Abstract
The activation of nuclear factor-kappaB (NFκB), a proinflammatory transcription factor, is a commonly observed phenomenon in breast cancer. It facilitates the development of a hormone-independent, invasive, high-grade, and late-stage tumor phenotype. Moreover, the commonly used cancer chemotherapy and radiotherapy approaches activate NFκB, leading to the development of invasive breast cancers that show resistance to chemotherapy, radiotherapy, and endocrine therapy. Inhibition of NFκB results in an increase in the sensitivity of cancer cells to the apoptotic effects of chemotherapeutic agents and radiation and restoring hormone sensitivity, which is correlated with increased disease-free survival in patients with breast cancer. In this review article, we focus on the role of the NFκB signaling pathways in the development and progression of breast cancer and the validity of NFκB as a potential target for breast cancer prevention and therapy. We also discuss the recent findings that NFκB may have tumor suppressing activity in certain cancer types. Finally, this review also covers the state-of-the-art development of NFκB inhibitors for cancer therapy and prevention, the challenges in targeting validation, and pharmacology and toxicology evaluations of these agents from the bench to the bedside.
Keywords: Breast cancer, inflammation, NFκB, transcription factor
1. INTRODUCTION
Inflammation is intimately associated with cancer, and chronic inflammation increases the risk for several cancer types [1–3]. It has been long recognized that a strong correlation exists between the presence of inflammation and the occurrence of pre-malignant lesions at various sites [4]. For example, recent cellular and epidemiological evidences indicate that there is a higher risk of colorectal cancer due to Crohn’s disease and ulcerative colitis [5–8], while gastric Helicobacter pylori infection is the leading cause of gastric cancers [9–11]. The presence of inflammation, even in the absence of infection, may also contribute to carcinogenesis [1–3, 12–14], as seen in esophageal cancer [15], pancreatic cancer [16] and prostate cancer [17], because the development of these cancers is enhanced by inflammatory conditions, such as esophagitis, chronic pancreatitis, and chronic prostatitis, respectively.
Chronic inflammation is characterized by the generation of reactive oxygen and nitrogen species, the infiltration of inflammatory cells such as leukocytes, lymphocytes, and macrophages, tissue destruction, fibrosis, and enhanced vasculogenesis. The high levels of reactive oxygen species (ROS)/reactive nitrogen species (RNS) cause mutagenic insults, initiating tumorigenesis, and leading to cellular hyper-proliferation, the inhibition of apoptosis, and the promotion of angiogenesis and cell invasion [4,18–20]. Thus, the development of cancer in association with inflammation is essentially a process driven by inflammatory cells and pro-inflammatory mediators, which together establish a microenvironment conducive to carcinogenesis. This process is associated with the activation of multiple signaling pathways, including the nuclear factor-κB (NFκB) pathways, which have functions in both the inflammatory responses and cancer development [21–29].
NFκB is a transcription factor that was discovered in 1986 as a nuclear factor binding to the enhancer element of the immunoglobulin kappa light-chain of activated B cells (thus, the abbreviation NFκB) [30, 31]. The NFκB family of transcription factors includes five members: RelA (p65), c-Rel, RelB, NFκB1 (p50) and NFκB2 (p52), which are expressed in nearly all cell types and regulate genes with different functions [32]. The N-termini of these transcription factors contain a Rel homology domain (RHD) responsible for sequence-specific DNA binding and translocation, while the C-termini contain domains responsible for either transcriptional activation (RelA, c-Rel and RelB) or inhibition (p105 and p100) [32, 33]. Proteolytic cleavages of the p105 and p100 proteins into p50 and p52, respectively, occur at C-terminal to the glycine-rich regions (GRRs) present in the N-terminal region of both p105 and p100 [34]. The Rel family members form different hetero/homodimeric combinations, with the most common being the NFκB complex made up of a p65/p50 heterodimer [32]. In most cell types, NFκB is present in an inactive form, where it is complexed with the inhibitory κB protein (IκB) in the cytoplasm [35].
Although it is essential for innate and humoral immunity, the activation of NFκB in organs other than the immune system can lead to various disorders. This is because NFκB regulates more than 500 genes involved in inflammation, cellular transformation, survival, proliferation, angiogenesis, invasion, and metastasis [36, 37]. Constitutive activation of NFκB has been observed in breast cancer [30, 38–42] and several other cancer types, and is associated with oncogenesis, cell survival, proliferation, angiogenesis, metastasis, and chemo- and radio-resistance [43–64]. The existence of crosstalk between NFκB and various other transcription factors and regulatory molecules is well established, with most tumor cells being highly “addicted” to the activated form of NFκB [26].
Although NFκB is required for normal mammary gland morphogenesis [63, 64], abnormal constitutive expression of NFκB subunits (such as c-Rel, p65, and p50) has been widely reported in breast cancers [65–67]. NFκB activation has been demonstrated to drive breast cancer development and progression [39, 68, 69], and its activation is specifically associated with a particularly aggressive estrogen receptor (ER)-negative and human epidermal growth factor receptor 2 (HER2)-positive breast cancer subtype known as inflammatory breast cancer (IBC) [70, 71]. The upregulation of NFκB signaling alone and/or in conjunction with other signaling pathways, promotes angiogenic neovascularization, the epithelial-mesenchymal transition (EMT), increases cancer cell “stemness”, and leads to chemoresistance, radioresistance, and endocrine resistance. All of these are associated with invasive phenotypes that lead to early relapse, advanced forms of the disease, and reduced overall survival [72–76]. How NFκB affects all of these processes and whether it may represent a valid target for breast cancer therapy form the crux of this review.
2. THE BIOLOGY AND REGULATION OF THE NFκB SIGNALING PATHWAYS
2.1. The Canonical Pathway
In normal cells, NFκB is cytoplasmically sequestered in a latent, inactive form that is bound to the inhibitor of κB (IκB) proteins, which include IκBα, IκBβ, IκBε and IκBζ [40]. Cellular stimulation by tumor necrosis factor alpha (TNFα) or its activation by various inducers, such as cytokines, mitogens, growth factors, bacterial and viral genes, ultraviolet radiation, etc., leads to the activation of the inhibitory κB kinases (IκKs). These activated kinases then phosphorylate the IκBs, targeting them for proteasomal degradation [77]. This releases the sequestered NFκB dimers, which then translocate into the nucleus and bind to specific DNA sequences in the promoter or enhancer regions of target genes to transactivate them, including those encoding IκB and the A20 protein [78, 79]. The newly synthesized IκB translocates to the nucleus, attaches to the NFκB dimers and eliminates them from the nucleus, while A20 protein stays in the cytoplasm and suppresses the activity of TNFα receptors. Thus, the NFκB system consists of at least two negative feedback loops: one is involved in IκB-mediated cytoplasmic localization and another is associated with A20 protein [78, 79].
2.2. The Non-Canonical Pathway
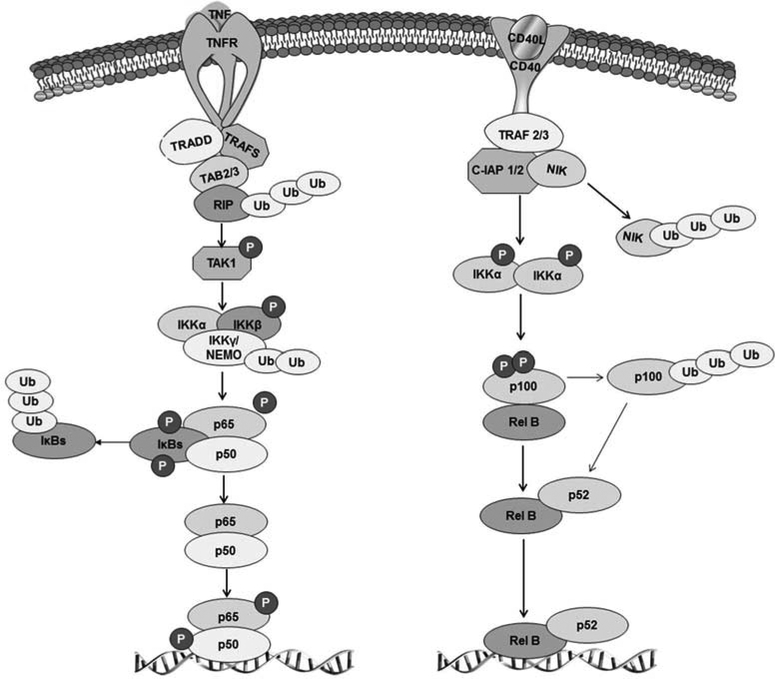
A parallel non-canonical pathway exists for the activation of specific Rel proteins in response to various stimuli, such as viruses, cellular stress, growth factors, lipopolysaccharides (LPS), etc. In contrast to the canonical pathway, in this case, the RelB/NFκB2 dimer is formed via the inducible proteolytic processing of the NFκB2 gene product. The TNF-receptor superfamily members, such as CD40 and B-cell activating factor, selectively activate NFκB-inducing kinase (NIK) and IκB kinase 1 (IKK1), leading to the phosphorylation and ubiquitination of p100, resulting in its partial proteolytic processing to yield p52 [80, 81]. Both the canonical and non-canonical pathways contribute to cancer development and progression [53, 82]. Fig. (1) summarizes the different NFκB pathways.
Fig. (1). The main pathways of NFκB activation.
On the left is the TNFα-dependent canonical signaling pathway. The binding of TNFα to the TNF receptor, TNFR1, triggers the sequential recruitment of the adaptors, TRADD (TNFR1-associated death domain protein), RIP (Receptor-interacting protein) and TRAF2 (TNF receptor-associated factor 2), to the membrane. Then, TRAF2 mediates the recruitment of the IκB kinase (IKK) complex, composed of IKKα, IKKβ and NEMO (NF-kappa-B essential modulator), to the TNFR1 signaling complex, which causes IKKβ activation. The activation of IKKβ leads to IκBα phosphorylation on specific residues, which induces polyubiquitination through the binding of ubiquitin proteins, finally leading to its degradation through the proteasome pathway. The p50-p65 heterodimer then binds to specific κB sites and activates a variety of NFκB target genes coding for pro-inflammatory cytokines (such as IL-6) and chemokines. On the right is the alternative, non-canonical, pathway of NFκB activation. This pathway relies on the recruitment of the TRAF2-TRAF3 heterodimer to the CD40 receptor. TRAF3 links the E3 ligases c-IAP1/2 (cellular inhibitor of apoptosis 1/2) to the kinase, NIK (NFκB-inducing kinase). NIK is activated by phosphorylation, and is also subjected to a c-IAP1/2-dependent degradative polyubiquitination. IKKα homodimers are activated by NIK and phosphorylate the inhibitory molecule, p100, the partial processing (via proteasomal degradation) of which generates the NFκB protein, p52. p52 moves into the nucleus as a heterodimer with RelB to regulate the expression of genes involved in lymphoid organogenesis or coding for chemokines.
2.3. Regulation of the NFκB Signaling
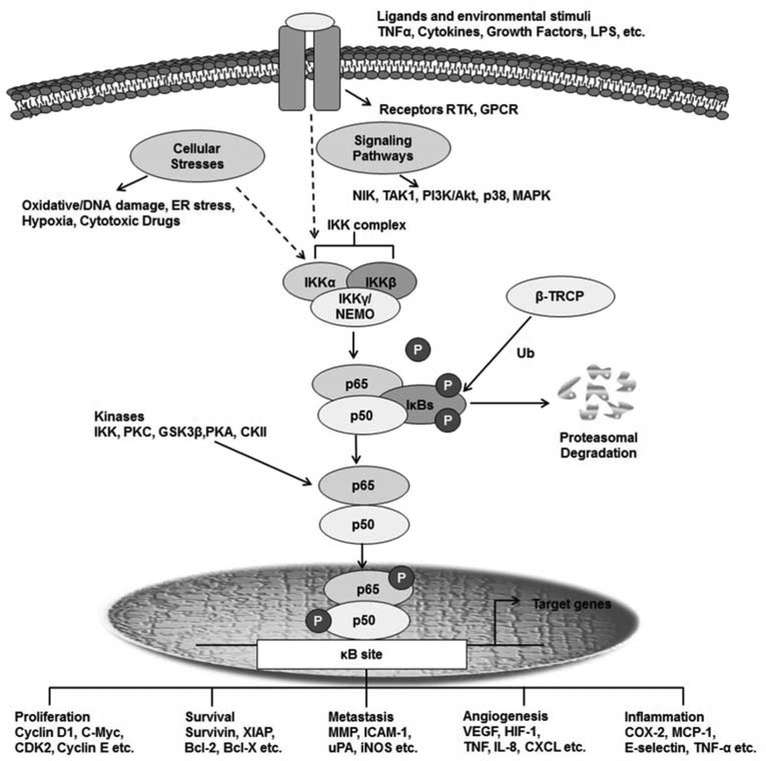
Besides the canonical and non-canonical pathways, additional atypical pathways of NFκB activation exist. For example, subsequent to genotoxic stress, the IKK complex can be activated via the ataxia-telangiectasia mutated (ATM) kinase, leading to the ubiquitination of NEMO (IKK-γ) [83]. Other pathways that can activate NFκB include the epidermal growth factor receptor (EGFR)-mediated NFκB-dependent transcription [58]; the ultraviolet (UV) radiation-mediated IKK-independent NFκB activation pathway that occurs via casein kinase 2 (CK2) phosphorylation [84]; and hydrogen peroxide-mediated NFκB activation through the induction of IκB phosphorylation at Tyr42 by c-Src [85]. The common feature of all of these pathways is the liberation of various NFκB dimers following the activation of IKKs, their nuclear translocation, and the subsequent binding of the RHD to cognate DNA sequences in the enhancer elements of NFκB target genes, followed by their activation [86]. This activation is further controlled by interactions with other co-activators, co-repressors, and transcription factors, in addition to crosstalk with other signaling pathways [24]. Various post-translational modifications (specifically phosphorylation and acetylation), especially of the RelA subunit, control the transcriptional activity of NFκB and add multiple layers of complexity to NFκB signaling [87]. Both the phosphorylation and acetylation of RelA (especially phosphorylation on the S276 or S536 residues) contributes to the inflammatory response and tumorigenesis [88–90]. Table 1 presents a list of representative proteins that physically interact with NFκB family members and augment or attenuate their activity. Fig. (2) shows the physiological and pathological stimuli and kinases involved in NFκB activation, and the downstream targets of NFκB.
Table 1.
NFκB-interactive proteins and the biological effects of the interactions.
| Protein Name | Interacting Partner | Biological Consequence(s) of the Protein’s Interaction with the NFκB Family Subunit | References |
|---|---|---|---|
| Androgen receptor | c-Rel | Decreases androgen sensitivity | [91] |
| Aryl-hydrocarbon receptor | Rel B | Increases transcription of IL-8 | [92] |
| β-arrestin | IκBα, p105 | Stabilizes IκBα; β-arrestin binding to p105 negatively regulates TLR4 signaling | [93,94] |
| β-TRCP | IκBα | Facilitates recognition of IκBα by the ubiquitin-proteasome system, promoting its proteasomal degradation and NFκB activation | [95] |
| Calmodulin | c-Rel | Inhibits c-rel translocation and inhibits the secretion of IL-12 | [96] |
| Cdk2/cyclin E | c-Rel | Promotes G1/S cell cycle arrest | [97] |
| c-Jun/c-FOS | p65, NEMO | Stimulates the κB enhancer element; the interaction with NEMO regulates TNFα signaling | [98,99] |
| CK-II | p65 | CK-II phosphorylates p65 at serine 529, increasing its transcriptional activity | [100] |
| c-SRC | p65 | c-SRC phosphorylates RelA/p65, promoting ICAM-1 expression | [101] |
| DAXX | p65 | Inhibits the acetylation of NFκB and inhibits its transcriptional activity | [102] |
| E2F1 | p65 | Increases the transcriptional activity of NFκB | [103] |
| EZH2 | p65/RelB | Increases the transcriptional activity of NFκB in ER-negative breast cancer cells, while EZH2 represses NFκB transcriptional activity in ER-positive breast cancer cells | [104] |
| Estrogen Receptor | p65 | Estrogen receptors and the p65 subunit of NFκB mutually inhibit each other | [105–107] |
| Gankyrin | p65 | Suppresses the transcriptional activity of NFκB by modulating its acetylation by SIRT1. | [108] |
| HSP27 | IKKα/β | Suppresses NFκB activation | [109] |
| HDAC | p65 | HDAC1 suppresses NFκB activation; interacts with Sp1/NFκB complex to repress transcription of KIT | [110–113] |
| JNK-1 | c-Rel | Stimulates the κB enhancer element | [114] |
| mTOR | IKKα/β | Controls NFκB activation downstream of Akt | [115] |
| NFATc | c-Rel | Interaction with NFκB leads to activation of CD154 gene transcription and survival in B-cell lymphoma | [116] |
| Notch-I | IKKα | Increases NFκB activity | [117, 118] |
| PARP-I | p65 | Leads to the transactivation of NFκB-dependent ERBB2, promoting cell hyper-proliferation | [119] |
| NRF | p65 | Inhibits NFκB’s transcriptional activity | [120] |
| PML | p65 | Inhibits NFκB activity and promotes TNFα-mediated apoptosis | [121] |
| p38-MAPK | p65 | Forms part of a transcription complex that controls increases in NFκB activity. | [122] |
| p53 | IκBα | Interaction with NFκB increases the p53-mediated apoptosis | [123] |
| RP S3 | p65 | Interacts with the non-Rel subunit of the NFκB p65 homodimer and the p65-p50 heterodimer DNA-binding complexes, enhances NFκB transcriptional activity | [124] |
| S6 Kinase | IκBα | Not reported | [126] |
| Sp1 | Igκ binding site | Leads to transactivation of NFκB target genes | [126] |
| Stat-3 | p65 | Activates the catalytic subunit of telomerase (hTERT); Directly interacts with NFκB components, inhibiting transcriptional activation of the iNOS gene; p65 homodimers cooperate with unphosphorylated STAT3 to affect transcription of several target genes in vivo | [127–129] |
Fig. (2). The components, functions, and regulation of NFκB.
This schematic diagram shows the upstream physiological and pathological stimuli and kinases involved in NFκB activation in the cytoplasm, and representative transcriptional activities in the nucleus.
3. THE NFκB SIGNALING PATHWAYS IN BREAST CANCER DEVELOPMENT AND PROGRESSION
Breast cancer is one of the major causes of cancer-related death in women worldwide [130]. The steroidal sex hormone estrogen is crucial for the initiation and progression of breast cancer. The majority of breast cancers express the estrogen receptor, which mediates the actions of estrogen, and is required for estrogen-dependent tumor growth [131–133]. However, in many cases, breast cancer eventually progresses from a hormone-dependent, localized, estrogen-sensitive phenotype, to a highly invasive, hormone-independent and chemoresistant phenotype [132,134–135]. This progression occurs concomitantly with altered ER function or the development of ER-negative cancer cells [132,133–136]. As aforementioned, the IBC phenotype typically exhibits high constitutive NFκB activity. IBCs are at an advanced stage at the time of diagnosis, and are mostly ER-negative and HER2-positive [70,71, 137]. Another breast cancer subtype with high levels of constitutively active NFκB signaling is triple negative breast cancer (TNBC), so termed because of the lack of the ER, progesterone receptor (PR), and HER2 receptor. TNBC cells are characterized by the basal cell type, and often possess p53 mutations, indicating possible crosstalk between p53 and NFκB [138]. In clinical studies, the constitutive activation of NFκB in breast cancers has been found to be associated with larger breast tumor size, increased metastases to pulmonary and brain sites, and overexpression of the HER2 oncoprotein [139].
3.1. NFκB Activation in Breast Cancer: Role of IKKε
A recent study of different breast cancer cell lines indicated that the aggressive basal subtypes, which lack the ER, typically exhibit high constitutive NFκB activity [140]. Human breast cancers display nuclear accumulation of the classic form (p50/p65), as well as p52 and B-cell lymphoma 3 (Bcl-3), along with c-Rel [141]. These activated NFκB dimers enhance cellular proliferation and cause decreased apoptosis, but what triggers the activation of NFκB in breast cancer is still unknown. Unlike lymphoid malignancies where oncogenic mutations in RelA, c-Rel, or other NFκB proteins have been identified [42], the activation of NFκB in solid tumors, such as breast cancer, is not generally accompanied by any loss-of-function IκB mutations or gain-of-function IKK mutations [39]. In fact, Karin et al. suggested that the NFκB activation in solid tumors may be either caused by the inflammatory tumor microenvironment or activation of mutated upstream components in the IKK–NFκB signaling pathways [39]. In 2007, Boehm et al. revealed that IKKε was amplified in least one-third of breast cancers. Using complementary genomic approaches, they demonstrated the amplification and overexpression of IKKε in both breast cancer cell lines and tumors derived from patients [41]. Further, their study showed that IKKε increased the transcriptional activity of NFκB and upregulated downstream targets, such as matrix metallopeptidase 9 (MMP9) and Bcl-2. The suppression of IKKε expression in these breast cancer cell lines induced cell death. IKKε was also found to promote malignant transformation via Akt (also known as protein kinase B or PKB), thus implicating the NFκB pathway as a downstream mediator of phosphatidylinositol-3 kinase (PI3K) signaling [41]. In another study, TNFα and IL-1β stimulation induced K63-linked polyubiquitination of IKKε at lysines 30 and 401 via an IAP1/cIAP2/TRAF2 E3 ligase complex. This modification is essential for IKKε-mediated NFκB activation and toll-like receptor (TLR) signaling. Disruption of this polyubiquitination impairs the recruitment of canonical NFκB proteins and prevents cellular transformation [142–144].
