndrome. J Comp Pathol 71:101–9 [DOI] [PubMed] [Google Scholar]
- 101.Pedersen JS, Otzen DE. 2008. Amyloid-a state in many guises: Survival of the fittest fibril fold. Protein Sci 17:2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, et al. 2015. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–4 [DOI] [PubMed] [Google Scholar]
- 103.Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, et al. 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921–32 [DOI] [PubMed] [Google Scholar]
- 104.Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. 2005. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science 307:262–5 [DOI] [PubMed] [Google Scholar]
- 105.Phinney AL, Deller T, Stalder M, Calhoun ME, Frotscher M, et al. 1999. Cerebral amyloid induces aberrant axonal sprouting and ectopic terminal formation in amyloid precursor protein transgenic mice. J Neurosci 19:8552–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piccini A, Russo C, Gliozzi A, Relini A, Vitali A, et al. 2005. Beta-Amyloid is different in normal aging and in Alzheimer disease. J Biol Chem 34:186–92 [DOI] [PubMed] [Google Scholar]
- 107.Portelius E, Bogdanovic N, Gustavsson MK, Volkmann I, Brinkmalm G, et al. 2010. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol 120:185–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Portelius E, Lashley T, Westerlund A, Persson R, Fox NC, et al. 2015. Brain amyloid-beta fragment signatures in pathological ageing and Alzheimer’s disease by hybrid immunoprecipitation mass spectrometry. Neurodegener Dis 15:50–7 [DOI] [PubMed] [Google Scholar]
- 109.Potter H, Wisniewski T. 2012. Apolipoprotein e: essential catalyst of the Alzheimer amyloid cascade. Int J Alzheimers Dis 2012:489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prusiner SB. 1998. Prions. Proc Natl Acad Sci U S A 95:13363–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prusiner SB. 2013. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47:601–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Puchtler H, Sweat F. 1966. A review of early concepts of amyloid in context with contemporary chemical literature from 1839 to 1859. J Histochem Cytochem 14:123–34 [DOI] [PubMed] [Google Scholar]
- 113.Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, et al. 2006. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO reports 7:940–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reitz C, Brayne C, Mayeux R. 2011. Epidemiology of Alzheimer disease. Nat Rev Neurol 7:137–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Requena JR, Wille H. 2014. The structure of the infectious prion protein: Experimental data and molecular models. Prion 8:60–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Resnick SM, Bilgel M, Moghekar A, An Y, Cai Q, et al. 2015. Changes in Abeta biomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiol Aging 36:2333–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Revesz T, Ghiso J, Lashley T, Plant G, Rostagno A, et al. 2003. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol 62:885–98. [DOI] [PubMed] [Google Scholar]
- 118.Roberts HL, Brown DR. 2015. Seeking a mechanism for the toxicity of oligomeric alpha-synuclein. Biomolecules 5:282–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson JL, Geser F, Stieber A, Umoh M, Kwong LK, et al. 2013. TDP-43 skeins show properties of amyloid in a subset of ALS cases. Acta Neuropathol 125:121–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rodriguez JA, Ivanova MI, Sawaya MR, Cascio D, Reyes FE, et al. 2015. Structure of the toxic core of alpha-synuclein from invisible crystals. Nature 525:486–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosen RF, Ciliax BJ, Wingo TS, Gearing M, Dooyema J, et al. 2010. Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer’s disease. Acta Neuropathol 119:221–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rosen RF, Tomidokoro Y, Farberg AS, Dooyema J, Ciliax B, et al. 2016. Comparative pathobiology of beta-amyloid and the unique susceptibility of humans to Alzheimer’s disease. Neurobiol Aging 44:185–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosen RF, Walker LC, Levine H 3rd. 2011. PIB binding in aged primate brain: enrichment of high-affinity sites in humans with Alzheimer’s disease. Neurobiol Aging 32:223–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Safar J, Wille H, Itri V, Groth D, Serban H, et al. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4:1157–65 [DOI] [PubMed] [Google Scholar]
- 125.Safar JG, Xiao X, Kabir ME, Chen S, Kim C, et al. 2015. Structural determinants of phenotypic diversity and replication rate of human prions. PLoS Pathogens 11:e1004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanders DW, Kaufman SK, Holmes BB, Diamond MI. 2016. Prions and protein assemblies that convey biological information in health and disease. Neuron 89:433–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Selkoe DJ. 1999. Biology of beta-amyloid precursor protein and the mechanism of Alzheimer disease In Alzheimer Disease, ed. Terry RD, Katzman R, Bick KL, Sisodia SS: 293–310. Philadelphia: Lippincott Williams and Wilkins. [Google Scholar]
- 128.Selkoe DJ. 2011. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med 17:1060–5 [DOI] [PubMed] [Google Scholar]
