Abstract
The clinical need for organ replacement therapies has inspired the idea of growing human organs in animal hosts. The injection of human pluripotent stem cells into animal blastocysts provides a possible strategy to accomplish this goal. A recent study in Cell tests the feasibility of this approach by creating chimeric embryos between humans and large domestic animals, including pigs and cattle. The study further examines the potential of combining CRISPR/Cas9 gene editing with blastocyst complementation to grow fully foreign organs in chimeric hosts. Here, we consider what this report and related studies reveal about the likelihood of human-animal chimeras reaching the clinic and translating into therapies. A careful look suggests hope for eventual success in this area, but also underscores important challenges that will require dedicated effort to resolve.
Keywords: kidney transplantation, gene editing, xenotransplantation, embryonic stem cell, induced pluripotent stem cell, organ farming, IBC, morula
“Ultimately, these observations also raise the possibility of xeno-generating transplantable human tissues and organs towards addressing the worldwide shortage of organ donors” – so concludes Interspecies Chimerism with Mammalian Pluripotent Stem Cells, one of the first studies to investigate the potential of creating human-animal chimeras in embryos of large domestic species [1]. Studies in this field may one day culminate in the farming of human organs grown inside animals, for transplantation purposes. An exciting and lofty goal – but how realistic is it? Here, taking a critical eye to the data, we consider what this study reveals about the likelihood of human organs grown inside animals eventually reaching the clinic.
It should be noted at the outset that these are difficult experiments. Speciation is a difficult paradigm to shift, and early embryos have little tolerance for error. Previous work in this area has primarily focused on injection of human pluripotent stem cells (hPSCs, including both embryonic and induced pluripotent stem cells) into rat or mouse blastocyst-stage embryos. Chimera formation is routinely very low in these experiments, but whether this is also true in larger animals has not yet been investigated. Few laboratories have the resources and dedication required to pursue such experiments, which are currently subject to a funding moratorium by the National Institutes of Health (https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15–158.html). The successful completion of these studies is therefore a significant accomplishment.
Chimera formation in rodent species using CRISPR-IBC
The formation of human-animal chimeras is not a new concept. There are numerous examples in the literature of hPSCs contributing to chimeric embryo formation in rodent hosts [2–6]. However, none of these has achieved high levels of chimerism in an embryo, much less produce a viable chimera. Indeed, generation of chimeras between any two species, even related ones, is a challenge, and has only been successful between mouse species, between rats and mice, and between goats and sheep [7–9]. Such chimeras have been formed by combining blastocyst cells from the two species into a single embyro, either by aggregation or injection.
Based on these previous studies, Wu et al. begin their experiments in rodent blastocysts. To distinguish donor cells from host, the authors label rat pluripotent stem cells with a genetically-encoded red fluorescent marker (humanized Kusabira Orange, or hKO), introduced by transfection or viral transduction. This labeling method is convenient, as it can be applied to any cell line of interest. However, because the hKO DNA is introduced exogenously, there is a risk that it might label non-donor cells (for instance, feeder cells in hPSC cultures, or cells of the recipient embryo), resulting in false positives. It is therefore significant that the authors utilize secondary methods, such as species-specific RT-PCR and immunostaining, to confirm their findings.
In the first set of experiments, the hKO-labeled rat stem cells are injected into mouse blastocysts followed by embryo transfer into surrogate mice. “Robust” chimerism is observed, which means that ~ 20 % of embryos show some evidence of chimerism. However, when quantified by RT-PCR, the actual contribution of rat cells to mouse tissues is low, ranging from less than 0.01 % up to a maximum of 10 % of all cells within an organ, and typically less than 1 %. This quantitative analysis appears to have been performed on a single mouse, making it difficult to generalize. Red fluorescent cells are also observed in the gall bladder, an organ that does not form in rats, although confirmatory RT-PCR data for this is not presented.