3.2. NFκB Activation in Breast Cancer: Role of Inflammation
The origins of several human cancers can be traced to a chronic inflammatory process [1]. Inflammation, especially chronic inflammation, produces numerous changes in the cellular environment: changes in metabolism, the generation of inflammatory byproducts, the production of reactive oxygen and nitrogen species, DNA damage, etc. [19]. The inflammatory response involves the release of cytokines and activation of the canonical NFκB signaling pathway. However, the role of NFκB in promoting the malignant transformation of a cell can be complex. While activation of NFκB, as part of the immune surveillance against tumors, can lead to the destruction of transformed cells [145–147], constitutive activation of NFκB in different cancer types also exerts a variety of oncogenic functions [145–147]. This is probably due to the fact that the physiological immune defense against cancer cells is insufficient to eliminate all abnormal cells, resulting in a subset of cells that “escapes” the surveillance and outperforms the immune system. This phenomenon is often seen under chronic inflammatory conditions accompanied only by moderately elevated NFκB activity [147]. In breast cancer, accumulating evidence suggests that tumor-infiltrating leukocytes in the tumor stroma may promote cancer progression and/or increase the metastatic capability of malignant breast epithelial cells [148]. The significance of the inflammatory leukocytes and the immune system in oncogenesis can also be gauged by the increased cancer incidence and rate of metastasis in immunocompromised subjects [149,150]. Immunohistochemical studies indicated that breast carcinogenesis and metastatic progression are accompanied by the infiltration of lymphocytes into neoplastic tissue, increased immunoglobulin-mediated release of vascular endothelial growth factor (VEGF) into the tumor interstitium, and the release of cytokines that promote a Th2 polarized immune response. The mutual activation of NFκB and the cytokines makes NFκB an important player in the inflammation-associated development of cancer [148]. Of note, prolonged exposure to estrogen increases the risk of breast cancer via the generation of copious amounts of ROS, which facilitate continued NFκB activity, typically through IKK [151].
3.3. NFκB Activation in Breast Cancer: Tumorigenesis, Cell Cycle Regulation, and Apoptosis
Studies using breast cancer cell lines, animal models, and patient specimens have identified that: a) RelA/p65 [66] and c-Rel [39], as well as b) NFκB transcriptional activity are enhanced prior to malignant transformation in the breast [123]. As discussed previously in Section 3.1, this malignant transformation is mediated by IKKε via the activation of NFκB. The involvement of NFκB in tumorigenesis has been further validated in different transgenic murine models where a genetic deletion of IKK β significantly reduced tumor growth. Further, growth factors such as interleukin-6 (IL-6) are also dramatically decreased when NFκB signaling is disrupted [149]. NFκB activation in cancer leads to the upregulation of antiapoptotic and cell proliferation-associated genes, kicking in a survival mechanism that helps the cell to withstand the physiological stress triggering the inflammatory response. The activation of NFκB in breast cancer has been reported to upregulate the expression of Cyclin D1, Cyclin-dependent kinase 2 (CDK2), and c-Myc [152–154], which drive cell cycle progression and cause uncontrolled cell proliferation. NFκB also regulates the expression and the function of growth stimulating cytokines, such as IL-1β and TNFα [78], while growth factors such as the EGFR and HER2, which promote solid tumor growth, activate NFκB [155]. Dysregulation of NFκB activity alters the expression of cell death-regulating genes, leading to the upregulation of antiapoptotic and pro-survival genes, such as members of the Bcl-2 family [155], IAP proteins (XIAP, cIAP-1/2) [156], and TNF receptor-associated factor (TRAF)1/2 [157], and inhibiting the apoptotic response to chemotherapeutic agents.
3.4. NFκB Activation in Breast Cancer: EMT, Invasion, and Metastasis
Apart from initiating tumorigenesis in the mammary gland, NFκB also plays a role in the progression of malignancy and the acquisition of aggressive behavior. Cellular migration and invasion, which are essential for tumor progression, are regulated by NFκB-dependent genes, including matrix metalloproteinases (MMPs), annexin 1, the urokinase type of plasminogen activator (uPA), IL-8, VCAM-1 (an adhesion molecule), and chemokine receptors such as chemokine receptor type 4 (CXCR4) [158–163]. The redox protein, thioredoxin (Trx-1), has been reported to promote invasion in the MDA-MB-231 cell line by augmenting MMP-9 transcription by activating NFκB, thus showing the intimate association between the oxidative state and NFκB [164].
Overexpression of the p65 subunit in the immortalized, but non-malignant, MCF-10A cell line facilitates the EMT, causing a decrease in the expression of epithelial markers such as E-cadherin and desmoplakin, accompanied by a concomitant increase in mesenchymal markers, such as vimentin [165]. This process is postulated to occur via the NFκB-dependent expression of zinc finger E-box-binding homeobox 1 (ZEB-1/ZFHX1A) and ZEB-2/ZFHX1B/Smad-interacting protein (SIP1), two transcriptional regulators that downregulate E-cadherin expression and promote the EMT [165]. NFκB has also been reported to induce and stabilize the expression of EMT markers such as Snail and twist-related protein 1 (Twist1), respectively. NFκB induces COP9 signalosome 2 (CSN2), which, in turn, blocks the ubiquitination and degradation of Snail [166–168]. On the other hand, chronic treatment of cells with TNF-α rapidly induces Twist1 mRNA and protein expression in normal breast epithelial cells and breast cancer cells [168]. NFκB also promotes angiogenic neovascularization following radiation treatment [74], while inhibition of NFκB activation attenuates the VEGF and fibroblast growth factor 2 (FGF-2) levels [169]. Inhibiting the DNA-binding activity of NFκB leads to a decrease in the expression of VEGF, IL-8 and MMP-9, thus indicating that NFκB exerts transcriptional control on these factors [170].
In a metastasis model of breast cancer using rat sarcoma (Ras)-transformed mammary epithelial cells, NFκB has been shown to cause the induction and maintenance of the EMT via transforming growth factor beta (TGFβ) [42]. Moreover, in a recent study of patients with infiltrating ductal carcinoma, NFκB was seen to play a role in the initiation and development of the disease, while VEGF-C appeared to promote lymph node metastasis [171]. A recent report indicated a hitherto undescribed non-canonical crosstalk mechanism in the highly tumorigenic MDA-MB-231 xenograft model involving Jagged, Notch, Akt and IKKα [172,173]. MDA-MB-231 cells, which are basal-like, exhibited an NFκB-dependent induction of jagged 1 (Jag1) and a Notch-dependent increase in the cancer stem cell population [172]. Further evidence of the involvement of NFκB is provided by the fact that noscapine, an alkaloid compound, synergistically increased the anticancer activity of doxorubicin in basal-like breast cancer cells via the inactivation of the NFκB and angiogenic pathways and the stimulation of apoptosis [174].
In TNBC cells, an NFκB signaling cascade involving the histone methyltransferase enhancer of zeste homologue 2 (EZH2) was required for the expression of IL-6, IL-8 and Chemokine (C-X-C motif) ligand 1 (CXCL1). These cytokines promote colony formation and cell survival in vitro, and tumor engraftment and growth in vivo [175]. In fact, a Cox multivariable analysis of patient specimens revealed that the expression levels of IL-6 and IL-8 predict the length of patient survival, indicating that NFκB is an important prognostic indicator in breast cancer [175].
The induction of the urokinase-type plasminogen activator (uPA) by PI3K-activated NFκB promoted the metastasis of breast cancer cells; and this invasive behavior could be curtailed by pretreatment with PI3K inhibitors, such as wortmannin and LY294002. This indicates that the NFκB activation in breast cancer occurs downstream of PI3K. Additionally, uPA can serve as a major biomarker of breast cancer metastasis in the clinical setting [176,177].
3.5. NFκB Activation in Breast Cancer: Stem Cells
As they undergo the EMT, cancer cells gain stem cell properties that facilitate their survival in response to the cytotoxic chemotherapeutic drugs. Several studies have demonstrated how NFκB integrates proinflammatory signals from the tumor microenvironment to regulate these properties [26–28]. Inflammatory breast cancer, a particularly aggressive form of breast cancer with increased invasive and metastatic potential, is an example of this process [70, 71]. NFκB is hyperactive in IBC, and IBC tumor cells exhibit more stem cell characteristics compared to tumor cells from non-IBC subsets [178].
HER2, a membrane-bound receptor tyrosine kinase, is overexpressed in one-third of all breast cancers, and is a key modulator of the cancer stem cell population [54,178]. As HER2 activates NFκB through the canonical pathway [179,180], it is reasonable to expect that the NFκB family may be involved in the growth and expansion of breast cancer stem cells [40]. In fact, in a mouse model of HER2 breast tumorigenesis with selective suppression of NFκB in the mammary gland, it was demonstrated that NFκB controlled tumor initiation, cell proliferation, colony formation, inflammation, recruitment of tumor-associated macrophages (TAMs), angiogenesis, and invasion [40]. Interestingly, NFκB suppression drastically reduced the proportion of CD44-positive cells in HER2-dependent tumors, indicating that NFκB is responsible for the maintenance and expansion of the progenitor cell population [40]. Studies also indicated that IKKα led to the self-renewal of tumor-initiating cells in a HER2 breast cancer model via the receptor activator of NFκB ligand (RANKL)/RANK pathway, with cell proliferation occurring through the Cyclin D1 gene [181,182]. NFκB activation is also seen during the differentiation of the mammary colony-forming cells derived from luminal progenitor cells, but not in the cells that were located basally [183].
3.6. NFκB Activation in Breast Cancer: DNA Repair
NFκB activation regulates the DNA repair process protecting cells from apoptosis following DNA damage. The DNA damage generated by cytotoxic agents, such as camptothecin, activates ATM kinase and NFκB essential modifier (NEMO), leading to the induction of the NFκB p50/p65 heterodimer [184]. ROS can also be generated in a parallel signaling pathway in sufficient quantities to activate the NFκB pathway. Physical genotoxic agents, such as UV or hydrogen peroxide, lead to extensive cytoplasmic oxidative damage that activates the NFκB pathway in the absence of DNA damage [84, 85]. Among the various types of DNA damage, repairing double strand breaks can be particularly challenging to cells, contributing to the genomic instability associated with most cancers [185–187]. NFκB is involved in double strand removal and repair via a stimulatory action on homologous repair, involving the targets ATM and the tumor suppressor gene, breast cancer susceptibility gene 2 (BRCA2) [188]. The activation of NFκB by ATM results in an antiapoptotic signal in the cells. NFκB utilizes multiple mechanisms to enhance homologous recombination, including stimulation of the activity of CtIP–BRCA1 complexes to trigger DNA end-processing, and the upregulation of ATM and BRCA2 for strand transfer [188].
3.7. Crosstalks between NFκB and Other Signaling Pathways
There is a large array of interactions between the NFκB signaling cascade and other transcription factors/signaling pathways that modulate the transcriptional activity of NFκB [189].
3.7.1. STAT3 (Signal Transducer and Activator of Transcription 3)
NFκB and STAT3 regulate a number of genes involved in cell cycle progression and survival pathways, in addition to regulating a collective set of genes encoding cytokines and chemokines [24,190–192]. In breast cancer stem cells, STAT3 is shown to physically associate with CD44 and NFκB and activates the catalytic subunit of telomerase (hTERT) [129]. The hTERT expression levels are closely correlated with clinical aggressiveness and poor prognosis of breast cancer [193, 194]. Recently, Yu et al suggest that the non-canonical NFκB pathway regulates the STAT3-dependent upregulation of the intracellular enzyme, indoleamine 2, 3-dioxygenase (IDO), in breast cancer–derived myeloid-derived suppressor cells (MDSCs) [195]. MDSCs are a hetrogenous cell population in which IDO mediates T-cell immunotolerance and immunosuppression, thus promoting lymph node metastasis in patients with breast cancer. Additionally, selective and specific blocking of the non-canonical NFκB pathway in breast cancer MDSCs can improve the clinical efficiency of immunotherapy [195].
3.7.2. GSK3-β (Glycogen Synthase Kinase 3 Beta)
GSK3-β is a serine/threonine kinase that regulates the NFκB complex post-transcriptionally through histone methylation. While GSK-3β has no effect on the nuclear accumulation of NFκB, it modulates the transcriptional activity of the NFκB complex by preventing its binding to certain target promoters [196–199]. It has recently been reported that stabilization of β-catenin by treatment with lithium chloride, a well-known GSK-3β inhibitor, leads to the downregulation of uPA, uPAR and plasminogen activator inhibitor 1 (PAI-1) mRNA expression in the highly metastatic MDA-MB-231 cells, inhibiting their invasive capacity [200].
3.7.3. Tumor Suppressor p53
In contrast to the positive feedback between NFκB and STAT3, a mutual inhibition has been reported for NFκB and the tumor suppressor p53, with both of the transcription factors mutually inhibiting each other’s capacity to transactivate gene expression [201]. Interestingly, the initial reports of the relationship between the proteins indicated otherwise [202], and reactivation of p53 was actually seen to activate NFκB via the MEK1 and Ribosomal S6 kinase (RSK) serine/threonine kinase pathways [202]. In fact, the loss of p53 led to resistance against p53-activated death signals. Mutations in TP53 (encoding p53) cause the protein to lose its ability to regulate NFκB-mediated transcription, abrogating its proapoptotic properties. Interestingly, p53 deficiency and presence of nuclear NFκB/p65 correlate with decreased disease free-survival in patients with breast cancer [203]. Mutant p53 also augments TNFα-induced NFκB activity, preventing cells from TNFα-induced apoptosis; and mutant p53/NFκB cross signaling drives cell cycle progression via the MAPK pathways and is associated with EMT and metastasis [204]. Data from gain- and loss-of-function studies indicate that antiapoptotic NFκB p65 activity is constitutively induced by a p53 hot-spot mutation that is frequently observed in breast cancer [205].
3.7.4. MDM2 (Mouse Double Minute 2 Homolog)
The MDM2 oncoprotein (a negative regulator of p53) is known to act as a co-factor for NFκB binding to its target gene promoter binding sites, while the upstream signaling following NFκB activation is independent of MDM2 [206–208]. In addition to the above interactions between NFκB and p53, NFκB also suppresses p53 signaling by inducing MDM2 through the transcriptional activation of sp1 sites [206], while p53 negatively regulates both NFκB signaling and MDM2 expression [207, 208]. Considering that MDM2 is known to be amplified in breast cancer and contributes to a poor prognosis, it will be interesting to elucidate how these two contribute to each other’s pro-cancer effects.
3.7.5. EGFR
Breast cancer often exhibits overexpression of the EGFR family members (HER1 and HER2/neu), along with concomitant NFκB activation [209]. Studies have shown that NFκB activation in breast cancer occurs downstream of EGFR (erbB1/HER1) signaling, particularly in the ER-negative subtype [193]. Overexpression of HER-2/neu leads to constitutive activation of PI3K/Akt and induction of NFκB (p50/p65). Stimulation of such EGFR-expressing cell lines with EGF promptly activates NFκB signaling, which can be blocked by IKK inhibitors, or the by inhibition of PI3K signaling by LY294 [209]. Inhibiting the activation of NFκB prevents breast tumor growth in mice, while IKK inhibition prevents tumorigenesis. It is interesting to note that p53 mutations in breast cancer cell lines contribute to EGFR/Akt activation and increase the levels of TGFβ and platelet-derived growth factor A (PDGF-a); all of which can facilitate the EMT and angiogenesis [210].
3.8. Crosstalks Between NFκB and miRNAs
MicroRNAs (miRNAs) are small (~22 nucleotide), non-coding, single stranded RNAs that bind to the 3′UTR of protein-coding mRNAs and cause mRNA cleavage or translational repression of their corresponding targets [211]. A single miRNA may have multiple target genes, while sometimes, the miRNAs are themselves transcriptional targets. Several miRNAs are known to be transcriptional targets of NFκB, including miR-143, miR-146, miR-224, miR-9, and miR-21 [212,213]. These miRNAs target either upstream signaling molecules or members of the NFκB family. For example, both miR-146a and miR-146b (miR-146a/b) negatively regulate NFκB activity in the highly metastatic human breast cancer cell line, MDA-MB-231 [214]. Following the exogenous expression of miR-146a/b, the expression levels of positive regulators of NFκB activity, such as IL-1 receptor-associated kinase (IRAK) and TNF receptor-associated factor 6 (TRAF6), are downregulated. Further, miR-146a/b-expression significantly impairs the invasion and migration capacity of the MDA-MB-231 cells [214]. Moreover, NFκB can induce the synthesis of proteins that regulate miRNAs. It is shown that miR-155 is upregulated in breast cancer, and is an NFκB transactivational target, which participates in a negative feedback loop through the downregulation of IKKs and other genes [215]. Another oncogenic microRNA, miR-21, is transactivated by genotoxic NFκB/STAT3 activation, and facilitates cellular evasion of DNA damage-induced apoptosis and increases the metastatic potential of breast cancer cells via the downregulation of phosphatase and tensin homolog (PTEN) and programmed cell death protein 4 (PDCD4) [216]. Conversely, miR-200c, which suppresses the EMT, is lost in invasive triple negative breast cancers. Neurotrophin 3 (NTF3), a ligand of the TrkB receptor tyrosine kinase, is a direct target of miR-200c, and NTF3 mediates anoikis resistance in TNBC cell lines via NFκB [217]. In fact, the inhibition of NFκB activity represses the cellular resistance to anoikis. On the other hand, miR-520/373 exerts a metastasis-suppressive role by strongly downregulating TGFβ signaling in breast cancer cells. There is an inverse correlation between the expression of miR-520c and transforming growth factor, beta receptor II (TGFBR2) in ER-negative breast cancer patients, revealing that the miR-520/373 family suppresses cellular invasion in ER-negative breast cancer by acting as a link between the NFκB and TGFβ pathways [218].
3.9. NFβB Activation in Breast Cancer: Interaction with ER
Gene expression profiling has indicated that breast cancer is a heterogeneous disease comprising at least five subtypes, categorized by the presence/absence of hormone receptors and growth receptors such as the ER and PR, and HER2, respectively [219–223]. ER-positive breast cancers originate in the luminal cell layer of the mammary tissue and are further subdivided into luminal A and luminal B tumors, based on differences in their gene expression [219]. Luminal B tumors have overexpression of genes leading to proliferation, and exhibit resistance to tamoxifen [224]. Although the luminal A-type breast tumors proliferate more slowly than luminal B tumors, a significant fraction (up to 30%) of these tumors exhibit high recurrence rates. These findings of aggressive and resistant ER-positive breast cancers, suggests that other factors contribute to the decreased response of these cells to hormone therapy [225,226]. NFκB is known to be intimately linked to ER signaling in breast cancer cells, although the exact nature of the interaction remains unclear. The ER and NFκB are known to mutually mitigate each other’s activities, and thus, ER inhibition by anti-estrogens might actually drive NFκB-mediated tumor progression by uncoupling NFκB from the ER’s inhibitory control [227]. Increased DNA-binding activity of NFκB coupled with expression of downstream targets, such as IL-6, causes a shift from estrogen dependence to estrogen independence in breast cancer [228]. Thus, treatment with estrogen restores the sensitivity of these malignant cells to apoptosis and reduces the invasive characteristics of breast tumors that are resistant to anti-estrogen treatment, which is accompanied by a reduction in NFκB activity [229]. This suggests that the proapoptotic effects of estrogen in these tumors maybe mediated, at least partly, through the inhibition of NFκB [229].
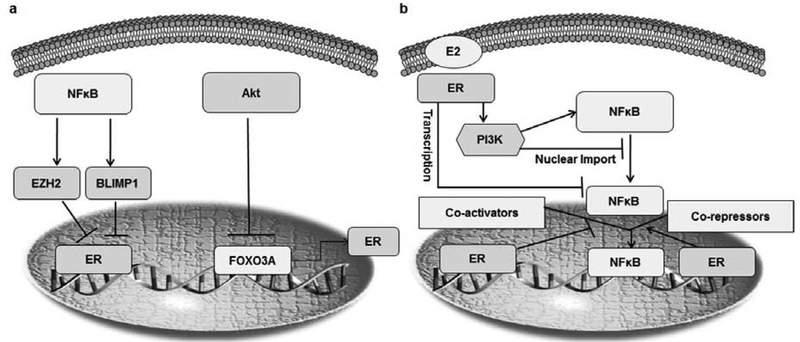
One possible mechanism underlying the activation of NFκB in breast cancer is the loss of ER expression and over-expression of HER2, which occur via the EGFR, Mitogen-activated protein kinase (MAPK) and IKKα pathways [71,179]. A loss of ER function is correlated with constitutive NFκB activity and hyperactive MAPK, leading to hormone-insensitive and advanced forms of breast cancer [71,227]. NFκB can also attenuate ER expression and/or activity, leading to ER-negative cell populations, which are naturally resistant to endocrine therapy [71,155,227]. It has also been demonstrated that the c-Rel mediated upregulation of forkhead box O3 (FOXO3A) leads to decreased synthesis of the ER [230]. The Rel B NFκB subunit can repress ER expression via the zinc finger protein, B lymphocyte induced maturation protein 1 (BLIMP1), which inhibits ER transcription [231]. On the other hand, the EZH2 histone methyl transferase (activated by TNFα in an NFκB-dependent manner) interacts with p65/ RELB and regulates the NFκB-dependent gene expression in breast cancer (Fig. 3a) [232].