- 129.Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, et al. 2014. Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 21:221–4 [DOI] [PubMed] [Google Scholar]
- 130.Sipe JD, Cohen AS. 2000. Review: history of the amyloid fibril. J Struct Biol 130:88–98 [DOI] [PubMed] [Google Scholar]
- 131.Smethurst P, Sidle KC, Hardy J. 2015. Review: Prion-like mechanisms of transactive response DNA binding protein of 43 kDa (TDP-43) in amyotrophic lateral sclerosis (ALS). Neuropathol Appl Neurobiol 41:578–97 [DOI] [PubMed] [Google Scholar]
- 132.Spillantini MG, Goedert M. 2013. Tau pathology and neurodegeneration. Lancet Neurol 12:609–22 [DOI] [PubMed] [Google Scholar]
- 133.Spirig T, Ovchinnikova O, Vagt T, Glockshuber R. 2014. Direct evidence for self-propagation of different amyloid-beta fibril conformations. Neurodegener Dis 14:151–9 [DOI] [PubMed] [Google Scholar]
- 134.Stohr J, Condello C, Watts JC, Bloch L, Oehler A, et al. 2014. Distinct synthetic Abeta prion strains producing different amyloid deposits in bigenic mice. Proc Natl Acad Sci U S A 111:10329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, et al. 1997. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A 94:13287–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Surmacz-Chwedoruk W, Nieznanska H, Wojcik S, Dzwolak W. 2012. Cross-seeding of fibrils from two types of insulin induces new amyloid strains. Biochemistry 51:9460–9 [DOI] [PubMed] [Google Scholar]
- 137.Tanaka M, Collins SR, Toyama BH, Weissman JS. 2006. The physical basis of how prion conformations determine strain phenotypes. Nature 442:585–9 [DOI] [PubMed] [Google Scholar]
- 138.Taniguchi-Watanabe S, Arai T, Kametani F, Nonaka T, Masuda-Suzukake M, et al. 2015. Biochemical classification of tauopathies by immunoblot, protein sequence and mass spectrometric analyses of sarkosyl-insoluble and trypsin-resistant tau. Acta Neuropathol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tanzi RE. 2012. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, et al. 1987. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235:880–4 [DOI] [PubMed] [Google Scholar]
- 141.Tycko R 2014. Physical and structural basis for polymorphism in amyloid fibrils. Protein Sci 23:1528–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tycko R 2015. Amyloid polymorphism: structural basis and neurobiological relevance. Neuron 86:632–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Broeckhoven C, Haan J, Bakker E, Hardy JA, Van Hul W, et al. 1990. Amyloid beta protein precursor gene and hereditary cerebral hemorrhage with amyloidosis (Dutch). Science 248:1120–2 [DOI] [PubMed] [Google Scholar]
- 144.Vasconcelos B, Stancu IC, Buist A, Bird M, Wang P, et al. 2016. Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol 131:549–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Walker L, Levine H, Jucker M. 2006. Koch’s postulates and infectious proteins. Acta Neuropathol 112:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walker LC, Jucker M. 2015. Neurodegenerative diseases: expanding the prion concept. Annu Rev Neurosci 38:87–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Walker LC, Pahnke J, Madauss M, Vogelgesang S, Pahnke A, et al. 2000. Apolipoprotein E4 promotes the early deposition of Abeta42 and then Abeta40 in the elderly. Acta Neuropathol 100:36–42 [DOI] [PubMed] [Google Scholar]
- 148.Warzok RW, Kessler C, Apel G, Schwarz A, Egensperger R, et al. 1998. Apolipoprotein E4 promotes incipient Alzheimer pathology in the elderly. Alzheimer Dis Assoc Disord 12:33–9 [DOI] [PubMed] [Google Scholar]
- 149.Watts JC, Condello C, Stohr J, Oehler A, Lee J, et al. 2014. Serial propagation of distinct strains of Abeta prions from Alzheimer’s disease patients. Proc Natl Acad Sci U S A 111:10323–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weissmann C 2004. The state of the prion. Nat Rev Microbiol 2:861–71 [DOI] [PubMed] [Google Scholar]
- 151.Weissmann C 2012. Mutation and selection of prions. PLoS Pathogens 8:e1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Westermark GT, Fandrich M, Westermark P. 2015. AA amyloidosis: pathogenesis and targeted therapy. Annu Rev Pathol 10:321–44 [DOI] [PubMed] [Google Scholar]
- 153.Whitfield JT, Pako WH, Collinge J, Alpers MP. 2008. Mortuary rites of the South Fore and kuru. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 363:3721–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, et al. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921–5 [DOI] [PubMed] [Google Scholar]
- 155.Wingo TS, Lah JJ, Levey AI, Cutler DJ. 2012. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch Neurol 69:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ye L, Fritschi SK, Schelle J, Obermuller U, Degenhardt K, et al. 2015. Persistence of Abeta seeds in APP null mouse brain. Nat Neurosci 18:1559–61 [DOI] [PubMed] [Google Scholar]
- 157.Ye L, Hamaguchi T, Fritschi SK, Eisele YS, Obermuller U, et al. 2015. Progression of seed-induced Abeta deposition within the limbic connectome. Brain Pathol 25:743–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yu JT, Tan L, Hardy J. 2014. Apolipoprotein E in Alzheimer’s disease: An update. Annu Rev Neurosci 37:79–100 [DOI] [PubMed] [Google Scholar]