Having established this baseline for rat-mouse chimerism, the authors proceed to test a technique called interspecies blastocyst complementation (IBC), which was introduced in 2010 as a variation on the classic blastocyst complementation technique [8,10]. In IBC, the recipient blastocyst carries a mutation that makes it deficient in a particular type of tissue or organ. This creates a genetic vacuum (niche) that can only be filled by the interspecies cells, greatly increasing the level of chimerism within that organ. Here, the use of the CRISPR-Cas9 gene editing system is developed for this purpose. Cas9 is a programmable endonuclease that can be co-expressed with a guide RNA to introduce double-stranded breaks at specific genetic loci [11]. Use of CRISPR enables testing of IBC in a variety of different organs. The authors state that ~ 50 % of their CRISPR-targeted mice were knockouts (homozygous nulls), as opposed to just ~ 20 % mosaics, which is a very impressive rate of mutagenesis. As even a low degree of mosiacism in the target organs could drastically affect the outcome of these experiments, including the raw genotyping data would have been useful here.
Armed with the CRISPR-IBC technique, the authors perform IBC experiments at a scale not previously been attained. They succeed not only in reproducing the ‘rat pancreas in a mouse’ experiment by targeting Pdx1, but also in generating rat heart and eye in a mouse by targeting Nkx2.5 and Pax6, respectively. The resultant organs are ‘enriched’ for rat cells, meaning that they contained donor cells but are not pure. For instance, endothelial cells within the IBC pancreas are derived from host and not donor. It seems likely that knockout of multiple genes simultaneously will be required to enable generation of a ‘pure’ human organ in an animal (Fig. 1).
Fig. 1.
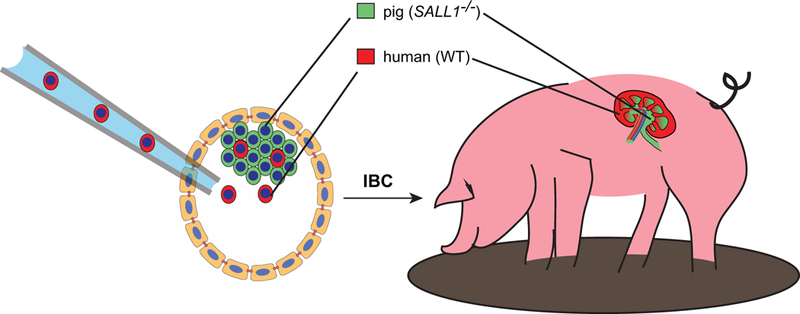
Growth of human organs in pigs. A conceptual rendering is shown depicting the possible use of IBC to produce organ-specific chimeras in large domestic species. Implantation of naive hPSCs into a SALL1−/− porcine embryo (left) results in the formation of a blastocyst chimera. The ‘wild-type’ (WT) human cells establish an organogenic niche within the developing embryo, enabling it to grow a kidney. Human cells contribute negligibly to the rest of the animal. As the kidney is a complex organ, involving many different cell types, only part of the kidney is human, whereas other parts are pig and would be highly vulnerable to immune rejection in patient recipients.
Notably, although chimeras with hearts enriched with rat cells can be obtained in CRISPR-mutant Nkx2.5 embryos, and the hearts appear normal, these embryos fail to survive to birth (12/12 embryos). Similarly, it has previously been observed that kidney agenesis in Sal1−/− mouse embryos can be rescued using blastocyst complementation using cells from wild-type mice, but the resultant animals do not survive until adulthood [12]. Furthermore, rat cells do not succeed in rescuing kidney development in these embryos, pointing to a specific incompatibility between mouse and rat [12]. Collectively, these findings indicate that IBC in certain organs may be incompatible with life, for reasons that are not clear.
Human chimerism in embryos of large domestic species
Having introduced techniques to generate interspecific chimeras in rodent species, the authors shift gears to attempting chimerism between humans and larger domestic species, including cows and pigs. The use of such larger host animals may be required for the vision of ‘farming organs’ to become a reality, as the size of their organs is much closer to that of humans. Here, the experiments in larger animals are limited to formation of ‘wild-type’ chimeras, whereas IBC is not attempted. Nor is the goal to be to produce a viable chimera, as in all cases the experiments are terminated early in gestation. Rather, the aim of these experiments is to determine which cells, if any, can engraft in domestic species during the early stages of embryogenesis.