Fig. (3). The interaction between ER and NFκB in breast cancer.
(a) Mutual transrepression of the ER and NFκB in mammary epithelial tissue. NFκB can inhibit the estrogen receptor (ER) in different ways. The activation of Akt inhibits the activity of FOXO3A, which plays an important role in the synthesis of the ER. Consequently, blocking FOXO3A activity leads to a reduction in the transcription of the ER. Another mechanism by which NFκB can inhibit the ER is by stimulating the activity of the enhancer of zeste homolog 2 (EZH2), which then inhibits the ER. Finally, NFκB (RelB) can also inhibit ER transcription by upregulating Blimp1. (b) The ER represses NFκB by blocking its nuclear translocation by increasing the transcription of the cytoplasmic NFκB subunit. ER signaling can activate the PI3K signaling pathway, leading to cytoplasmic accumulation of NFκB while inhibiting its nuclear translocation. Another mechanism by which the ER inhibits NFκB activity is by preventing it from binding to DNA.
Of note, the estrogen receptor can prevent NFκB from binding to DNA in human breast cancer cells via its interaction with the Rel homology domain of NFκB [233]. In addition, the main ER-activating ligand, 17β-estradiol, has been shown to inhibit NFκB activation by blocking the nuclear translocation of NFκB’s p105 subunit in the MCF-7 breast cancer cell line [233]. This inhibitory action of ER is limited to NFκB family members possessing a transactivation domain (RelA, RelB and c-Rel) and is cell-type dependent [233–235]. Another mechanism via which the ER modulates NFκB activation is through its interaction with transcriptional activators or repressors [227]. In MCF-7 cells, the ER either competes with NFκB for binding to transcriptional co-activators such as cAMP-response element-binding protein (CREBP), or recruits co-repressors, such as glucocorticoid receptor interacting protein 1 (GRIP1) to NFκB complexes [236,237]. The ER also has been found to control NFκB at the transcriptional level by inhibiting the de novo synthesis of RelB in the MCF-7 cell line, preventing the epithelial to mesenchymal transition and the development of an invasive cancer type (Fig. 3b) [229].
Contrarily, evidence also suggests that there is synergy between the activity of the ER and NFκB, leading to increased transcription of pro-survival and pro-invasion genes [71,155,227,229]. A gene expression profiling study reported by Frasor et al. on the ER-positive MCF-7 cell line indicated that the level of crosstalk between NFκB and ER is more prominent than their mutual transrepression [238]. This positive crosstalk is restricted to only certain ER and NFκB target genes, suggesting that there is a dependency on supplementary regulatory mechanisms. It is believed that both transcription factors stabilize each other’s interactions with their respective response elements, and enhance the activity of downstream targets. The synergistic stimulation of both the NFκB and ER pathways also affects NFκB dimerization and selectively enhances the formation of transcriptionally active dimers, such as RelA/NFκB 1 [238]. Finally, the interaction between the ER and NFκB is also facilitated by the IKK family, which is known to modulate the expression of several ER responsive genes via direct physical interactions and/or post-translational modifications, such as phosphorylation [87]. In the clinical setting, the activation of NFκB correlates with ER-positive primary breast cancers that relapse early despite adjuvant therapy with tamoxifen [75,239].
4. INVESTIGATIONS OF THE ROLES OF NFκB IN BREAST CANCER USING IN VIVO ANIMAL MODELS
A range of murine genetic models (transgenic/knockout) have been developed to elucidate the biological roles of the core components of the NFκB signaling cascade. The most common strategies have included: a) transgenic expression of dominant-negative or constitutively active forms of IKK and IκB proteins (tissue-specific or ubiquitous); b) systemic knockout of single or multiple components, focusing on IκB and IKK proteins; c) conditional knockout animals generated via Cre/loxP recombination; d) gene knock-ins to examine specific aspects of NFκB pathway function and e) κB-site reporter mice to study NFκB’s transcriptional activity [240, 241]. The biggest challenge faced is the embryonic lethality that results from a lack of IKKβ or RelA (p65) function. This issue has been resolved to some extent by the use of conditional knockout models [240, 241]. In addition, due to the extensive protein interactions and crosstalk between NFκB, IKKs, etc., disruption of other signaling pathways resulting from the loss of NFκB activity can complicate the interpretation of data obtained from these models. In the following paragraph, we give a brief overview of some models that explain the role of NFκB in cancers, specifically breast cancer. A discussion on the various NFκB transgenic models is outside the scope of the present report, but interested readers are directed to a comprehensive review by Gerondakis et al [241].
The loss of p52/p100 (the major dimer partner of RelB) is not lethal, but leads to several immune defects, including impaired B-lymphocyte maturation, disruption of the splenic architecture, aberrant T-cell function and a failure to develop normal secondary lymphoid structures [242]. On the other hand, targeted deletion of the p100 C-terminal ankyrin repeats (containing the transrepressor domain) leads to several hyperproliferative defects, such as gastric hyperplasia, cardiac and splenic hyperkeratosis [242]. These findings underscore the importance of tight control of the nuclear p52 dimer levels to maintain normal cellular proliferation. The c-Rel subunit has been implicated as an oncogene, and promotes the development of several cancer types [243]. A c-Rel transgenic mouse has been developed, in which the hormone-responsive mouse mammary tumor virus (MMTV) promoter transcriptionally controls c-Rel expression [39]. These mice develop breast tumors, exhibiting increased expression of several NFκB target genes, including Cyclin D1, c-Myc and B-cell lymphoma-extra large (Bcl-xL). These findings are consistent with the effects of c-Rel overexpression in human breast cancers.
Germline NFκB (p65) deletion results in embryonic lethality [244]. In an interesting study, Liu et al. developed a model in which they selectively inactivated p65 in breast tissue by modulating the inflammatory environment therein. The model demonstrates that the canonical NFκB pathway drives breast cancer development, with the initial “insult” provided by the ERBB2 (encoding HER2) oncogene. This activates NFκB, which then stimulates pro-inflammatory, pro-survival, and pro-growth pathways via tumor-associated macrophages (TAM). Inactivation of this inflammatory NFκB pathway in the breast epithelium inhibits the initiation and progression of breast cancer in murine models, thus demonstrating that NFκB inhibition may have clinical implications in the treatment and management of breast cancer [54]. On the other hand, an IKKα knock-in murine model (with mutant IKKα containing alanines instead of serines in the activation loop) do not present any issues with viability; instead, the female mice exhibit impaired proliferation of mammary epithelial cells, leading to a severe lactation defects. Thus, the IKKα and the Rel-B/p52 complex both contribute to mammary gland development, indicating the specific roles of different NFκB subunits in mammary gland organogenesis and oncogenesis [245].
5. NFκB ACTIVATION AND THE RESISTANCE TO BREAST CANCER THERAPY
NFκB activation in breast cancer cells leads to increased transcription of pro-proliferation and pro-survival factors, such as Cyclin D1, Inhibitors of apoptosis (IAPs) and Bcl-xL [139–141]. This augmented antiapoptotic signaling in the malignant breast cells contributes to endocrine, chemotherapeutic and radiation resistance [246].
As described earlier, NFκB activation also plays an important role in ER-positive endocrine-resistant breast cancer and the acquisition of anti-estrogen (specifically tamoxifen) resistance, which correlates with earlier relapse, metastasis and a reduced overall survival [230]. Indeed, in IBC patients exhibiting an ER-positive phenotype, almost complete resistance to endocrine therapy is observed [227]. The fact that ER transrepresses NFκB may explain the mechanisms underlying the resistance to aromatase inhibitors, selective estrogen receptor downregulators (SERDs), and estrogen withdrawal in these tumors [247]. Decreased ER activation resulting from estrogen withdrawal or aromatase inhibition releases NFκB from the ER-mediated inhibition, leading to NFκB-driven tumor progression. Endocrine resistance in tumor cells leads to an aggressive phenotype, characterized by the expression of genes associated with the EMT and stemness [227]. Endocrine resistance, in conjunction with NFκB activation, leads to an additive effect on the expression of several pro-survival genes (i.e. genes encoding the IAPs and Bcl-xL) and multidrug transporter proteins, such as breast cancer resistance protein (BCRP, also known as ABCG2). The presence of a polymorphism in the ABCG2 gene is a prognostic factor for breast cancer patients treated with tamoxifen [248]. Apart from the ER, NFκB also activates the expression of resistance-mediating proteins such as BRCP and clusterin [249–251]. The NFκB p50 subunit causes BRCP activation at the transcriptional level, although wild-type p53 antagonizes this effect. Similarly, the anti-apoptotic protein S-clusterin, which confers resistance to TNFα-mediated apoptosis, is induced by NFκB [250].
NFκB activation by chemotherapeutic agents is associated with chemoresistance. Cytotoxic agents, such as doxorubicin, are shown to activate the IKK complex, leading to NFκB nuclear translocation and subsequent activation of downstream targets [85]. However, an IKK-independent PI3K-dependent pathway that causes late activation of NFκB by doxorubicin is reported [246]. Lopez et al. demonstrate that doxorubicin therapy causes atypical NFκB activation through c-Abl kinase activity in breast cancer cells, mediating resistance [252]. On the other hand, Ho et al., show that NFκB induced by doxorubicin is deficient in RelA phosphorylation and acetylation; and actually suppresses NFκB mediated downstream gene expression in breast cancer cells [253]. A recent study identifies another mechanism of drug resistance, wherein trastuzumab resistance in PTEN knockdown breast cancer cells is mediated by activation of an NFκB -IL-6 inflammatory feedback loop with expansion of cancer stem cell (CSC) population [254]. Lapatinib, an HER2 inhibitor, upregulates NFκB transcriptional activity by increasing the calcium-dependent phosphorylation of p65/RelA at Ser529 [255]. IKKα then phosphorylates the ER and promotes the expression of its responsive genes, thus promoting the proliferation of breast cancer cells [256]. In a model of in situ breast cancer, Akt-driven lesions that survived radiation treatment are seen to acquire an invasive phenotype mediated by Beta1-integrin via an NFκB-dependent signaling pathway [257].
Similarly, microtubule disrupting agents, such as paclitaxel, vinca alkaloids, and platinum compounds, also activate NFκB [253]. Paclitaxel down-regulates IκBα, promoting the nuclear translocation of NFκB [242]. On the other hand, cisplatin activates NFκB by activating the mitogen-activated protein kinase kinase (MEK)/extracellular-signal-regulated kinase (ERK) signaling cascade [246]. NFκB activation also plays an important role in the resistance to 5-Fluorouracil (FU) and gemcitabine [246].
Ionizing radiation (IR) has also been shown to activate NFκB in both in vitro and in vivo models [258–263]. IR facilitates the DNA binding of NFκB, thus increasing its mRNA levels [226]. Radiation therefore leads to NFκB activation resulting from the degradation of IκBα via post-translational modifications, such as phosphorylation or nitration, which allow for degradation of the IκBα-NFκB complex [260,261]. IR also leads to transactivation of pro-invasion genes such as β1-integrin by NFκB. Since β1-integrin itself is known to activate NFκB, this may indicate a novel forward feedback pathway for cancer progression and resistance in breast cancer [259]. Thus, in addition to the basal level of NFκB activation in breast cancers, HER2 activity and radiation treatment can also induce NFκB activity [262, 263].
6. TARGETING NFκB IN BREAST CANCER PREVENTION AND THERAPY
6.1. Prevention
Although a steady decrease in breast cancer mortality has been observed over the past two decades, it is estimated that approximately 40,000 women will die of breast cancer this year in the U.S. [130]. Preventing breast cancer prior to its development remains the most effective way to reduce mortality resulting from this disease. Increasing evidence demonstrating the key role(s) of NFκB in breast cancer development suggests that NFκB may represent a target for breast cancer chemoprevention [38–42]. Interestingly, several agents with breast cancer preventive potential, including dietary compounds, possess NFκB inhibitory activity [264]. Curcumin, one of the most extensively studied chemopreventive phytochemicals, blocks angiogenesis via inhibition of the NFκB downstream target, cyclooxygenase-2 (COX-2). Preclinical studies show that curcumin blocks NFκB activation by inhibiting the upstream activator complex consisting of NFκB-inducing kinase and the IκBα kinase enzymes in breast cancer cells [265]. Since COX-2-derived prostaglandins also stimulate aromatase activity in an organ-specific manner (generating estradiol), curcumin supplementation, along with traditional anti-estrogen therapies, may lead to a better therapeutic response. A synthetic COX-2 inhibitor, celecoxib, is the focus of several studies investigating its effectiveness for the prevention of ER-negative breast cancer. Celecoxib significantly delays the onset of tumor formation in MMTV-erbB2 transgenic mice, which develop primarily ER-negative tumors [266]. This observation is particularly relevant for the prevention of both ER-negative and TNBCs.
Ginseng, a staple of traditional Chinese medicine, has been reported to have excellent chemopreventive and chemotherapeutic effects. The active principles of the ginseng plant are considered to be the steroidal saponin glycosides known as ginsenosides, and more than 40 ginsenosides have been characterized so far [267]. One of the major biological activities of the ginseng saponins is their inhibition of inflammation. In a recent study, Li and colleagues demonstrate that the ginsenoside Rg1 inhibits Phorbol myristate acetate (PMA) induced invasion and migration. They further show that this invasive process is regulated in breast cancer cells through the NFκB-mediated transcriptional control of MMP-9 expression [268]. The ginsenoside Rg3, one of the main chemical constituents of heat-processed ginseng, is shown to exert its anti-proliferative and pro-apoptotic effects in breast cancer cells by transcriptional inactivation of NFκB along with destabilization of the oncogenic mutant p53 and inactivation of upstream molecules such as ERK and Akt [269]. Similarly, American ginseng is shown to inhibit the activation of COX-2 and NFκB in the MDA-MB-231 and MCF-7 cell lines [270]. However, clinical studies on the utility of ginseng have yielded confounding results. One case-control study finds no significant association between breast cancer risk and ginseng [271]. Another large cohort study (Shanghai Breast Cancer Study) conclusively proves that use of ginseng can improve both overall and disease-free survival and enhance the quality of life of breast cancer pateints [272]. Rationally developed combination treatments involving natural products, along with conventional chemotherapeutic agents, may be a better choice for breast cancer chemoprevention [265]. This strategy may improve the efficacy of cancer prevention while eliminating possible side effects. The key question that remains unanswered is whether NFκB inhibition can decrease the human breast cancer incidence and reduce the tumor burden.
6.2. Therapy
All of the information described above suggests that the inhibition of NFκB activity in advanced and resistant forms of breast cancer is associated with decreased proliferation, increased apoptosis, and (re)sensitization following radiation and chemotherapeutic treatment. These observations indicate that NFκB is a valuable pharmacological target for breast cancer therapy. Different points in the NFκB pathway have been targeted to inhibit or regulate NFκB activation in breast cancer. In the past few years, much effort has been devoted to the development and characterization of NFκB blocking agents, including natural, as well as synthetic compounds. The key events targeted in the NFκB signaling pathway include: IKK activation, IκB degradation and NFκB nuclear translocation/DNA binding (Table 2). A significant amount of progress has been made in the preclinical and clinical studies, and some anticancer compounds with NFκB-inhibiting properties, such as bortezomib, are already being used clinically. The main strategies presently used to target the NFκB signaling pathways are described in Table 2. In the following section, we discuss in further detail some of the most promising approaches.
Table 2.
Strategies used to inhibit constitutive NFκB activation in cancers.
| Strategy | Mechanisms of Action | Prototype Agent | Status as Anticancer Agent | Use in Breast Cancer | References |
|---|---|---|---|---|---|
| Genetic/RNA interference/Peptides | |||||
| Gene Therapy | Gene transfer of IκBα that can inhibit NFκB activation | Nonphosphorylatable form of IκBα that cannot be degraded, and therefore prevents the activation of NFκB | Preclinical testing; combination with anticancer agents caused chemosensitization. | Not yet reported | [273] |
| siRNA | Antisense oligonucleo-tides against NFκB genes, interfering with their expression | Rel-A antisense oligonucleotides | Preclinical testing; pronounced inhibition of tumorigenesis in murine models | Blocks cell adhesin | [274] |
| Cell-Permeable Peptide Inhibitors | Small peptides that inhibit the nuclear trans-location of NFκB | SN-50/52 peptides (contain the p50/p52 NLS, thereby inhibiting the nuclear import of NFκB), | Preclinical testing in ovarian and prostate cancer models | Used as a pharmacological agent in breast cancer | [275–277] |
| NEMO-binding domain (NBD) peptide which inhibits the IKK complex that activates NFκB | Preclinical testing | Blocks proliferation and induces apoptosis | [278] | ||
| Inhibitors of NFκB DNA binding | |||||
| Dual Inhibitor | Inhibits both canonical and non-canonical NFκB pathways, preventing RelA, RelB, and c-Rel DNA binding | PBS-1086 | Preclinical testing; potent cytotoxicity demonstrated in multiple myeloma cell lines. | Promotes apoptosis and potentiates radioactivity | [279, 280] |
| Sesquiterpene Lactone | Inhibits RelA (p65) DNA binding by binding to reactive cysteines in p65 | Parthenolide | Preclinical testing | Preclinically tested; promotes apoptosis and inhibits angiogenesis; inhibits cancer stem cells | [281,282] |
| Quinomycin Antibiotic | The epoxide group in DHMEQ covalently binds to the thiol group of Cys 38 in p65 | DHMEQ | Preclinical testing; potent cytotoxicity demonstrated in several different cell lines. | Preclinically tested; promotes apoptosis | [283,284] |
| Modulators of NFκB post-translational modifications | |||||
| Acetylation Inhibitors | Inhibition of NFκB acetylation, which regulates its activity. | Vorinostat | FDA-approved for cutaneous T-cell lymphoma (2006) | Increases apoptosis but also activates NFκB | [285] |
| Romidepsin | FDA-approved for cutaneous T-cell lymphoma (2009) | Not yet reported | [286,287] | ||
| Sirtuin inhibitors | Preclinical testing. Recent report of the discovery of thieno[3,2-d]pyrimidine- 6-carboxamides as potent inhibitors of SIRT1, 2, and 3a | Not yet reported | [288,289] | ||
| Inhibition of components of the NFκB signaling cascade | |||||
| Proteasome Inhibitors | The activation of NFκB is mediated through the proteasomal degradation of IκBα which is inhibited by bortezomib | Bortezomib | FDA-approved for multiple myeloma (2003) | Both preclinically and clinically tested in primary/metastatic breast cancer, alone and with other chemotherapeutic agents. Phase II studies showed limited clinical activity in metastatic breast cancer () | [290,291] |
| These agents target the 26S proteasome, preventing the ubiquitination and degradation of IκBα thus decreasing the NFκB in the cytoplasm. | Carfilzomib | FDA-approved for multiple myeloma (2012) | Not yet reported | [292,293] | |
| Delanzomib | Phase I/II trials in multiple myeloma () | Not yet reported | [294] | ||
| Marizomib | Phase I trials in multiple myeloma (), phase I trials in advanced solid tumors or refractory lymphoma (, ) | Not yet reported | [295] | ||
| MLN-4924 | Phase I and I/II trials in patients with lymphoma and multiple melanoma (, ) | Preclinical testing in the MDA-MB-231 cell line. Submicromolar IC50 seen | [296] | ||
| IKK Inhibitors | Block the phosphorylation and subsequent degradation of IκBα either directly or by binding components of IKKβ/α. | IMD-0354 | No clinical translation | Combination with doxorubicin targeted nanoparticles; inhibits CSCs | [297] |
| BAY-11-7082 | No clinical translation | Blocks metastasis via ICAM1 downregulation | [298] | ||
| BAY-11-7085 | No clinical translation | Promotes apoptosis in combination with HDAC1 inhibitors | [286,299] | ||
| MLN120B | No clinical translation | Inhibits the transcription of NFKB-dependent genes | [300] | ||
| PS-1145 | No clinical translation | Blocks HER2-mediated cell growth and promotes apoptosis | [301] | ||
| Inhibition of Upstream Signaling Components | Target signaling pathways upstream of NFκB to inhibit NFκB activation | Denosumab (RANK ligand inhibitor) | FDA-approved for the prevention of skeleton-related events in patients with bone metastases from breast cancer and other solid tumors (2010) | Clinically-approved for the treatment of bone metastases | [302] |
| Miscellaneous | |||||
| Natural Compounds | A number of natural compounds have been shown to inhibit NFκB activation. | Curcumin (polyphenol) | Phase II trials in advanced breast cancer () -Used curcumin complexed with soy lecithin | Inhibits lung metastasis | [65,303] |
| Resveratrol (polyphenol) | Completed phase I studies in colon cancer () | Inhibits Bc12 and NFκB, induces apoptosis | [304] | ||
| Ginsenosides | No significant correlation seen | Inhibits Bc12/iNOS/VEGF and NFκb, induces apoptosis | [267] | ||
| Thymoquinone | No clinical translation | Induces apoptosis and inhibits cell proliferation | [305] | ||
| Vitamin E and derivatives | Clinically tested; however a relationship between NFκB binding and anticancer activity not established | Induces apoptosis and inhibits cell proliferation | [306] | ||
| NSAIDS | HS-donating aspirin | Clinically tested and validated | Induces G0/G1 arrest and apoptosis | [307–309] | |
| Glucocorticoids | Dexamethasone | Clinically tested | Chemosensitization of MDA-MB-231 cells to adriamycin | [310] | |
| Statins | Cerivastatin | Clinically tested | Induces apoptosis and inhibits cell proliferation | [311,312] | |
| Monoclonal Antibodies | Anti-EGFR | Cetuximab | Clinically tested in combination with carboplatin in metastatic TNBC; low efficacy | Induces apoptosis and inhibits cell proliferation | [313] |
| Anti PD1 | Pembrolizumab (MK-3475) | Phase I Clinical trials started () | Increase T lymphocyte immune responses and modulates the level of IL-2, TNFα, IFNγ and other cytokines. | [314,315] | |
6.2.1. Direct Targeting of NFκB Subunits
Direct inhibition of NFκB-DNA binding is theoretically a good approach to inhibit the activity of NFκB, as it would prevent the transactivation of the pro-survival and antiapoptotic downstream targets, and also be highly selective. Certain natural products, such as sesquiterpene lactones and quinomycin derivatives, and synthetic compounds such as PBS-1086, target the reactive cysteine residues such as Cys38 in the RHD of RelA directly inhibit the NFκB subunit DNA binding [279–284]. Recently, sesquiterpene lactone dimers from the Inula species are found to be active in breast cancer and other cancers [316, 317].
6.2.2. Regulating the Oxidative State
ROS and RNS, which are both generated and destroyed by NFκB target genes, also activate NFκB through multiple mechanisms. Aberrant activation of ROS-associated transcription factors, such as hypoxia-inducible factor 1 (HIF-1), contributes to oncogenesis by driving cell growth, cell survival and angiogenesis [318]. The generation of intermediate levels of ROS induces NFκB activity, and the activated NFκB regulates ROS and RNS-generating enzymes, such as COX-2 and iNOS, as well as antioxidant enzymes, like MnSOD, in a way that supports continued NFκB activity, preventing apoptosis [319]. Several chemotherapeutic and radiotherapeutic modalities depend upon ROS generation to induce cell death, and therefore, NFκB-mediated regulation of oxidative stress may contribute to chemo/radioresistance. Prolonged ROS formation during chronic inflammation in untransformed cells may also contribute to genetic mutations leading to tumorigenesis [320].
Antioxidant compounds inhibit NFκB signaling via ROS scavenging or prevention [25], or by stimulating antioxidant enzymes. On the other hand, certain compounds, such as theaflavins (present in black tea), impede the migration of cancer cells by increasing the formation of p53-dependent reactive oxygen species that induce p53-phosphorylation and inhibit NFκB nuclear translocation. These anti-migratory effects of theaflavins are abrogated by p53 knockdown, ROS inhibitors and NFκB overexpression [321].
6.2.3. Proteasome Inhibition
The main step in NFκB activation involves the phosphorylation, ubiquitination, and degradation of IκBα by the 26S proteasome, which is followed by the nuclear import of the NFκB subunit [39]. Thus, proteasome inhibitors are attractive therapeutic agents for the inhibition of NFκB activation. The 26S proteasome is a multiunit, adenosine triphosphate (ATP)-dependent complex with multiple catalytic sites, including caspase-like (B1), trypsin-like (B2), and chymotrypsin-like (B5) proteases that form the main sites of attack for proteasomal inhibitors [322,323]. Based on their chemical structure, ability to form a covalent or non-covalent bond with the active site(s), synthetic or natural origin, etc. the proteasome inhibitors are classified into various categories [324].
Proteasome inhibitors show minimal effects on normal cells, while the prototype compound of this class, bortezomib (PS-341), is shown to possess impressive cytotoxicity against a range of human cancer cell lines, including breast cancer cells [290,291,325], and to synergistically enhance the effects of trastuzumab via inhibition of NFκB activation and the nuclear accumulation of the cell cycle inhibitory molecule, p27 [326]. A clinical trial in advanced breast cancer patients is performed in an attempt to replicate these findings [], however, recent evidence suggests that the anticancer effects of bortezomib and other proteasome inhibitors are highly complex, and these compounds have many NFκB-independent effects. Nevertheless, proteasome inhibitors appear to show great promise as part of multidrug therapy, with several agents currently being evaluated in clinical trials.
6.2.4. Anti-Inflammatory Compounds (Steroidal and Nonsteroidal)
Nonsteroidal Anti-inflammatory Drugs (NSAIDS) such as aspirin, ibuprofen, naproxen, indomethacin and sulindac prevent tumor formation and development partly via their inhibition of COX-2, and as a result of decreased inflammatory signaling and decreased NFκB activity [307–309]. NFκB and COX-2 activate each other in a feedforward fashion, with NFκB regulating the COX-2 promoter [25]. COX-2 is frequently overexpressed in primary breast cancer, and contributes to tumorigenesis in transgenic models [327]. A recent phase II randomized clinical trial demonstrates that short-term COX-2 inhibition by celecoxib led to anti-tumor changes in gene expression in breast carcinoma tissue [327]. In the MDA-MB-231 triple negative breast cancer cell line, celecoxib increases cytoxicity to doxorubicin and promoted apoptosis by downregulating the NFκB pathway [328, 329]. On the other hand, salicylates and aspirin directly compete with ATP for IKKβ, inhibiting IKKβ function and preventing NFκB activation [330].
Glucocorticoids (GCs) exert their anti-inflammatory effects by downregulating inflammatory cytokines, and by directly inhibiting the NFκB pathway [331,332]. Dexamethasone (DEX) activates the endogenous glucocorticoid receptor, inhibiting NFκB’s DNA binding and transactivation. It is shown that the zinc-finger component of the activated glucocorticoid receptor (GR) is capable of directly binding to and inhibiting p65 in the nucleus. DEX pretreatment in a murine breast cancer model leads to significantly enhanced cytotoxicity following Adriamycin treatment [310]. This finding is associated with decreased IL-1β and VEGF expression, the cytoplasmic accumulation of Adriamycin, and NFκB inhibition. DEX pretreatment also sensitizes breast cancer xenograft tumors to carboplatin and gemcitabine [310].
6.2.5. Inhibition of IKKs
Considering that IKKα and IKKβ are key modulators of non-canonical and canonical NFκB signaling, respectively, several IKK inhibitors have been developed. Most of them are specific for of IKKβ, although some also have a degree of affinity for IKKα. These compounds either compete for the ATP-binding region, because ATP is required for IKKβ activation, or allosterically decrease the IKK activity [333]. Several synthetic inhibitors are shown to be effective in human breast cancer cell lines, including IMD0354, PS-1145, and MLN120B [297–301]. However, these agents have multiple off-target effects, possibly due to their binding to ATP, and therefore, these compounds need to be carefully evaluated before this class can advance to the clinic.
7. DISCUSSION AND FUTURE DIRECTIONS
Since its discovery almost 30 years ago, NFκB has been revealed to be a key regulator of various inflammatory and carcinogenic pathways. The NFκB pathways drive tumor development, progression, and chemo- and radio-resistance in diverse cancer types, especially hormone-independent forms of breast cancer [155,334]. In addition, NFκB regulates multiple physiological functions, including neurological development, immune responses, and cell cycle control [335]. In breast cancer, NFκB is a key mediator of the resistance to endocrine therapy. Thus, from a theoretical perspective, inhibition of NFκB presents a viable therapeutic strategy for breast cancer. Indeed, inhibition of NFκB by both pharmacological and molecular techniques has already been established by proof-of-concept studies in several cellular and animal models [273–315].
However, it is necessary to exercise caution when considering NFκB inhibition as a broad therapeutic strategy in breast cancer. Although the NFκB signaling cascade is inherently oncogenic, several lines of evidence in various cancer types indicate that NFκB may act as a tumor suppressor in cooperation with different signaling molecules such as p53 and JNK and that inhibition of NFκB can lead to spontaneous tumor formation and increase angiogenic potential of the tumors [336–341]. Recent studies indicate that NFκB sensitizes tumor cells to apoptosis and senescence [202, 341–345]. The canonical NFκB pathway is shown to be a Fas (Fas cell surface death receptor) transcription activator and the inhibition of NFκB can suppress Fas-mediated apoptosis, impairing the host immune cell-mediated tumor suppression [341]. Similarly, Ryan et al. demonstrate that the p65 subunit is required for p53-dependent apoptosis [202]. In another study, the tumor suppressor ADP-ribosylation factor (ARF) is seen to facilitate the interaction of p65 with Histone deacetylase 1 (HDAC1), thereby turning it into a corepressor. NFκB is not proapoptotic under these circumstances; rather it acts as a facilitator of apoptosis by repressing the expression of antiapoptotic genes [340, 341]. Recently, Chien et al. demonstrate that the p65 subunit of NFκB is particularly enriched in senescent chromatin and that the cytotoxic therapy is unable to induce a senescence response in p65-deficient murine lymphomas [343], while a related study shows that NFκB target genes, especially those encoding secreted cytokines, are upregulated during senescence, in cmyc overexpressing murine lymphoma cells [344]. These studies provide compelling evidence that functional NFκB signaling may be necessary for inducing cytotoxic drug-mediated senescence and/or toxicity in certain tumor types, suggesting that inhibition of NFκB signaling may actually decrease chemosensitivity, instead of promoting cell death [341,343,344]. In addition, this observation may provide a rationale strategy for cancer therapy. For example, Chen et al. suggest that lapatinib co-treatment with bortezomib in breast cancer increases the addiction of these cancer cells to NFκB, potentiating the effect of the NFκB inhibitors such as bortezomib [345]. Therefore, further in-depth research is needed to identify the precise mechanisms of NFκB in onco-genesis. Finally, these findings implicate a more complex role of NFκB in oncogenesis, suggesting that NFκB as a tumor suppressor has significant clinical ramifications and the use of NFκB inhibitors requires extensive assessment in the clinic. In breast cancer, though NF-κB has been definitively shown to increase chemo- and radio-resistance, it is still important to determine and understand the specific role of NFκB in various cellular contexts when NFκB inhibitors are used.
Moreover, recent research has yielded new insights into NFκB signaling pathways that reveal the complexity and difficulty of effectively targeting this pathway. NFκB, being a master regulator of different cellular processes, regulates, and is regulated by various other signaling pathways. Apart from the canonical/non-canonical pathways of NFκB activation, constitutive NFκB activation in cancer cells can result from crosstalk with oncogenic pathways, such as those involving the EGFR, RAS, and PI3K/Akt [346, 347]. Its transcriptional activity can be modulated by post-translational modifications of NFκB, variable dimerization of the NFκB subunits, the expression of transcriptional coactivators/corepressors, chromatin remodeling, and other epigenetic factors also regulate [25,87]. Thus, NFκB gene expression may induce different phenotypes, depending upon the selective expression of target genes.
More than five hundred NFκB inhibitors are known, and the number is growing rapidly [25]. Most of these inhibitors work primarily by preventing the ubiquitination and proteasomal degradation of the IκB proteins, confining NFκB to the cytoplasm. However, the complexity of the NFκB signaling pathway, the absence of appropriate bio-markers, poor drug specificity, and inadequate drug delivery complicate the targeting of NFκB. The mechanism(s) underlying the NFκB inhibition by most drugs is poorly understood, and multiple mechanisms involving the IκBα phosphorylation status, NFκB nuclear translocation, and NFκB DNA binding are often proposed. Efforts must be made to develop NFκB inhibitors which are specific for (or at least are able to modulate) one or more of the various pathway components, including upstream activators, IKK, IκB, NFκB subunits, oncogenic mutations linked to NFκB activation, novel NFκB signaling intermediates (e.g. HSP90 (Heat shock protein 90)), transcriptional co-activators, etc. Since ubiquitination and proteasomal degradation play an important role in NFκB signaling, targeting E3 ligases and/or deubiquitinating enzymes may also contribute to NFκB inhibition. However, it should be kept in mind that ubiquitin-regulating enzymes participate in diverse cellular functions, and the implications of their inhibition may be far-reaching, with unexpected effects and potentially detrimental adverse effects.
As suggested above, different targets for NFκB inhibition must be considered. For example, the currently used IKK inhibitors selectively target the β isoform, and only a few IKKα-specific inhibitors have been developed. The IKKβ-mediated activation of RelA/p65 was initially thought to be the main driver of oncogenic phenotypes, but IKKα-mediated activity has now also been implicated in some cancers, notably in HER2-driven mammary tumorigenesis [245]. Thus, targeting IKKα may be helpful, especially for breast cancer treatment. In addition, as mentioned previously, the simultaneous targeting of parallel oncogenic pathways that activate NFκB, such as those involving the EGFR, Ras, and PI3K/Akt, may be of additional use, and several of these oncogenes already have multiple clinically used inhibitors.
Biochemical assays to detect increased expression or activity of the signaling components influencing NFκB activity should be developed. Most NFκB inhibitors have demonstrated limited chemotherapeutic efficacy in vivo, and work best as chemosensitizers for other cytotoxic agents. This is probably due to the multiple concomitant mechanisms contributing to the constitutive activation of NFκB, which renders a single targeted therapy ineffective. A multimodal approach to NFκB inhibition based on targeting specific pathways that most strongly contribute to the NFκB activation in a given tumor type would be beneficial. For example, in breast cancer cells, Akt activity is known to correlate with I B phosphorylation, NFκB-DNA binding and tamoxifen resistance in vivo [239]. The pharmacological and molecular inhibition of NFκB restores estrogen sensitivity in cells expressing high levels of Akt [239]. Thus, in breast cancer, concomitant administration of a proteasome inhibitor, an anti-inflammatory agent, and an Akt inhibitor may effectively prevent NFκB induction. This approach should maximize the NFκB inhibition, particularly after stimulation by chemotherapy and radiotherapy, as well as minimizing the inflammatory tumor microenvironment, and may improve the chemosensitivity by limiting the contribution of NFκB to tumor development and progression.
Finally, although NFκB is a crucial player in cancer development and there exists a solid rationale for development of anticancer therapy that suppresses NFκB signaling, several key challenges still exist before a successful transition for the myriad NFκB inhibitors to the clinic. Due to the ubiquituousness of the NFkB signaling cascade, it is not surprising that NFκB inhibitors affect other cellular processes, resulting in adverse effects. A solution in this regard would be to develop NFκB inhibitors for local application rather than systemic administration. In addition, several important factors such as the lack of adequate drug delivery and the low bioavailability limit the clinical utility of several NFκB inhibitors. In this context, the results of the recent phase I clinical trial for a nanoparticulate curcumin formulation are encouraging, as it has demonstrated increased water solubility and increased serum drug levels, with minimal host toxicity [348, 349]. Several structural analogues of curcumin and other natural NFκB inhibitors are under development, which may possess better pharmacokinetic and drug-like properties, improving their clinical utility as anticancer agents. Therefore, addressing pharmacological, pharmaceutical, and toxicological issues is critical in the development of effective NFκB inhibitors as anticancer agents.
ACKNOWLEDGEMENTS
This work was supported in part by NIH/NCI grants R01 CA112029, R01 CA121211, and R01 CA186662. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the current and former members of our laboratories for their contributions to the publications cited in this work. The research field reviewed in this article is rapidly expanding; we apologize for not being able to cite all of the references published in recent years due to space limitations.