Initially, the authors attempt to implant mouse and rat pluripotent stem cells into pig embryos, but no contribution from these cells is observed after three weeks of gestation, in a total of forty-five embryos. A cohort of human iPS cells is then tested for their ability to survive in pig and cattle blastocysts. These represent a spectrum of pluripotent states, ranging from ‘naïve’ hPSCs modeling the inner cell mass of the pre-implantation blastocyst, to ‘primed’ hPSCs modeling the implantation-stage embryonic epiblast (‘primed’). Although hPSCs in cell culture favor the primed state, naïve (also called ground state) hPSCs have been isolated by several groups using chemically-defined conditions [4,13,14]. In this case, the cohort examined includes iPS cells derived in four different conditions, known as NHSM, FAC, 4i, and 2iLD. The first three of these represent the use of different media formulations for iPS cell derivation, whereas the 2iLD condition also involves exogenous expression of pluripotency genes in primed hPSCs to ‘revert’ these to a naïve state.
Importantly, all of these hPSCs appear to have been freshly derived by the authors from the same parental human fibroblast cell line – otherwise, these lines would be impossible to compare to one another due to differences in history and genetic background. Non-integrating episomes are used to reprogram these fibroblasts, which reduces the likelihood that differences between lines might be due to off-target effects of genetic integration. Each of the new lines is shown to form teratomas in immunodeficient mice, demonstrating that they are bona fide pluripotent cells. However, the purity of the undifferentiated cultures is not reported, and the images suggest possible contamination with differentiating descendant cells. It is also unclear whether the authors used multiple hPSC lines for each condition, or only a single clone. As every hPSC line is different, the use of multiple lines for each condition is a prerequisite for comparing them in a robust and reliable way. Inclusion of these data and details would have made this experiment more compelling and easier to interpret.
The cohort of hPSCs is labeled with a fluorescence tracer, similar to the rodent cells. When primed hPSC lines are injected into pre-implantation blastocysts (10 cells/embryo), they do not survive. This is not surprising, given the mismatch between developmental stage between cell and embryo, and the propensity of primed hPSCs to undergo apoptosis upon dissociation [3,15]. In contrast, when the naïve hPSC lines are injected, they are detected in the embryos after 48 hours of incubation in vitro, and maintain pluripotency marker expression. Although in most cases the number of human cells in the embryos decreases over 48 hours, a modest ability to proliferate is also observed in some of the experiments, particularly the NHSM cells in cattle embryos.
Using these optimization experiments as a baseline, the researchers further test whether these naïve human cell lines can contribute chimerically to pig embryo tissues. To do this, they perform injections of over 2,000 blastocysts, resulting in ~ 200 embryos for analysis three weeks later. One-third of these embryos showed some evidence of fluorescent cell contribution, and showed increased growth retardation, compared to non-fluorescent controls. In only one of the conditions (FAC), thought to represent an ‘intermediate’ cell type between naïve and primed pluripotency, could human cells be clearly detected in the resultant embryonic tissues, which could be co-localized with lineage-specific markers such as smooth muscle alpha actin and neuron-specific beta tubulin. However, human-pig chimerism was observed to be much lower than in rat-mouse chimeric embryos. In this regard, it would be interesting to perform these assays in ‘EPS’ cells, a recently-derived condition that is transcriptionally distinct from naïve and primed, and which promotes hPSC chimerism in rodent recipient embryos [16].
Conclusions and outlook
Overall, the studies described in this paper are bold and advance the state of the art of human-animal chimerism. Interspecies chimerism remains highly inefficient, which makes the resulting numbers of embryos for analysis rather low. It is therefore difficult to draw strong, quantitative conclusions regarding which methodologies work best. At both the technical and conceptual level, there are also certain deficiencies. The experiments presented are highly descriptive, and the link between the first and second halves of the paper is tenuous. It is not always clear how representative some of the images and charts are, and some analytical data appear to have been gathered from just one sample, even though they are depicted with error bars. There are also several examples of ‘data not shown,’ including the substitution of summary charts for raw data that would have been useful to see, and omission of certain important details. Despite these limitations, the experiments presented are convincing in their general conclusions, which are: (1) chimerism can occur between humans and large animals, although it is highly inefficient; (2) CRISPR-IBC can be used to increase chimerism levels between rodent species.