Biography

Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Shacter E; Weitzman SA Chronic inflammation and cancer. Oncology (Williston Park), 2002, 16(2), 217–226, 229; discussion 230–232. [PubMed] [Google Scholar]
- [2].Grivennikov SI; Greten FR; Karin M Immunity, inflammation, and cancer. Cell, 2010, 140(6), 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lu H; Ouyang W; Huang C Inflammation, a key event in cancer development. Mol. Cancer Res, 2006, 4(4), 221–233. [DOI] [PubMed] [Google Scholar]
- [4].Balkwill F; Mantovani A Inflammation and cancer: back to Virchow? Lancet, 2001, 357 (9255), 539–545. [DOI] [PubMed] [Google Scholar]
- [5].Danese S Inflammatory bowel disease and inflammation-associated colon cancer: partners in crime. Curr. Drug Targets, 2008, 9(5), 360. [DOI] [PubMed] [Google Scholar]
- [6].Erdman SE; Poutahidis T Roles for inflammation and regulatory T cells in colon cancer. Toxicol. Pathol, 2010, 38 (1), 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Terzic J; Grivennikov S; Karin E; Karin M, Inflammation and colon cancer. Gastroenterology, 2010, 138 (6), 2101–2114. e5. [DOI] [PubMed] [Google Scholar]
- [8].Klampfer L Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets, 2011, 11 (4), 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carrasco G; Corvalan AH Helicobacter pylori-Induced Chronic Gastritis and Assessing Risks for Gastric Cancer. Gastroenterol. Res. Pract, 2013, 2013, 393015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kong Y; Ma LQ; Bai PS; Da R; Sun H; Qi XG; Ma JQ; Zhao RM; Chen NZ; Nan KJ Helicobacter pylori promotes invasion and metastasis of gastric cancer cells through activation of AP-1 and up-regulation of CACUL1. Int. J. Biochem. Cell Biol, 2013, 45 (11), 2666–2678. [DOI] [PubMed] [Google Scholar]
- [11].Watanabe T; Takahashi A; Suzuki K; Kurusu-Kanno M; Yamaguchi K; Fujiki H; Suganuma M Epithelial-mesenchymal transition in human gastric cancer cell lines induced by TNF-alpha-inducing protein of Helicobacter pylori. Int. J. Cancer, 2014, 134(10), 2373–2382. [DOI] [PubMed] [Google Scholar]
- [12].de Visser KE; Korets LV; Coussens LM De novo carcino-genesis promoted by chronic inflammation is B lymphocyte dependent. Cancer cell, 2005, 7 (5), 411–423. [DOI] [PubMed] [Google Scholar]
- [13].Meira LB; Bugni JM; Green SL; Lee CW; Pang B; Borenshtein D; Rickman BH; Rogers AB; Moroski-Erkul CA; McFaline JL; Schauer DB; Dedon PC; Fox JG; Samson LD DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest, 2008, 118 (7), 2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Niwa T; Ushijima T Induction of epigenetic alterations by chronic inflammation and its significance on carcinogenesis. Adv. Genet, 2010, 71, 41–56. [DOI] [PubMed] [Google Scholar]
- [15].Zhang M; Zhou S; Zhang L; Ye W; Wen Q; Wang J Role of cancer-related inflammation in esophageal cancer., Crit. Rev. Eukaryot. Gene Expr, 2013, 23 (1), 27–35. [DOI] [PubMed] [Google Scholar]
- [16].Hamada S; Masamune A; Shimosegawa T Inflammation and pancreatic cancer: disease promoter and new therapeutic target. J.Gastroenterol, 2014, 49 (4), 605–617. [DOI] [PubMed] [Google Scholar]
- [17].Kwon OJ; Zhang L; Ittmann MM; Xin L Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl. Acad. Sci. USA, 2014, 111 (5), E592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marshall HE; Hess DT; Stamler JS S-nitrosylation: physiological regulation of NF-kappaB. Proc. Natl. Acad. Sci. USA, 2004, 101 (24), 8841–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kawanishi S; Hiraku Y; Pinlaor S; Ma N Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem, 2006, 387 (4), 365–372. [DOI] [PubMed] [Google Scholar]
- [20].Hussain SP; Hofseth LJ; Harris CC Radical causes of cancer. Nat. Rev. Cancer, 2003, 3 (4), 276–285. [DOI] [PubMed] [Google Scholar]
- [21].Rayet B; Gelinas C Aberrant rel/nfkb genes and activity in human cancer. Oncogene, 1999, 18 (49), 6938–6947. [DOI] [PubMed] [Google Scholar]
- [22].Ben-Neriah Y; Karin M Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol, 2011, 12 (8), 715–723. [DOI] [PubMed] [Google Scholar]
- [23].Karin M NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol, 2009, 1 (5), a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perkins ND The diverse and complex roles of NF-kappaB subunits in cancer, Nat. Rev. Cancer, 2012, 12, 121–132. [DOI] [PubMed] [Google Scholar]
- [25].Erstad DJ; Cusack JC Jr. Targeting the NFκB Pathway in Cancer Therapy, Surg. Oncol. Clin. N. Am, 2013, 22, 705–746. [DOI] [PubMed] [Google Scholar]
- [26].Chaturvedi MM; Sung B; Yadav VR; Kannappan R; Aggarwal BB NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene, 2011, 30 (14), 1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maeda S; Hikiba Y; Sakamoto K; Nakagawa H; Hirata Y; Hayakawa Y; Akanuma M Colon cancer-derived factors activate NF-kappaB in myeloid cells via TLR2 to link inflammation and tumorigenesis. Mol. Med. Rep, 2011, 4 (6), 1083–1088. [DOI] [PubMed] [Google Scholar]
- [28].Pikarsky E; Porat RM; Stein I; Abramovitch R; Amit S; Kasem S; Gutkovich-Pyest E; Urieli-Shoval S; Galun E; Ben-Neriah Y NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature, 2004, 431 (7007), 461–466. [DOI] [PubMed] [Google Scholar]
- [29].Setia S; Nehru B; Sanyal SN Activation of NF-kappaB: Bridging the gap between inflammation and cancer in colitis-mediated colon carcinogenesis. Biomed. Pharmacother, 2014, 68 (1), 119–128. [DOI] [PubMed] [Google Scholar]
- [30].Ling J; Kumar R Crosstalk between NFκB and glucocorticoid signaling: a potential target of breast cancer therapy, Cancer Lett, 2012, 322, 119–126. [DOI] [PubMed] [Google Scholar]
- [31].Sen R; Baltimore D Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell, 1986, 46 (5), 705–716. [DOI] [PubMed] [Google Scholar]
- [32].Ghosh S; May MJ; Kopp EB NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Ann. Rev. Immunol, 1998, 16, 225–260. [DOI] [PubMed] [Google Scholar]
- [33].Verma IM; Stevenson JK; Schwarz EM; Van Antwerp D; Miyamoto S Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes. Dev, 1995, 9 (22), 2723–2735. [DOI] [PubMed] [Google Scholar]
- [34].Lin L; Ghosh S A glycine-rich region in NF-kappaB p105 functions as a processing signal for the generation of the p50 subunit. Mol. Cell Biol, 1996, 16 (5), 2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Baeuerle PA; Baltimore D I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science, 1988, 242 (4878), 540–546. [DOI] [PubMed] [Google Scholar]
- [36].Gupta SC; Kim JH; Prasad S; Aggarwal BB Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metas. Rev, 2010, 29 (3), 405–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gilmore TD Multiple myeloma: lusting for NF-kappaB. Cancer cell 2007, 12 (2), 95–97. [DOI] [PubMed] [Google Scholar]
- [38].Wu JT; Kral JG The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J. Surg. Res, 2005, 123(1), 158–169. [DOI] [PubMed] [Google Scholar]
- [39].Romieu-Mourez R; Kim DW; Shin SM; Demicco EG; Landesman-Bollag E; Seldin DC; Cardiff RD; Sonenshein GE Mouse mammary tumor virus c-rel transgenic mice develop mammary tumors. Mol. Cell Biol, 2003, 23 (16), 5738–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu M; Sakamaki T; Casimiro MC; Willmarth NE; Quong AA; Ju X; Ojeifo J; Jiao X; Yeow WS; Katiyar S; Shirley LA; Joyce D; Lisanti MP; Albanese C; Pestell RG The canonical NF-kappaB pathway governs mammary tumorigenesis in transgenic mice and tumor stem cell expansion. Cancer Res, 2010, 70 (24), 10464–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Boehm JS; Zhao JJ; Yao J; Kim SY; Firestein R; Dunn IF; Sjostrom SK; Garraway LA; Weremowicz S; Richardson AL; Greulich H; Stewart CJ; Mulvey LA; Shen RR; Ambrogio L; Hirozane-Kishikawa T; Hill DE; Vidal M; Meyerson M; Grenier JK; Hinkle G; Root DE; Roberts TM; Lander ES; Polyak K; Hahn WC Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell, 2007, 129 (6), 1065–1079. [DOI] [PubMed] [Google Scholar]
- [42].Huber MA; Azoitei N; Baumann B; Grunert S; Sommer A; Pehamberger H; Kraut N; Beug H; Wirth T NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest, 2004, 114 (4), 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Karin M; Cao Y; Greten FR; Li ZW NF-kappaB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer, 2002, 2(4), 301–310. [DOI] [PubMed] [Google Scholar]
- [44].DiDonato JA; Mercurio F; Karin M NF-kappaB and the link between inflammation and cancer. Immunol Rev, 2012, 246 (1), 379–400. [DOI] [PubMed] [Google Scholar]
- [45].Cabannes E; Khan G; Aillet F; Jarrett RF; Hay RT Mutations in the IkBa gene in Hodgkin’s disease suggest a tumour suppressor role for IkappaBalpha. Oncogene, 1999, 18, 3063–3070. [DOI] [PubMed] [Google Scholar]
- [46].Pacifico F; Leonardi A NF-kappaB in solid tumors. Biochem. Pharmacol, 2006, 72 (9), 1142–1152. [DOI] [PubMed] [Google Scholar]
- [47].Dos Santos NR; Ghezzo MN; da Silva RC, Fernandes MT NFκB in T-cell Acute Lymphoblastic Leukemia: Oncogenic Functions in Leukemic and in Microenvironmental Cells, Cancers (Basel), 2010, 2, 1838–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pavan A; Spina M; Canzonieri V; Sansonno S; Toffoli G; De Re V Recent prognostic factors in diffuse large B-cell lymphoma indicate NF-kappaB pathway as a target for new therapeutic strategies, Leuk. Lymphoma, 2008, 49, 2048–2058. [DOI] [PubMed] [Google Scholar]
- [49].Gilmore TD; Gerondakis S; The c-Rel Transcription Factor in Development and Disease, Genes Cancer, 2011, 2, 695–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mathas S; Johrens K; Joos S; Lietz A; Hummel F; Janz M; Jundt F; Anagnostopoulos I; Bommert K; Lichter P; Stein H; Scheidereit C; Dorken B Elevated NF-kappaB p50 complex formation and Bcl-3 expression in classical Hodgkin, anaplastic large-cell, and other peripheral T-cell lymphomas, Blood, 2005, 106, 4287–4293. [DOI] [PubMed] [Google Scholar]
- [51].Neri A; Chang CC; Lombardi L; Salina M; Corradini P; Maiolo AT; Chaganti RS; Dalla-Favera R B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50, Cell, 1991, 67,1075–1087. [DOI] [PubMed] [Google Scholar]
- [52].Liptay S; Schmid RM; Perkins ND; Meltzer P; Altherr MR; McPherson JD; Wasmuth JJ; Nabel GJ, Related subunits of NF-kappa B map to two distinct loci associated with translocations in leukemia, NFKB1 and NFKB2, Genomics, 1992, 13, 287–292. [DOI] [PubMed] [Google Scholar]
- [53].Keats JJ; Fonseca R; Chesi M; Schop R; Baker A; Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Brag-gio E, Henry T, Zhu YX, Fogle H, Price-Troska T, Ahmann G, Mancini C, Brents LA, Kumar S, Greipp P, Dispenzieri A, Bryant B, Mulligan G, Bruhn L, Barrett M, Valdez R, Trent J, Stewart AK, Carpten J, Bergsagel PL, Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma, Cancer cell, 2007,12, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hamdane M; David-Cordonnier MH; D’Halluin JC Activation of p65 NF-kappaB protein by p210BCR-ABL in a myeloid cell line (P210BCR-ABL activates p65 NF-kappaB). Oncogene, 1997, 15 (19), 2267–2275. [DOI] [PubMed] [Google Scholar]
- [55].Stirewalt DL; Meshinchi S; Radich JP Molecular targets in acute myelogenous leukemia. Blood Rev, 2003, 17 (1), 15–23. [DOI] [PubMed] [Google Scholar]
- [56].Molinolo AA; Amornphimoltham P; Squarize CH; Castilho RM; Patel V; Gutkind JS Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol, 2009, 45 (4–5), 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Annunziata CM; Stavnes HT; Kleinberg L; Berner A; Hernandez LF; Birrer MJ; Steinberg SM; Davidson B; Kohn EC Nuclear factor kappaB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer, 2010, 116(13), 3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Alberti C; Pinciroli P; Valeri B; Ferri R; Ditto A; Umezawa K; Sensi M; Canevari S; Tomassetti A Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer. Oncogene, 2012, 31 (37), 4139–4149. [DOI] [PubMed] [Google Scholar]
- [59].Guo RX; Qiao YH; Zhou Y; Li LX; Shi HR; Chen KS Increased staining for phosphorylated AKT and nuclear factor-kappaB p65 and their relationship with prognosis in epithelial ovarian cancer. Pathol. Int, 2008, 58 (12), 749–756. [DOI] [PubMed] [Google Scholar]
- [60].Fujioka S; Sclabas GM; Schmidt C; Frederick WA; Dong QG; Abbruzzese JL; Evans DB; Baker C; Chiao PJ Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin. Cancer Res, 2003, 9 (1), 346–354. [PubMed] [Google Scholar]
- [61].Wilson W 3rd; Baldwin AS Maintenance of constitutive IkappaB kinase activity by glycogen synthase kinase-3alpha/beta in pancreatic cancer. Cancer Res, 2008, 68 (19), 8156–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gasparian AV; Yao YJ; Kowalczyk D; Lyakh LA; Karseladze A; Slaga TJ; Budunova IV The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J. Cell Sci, 2002, 115 (Pt 1), 141–151. [DOI] [PubMed] [Google Scholar]
- [63].Brantley DM; Yull FE; Muraoka RS; Hicks DJ; Cook CM; Kerr LD Dynamic expression and activity of NF-kappaB during post-natal mammary gland morphogenesis. Mech. Dev, 2000, 97 (1–2), 149–155. [DOI] [PubMed] [Google Scholar]
- [64].Brantley DM; Chen CL; Muraoka RS; Bushdid PB; Bradberry JL; Kittrell F; Medina D; Matrisian LM; Kerr LD; Yull FE Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol. Biol. Cell, 2001, 12 (5), 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cogswell PC; Guttridge DC; Funkhouser WK; Baldwin AS Jr. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Onco-gene, 2000, 19 (9), 1123–1131. [DOI] [PubMed] [Google Scholar]
- [66].Nakshatri H; Bhat-Nakshatri P; Martin DA; Goulet RJ Jr.; Sledge GW Jr. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol. Cell Biol, 1997, 17 (7), 3629–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sovak MA; Bellas RE; Kim DW; Zanieski GJ; Rogers AE; Traish AM; Sonenshein GE Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J. Clin. Invest, 1997, 100 (12), 2952–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Demicco EG; Kavanagh KT; Romieu-Mourez R; Wang X; Shin SR; Landesman-Bollag E; Seldin DC; Sonenshein GE RelB/p52 NF-kappaB complexes rescue an early delay in mam-mary gland development in transgenic mice with targeted super-repressor IkappaB-alpha expression and promote carcinogenesis of the mammary gland. Mol. Cell Biol, 2005, 25 (22), 10136–10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Srivastava S; Matsuda M; Hou Z; Bailey JP; Kitazawa R; Herbst MP; Horseman ND Receptor activator of NF-kappaB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J. Biol. Chem, 2003, 278 (46), 46171–46178. [DOI] [PubMed] [Google Scholar]
- [70].Van Laere SJ; Van der Auwera I; Van den Eynden GG; Elst HJ; Weyler J; Harris AL; van Dam P; Van Marck EA; Vermeulen PB; Dirix LY Nuclear factor-kappaB signature of inflammatory breast cancer by cDNA microarray validated by quantitative real-time reverse transcription-PCR, immunohisto-chemistry, and nuclear factor-kappaB DNA-binding. Clin. Cancer Res, 2006, 12 (11 Pt 1), 3249–3256. [DOI] [PubMed] [Google Scholar]
- [71].Van Laere SJ; Van der Auwera I; Van den Eynden GG; van Dam P; Van Marck EA; Vermeulen PB; Dirix LY NF-kappaB activation in inflammatory breast cancer is associated with oestrogen receptor downregulation, secondary to EGFR and/or ErbB2 overexpression and MAPK hyperactivation. Br. J. Cancer, 2007, 97 (5), 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Romagnoli M; Belguise K; Yu Z; Wang X; Landesman-Bollag E; Seldin DC; Chalbos D; Barille-Nion S; Jezequel P; Seldin ML; Sonenshein GE Epithelial-to-mesenchymal transition induced by TGF-beta1 is mediated by Blimp-1-dependent repression of BMP-5. Cancer Res, 2012, 72 (23), 6268–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shostak K; Chariot A NF-kappaB, stem cells and breast cancer: the links get stronger. Breast Cancer Res, 2011, 13 (4), 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yu H; Mohan S; Natarajan M Radiation-Triggered NF-kappaB Activation is Responsible for the Angiogenic Signaling Pathway and Neovascularization for Breast Cancer Cell Proliferation and Growth. Breast Cancer Res, 2012, 6, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhou Y; Eppenberger-Castori S; Eppenberger U; Benz CC The NFkappaB pathway and endocrine-resistant breast cancer. Endocr. Relat. Cancer, 2005, 12 Suppl 1, S37–46. [DOI] [PubMed] [Google Scholar]
- [76].Tobar N; Villar V; Santibanez JF ROS-NFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol. Cell Biochem, 2010, 340 (1–2), 195–202. [DOI] [PubMed] [Google Scholar]
- [77].Oeckinghaus A; Ghosh S The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol, 2009, 1 (4), a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pahl HL Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene, 1999, 18 (49), 6853–6866. [DOI] [PubMed] [Google Scholar]
- [79].Pujari R; Hunte R; Khan WN; Shembade N A20-mediated negative regulation of canonical NF-κB signaling pathway. Immunol. Res, 2013, 57 (1–3), 166–171. [DOI] [PubMed] [Google Scholar]
- [80].Sun SC Non-canonical NF-kappaB signaling pathway. Cell Res, 2011, 21 (1), 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Razani B; Reichardt AD; Cheng G Non-canonical NF-kappaB signaling activation and regulation: principles and perspectives. Immunol. Rev, 2011, 244 (1), 44–54. [DOI] [PubMed] [Google Scholar]
- [82].Kendellen MF; Bradford JW; Lawrence CL; Clark KS; Baldwin AS Canonical and non-canonical NF-kappaB signaling promotes breast cancer tumor-initiating cells. Oncogene, 2014, 33(10), 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Huang TT; Wuerzberger-Davis SM; Wu ZH; Miyamoto S Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell, 2003, 115 (5), 565–576. [DOI] [PubMed] [Google Scholar]
- [84].Kato T Jr.; Delhase M; Hoffmann A; Karin M CK2 Is a C-Terminal IkappaB Kinase Responsible for NF-kappaB Activation during the UV Response. Mol. Cell, 2003, 12 (4), 829–839. [DOI] [PubMed] [Google Scholar]
- [85].Tergaonkar V; Bottero V; Ikawa M; Li Q; Verma IM IkappaB kinase-independent IkappaBalpha degradation pathway: functional NF-kappaB activity and implications for cancer therapy. Mol. Cell Biol, 2003, 23 (22), 8070–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tieri P; Termanini A; Bellavista E; Salvioli S; Capri M; Franceschi C Charting the NF-kappaB pathway interactome map. PloS one, 2012, 7 (3), e32678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Huang B; Yang XD; Lamb A; Chen LF Posttranslational modifications of NF-kappaB: another layer of regulation for NF-kappaB signaling pathway. Cell Signal, 2010, 22 (9), 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Liu Y; Smith PW; Jones DR Breast cancer metastasis suppressor 1 functions as a corepressor by enhancing histone deacetylase 1-mediated deacetylation of RelA/p65 and promoting apoptosis. Mol. Cell Biol, 2006, 26 (23), 8683–8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yde CW; Emdal KB; Guerra B; Lykkesfeldt AE NFkappaB signaling is important for growth of antiestrogen resistant breast cancer cells. Breast Cancer Res. Treat, 2012, 135 (1), 67–78. [DOI] [PubMed] [Google Scholar]
- [90].Perkins ND Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene, 2006, 25 (51), 6717–6730. [DOI] [PubMed] [Google Scholar]
- [91].Mukhopadhyay NK; Ferdinand AS; Mukhopadhyay L; Cinar B; Lutchman M; Richie JP; Freeman MR; Liu BC Unraveling androgen receptor interactomes by an array-based method: discovery of proto-oncoprotein c-Rel as a negative regulator of androgen receptor. Exp. Cell Res, 2006, 312 (19), 3782–3795. [DOI] [PubMed] [Google Scholar]
- [92].Vogel CF; Sciullo E; Li W; Wong P; Lazennec G; Matsumura F RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol, 2007, 21 (12), 2941–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gao H; Sun Y; Wu Y; Luan B; Wang Y; Qu B; Pei G Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol. Cell, 2004, 14(3), 303–317. [DOI] [PubMed] [Google Scholar]
- [94].Parameswaran N; Pao CS; Leonhard KS; Kang DS; Kratz M; Ley SC; Benovic JL Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J. Biol. Chem, 2006, 281 (45), 34159–34170. [DOI] [PubMed] [Google Scholar]
- [95].Kroll M; Margottin F; Kohl A; Renard P; Durand H; Concordet JP; Bachelerie F; Arenzana-Seisdedos F; Benarous R Inducible degradation of IkappaBalpha by the proteasome requires interaction with the F-box protein h-betaTrCP. J. Biol. Chem, 1999, 274 (12), 7941–7945. [DOI] [PubMed] [Google Scholar]