In the long term, the study provides perspective into the potential of ‘organ farming’ as a therapeutic approach, and highlights some of the challenges that will need to be overcome if this vision is to translate into clinical reality. Contemporaneously with this publication, it was demonstrated that mouse pancreatic islets, generated by IBC in rats, could be transplanted into diabetic mice to rescue blood glucose levels [17]. Blastocyst complementation has been shown to work in pigs, although this is technically challenging, and IBC has not yet been attempted [18]. A logical next step for the field is to test IBC in large animals.
As the human body will very rapidly reject any type of cell from a foreign species, IBC techniques will need to improve to the point where the organ is relatively pure, including endothelial, stromal, and possibly also resident immune cells. Thus, multiple genes will need to be targeted simultaneously, which will reduce the efficiency of chimera generation. Another issue raised by this paper is that IBC in certain organs may be incompatible with life. One reason for this may be that many organs arise from complex interactions between different cell types, which may fail in developing organs due to incompatibilities between cells of different species. For instance, researchers have been unsuccessful in using IBC to produce rat kidneys in Sall1−/− mice, possibly due to incompatibilities between mouse ureteric bud cells and rat metanephric mesenchyme cells, which would need to induce one another to form nephrons [12]. This issue might be overcome by performing IBC in compound-mutant mice lacking both ureteric bud and metanephric mesenchyme, both of which would need to derive from the donor species and would therefore be mutually compatible. For these reasons, a ‘pure’ human organ grown in an animal might develop more successfully, and be more compatible with life, than an organ that is part human and part animal. Generating ‘pure’ organs is therefore an important frontier that might improve both the efficiency of IBC organ generation, and their fitness for eventual use in the clinic (Fig. 1).
To date, no viable human-animal chimera has been produced via blastocyst injection. In addition to the technical challenge involved, scientists have been very cautious not to overstep ethical and legal boundaries surrounding the creation of such creatures. On the one hand, there are concerns that donor human cells could contribute to formation of chimeric brains or germ cells, which would raise questions related to human rights and dignity. On the other hand, society has an ethical responsibility to explore new sources of transplantable organs for the many patients in need, whose numbers far exceed the official waiting lists [19]. Ultimately, for this field to progress and translate into therapies, it will be necessary to break the viability barrier and produce live human-animal chimeras, in which the formation of human tissues can be appreciated by eye. IBC provides a strategy for doing this in a tissue-specific and ethically responsible way. Based on this study and related studies, we can predict that rates of human chimerism in non-targeted organs will be very low (less than one-tenth of one percent), whereas substantial chimerism will be observed only in the organ targeted by IBC [1,4,5]. It should therefore be possible to generate specific human organs, such as hearts or lungs, in animal hosts, without accidentally generating human brains or fetuses. Such an accomplishment will be an important step towards modeling and treating disease, to alleviate the suffering of all creatures.
ACKNOWLEDGEMENTS
The author thanks Rudolf Jaenisch for helpful discussions. The Freedman laboratory is supported by an NIH Career Development Award DK102826 (NIDDK), a PKD Foundation Research Award, an American Society of Nephrology Career Development Award, a gift from the Northwest Kidney Centers to the Kidney Research Institute, and start-up funds from the University of Washington.
Footnotes
DISCLOSURE STATEMENT
The author has no conflicts of interest to declare.
REFERENCES
- 1.Wu J, Platero-Luengo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, Suzuki K, Bogliotti YS, Cuello C, Morales Valencia M, Okumura D, Luo J, Vilarino M, Parrilla I, Soto DA, Martinez CA, Hishida T, Sanchez-Bautista S, Martinez-Martinez ML, Wang H, Nohalez A, Aizawa E, Martinez-Redondo P, Ocampo A, Reddy P, Roca J, Maga EA, Esteban CR, Berggren WT, Nunez Delicado E, Lajara J, Guillen I, Guillen P, Campistol JM, Martinez EA, Ross PJ, Izpisua Belmonte JC: Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 2017;168:473–486 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James D, Noggle SA, Swigut T, Brivanlou AH: Contribution of human embryonic stem cells to mouse blastocysts. Dev Biol 2006;295:90–102. [DOI] [PubMed] [Google Scholar]
- 3.Mascetti VL, Pedersen RA: Human-Mouse Chimerism Validates Human Stem Cell Pluripotency. Cell Stem Cell 2016;18:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, Rais Y, Shipony Z, Mukamel Z, Krupalnik V, Zerbib M, Geula S, Caspi I, Schneir D, Shwartz T, Gilad S, Amann-Zalcenstein D, Benjamin S, Amit I, Tanay A, Massarwa R, Novershtern N, Hanna JH: Derivation of novel human ground state naive pluripotent stem cells. Nature 2013;504:282–286. [DOI] [PubMed] [Google Scholar]
- 5.Theunissen TW, Friedli M, He Y, Planet E, O’Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M, Duc J, Cohen MA, Wert KJ, Castanon R, Zhang Z, Huang Y, Nery JR, Drotar J, Lungjangwa T, Trono D, Ecker JR, Jaenisch R: Molecular Criteria for Defining the Naive Human Pluripotent State. Cell Stem Cell 2016;19:502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Okamura D, Li M, Suzuki K, Luo C, Ma L, He Y, Li Z, Benner C, Tamura I, Krause MN, Nery JR, Du T, Zhang Z, Hishida T, Takahashi Y, Aizawa E, Kim NY, Lajara J, Guillen P, Campistol JM, Esteban CR, Ross PJ, Saghatelian A, Ren B, Ecker JR, Izpisua Belmonte JC: An alternative pluripotent state confers interspecies chimaeric competency. Nature 2015;521:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossant J, Frels WI: Interspecific chimeras in mammals: successful production of live chimeras between Mus musculus and Mus caroli. Science 1980;208:419–421. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui J, Knisely AS, Hirabayashi M, Nakauchi H: Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 2010;142:787–799. [DOI] [PubMed] [Google Scholar]
- 9.Fehilly CB, Willadsen SM, Tucker EM: Interspecific chimaerism between sheep and goat. Nature 1984;307:634–636. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Lansford R, Stewart V, Young F, Alt FW: RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A 1993;90:4528–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E: A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012;337:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H: Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol 2012;180:2417–2426. [DOI] [PubMed] [Google Scholar]
- 13.Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, Tesar PJ, Okada J, Margaretha L, Sperber H, Choi M, Blau CA, Treuting PM, Hawkins RD, Cirulli V, Ruohola-Baker H: Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A 2014;111:4484–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, Lungjangwa T, Imsoonthornruksa S, Stelzer Y, Rangarajan S, D’Alessio A, Zhang J, Gao Q, Dawlaty MM, Young RA, Gray NS, Jaenisch R: Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 2014;15:471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, Muguruma K, Nakano T, Suga H, Ueno M, Ishizaki T, Suemori H, Narumiya S, Niwa H, Sasai Y: Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell 2010;7:225–239. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Liu B, Xu J, Wang J, Wu J, Shi C, Xu Y, Dong J, Wang C, Lai W, Zhu J, Xiong L, Zhu D, Li X, Yang W, Yamauchi T, Sugawara A, Li Z, Sun F, Li X, Li C, He A, Du Y, Wang T, Zhao C, Li H, Chi X, Zhang H, Liu Y, Li C, Duo S, Yin M, Shen H, Belmonte JC, Deng H: Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 2017;169:243–257 e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi T, Sato H, Kato-Itoh M, Goto T, Hara H, Sanbo M, Mizuno N, Kobayashi T, Yanagida A, Umino A, Ota Y, Hamanaka S, Masaki H, Rashid ST, Hirabayashi M, Nakauchi H: Interspecies organogenesis generates autologous functional islets. Nature 2017;542:191–196. [DOI] [PubMed] [Google Scholar]
- 18.Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA, Nakauchi H: Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci U S A 2013;110:4557–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Church GM, Markmann JF, Sachs DH, Chandraker A, Wertheim JA, Rothblatt M, Boyden ES, Eidbo E, Lee WPA, Pomahac B, Brandacher G, Weinstock DM, Elliott G, Nelson D, Acker JP, Uygun K, Schmalz B, Weegman BP, Tocchio A, Fahy GM, Storey KB, Rubinsky B, Bischof J, Elliott JAW, Woodruff TK, Morris GJ, Demirci U, Brockbank KGM, Woods EJ, Ben RN, Baust JG, Gao D, Fuller B, Rabin Y, Kravitz DC, Taylor MJ, Toner M: The promise of organ and tissue preservation to transform medicine. Nat Biotechnol 2017;35:530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]