- [96].Khan N; Rahim SS; Boddupalli CS; Ghousunnissa S; Padma S; Pathak N; Thiagarajan D; Hasnain SE; Mukhopadhyay S Hydrogen peroxide inhibits IL-12 p40 induction in macrophages by inhibiting c-rel translocation to the nucleus through activation of calmodulin protein. Blood, 2006, 107 (4), 1513–1520. [DOI] [PubMed] [Google Scholar]
- [97].Chen E; Li CC Association of Cdk2/cyclin E and NF-kappa B complexes at G1/S phase. Biochem. Biophys. Res. Comm, 1998, 249 (3), 728–734. [DOI] [PubMed] [Google Scholar]
- [98].Stein B; Baldwin AS Jr.; Ballard DW; Greene WC; Angel P; Herrlich P Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J, 1993, 12 (10), 3879–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Shifera AS; Friedman JM; Horwitz MS IKK gamma (NEMO) is involved in the coordination of the AP-1 and NF-kappa B pathways. Mol. Cell Biol, 2008, 310 (1–2), 181–190. [DOI] [PubMed] [Google Scholar]
- [100].Wang D; Westerheide SD; Hanson JL; Baldwin AS Jr. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem, 2000, 275 (42), 32592–32597. [DOI] [PubMed] [Google Scholar]
- [101].Bijli KM; Minhajuddin M; Fazal F; O’Reilly MA; Platanias LC; Rahman A c-Src interacts with and phosphorylates RelA/p65 to promote thrombin-induced ICAM-1 expression in endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol, 2007, 292(2), L396–404. [DOI] [PubMed] [Google Scholar]
- [102].Park J; Lee JH; La M; Jang MJ; Chae GW; Kim SB; Tak H; Jung Y; Byun B; Ahn JK; Joe CO Inhibition of NF-kappaB acetylation and its transcriptional activity by Daxx. J. Mol. Biol,. 2007, 368 (2), 388–397. [DOI] [PubMed] [Google Scholar]
- [103].Lim CA; Yao F; Wong JJ; George J; Xu H; Chiu KP; Sung WK; Lipovich L; Vega VB; Chen J; Shahab A; Zhao XD; Hibberd M; Wei CL; Lim B; Ng HH; Ruan Y; Chin KC Genome-wide mapping of RELA(p65) binding identifies E2F1 as a transcriptional activator recruited by NF-kappaB upon TLR4 activation. Mol. Cell, 2007, 27 (4), 622–635. [DOI] [PubMed] [Google Scholar]
- [104].Lee ST; Li Z; Wu Z; Aau M; Guan P; Karuturi RK; Liou YC; Yu Q Context-specific regulation of NF-kappaB target gene expression by EZH2 in breast cancers. Mol. Cell, 2011, 43(5), 798–810. [DOI] [PubMed] [Google Scholar]
- [105].Quaedackers ME; van den Brink CE; van der Saag PT; Tertoolen LG Direct interaction between estrogen receptor alpha and NF-kappaB in the nucleus of living cells. Mol. Cell Endocrinol, 2007, 273 (1–2), 42–50. [DOI] [PubMed] [Google Scholar]
- [106].Feldman I; Feldman GM; Mobarak C; Dunkelberg JC; Leslie KK Identification of proteins within the nuclear factor-kappa B transcriptional complex including estrogen receptor-alpha. Am. J. Obstet. Gynecol, 2007, 196 (4), 394 e1–11; discussion 394 e11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kalaitzidis D; Gilmore TD Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trend Endocrinol. Metabol, 2005, 16 (2), 46–52. [DOI] [PubMed] [Google Scholar]
- [108].Higashitsuji H; Liu Y; Masuda T; Fujita T; Abdel-Aziz HI; Kongkham S; Dawson S; John Mayer R; Itoh Y; Sakurai T; Itoh K; Fujita J The oncoprotein gankyrin interacts with RelA and suppresses NF-kappaB activity. Biochem. Biophys. Res. Comm, 2007, 363 (3), 879–884. [DOI] [PubMed] [Google Scholar]
- [109].Kammanadiminti SJ; Chadee K Suppression of NF-kappaB activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J. Biol. Chem, 2006, 281 (36), 26112–26120. [DOI] [PubMed] [Google Scholar]
- [110].Ashburner BP; Westerheide SD; Baldwin AS Jr. The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell Biol, 2001, 21 (20), 7065–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Viatour P; Legrand-Poels S; van Lint C; Warnier M; Merville MP; Gielen J; Piette J; Bours V; Chariot A Cytoplasmic IkappaBalpha increases NF-kappaB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J. Biol. Chem, 2003, 278 (47), 46541–46548. [DOI] [PubMed] [Google Scholar]
- [112].Chen L; Fischle W; Verdin E; Greene WC Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science, 2001, 293 (5535), 1653–1657. [DOI] [PubMed] [Google Scholar]
- [113].Liu S; Wu LC; Pang J; Santhanam R; Schwind S; Wu YZ; Hickey CJ; Yu J; Becker H; Maharry K; Radmacher MD; Li C; Whitman SP; Mishra A; Stauffer N; Eiring AM; Briesewitz R; Baiocchi RA; Chan KK; Paschka P; Caligiuri MA; Byrd JC; Croce CM; Bloomfield CD; Perrotti D; Garzon R; Marcucci G Sp1/NFkappaB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer Cell, 2010, 17 (4), 333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Meyer CF; Wang X; Chang C; Templeton D; Tan TH Interaction between c-Rel and the mitogen-activated protein kinase kinase kinase 1 signaling cascade in mediating kappaB enhancer activation. J. Biol. Chem, 1996, 271 (15), 8971–8976. [DOI] [PubMed] [Google Scholar]
- [115].Dan HC; Cooper MJ; Cogswell PC; Duncan JA; Ting JP; Baldwin AS Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev, 2008, 22 (11), 1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Pham LV; Tamayo AT; Yoshimura LC; Lin-Lee YC; Ford RJ Constitutive NF-kappaB and NFAT activation in aggressive B-cell lymphomas synergistically activates the CD154 gene and maintains lymphoma cell survival. Blood, 2005, 106 (12), 3940–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Song LL; Peng Y; Yun J; Rizzo P; Chaturvedi V; Weijzen S; Kast WM; Stone PJ; Santos L; Loredo A; Lendahl U; Sonenshein G; Osborne B; Qin JZ; Pannuti A; Nickoloff BJ; Miele L Notch-1 associates with IKKalpha and regulates IKK activity in cervical cancer cells. Oncogene, 2008, 27 (44), 5833–5844. [DOI] [PubMed] [Google Scholar]
- [118].Kitamura T; Sekimata M; Kikuchi S; Homma Y Involvement of poly(ADP-ribose) polymerase 1 in ERBB2 expression in rheumatoid synovial cells. Am. J. Physiol. Cell Physiol, 2005, 289 (1), C82–88. [DOI] [PubMed] [Google Scholar]
- [119].Kalkhoven E; Wissink S; van der Saag PT; van der Burg B Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J. Biol. Chem, 1996, 271 (11), 6217–6224. [DOI] [PubMed] [Google Scholar]
- [120].Nourbakhsh M; Hauser H Constitutive silencing of IFN-beta promoter is mediated by NRF (NF-kappaB-repressing factor), a nuclear inhibitor of NF-kappaB. EMBO J, 1999, 18 (22), 6415–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wu WS; Xu ZX; Hittelman WN; Salomoni P; Pandolfi PP; Chang KS Promyelocytic leukemia protein sensitizes tumor necrosis factor alpha-induced apoptosis by inhibiting the NF-kappaB survival pathway. J. Biol. Chem, 2003, 278 (14), 12294–12304. [DOI] [PubMed] [Google Scholar]
- [122].Von Brandenstein MG; Ngum Abety A; Depping R; Roth T; Koehler M; Dienes HP; Fries JW A p38-p65 transcription complex induced by endothelin-1 mediates signal transduction in cancer cells. Biochim. Biophys. Acta, 2008, 1783 (9), 1613–1622. [DOI] [PubMed] [Google Scholar]
- [123].Chang NS The non-ankyrin C terminus of Ikappa Balpha physically interacts with p53 in vivo and dissociates in response to apoptotic stress, hypoxia, DNA damage, and transforming growth factor-beta 1-mediated growth suppression. J. Biol. Chem, 2002, 277(12), 10323–10331. [DOI] [PubMed] [Google Scholar]
- [124].Wan F; Anderson DE; Barnitz RA; Snow A; Bidere N; Zheng L; Hegde V; Lam LT; Staudt LM; Levens D; Deutsch WA; Lenardo MJ Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell, 2007, 131 (5), 927–939. [DOI] [PubMed] [Google Scholar]
- [125].Rual JF; Venkatesan K; Hao T; Hirozane-Kishikawa T; Dricot A; Li N; Berriz GF; Gibbons FD; Dreze M; Ayivi-Guedehoussou N; Klitgord N; Simon C; Boxem M; Milstein S; Rosenberg J; Goldberg DS; Zhang LV; Wong SL; Franklin G; Li S; Albala JS; Lim J; Fraughton C; Llamosas E; Cevik S; Bex C; Lamesch P; Sikorski RS; Vandenhaute J; Zoghbi HY; Smolyar A; Bosak S; Sequerra R; Doucette-Stamm L; Cusick ME; Hill DE; Roth FP; Vidal M Towards a proteome-scale map of the human protein-protein interaction network. Nature, 2005, 437 (7062), 1173–1178. [DOI] [PubMed] [Google Scholar]
- [126].Hirano F; Tanaka H; Hirano Y; Hiramoto M; Handa H; Makino I; Scheidereit C Functional interference of Sp1 and NF-kappaB through the same DNA binding site. Mol. Cell Biol, 1998, 18 (3), 1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yu Z; Zhang W; Kone BC Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J, 2002, 367 (Pt 1), 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Yoshida Y; Kumar A; Koyama Y; Peng H; Arman A; Boch JA; Auron PE Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique TRAF6- and p65-dependent mechanism. J. Biol. Chem, 2004, 279 (3), 1768–1776. [DOI] [PubMed] [Google Scholar]
- [129].Chung SS; Aroh C; Vadgama JV Constitutive activation of STAT3 signaling regulates hTERT and promotes stem cell-like traits in human breast cancer cells. PloS one, 2013, 8 (12), e83971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Desantis C; Ma J; Bryan L; Jemal A Breast cancer statistics, 2013. CA: Cancer J. Clin, 2013. 64 (2014), 52–62. [DOI] [PubMed] [Google Scholar]
- [131].Williams C; Lin CY Oestrogen receptors in breast cancer: basic mechanisms and clinical implications. Ecancermedicalscience, 2013, 7, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yue W; Yager JD; Wang JP; Jupe ER; Santen RJ Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids, 2013, 78 (2), 161–170. [DOI] [PubMed] [Google Scholar]
- [133].Yager JD; Davidson NE Estrogen carcinogenesis in breast cancer. N. Eng. J. Med, 2006, 354 (3), 270–282. [DOI] [PubMed] [Google Scholar]
- [134].Simstein R; Burow M; Parker A; Weldon C; Beckman B Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp. Biol. Med. (Maywood), 2003, 228(9), 995–1003. [DOI] [PubMed] [Google Scholar]
- [135].D’Abreo N; Hindenburg AA Sex hormone receptors in breast cancer. Vitam. Horm, 2013, 93, 99–133. [DOI] [PubMed] [Google Scholar]
- [136].Toy W; Shen Y; Won H; Green B; Sakr RA; Will M; Li Z; Gala K; Fanning S; King TA; Hudis C; Chen D; Taran T; Hortobagyi G; Greene G; Berger M; Baselga J; Chandarlapaty S ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nature genetics, 2013, 45 (12), 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cristofanilli M; Singletary ES; Hortobagyi GN Inflammatory breast carcinoma: the sphinx of breast cancer research. J. Clin. Oncol, 2004, 22 (2), 381–383; author reply 383. [DOI] [PubMed] [Google Scholar]
- [138].Peddi PF; Ellis MJ; Ma C Molecular basis of triple negative breast cancer and implications for therapy. Int. J. Breast Cancer, 2012, 2012, 217185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sarkar DK; Jana D; Patil PS; Chaudhari KS; Chattopadhyay BK; Chikkala BR; Mandal S; Chowdhary P Role of NFκB as a Prognostic Marker in Breast Cancer : A Pilot Study in Indian Patients. Indian J. Surg. Oncol, 2013, 4, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yamaguchi N; Ito T; Azuma S; Ito E; Honma R; Yanagisawa Y; Nishikawa A; Kawamura M; Imai J; Watanabe S; Semba K; Inoue J Constitutive activation of nuclear factor-kappaB is preferentially involved in the proliferation of basal-like subtype breast cancer cell lines. Cancer Sci, 2009, 100 (9), 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gilmore TD The Re1/NF-kappa B/I kappa B signal transduction pathway and cancer. Cancer Treat. Res, 2003, 115, 241–265. [PubMed] [Google Scholar]
- [142].Baldwin AS Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Invest, 2001, 107 (3), 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhou AY; Shen RR; Kim E; Lock YJ; Xu M; Chen ZJ; Hahn WC IKKepsilon-mediated tumorigenesis requires K63-linked polyubiquitination by a cIAP1/cIAP2/TRAF2 E3 ubiquitin ligase complex. Cell Rep, 2013, 3 (3), 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Shen RR; Hahn WC Emerging roles for the non-canonical IKKs in cancer. Oncogene, 2011, 30 (6), 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hagemann T; Biswas SK; Lawrence T; Sica A; Lewis CE Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood, 2009, 113 (14), 3139–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Smyth MJ; Dunn GP; Schreiber RD Cancer immunosurveil-lance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol, 2006, 90, 1–50. [DOI] [PubMed] [Google Scholar]
- [147].Dunn GP; Old LJ; Schreiber RD The three Es of cancer immunoediting. Ann. Rev. Immunol, 2004, 22, 329–360. [DOI] [PubMed] [Google Scholar]
- [148].DeNardo DG; Coussens LM Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res, 2007, 9 (4), 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Roy LD; Curry JM; Sahraei M; Besmer DM; Kidiyoor A; Gruber HE; Mukherjee P Arthritis augments breast cancer metastasis: role of mast cells and SCF/c-Kit signaling. Breast Cancer Res, 2013, 15 (2), R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Stewart T; Tsai SC; Grayson H; Henderson R; Opelz G Incidence of de-novo breast cancer in women chronically immuno-suppressed after organ transplantation. Lancet, 1995, 346 (8978), 796–798. [DOI] [PubMed] [Google Scholar]
- [151].Okoh V; Deoraj A; Roy D Estrogen-induced reactive oxygen species-mediated signalings contribute to breast cancer. Biochim. Biophys. Acta, 2011, 1815 (1), 115–133. [DOI] [PubMed] [Google Scholar]
- [152].Hinz M; Krappmann D; Eichten A; Heder A; Scheidereit C; Strauss M NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol. Cell Biol, 1999, 19 (4), 2690–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Perkins ND; Felzien LK; Betts JC; Leung K; Beach DH; Nabel GJ Regulation of NF-kappaB by cyclin-dependent kinases associated with the p300 coactivator. Science, 1997, 275 (5299), 523–527. [DOI] [PubMed] [Google Scholar]
- [154].Kim DW; Gazourian L; Quadri SA; Romieu-Mourez R; Sherr DH; Sonenshein GE The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene, 2000, 19 (48), 5498–5506. [DOI] [PubMed] [Google Scholar]
- [155].Biswas DK; Cruz AP; Gansberger E; Pardee AB Epidermal growth factor-induced nuclear factor kappa B activation: A major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc. Natl. Acad. Sci. U S A, 2000, 97 (15), 8542–8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Neil JR; Tian M; Schiemann WP X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-{beta}-mediated nuclear factor-{kappa}B activation during breast cancer progression. J. Biol. Chem, 2009, 284 (32), 21209–21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wang CY; Mayo MW; Korneluk RG; Goeddel DV; Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science, 1998, 281 (5383), 1680–1683. [DOI] [PubMed] [Google Scholar]
- [158].Nanda DP; Sil H; Moulik S; Biswas J; Mandal SS; Chat-terjee A Matrix metalloproteinase-9 as a potential tumor marker in breast cancer. J. Environ. Pathol. Toxicol, 2013, 32 (2), 115–129. [DOI] [PubMed] [Google Scholar]
- [159].Bist P; Leow SC; Phua QH; Shu S; Zhuang Q; Loh WT; Nguyen TH; Zhou JB; Hooi SC; Lim LH Annexin-1 interacts with NEMO and RIP1 to constitutively activate IKK complex and NF-kappaB: implication in breast cancer metastasis. Oncogene, 2011, 30 (28), 3174–3185. [DOI] [PubMed] [Google Scholar]
- [160].Sliva D; Rizzo MT; English D Phosphatidylinositol 3-kinase and NF-kappaB regulate motility of invasive MDA-MB-231 human breast cancer cells by the secretion of urokinase-type plasminogen activator. J. Biol. Chem, 2002, 277 (5), 3150–3157. [DOI] [PubMed] [Google Scholar]
- [161].Chavey C; Muhlbauer M; Bossard C; Freund A; Durand S; Jorgensen C; Jobin C; Lazennec G Interleukin-8 expression is regulated by histone deacetylases through the nuclear factor-kappaB pathway in breast cancer. Mol. Pharmacol, 2008, 74 (5), 1359–1366. [DOI] [PubMed] [Google Scholar]
- [162].Chen Q; Massague J Molecular pathways: VCAM-1 as a potential therapeutic target in metastasis. Clin. Cancer Res, 2012, 18(20), 5520–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Helbig G; Christopherson KW; Bhat-Nakshatri P; Kumar S; Kishimoto H; Miller KD; Broxmeyer HE; Nakshatri H NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J. Biol. Chem, 2003, 278 (24), 21631–21638. [DOI] [PubMed] [Google Scholar]
- [164].Farina AR; Cappabianca L; DeSantis G; Di Ianni N; Ruggeri P; Ragone M; Merolle S; Tonissen KF; Gulino A; Mackay AR Thioredoxin stimulates MMP-9 expression, de-regulates the MMP-9/TIMP-1 equilibrium and promotes MMP-9 dependent invasion in human MDA-MB-231 breast cancer cells. FEBS Lett, 2011, 585 (20), 3328–3336. [DOI] [PubMed] [Google Scholar]
- [165].Chua HL; Bhat-Nakshatri P; Clare SE; Morimiya A; Badve S; Nakshatri H NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene, 2007, 26 (5), 711–724. [DOI] [PubMed] [Google Scholar]
- [166].Wu Y; Deng J; Rychahou PG; Qiu S; Evers BM; Zhou BP Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell, 2009, 15(5), 416–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Li S; Kendall SE; Raices R; Finlay J; Covarrubias M; Liu Z; Lowe G; Lin YH; Teh YH; Leigh V; Dhillon S; Flanagan S; Aboody KS; Glackin CA TWIST1 associates with NF-kappaB subunit RELA via carboxyl-terminal WR domain to promote cell autonomous invasion through IL8 production. BMC Biol, 2012, 10, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Li CW; Xia W; Huo L; Lim SO; Wu Y; Hsu JL; Chao CH; Yamaguchi H; Yang NK; Ding Q; Wang Y; Lai YJ; LaBaff AM; Wu TJ; Lin BR; Yang MH; Hortobagyi GN; Hung MC Epithelial-mesenchymal transition induced by TNF-α requires NFκB-mediated transcriptional upregulation of Twist1. Cancer Res, 2012, 72, (5), 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Shibata A; Nagaya T; Imai T; Funahashi H; Nakao A; Seo H Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res. Treat, 2002, 73 (3), 237–243. [DOI] [PubMed] [Google Scholar]
- [170].Li C; Guo S; Shi T Role of NF-kappaB activation in matrix metalloproteinase 9, vascular endothelial growth factor and inter-leukin 8 expression and secretion in human breast cancer cells. Cell Biochem Funct, 2013, 31 (3), 263–268. [DOI] [PubMed] [Google Scholar]
- [171].Dai XL; Zhou SL; Qiu J; Liu YF; Hua H Correlated expression of Fas, NF-kappaB, and VEGF-C in infiltrating ductal carcinoma of the breast. Eur. J. Gynaecol. Oncol, 2012, 33, 633–639. [PubMed] [Google Scholar]
- [172].Yamamoto M; Taguchi Y; Ito-Kureha T; Semba K; Yamaguchi N; Inoue J NF-kappaB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nat. Commun, 2013, 4, 2299. [DOI] [PubMed] [Google Scholar]
- [173].Penault-Llorca F; Viale G Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann. Oncol, 2012, 23 (Suppl 6), vi19–22. [DOI] [PubMed] [Google Scholar]
- [174].Chougule MB; Patel AR; Jackson T; Singh M Antitumor activity of Noscapine in combination with Doxorubicin in triple negative breast cancer. PloS on,e 2011, 6 (3), e17733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Hartman ZC; Poage GM; den Hollander P; Tsimelzon A; Hill J; Panupinthu N; Zhang Y; Mazumdar A; Hilsenbeck SG; Mills GB; Brown PH Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res, 2013, 73(11), 3470–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Aggarwal BB; Gehlot P, Inflammation and cancer: how friendly is the relationship for cancer patients. Curr. Opin. Pharmacol, 2009, 9 (4), 351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Han B; Nakamura M; Mori I; Nakamura Y; Kakudo K, Urokinase-type plasminogen activator system and breast cancer (Review). Oncol. Rep, 2005, 14 (1), 105–112. [PubMed] [Google Scholar]
- [178].Van Laere S; Limame R; Van Marck EA; Vermeulen PB; Dirix LY, Is there a role for mammary stem cells in inflammatory breast carcinoma?: a review of evidence from cell line, animal model, and human tissue sample experiments. Cancer, 2010, 116(11 Suppl), 2794–2805. [DOI] [PubMed] [Google Scholar]
- [179].Merkhofer EC; Cogswell P; Baldwin AS, Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene, 2010, 29 (8), 1238–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Makino K; Day CP; Wang SC; Li YM; Hung MC, Upregulation of IKKalpha/IKKbeta by integrin-linked kinase is required for HER2/neu-induced NF-kappaB antiapoptotic pathway. Oncogene, 2004, 23 (21), 3883–3887. [DOI] [PubMed] [Google Scholar]
- [181].Schramek D; Leibbrandt A; Sigl V; Kenner L; Pospisilik JA; Lee HJ; Hanada R; Joshi PA; Aliprantis A; Glimcher L; Pasparakis M; Khokha R; Ormandy CJ; Widschwendter M; Schett G; Penninger JM, Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature, 2010, 468 (7320), 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Gonzalez-Suarez E; Jacob AP; Jones J; Miller R; Roudier-Meyer MP; Erwert R; Pinkas J; Branstetter D; Dougall WC, RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature, 2010, 468 (7320), 103–107. [DOI] [PubMed] [Google Scholar]
- [183].Pratt MA; Tibbo E; Robertson SJ; Jansson D; Hurst K; Perez-Iratxeta C; Lau R; Niu MY, The canonical NF-kappaB pathway is required for formation of luminal mammary neoplasias and is activated in the mammary progenitor population. Oncogene, 2009, 28 (30), 2710–2722. [DOI] [PubMed] [Google Scholar]
- [184].Zhang JY; Tao S; Kimmel R; Khavari PA, CDK4 regulation by TNFR1 and JNK is required for NF-kappaB-mediated epidermal growth control. J. Cell. Bio, 2005, 168 (4), 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Halazonetis TD; Gorgoulis VG; Bartek J, An oncogene-induced DNA damage model for cancer development. Science, 2008, 319 (5868), 1352–1355. [DOI] [PubMed] [Google Scholar]
- [186].Brzoska K; Szumiel I, Signalling loops and linear pathways: NF-kappaB activation in response to genotoxic stress. Mutagenesis, 2009, 24 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- [187].Hartwig A; Blessing H; Schwerdtle T; Walter I, Modulation of DNA repair processes by arsenic and selenium compounds. Toxicology, 2003, 193 (1–2), 161–169. [DOI] [PubMed] [Google Scholar]
- [188].Volcic M; Karl S; Baumann B; Salles D; Daniel P; Fulda S; Wiesmuller L, NF-kappaB regulates DNA double-strand break repair in conjunction with BRCA1-CtIP complexes. Nucleic acids Res, 2012, 40 (1), 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Hoesel B; Schmid JA, The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer, 2013, 12, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Grivennikov SI; Karin M, Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev, 2010, 21 (1), 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Yang J; Liao X; Agarwal MK; Barnes L; Auron PE; Stark GR, Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev, 2007, 21 (11), 1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Lee H; Herrmann A; Deng JH; Kujawski M; Niu G; Li Z; Forman S; Jove R; Pardoll DM; Yu H, Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell, 2009, 15 (4), 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Divella R; Tommasi S; Lacalamita R; Daniele A; Abbate I; Garrisi VM; Savino E; Coviello M; Rubini V; Simone G; Paradiso A; Quaranta M, Circulating hTERT DNA in early breast cancer. Anticancer Res, 2009, 29 (7), 2845–2849. [PubMed] [Google Scholar]
- [194].Wang Y; Hu Z; Liang J; Wang Z; Tang J; Wang S; Wang X; Qin J; Shen H, A tandem repeat of human telomerase reverse transcriptase (hTERT) and risk of breast cancer development and metastasis in Chinese women. Carcinogenesis, 2008, 29 (6), 1197–1201. [DOI] [PubMed] [Google Scholar]
- [195].Yu J; Wang Y; Yan F; Zhang P; Li H; Zhao H; Yan C; Ren X, Noncanonical NF-kappaB Activation Mediates STAT3-Stimulated IDO Upregulation in Myeloid-Derived Suppressor Cells in Breast Cancer. J Immunol, 2014, 193 (5), 2574–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Hoeflich KP; Luo J; Rubie EA; Tsao MS; Jin O; Woodgett JR, Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature, 2000, 406 (6791), 86–90. [DOI] [PubMed] [Google Scholar]
- [197].Luo J, Glycogen synthase kinase 3beta (GSK3beta) in tumori-genesis and cancer chemotherapy. Cancer Lett, 2009, 273 (2), 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Deng J; Xia W; Miller SA; Wen Y; Wang HY; Hung MC, Crossregulation of NF-kappaB by the APC/GSK-3beta/beta-catenin pathway. Mol. Carcinog, 2004, 39 (3), 139–146. [DOI] [PubMed] [Google Scholar]
- [199].Wang Y; Lam JB; Lam KS; Liu J; Lam MC; Hoo RL; Wu D; Cooper GJ; Xu A, Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res, 2006, 66 (23), 11462–11470. [DOI] [PubMed] [Google Scholar]
- [200].Moreau M; Mourah S; Dosquet C, beta-Catenin and NF-kappaB cooperate to regulate the uPA/uPAR system in cancer cells. Int. J. Cancer, 2011, 128 (6), 1280–1292. [DOI] [PubMed] [Google Scholar]
- [201].Webster GA; Perkins ND, Transcriptional cross talk between NF-kappaB and p53. Mol. Cell Biol, 1999, 19 (5), 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Ryan KM; Ernst MK; Rice NR; Vousden KH, Role of NF-kappaB in p53-mediated programmed cell death. Nature, 2000, 404 (6780), 892–897. [DOI] [PubMed] [Google Scholar]
- [203].Dalmases A; Gonzalez I; Menendez S; Arpi O; Corominas JM; Servitja S; Tusquets I; Chamizo C; Rincon R; Espinosa L; Bigas A; Eroles P; Furriol J; Lluch A; Rovira A; Albanell J; Rojo F, Deficiency in p53 is required for doxorubicin induced transcriptional activation of NF-small ka, CyrillicB target genes in human breast cancer. Oncotarget, 2014, 5 (1), 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Schneider G; Kramer OH, NFkappaB/p53 crosstalk-a promising new therapeutic target. Biochim. Biophys. Acta, 2011, 1815 (1), 90–103. [DOI] [PubMed] [Google Scholar]
- [205].Schneider G; Henrich A; Greiner G; Wolf V; Lovas A; Wieczorek M; Wagner T; Reichardt S; von Werder A; Schmid RM; Weih F; Heinzel T; Saur D; Kramer OH, Cross talk between stimulated NF-kappaB and the tumor suppressor p53. Oncogene, 2010, 29 (19), 2795–2806. [DOI] [PubMed] [Google Scholar]
- [206].Zhou M; Gu L; Findley HW; Jiang R; Woods WG, PTEN reverses MDM2-mediated chemotherapy resistance by interacting with p53 in acute lymphoblastic leukemia cells. Cancer Res, 2003, 63 (19), 6357–6362. [PubMed] [Google Scholar]
- [207].Thomasova D; Mulay SR; Bruns H; Anders HJ, p53-independent roles of MDM2 in NF-kappaB signaling: implications for cancer therapy, wound healing, and autoimmune diseases. Neoplasia, 2012, 14 (12), 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Busuttil V; Droin N; McCormick L; Bernassola F; Candi E; Melino G; Green DR, NF-kappaB inhibits T-cell activation-induced, p73-dependent cell death by induction of MDM2. Proc. Natl. Acad. Sci. USA, 2010, 107 (42), 18061–18066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Pianetti S; Arsura M; Romieu-Mourez R; Coffey RJ; Sonenshein GE, Her-2/neu overexpression induces NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene, 2001, 20 (11), 1287–1299. [DOI] [PubMed] [Google Scholar]
- [210].Shapira I; Lee A; Vora R; Budman DR, P53 mutations in triple negative breast cancer upregulate endosomal recycling of epidermal growth factor receptor (EGFR) increasing its oncogenic potency. Crit. Rev. Oncol. Hematol, 2013, 88 (2), 284–292. [DOI] [PubMed] [Google Scholar]
- [211].Qian B; Nag SA; Su Y; Voruganti S; Qin JJ; Zhang R; Cho WC, miRNAs in cancer prevention and treatment and as molecular targets for natural product anticancer agents. Curr. Cancer Drug Targets, 2013, 13 (5), 519–541. [DOI] [PubMed] [Google Scholar]
- [212].Bazzoni F; Rossato M; Fabbri M; Gaudiosi D; Mirolo M; Mori L; Tamassia N; Mantovani A; Cassatella MA; Locati M, Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc. Natl. Acad. Sci. USA, 2009, 106 (13), 5282–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Ma X; Becker Buscaglia LE; Barker JR; Li Y, MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol, 2011, 3 (3), 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Bhaumik D; Scott GK; Schokrpur S; Patil CK; Campisi J; Benz CC, Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene, 2008, 27 (42), 5643–5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Iorio MV; Ferracin M; Liu CG; Veronese A; Spizzo R; Sabbioni S; Magri E; Pedriali M; Fabbri M; Campiglio M; Menard S; Palazzo JP; Rosenberg A; Musiani P; Volinia S; Nenci I; Calin GA; Querzoli P; Negrini M; Croce CM, MicroRNA gene expression deregulation in human breast cancer. Cancer Res, 2005, 65 (16), 7065–7070. [DOI] [PubMed] [Google Scholar]
- [216].Niu J; Shi Y; Tan G; Yang CH; Fan M; Pfeffer LM; Wu ZH, DNA damage induces NF-kappaB-dependent microRNA-21 up-regulation and promotes breast cancer cell invasion. J. Biol. Chem, 2012, 287 (26), 21783–21795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [217].Howe EN; Cochrane DR; Cittelly DM; Richer JK, miR-200c targets a NF-kappaB up-regulated TrkB/NTF3 autocrine signaling loop to enhance anoikis sensitivity in triple negative breast cancer. PloS one, 2012, 7 (11), e49987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Keklikoglou I; Koerner C; Schmidt C; Zhang JD; Heckmann D; Shavinskaya A; Allgayer H; Guckel B; Fehm T; Schnee-weiss A; Sahin O; Wiemann S; Tschulena U, MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-kappaB and TGF-beta signaling pathways. Oncogene, 2012, 31 (37), 4150–4163. [DOI] [PubMed] [Google Scholar]
- [219].Sorlie T; Perou CM; Tibshirani R; Aas T; Geisler S; Johnsen H; Hastie T; Eisen MB; van de Rijn M; Jeffrey SS; Thorsen T; Quist H; Matese JC; Brown PO; Botstein D; Lonning PE; Borresen-Dale AL, Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA, 2001, 98 (19), 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].The Cancer Genome Atlas Network, Comprehensive molecular portraits of human breast tumors. Nature, 2012, 490, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Perou CM; Sorlie T; Eisen MB; van de Rijn M; Jeffrey SS; Rees CA; Pollack JR; Ross DT; Johnsen H; Akslen LA; Fluge O; Pergamenschikov A; Williams C; Zhu SX; Lonning PE; Borresen-Dale AL; Brown PO; Botstein D, Molecular portraits of human breast tumours. Nature, 2000, 406 (6797), 747–752. [DOI] [PubMed] [Google Scholar]
- [222].Bertucci F; Birnbaum D, Reasons for breast cancer heterogeneity. J.Biol, 2008, 7 (2), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Hsiao YH; Chou MC; Fowler C; Mason JT; Man YG, Breast cancer heterogeneity: mechanisms, proofs, and implications. J.Cancer, 2010, 1, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Fan C; Oh DS; Wessels L; Weigelt B; Nuyten DS; Nobel AB; van’t Veer LJ; Perou CM, Concordance among gene-expression-based predictors for breast cancer. N. Eng. J. Med, 2006, 355 (6), 560–569. [DOI] [PubMed] [Google Scholar]
- [225].Sikora MJ; Cooper KL; Bahreini A; Luthra S; Wang G; Chandran UR; Davidson NE; Dabbs DJ; Welm AL; Oesterreich S, Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response. Cancer Res, 2014. 74 (5), 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Creighton CJ; Hilger AM; Murthy S; Rae JM; Chinnaiyan AM; El-Ashry D, Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res, 2006, 66 (7), 3903–3911. [DOI] [PubMed] [Google Scholar]
- [227].Sas L; Lardon F; Vermeulen PB; Hauspy J; Van Dam P; Pauwels P; Dirix LY; Van Laere SJ, The interaction between ER and NFkappaB in resistance to endocrine therapy. Breast Cancer Res, 2012, 14 (4), 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Galien R; Garcia T, Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic acids Res, 1997, 25 (12), 2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Wang X; Belguise K; Kersual N; Kirsch KH; Mineva ND; Galtier F; Chalbos D; Sonenshein GE, Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat. Cell Biol, 2007, 9 (4), 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Belguise K; Sonenshein GE, PKCtheta promotes c-Rel-driven mammary tumorigenesis in mice and humans by repressing estrogen receptor alpha synthesis. J. Clin. Invest, 2007, 117 (12), 4009–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Wang X; Belguise K; O’Neill CF; Sanchez-Morgan N; Romagnoli M; Eddy SF; Mineva ND; Yu Z; Min C; Trinkaus-Randall V; Chalbos D; Sonenshein GE, RelB NF-kappaB represses estrogen receptor alpha expression via induction of the zinc finger protein Blimp1. Mol. Cell. Biol, 2009, 29 (14), 3832–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Reijm EA; Jansen MP; Ruigrok-Ritstier K; van Staveren IL; Look MP; van Gelder ME; Sieuwerts AM; Sleijfer S; Foekens JA; Berns EM, Decreased expression of EZH2 is associated with upregulation of ER and favorable outcome to tamoxifen in advanced breast cancer. Breast Cancer Res. Treat, 2011, 125 (2), 387–394. [DOI] [PubMed] [Google Scholar]
- [233].Paimela T; Ryhanen T; Mannermaa E; Ojala J; Kalesnykas G; Salminen A; Kaarniranta K, The effect of 17beta-estradiol on IL-6 secretion and NF-kappaB DNA-binding activity in human retinal pigment epithelial cells. Immunol. Lett, 2007, 110 (2), 139–144. [DOI] [PubMed] [Google Scholar]
- [234].Dai R; Phillips RA; Ahmed SA, Despite inhibition of nuclear localization of NF-kappa B p65, c-Rel, and RelB, 17-beta estradiol up-regulates NF-kappa B signaling in mouse splenocytes: the potential role of Bcl-3. J. Immunol, 2007, 179 (3), 1776–1783. [DOI] [PubMed] [Google Scholar]
- [235].Ghisletti S; Meda C; Maggi A; Vegeto E, 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol. Cell. Biol, 2005, 25 (8), 2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Nettles KW; Gil G; Nowak J; Metivier R; Sharma VB; Greene GL, CBP Is a dosage-dependent regulator of nuclear factor-kappaB suppression by the estrogen receptor. Mol Endocrinol, 2008, 22 (2), 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Cvoro A; Tzagarakis-Foster C; Tatomer D; Paruthiyil S; Fox MS; Leitman DC, Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Mol. Cell, 2006, 21(4), 555–564. [DOI] [PubMed] [Google Scholar]
- [238].Frasor J; Weaver A; Pradhan M; Dai Y; Miller LD; Lin CY; Stanculescu A, Positive cross-talk between estrogen receptor and NF-kappaB in breast cancer. Cancer Res, 2009, 69 (23), 8918–8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].deGraffenried LA; Chandrasekar B; Friedrichs WE; Donzis E; Silva J; Hidalgo M; Freeman JW; Weiss GR, NF-kappa B inhibition markedly enhances sensitivity of resistant breast cancer tumor cells to tamoxifen. Ann. Oncol, 2004, 15 (6), 885–890. [DOI] [PubMed] [Google Scholar]
- [240].Gerondakis S; Grumont R; Gugasyan R; Wong L; Isomura I; Ho W; Banerjee A, Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene, 2006, 25 (51), 6781–6799. [DOI] [PubMed] [Google Scholar]
- [241].Gerondakis S; Grossmann M; Nakamura Y; Pohl T; Grumont R, Genetic approaches in mice to understand Rel/NF-kappaB and IkappaB function: transgenics and knockouts. Oncogene, 1999, 18(49), 6888–6895. [DOI] [PubMed] [Google Scholar]
- [242].Beinke S; Ley SC, Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem. J, 2004, 382 (Pt 2), 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Courtois G; Gilmore TD, Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene, 2006, 25(51), 6831–6843. [DOI] [PubMed] [Google Scholar]
- [244].Beg AA; Sha WC; Bronson RT; Ghosh S; Baltimore D, Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature, 1995, 376 (6536), 167–170. [DOI] [PubMed] [Google Scholar]
- [245].Cao Y; Bonizzi G; Seagroves TN; Greten FR; Johnson R; Schmidt EV; Karin M, IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell, 2001, 107 (6), 763–775. [DOI] [PubMed] [Google Scholar]
- [246].Li F; Sethi G, Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta, 2010, 1805 (2), 167–180. [DOI] [PubMed] [Google Scholar]
- [247].Musgrove EA; Sutherland RL, Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer, 2009, 9(9), 631–643. [DOI] [PubMed] [Google Scholar]
- [248].Pradhan M; Bembinster LA; Baumgarten SC; Frasor J, Proinflammatory cytokines enhance estrogen-dependent expression of the multidrug transporter gene ABCG2 through estrogen receptor and NF{kappa}B cooperativity at adjacent response elements. J. Biol. Chem, 2010, 285 (41), 31100–31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Wang X; Wu X; Wang C; Zhang W; Ouyang Y; Yu Y; He Z, Transcriptional suppression of breast cancer resistance protein (BCRP) by wild-type p53 through the NF-kappaB pathway in MCF-7 cells. FEBS letters, 2010, 584 (15), 3392–3397. [DOI] [PubMed] [Google Scholar]
- [250].Wang Y; Wang X; Zhao H; Liang B; Du Q, Clusterin confers resistance to TNF-alpha-induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J. Che-mother, 2012, 24 (6), 348–357. [DOI] [PubMed] [Google Scholar]
- [251].Wang QP; Wang Y; Wang XD; Mo XM; Gu J; Lu ZY; Pan ZL; Zhu YX, Survivin up-regulates the expression of breast cancer resistance protein (BCRP) through attenuating the suppression of p53 on NF-kappaB expression in MCF-7/5-FU cells. Int. J. Biochem. Cell Biol, 2013, 45 (9), 2036–2044. [DOI] [PubMed] [Google Scholar]
- [252].Esparza-Lopez J; Medina-Franco H; Escobar-Arriaga E; Leon-Rodriguez E; Zentella-Dehesa A; Ibarra-Sanchez MJ, Doxorubicin induces atypical NF-kappaB activation through c-Abl kinase activity in breast cancer cells. J. Cancer Res. Clin. Oncol, 2013, 139 (10), 1625–1635. [DOI] [PubMed] [Google Scholar]
- [253].Ho WC; Dickson KM; Barker PA, Nuclear factor-kappaB induced by doxorubicin is deficient in phosphorylation and acetylation and represses nuclear factor-kappaB-dependent transcription in cancer cells. Cancer Res, 2005, 65 (10), 4273–4281. [DOI] [PubMed] [Google Scholar]
- [254].Korkaya H; Kim GI; Davis A; Malik F; Henry NL; Ithimakin S; Quraishi AA; Tawakkol N; D’Angelo R; Paulson AK; Chung S; Luther T; Paholak HJ; Liu S; Hassan KA; Zen Q; Clouthier SG; Wicha MS, Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell, 2012, 47 (4), 570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Xia W; Bacus S; Husain I; Liu L; Zhao S; Liu Z; Moseley MA 3rd; Thompson JW; Chen FL; Koch KM; Spector NL, Resistance to ErbB2 tyrosine kinase inhibitors in breast cancer is mediated by calcium-dependent activation of RelA. Mol. Cancer Ther, 2010, 9 (2), 292–299. [DOI] [PubMed] [Google Scholar]
- [256].Park KJ; Krishnan V; O’Malley BW; Yamamoto Y; Gaynor RB, Formation of an IKKalpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol. Cell, 2005, 18 (1), 71–82. [DOI] [PubMed] [Google Scholar]
- [257].Nam JM; Ahmed KM; Costes S; Zhang H; Onodera Y; Olshen AB; Hatanaka KC; Kinoshita R; Ishikawa M; Sabe H; Shirato H; Park CC, Beta1-integrin via NF-kappaB signaling is essential for acquisition of invasiveness in a model of radiation treated in situ breast cancer. Breast Cancer Res, 2013, 15 (4), R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Huang Y; Fan W, IkappaB kinase activation is involved in regulation of paclitaxel-induced apoptosis in human tumor cell lines. Mol. Pharmacol, 2002, 61 (1), 105–113. [DOI] [PubMed] [Google Scholar]
- [259].Ahmed KM; Zhang H; Park CC, NF-kappaB regulates radioresistance mediated by beta1-integrin in three-dimensional culture of breast cancer cells. Cancer Res, 2013, 73 (12), 3737–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Brach MA; Hass R; Sherman ML; Gunji H; Weichselbaum R; Kufe D, Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J. Clin. Invest, 1991, 88 (2), 691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Li N; Karin M, Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms., Proc. Natl. Acad. Sci. USA, 1998, 95 (22), 13012–13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Ahmed KM; Dong S; Fan M; Li JJ, Nuclear factor-kappaB p65 inhibits mitogen-activated protein kinase signaling pathway in radioresistant breast cancer cells. Mol. Cancer Res, 2006, 4 (12), 945–955. [DOI] [PubMed] [Google Scholar]
- [263].Cao N; Li S; Wang Z; Ahmed KM; Degnan ME; Fan M; Dynlacht JR; Li JJ, NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiation Res, 2009, 171 (1), 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Aggarwal BB; Van Kuiken ME; Iyer LH; Harikumar KB; Sung B, Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumori-genesis. Exp. Biol. Med. (Maywood), 2009, 234 (8), 825–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Aggarwal BB; Shishodia S; Takada Y; Banerjee S; Newman RA; Bueso-Ramos CE; Price JE, Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res, 2005, 11 (20), 7490–7498. [DOI] [PubMed] [Google Scholar]
- [266].Howe LR; Subbaramaiah K; Patel J; Masferrer JL; Deora A; Hudis C; Thaler HT; Muller WJ; Du B; Brown AM; Dannenberg AJ, Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res, 2002, 62 (19), 5405–5407. [PubMed] [Google Scholar]
- [267].Nag SA; Qin JJ; Wang W; Wang MH; Wang H; Zhang R, Ginsenosides as Anticancer Agents: In vitro and in vivo Activities, Structure-Activity Relationships, and Molecular Mechanisms of Action. Front. Pharmacol, 2012, 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Li L; Wang Y; Qi B; Yuan D; Dong S; Guo D; Zhang C; Yu M, Suppression of PMA-induced tumor cell invasion and mi gration by ginsenoside Rg1 via the inhibition of NF-kappaB-dependent MMP-9 expression. Oncol. Rep, 2014, 32 (5), 1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Kim BM; Kim DH; Park JH; Surh YJ; Na HK, Ginsenoside Rg3 Inhibits Constitutive Activation of NF-κB Signaling in Human Breast Cancer (MDA-MB-231) Cells: ERK and Akt as Potential Upstream Targets. J. Cancer. Prev, 2014, 19, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Peralta EA; Murphy LL; Minnis J; Louis S; Dunnington GL, American Ginseng inhibits induced COX-2 and NFKB activation in breast cancer cells. J. Surg. Res, 2009, 157 (2), 261–267. [DOI] [PubMed] [Google Scholar]
- [271].Rebbeck TR; Troxel AB; Norman S; Bunin GR; DeMichele A; Baumgarten M; Berlin M; Schinnar R; Strom BL, A retrospective case-control study of the use of hormone-related supplements and association with breast cancer. Int. J. Cancer, 2007, 120 (7), 1523–1528. [DOI] [PubMed] [Google Scholar]
- [272].Cui Y; Shu XO; Gao YT; Cai H; Tao MH; Zheng W, Association of ginseng use with survival and quality of life among breast cancer patients. Am. J. Epedemiol, 2006, 163 (7), 645–653. [DOI] [PubMed] [Google Scholar]
- [273].Lee SY; Seol JY; Park KH; Park GM; Hwang YI; Kim CH; Jang SH; Kwon SY; Yoo CG; Kim YW; Han SK; Shim YS; Lee CT The Effect of IκBα-SR Gene Transfer on the Sensitivity of Human Lung Cancer Cell Lines to Cisplatin and Paclitaxel. Tuberc. Respir. Dis. (Seoul), 2001, 51(2), 122–134. [Google Scholar]
- [274].Higgins KA; Perez JR; Coleman TA; Dorshkind K; McComas WA; Sarmiento UM; Rosen CA; Narayanan R, Antisense inhibition of the p65 subunit of NF-kappa B blocks tumorigenicity and causes tumor regression. Proc. Natl. Acad. Sci. USA, 1993, 90 (21), 9901–9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Xu Y; Fang F; St Clair DK; Sompol P; Josson S; St Clair WH, SN52, a novel nuclear factor-kappaB inhibitor, blocks nuclear import of RelB:p52 dimer and sensitizes prostate cancer cells to ionizing radiation. Mol. Cancer Ther, 2008, 7 (8), 2367–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Mabuchi S; Ohmichi M; Nishio Y; Hayasaka T; Kimura A; Ohta T; Saito M; Kawagoe J; Takahashi K; Yada-Hashimoto N; Sakata M; Motoyama T; Kurachi H; Tasaka K; Murata Y, Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J. Biol. Chem, 2004, 279(22), 23477–23485. [DOI] [PubMed] [Google Scholar]
- [277].Vinod BS; Antony J; Nair HH; Puliyappadamba VT; Saikia M; Narayanan SS; Bevin A; Anto RJ, Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis, 2013, 4, e505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Orange JS; May MJ, Cell penetrating peptide inhibitors of nuclear factor-kappa B. Cell Mol. Life Sci, 2008, 65 (22), 3564–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Fabre C; Mimura N; Bobb K; Kong SY; Gorgun G; Cirstea D; Hu Y; Minami J; Ohguchi H; Zhang J; Meshulam J; Carrasco RD; Tai YT; Richardson PG; Hideshima T; Anderson KC, Dual inhibition of canonical and noncanonical NF-kappaB pathways demonstrates significant antitumor activities in multiple myeloma. Clin. Cancer Res, 2012, 18 (17), 4669–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Hunter JE; Willmore E; Irving JA; Sliskovic D; Monie D; Bobb K; Zhang J; Durkacz BW The radiosensitizing and cytotoxic effects of PBS-1086, a Rel inhibitor of NF-KB, in breast cancer cells. Cancer Res, 2010, 70 (8 Supplement), 3561. [Google Scholar]
- [281].Patel NM; Nozaki S; Shortle NH; Bhat-Nakshatri P; Newton TR; Rice S; Gelfanov V; Boswell SH; Goulet RJ Jr.; Sledge GW Jr.; Nakshatri H, Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene, 2000, 19 (36), 4159–4169. [DOI] [PubMed] [Google Scholar]
- [282].Zhou J; Zhang H; Gu P; Bai J; Margolick JB; Zhang Y, NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res. Treat, 2008, 111 (3), 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Matsumoto N; Ariga A; To-e S; Nakamura H; Agata N; Hirano S; Inoue J; Umezawa K, Synthesis of NF-kappaB activation inhibitors derived from epoxyquinomicin C. Bioorg. Med. Chem. Lett, 2000, 10 (9), 865–869. [DOI] [PubMed] [Google Scholar]
- [284].Matsumoto G; Namekawa J; Muta M; Nakamura T; Bando H; Tohyama K; Toi M; Umezawa K, Targeting of nuclear factor kappaB Pathways by dehydroxymethylepoxyquinomicin, a novel inhibitor of breast carcinomas: antitumor and antiangiogenic potential in vivo. Clin. Cancer Res, 2005, 11 (3), 1287–1293. [PubMed] [Google Scholar]
- [285].Takada Y; Gillenwater A; Ichikawa H; Aggarwal BB, Suberoylanilide hydroxamic acid potentiates apoptosis, inhibits in vasion, and abolishes osteoclastogenesis by suppressing nuclear factor-kappaB activation. J. Biol. Chem, 2006, 281 (9), 5612–5622. [DOI] [PubMed] [Google Scholar]
- [286].Domingo-Domenech J; Pippa R; Tapia M; Gascon P; Bachs O; Bosch M, Inactivation of NF-kappaB by proteasome inhibition contributes to increased apoptosis induced by histone deacetylase inhibitors in human breast cancer cells. Breast Cancer Res. Treat, 2008, 112 (1), 53–62. [DOI] [PubMed] [Google Scholar]
- [287].Grant S, Advances in Cancer Research Volume 116, Histone Deacetylase Inhibitors as Cancer Therapeutics Academic Press, 2012. [DOI] [PubMed] [Google Scholar]
- [288].Disch JS; Evindar G; Chiu CH; Blum CA; Dai H; Jin L; Schuman E; Lind KE; Belyanskaya SL; Deng J; Coppo F; Aquilani L; Graybill TL; Cuozzo JW; Lavu S; Mao C; Vlasuk GP; Perni RB, Discovery of thieno[3,2-d]pyrimidine-6-carboxamides as potent inhibitors of SIRT1, SIRT2, and SIRT3. J. Med. Chem, 2013, 56 (9), 3666–3679. [DOI] [PubMed] [Google Scholar]
- [289].Fernandes CA; Fievez L; Neyrinck AM; Delzenne NM; Bureau F; Vanbever R, Sirtuin inhibition attenuates the production of inflammatory cytokines in lipopolysaccharide-stimulated macrophages. Biochem. Biophys. Res. Comm, 2012, 420 (4), 857–861. [DOI] [PubMed] [Google Scholar]
- [290].Yang CH; Gonzalez-Angulo AM; Reuben JM; Booser DJ; Pusztai L; Krishnamurthy S; Esseltine D; Stec J; Broglio KR; Islam R; Hortobagyi GN; Cristofanilli M, Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann. Oncol, 2006, 17 (5), 813–817. [DOI] [PubMed] [Google Scholar]
- [291].Chen D; Frezza M; Schmitt S; Kanwar J; Dou QP, Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr. Cancer Drug Targets, 2011, 11 (3), 239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Kuhn DJ; Chen Q; Voorhees PM; Strader JS; Shenk KD; Sun CM; Demo SD; Bennett MK; van Leeuwen FW; Chanan-Khan AA; Orlowski RZ, Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood, 2007, 110 (9), 3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Shank BR; Brown VT; Schwartz RN, Multiple myeloma maintenance therapy: A review of the pharmacologic treatment. J. Oncol. Pharm. Pract, 2014. [DOI] [PubMed] [Google Scholar]
- [294].Molineaux SM, Molecular pathways: targeting proteasomal protein degradation in cancer. Clin. Cancer Res, 2012, 18 (1), 15–20. [DOI] [PubMed] [Google Scholar]
- [295].Fuchs O, Targeting of NF-kappaB signaling pathway, other signaling pathways and epigenetics in therapy of multiple myeloma. Cardiovasc. Hematol Disord. Drug Targets, 2013, 13 (1), 16–34. [DOI] [PubMed] [Google Scholar]
- [296].Soucy TA; Smith PG; Milhollen MA; Berger AJ; Gavin JM; Adhikari S; Brownell JE; Burke KE; Cardin DP; Critchley S; Cullis CA; Doucette A; Garnsey JJ; Gaulin JL; Gershman RE; Lublinsky AR; McDonald A; Mizutani H; Narayanan U; Olhava EJ; Peluso S; Rezaei M; Sintchak MD; Talreja T; Thomas MP; Traore T; Vyskocil S; Weatherhead GS; Yu J; Zhang J; Dick LR; Claiborne CF; Rolfe M; Bolen JB; Langston SP, An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature, 2009, 458 (7239), 732–736. [DOI] [PubMed] [Google Scholar]
- [297].Gomez-Cabrero A; Wrasidlo W; Reisfeld RA, IMD-0354 targets breast cancer stem cells: a novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PloS one, 2013, 8 (8), e73607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Viola K; Kopf S; Huttary N; Vonach C; Kretschy N; Teich-mann M; Giessrigl B; Raab I; Stary S; Krieger S; Keller T; Bauer S; Hantusch B; Szekeres T; de Martin R; Jager W; Mikulits W; Dolznig H; Krupitza G; Grusch M, Bay11–7082 inhibits the disintegration of the lymphendothelial barrier triggered by MCF-7 breast cancer spheroids; the role of ICAM-1 and adhesion. Br. J. Cancer, 2013, 108 (3), 564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Peinado C; Kang X; Hardamon C; Arora S; Mah S; Zhang H; Ngolab J; Bui JD, The nuclear factor-kappaB pathway down-regulates expression of the NKG2D ligand H60a in vitro: implications for use of nuclear factor-kappaB inhibitors in cancer therapy. Immunology, 2013, 139 (2), 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Hideshima T; Neri P; Tassone P; Yasui H; Ishitsuka K; Raje N; Chauhan D; Podar K; Mitsiades C; Dang L; Munshi N; Richardson P; Schenkein D; Anderson KC, MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clinical Cancer Res, 2006, 12 (19), 5887–5894. [DOI] [PubMed] [Google Scholar]
- [301].Singh S; Shi Q; Bailey ST; Palczewski MJ; Pardee AB; Iglehart JD; Biswas DK, Nuclear factor-kappaB activation: a molecular therapeutic target for estrogen receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer. Mol. Cancer Ther, 2007, 6 (7), 1973–1982. [DOI] [PubMed] [Google Scholar]
- [302].Casas A; Llombart A; Martin M, Denosumab for the treatment of bone metastases in advanced breast cancer. Breast, 2013, 22 (5), 585–592. [DOI] [PubMed] [Google Scholar]
- [303].Liu D; Chen Z, The effect of curcumin on breast cancer cells. J. Breast Cancer, 2013, 16 (2), 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Pozo-Guisado E; Merino JM; Mulero-Navarro S; Lorenzo-Benayas MJ; Centeno F; Alvarez-Barrientos A; Fernandez-Salguero PM, Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int. J. Cancer, 2005, 115(1), 74–84. [DOI] [PubMed] [Google Scholar]
- [305].Sethi G; Ahn KS; Aggarwal BB, Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol. Cancer Res, 2008, 6 (6), 1059–1070. [DOI] [PubMed] [Google Scholar]
- [306].Loganathan R; Selvaduray KR; Nesaretnam K; Radhakrishnan AK, Tocotrienols promote apoptosis in human breast cancer cells by inducing poly(ADP-ribose) polymerase cleavage and inhibiting nuclear factor kappa-B activity. Cell Prolif, 2013, 46 (2), 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Chattopadhyay M; Kodela R; Nath N; Barsegian A; Boring D; Kashfi K, Hydrogen sulfide-releasing aspirin suppresses NF-kappaB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem. Pharmacol 2012, 83 (6), 723–732. [DOI] [PubMed] [Google Scholar]
- [308].DuBois RN, Aspirin and breast cancer prevention - The estrogen connection. J. Am. Med. Assoc, 2004, 291 (20), 2488–2489. [DOI] [PubMed] [Google Scholar]
- [309].Lazzeroni M; Petrera M; Marra D; DeCensi A, Aspirin and Breast Cancer Prevention. Curr. Breast Cancer Rep, 2013, 5 (3), 202–207. [Google Scholar]
- [310].Wang H; Wang Y; Rayburn ER; Hill DL; Rinehart JJ; Zhang R, Dexamethasone as a chemosensitizer for breast cancer chemotherapy: potentiation of the antitumor activity of adriamycin, modulation of cytokine expression, and pharmacokinetics. Int. J. Oncol, 2007, 30 (4), 947–953. [PubMed] [Google Scholar]
- [311].Campbell MJ; Esserman LJ; Zhou Y; Shoemaker M; Lobo M; Borman E; Baehner F; Kumar AS; Adduci K; Marx C; Petricoin EF; Liotta LA; Winters M; Benz S; Benz CC, Breast cancer growth prevention by statins. Cancer Res, 2006, 66(17), 8707–8714. [DOI] [PubMed] [Google Scholar]
- [312].Denoyelle C; Vasse M; Korner M; Mishal Z; Ganne F; Vannier JP; Soria J; Soria C, Cerivastatin, an inhibitor of HMGCoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis, 2001, 22 (8), 1139–1148. [DOI] [PubMed] [Google Scholar]
- [313].Carey LA; Rugo HS; Marcom PK; Mayer EL; Esteva FJ; Ma CX; Liu MC; Storniolo AM; Rimawi MF; Forero-Torres A; Wolff AC; Hobday TJ; Ivanova A; Chiu WK; Ferraro M; Burrows E; Bernard PS; Hoadley KA; Perou CM; Winer EP, TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J. Clin. Oncol, 2012, 30 (21), 2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Krempski J; Karyampudi L; Behrens MD; Erskine CL; Hartmann L; Dong H; Goode EL; Kalli KR; Knutson KL, Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J.Immunol, 2011, 186 (12), 6905–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Ascierto Paolo A.; Munn DH; Palucka AK; Sondel PM, Highlights and summary of the 28th annual meeting of the Society for Immunotherapy of Cancer. J. Immunother. Cancer, 2014, 2, 15. [Google Scholar]
- [316].Qin JJ; Jin HZ; Huang Y; Zhang SD; Shan L; Voruganti S; Nag S; Wang W; Zhang WD; Zhang R, Selective cytotoxicity, inhibition of cell cycle progression, and induction of apoptosis in human breast cancer cells by sesquiterpenoids from Inula lineariifolia Turcz. Eur. J. Med. Chem, 2013, 68, 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Li X; Yang X; Liu Y; Gong N; Yao W; Chen P; Qin J; Jin H; Li J; Chu R; Shan L; Zhang R; Zhang W; Wang H, Japonicone A suppresses growth of Burkitt lymphoma cells through its effect on NF-kappaB. Clin. Cancer Res, 2013, 19 (11), 2917–2928. [DOI] [PubMed] [Google Scholar]
- [318].Galanis A; Pappa A; Giannakakis A; Lanitis E; Dangaj D; Sandaltzopoulos R, Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett, 2008, 266 (1), 12–20. [DOI] [PubMed] [Google Scholar]
- [319].Siomek A, NF-kappaB signaling pathway and free radical impact. Acta Biochim. Pol, 2012, 59 (3), 323–331. [PubMed] [Google Scholar]
- [320].Epinat JC; Gilmore TD, Diverse agents act at multiple levels to inhibit the Rel/NF-kappaB signal transduction pathway. Onco-gene, 1999, 18 (49), 6896–6909. [DOI] [PubMed] [Google Scholar]
- [321].Adhikary A; Mohanty S; Lahiry L; Hossain DM; Chakraborty S; Das T, Theaflavins retard human breast cancer cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS Lett, 2010, 584 (1), 7–14. [DOI] [PubMed] [Google Scholar]
- [322].Eytan E; Ganoth D; Armon T; Hershko A, ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc. Natl. Acad. Sci. USA, 1989, 86 (20), 7751–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Tanaka K; Mizushima T; Saeki Y, The proteasome: molecular machinery and pathophysiological roles. Biol. Chem, 2012, 393(4), 217–234. [DOI] [PubMed] [Google Scholar]
- [324].Kisselev AF; van der Linden WA; Overkleeft HS, Protea-some inhibitors: an expanding army attacking a unique target. Chem. Biol, 2012, 19 (1), 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Russo SM; Tepper JE; Baldwin AS Jr.; Liu R; Adams J; Elliott P; Cusack JC Jr., Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int. J. Rad. Oncol. Biol. Phys, 2001, 50 (1), 183–193. [DOI] [PubMed] [Google Scholar]
- [326].Cardoso F; Durbecq V; Laes JF; Badran B; Lagneaux L; Bex F; Desmedt C; Willard-Gallo K; Ross JS; Burny A; Piccart M; Sotiriou C, Bortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER-2-positive breast cancer cells in a synergistic manner. Mol. Cancer Ther, 2006, 5(12), 3042–3051. [DOI] [PubMed] [Google Scholar]
- [327].Brandao RD; Veeck J; Van de Vijver KK; Lindsey P; de Vries B; van Elssen CH; Blok MJ; Keymeulen K; Ayoubi T; Smeets HJ; Tjan-Heijnen VC; Hupperets PS, A randomised controlled phase II trial of pre-operative celecoxib treatment reveals anti-tumour transcriptional response in primary breast cancer. Breast Cancer Res, 2013, 15 (2), R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].van Wijngaarden J; van Beek E; van Rossum G; van der Bent C; Hoekman K; van der Pluijm G; van der Pol MA; Broxterman HJ; van Hinsbergh VW; Lowik CW, Celecoxib enhances doxorubicin-induced cytotoxicity in MDA-MB231 cells by NF-kappaB-mediated increase of intracellular doxorubicin accumulation. Eur. J. Cancer, 2007, 43 (2), 433–442. [DOI] [PubMed] [Google Scholar]
- [329].Wang L; Kang F; Li J; Zhang J; Shan B, Overexpression of p65 attenuates celecoxib-induced cell death in MDA-MB-231 human breast cancer cell line. Cancer Cell Int, 2013, 13 (1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Yamamoto Y; Yin MJ; Lin KM; Gaynor RB, Sulindac inhibits activation of the NF-kappaB pathway. J. Biol. Chem, 1999, 274 (38), 27307–27314. [DOI] [PubMed] [Google Scholar]
- [331].Scheinman RI; Gualberto A; Jewell CM; Cidlowski JA; Baldwin AS Jr., Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol. Cell Biol, 1995, 15 (2), 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].McKay LI; Cidlowski JA, CBP (CREB binding protein) integrates NF-kappaB (nuclear factor-kappaB) and glucocorticoid receptor physical interactions and antagonism. Mol. Endocrinol, 2000, 14 (8), 1222–1234. [DOI] [PubMed] [Google Scholar]
- [333].Gamble C; McIntosh K; Scott R; Ho KH; Plevin R; Paul A, Inhibitory kappa B Kinases as targets for pharmacological regulation. Br. J. Pharmacol, 2012, 165 (4), 802–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Biswas DK; Shi Q; Baily S; Strickland I; Ghosh S; Pardee AB; Iglehart JD, NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc. Natl. Acad. Sci. USA, 2004, 101 (27), 10137–10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Meffert MK; Chang JM; Wiltgen BJ; Fanselow MS; Baltimore D, NF-kappa B functions in synaptic signaling and behavior. Nature Neuroscience 2003, 6 (10), 1072–1078. [DOI] [PubMed] [Google Scholar]
- [336].Luedde T; Beraza N; Kotsikoris V; van Loo G; Nenci A; De Vos R; Roskams T; Trautwein C; Pasparakis M, Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell, 2007, 11 (2), 119–132. [DOI] [PubMed] [Google Scholar]
- [337].Maeda S; Kamata H; Luo JL; Leffert H; Karin M, IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell, 2005, 121 (7), 977–990. [DOI] [PubMed] [Google Scholar]
- [338].Chen F; Castranova V, Nuclear factor-kappaB, an unappreciated tumor suppressor. Cancer Res, 2007, 67 (23), 11093–11098. [DOI] [PubMed] [Google Scholar]
- [339].Klein U; Ghosh S, The Two Faces of NF-kappaB Signaling in Cancer Development and Therapy. Cancer Cell, 2011, 20 (5), 556–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Mowla SN; Perkins ND; Jat PS, Friend or foe: emerging role of nuclear factor kappa-light-chain-enhancer of activated B cells in cell senescence. Onco. Targets Ther, 2013, 6, 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Perkins ND, NF-kappaB: tumor promoter or suppressor? Trends Cell Biol, 2004, 14 (2), 64–69. [DOI] [PubMed] [Google Scholar]
- [342].Liu F; Bardhan K; Yang D; Thangaraju M; Ganapathy V; Waller JL; Liles GB; Lee JR; Liu K, NF-kappaB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J. Biol. Chem, 2012, 287 (30), 25530–25540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Chien Y; Scuoppo C; Wang X; Fang X; Balgley B; Bolden JE; Premsrirut P; Luo W; Chicas A; Lee CS; Kogan SC; Lowe SW, Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev, 2011, 25 (20), 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Jing H; Kase J; Dorr JR; Milanovic M; Lenze D; Grau M; Beuster G; Ji S; Reimann M; Lenz P; Hummel M; Dorken B; Lenz G; Scheidereit C; Schmitt CA; Lee S, Opposing roles of NF-kappaB in anti-cancer treatment outcome unveiled by cross-species investigations. Genes Dev, 2011, 25 (20), 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Chen YJ; Yeh MH; Yu MC; Wei YL; Chen WS; Chen JY; Shih CY; Tu CY; Chen CH; Hsia TC; Chien PH; Liu SH; Yu YL; Huang WC, Lapatinib-induced NF-kappaB activation sensitizes triple-negative breast cancer cells to protea-some inhibitors. Breast Cancer Res, 2013, 15 (6), R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Klarenbeek S; van Miltenburg MH; Jonkers J, Genetically engineered mouse models of PI3K signaling in breast cancer. Mol. Oncol, 2013, 7 (2), 146–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Hutti JE; Pfefferle AD; Russell SC; Sircar M; Perou CM; Baldwin AS, Oncogenic PI3K mutations lead to NF-kappaB-dependent cytokine expression following growth factor deprivation. Cancer Res, 2012, 72 (13), 3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Marczylo TH; Verschoyle RD; Cooke DN; Morazzoni P; Steward WP; Gescher AJ, Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chem. Pharmacol, 2007, 60 (2), 171–177. [DOI] [PubMed] [Google Scholar]
- [349].Kanai M; Imaizumi A; Otsuka Y; Sasaki H; Hashiguchi M; Tsujiko K; Matsumoto S; Ishiguro H; Chiba T, Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chem. Pharmacol, 2012, 69 (1), 65–70. [DOI] [PubMed] [Google Scholar]