Abstract
Background
Neurocognitive dysfunction commonly occurs after solid organ transplantation and affects 15–30% of liver transplant recipients. The aim of this study was to evaluate the neurocognitive changes pre- and post-operation and the relative factors affecting those changes.
Material/Methods
Children with biliary atresia who underwent pediatric living donor-related liver transplantation before the age of 2 years were given Bayley Scale of Infant Development-II test (BSID-II), including Mental Development Index (MDI) and Psychomotor Development Index (PDI) the week before and again half a year after transplantation to assess the effect of transplantation on neurocognition. According to the test outcome, the children were divided into a normal group and an abnormal group. The association of clinical data with neurocognitive development between the 2 groups was analyzed by logistic regression analysis.
Results
There was a certain degree of improvement in neurocognition half a year after surgery compared with preoperative. The BSID-II subscales were significantly lower than expected before and after transplantation. Preoperative blood ammonia and bilirubin levels were independent risk factors for MDI half a year after transplantation, and preoperative albumin and bilirubin levels were risk factors for PDI.
Conclusions
Liver transplantation clearly improves children’s neurocognitive function. The postoperative neurocognition is closely related to pre-operation nutritional development.
MeSH Keywords: Cognition, Liver Transplantation, Pediatrics
Background
The first 1 or 2 years of life are crucial to a child’s brain development for the formation of cognitive, social, and emotional health [1], and neurocognitive function is vulnerable to the impact of some factors such as severe disease, surgical intervention, anesthesia, and medications [2–4]. Biliary atresia is the most common cause of end-stage liver disease in children, with morbidity as high as 1/8000~18 000 [5]. Children with end-stage liver diseases are vulnerable to cognitive deficits and have been reported to have lower performance intelligence quotients (IQs) compared with age-matched children [2]. Susan et al. have indicated that language and motor skills are degressive in infants with biliary atresia [4]. Since the 1960s, with the successful development of liver transplantation, it has become an important treatment for end-stage liver disease, with the postoperative 1-year, 5-year, and 10-year survival rates of up to 95%, 80%, and 60%, respectively [6,7]. It is undeniable that there are still some serious complications after liver transplantation in children affecting the liver and some remote organs such as kidney, heart, and lung [8,9]. It is worth noting that there can be neurological complications, with the incidence rate varying from 8% to 46% [10]. Some studies have found that neurocognition has a certain degree of injury in the preschool period [2,11]. The changes in neurocognitive function before and after liver transplantation and the effects of liver transplantation on neurocognitive function in patients with biliary atresia are not clear. We hypothesized that the neurocognition of children improved after liver transplantation. Therefore, the aim of the current study was to evaluate pediatric neurocognitive function pre- and post-liver transplantation and to analyze the related factors.
Material and Methods
Participants
Approval was obtained from the Ethics Committee of Tianjin First Center Hospital in China (Approval Number: 2016N0039KY), and written informed consent was obtained from eligible guardians. This study has been registered in clinical trial (ClinicalTrials.gov ID: NCT03024840). All processes were ethical.
This trial was carried out in Tianjin First Center Hospital in China, which has extensive clinical experience in transplantation. Children (ages ranging from 5 months to 2 years; American Society of Anesthesiologist physical status III or IV; October 2016 to May 2017) who were scheduled to undergo elective pediatric living related donor liver transplantation were recruited. Exclusion criteria were: children with congenital heart disease, central nervous system disease, or re-transplantation. All the living donors were family members (father or mother). Every case of transplantation passed the ethical review and approval process in Tianjin First Center Hospital. The ethical information was attached in the appendix, which included the Chinese names of recipients and donors, their ID numbers, their relationships, and the ethical approval numbers.
Clinical and biochemical data collection
The clinical and laboratory information were collected by a dedicated experienced research nurse as the part of the standard care in our hospital. The preoperative variables included the most recent tests of ammonia, total bilirubin, creatinine, pediatric end-stage liver disease model (PELD), international normalized ratio (INR), albumin, and white blood cell (WBC) count before the liver transplantation surgery. Intraoperative data included anhepatic time, operation time, anesthesia duration, infusion volume, and urinary volume. Postoperative data included age, gender, weight, height, and levels of ammonia, total bilirubin, and creatinine half a year after operation as well as complications, such as reoperation, biliary complications without surgeries, and acute pneumonia (defined as lasting up to the first month after transplantation), during the 6-month period after the surgery.
The PELD score was calculated as follows: PELD Score=0.436 (age <1 year)–0.687·loge albumin (g/dL)+0.480·loge total bilirubin (mg/dL)+1.875·loge INR+0.667 (growth failure height or weight ≥2 standard deviations below age- and sex-adjusted means).
Psychometric measures
General anesthesia was administered to all the children. The surgeons and anesthesiologist’s were the same for all patients. Test measures were administered by 2 trained attending physicians. The neurocognitive conditions were evaluated within 1 week before the operation and half a year after the operation using the Bayley Scale of Infant Development-II (BSID-II), which is expressed as mental development index (MDI) and psychomotor development index (PDI) [12]. BSID-II is a standardized tool to assess toddler and infant development, and it was conducted in a quiet room by trained examiners over the course of 30–45 minutes per child. All children were hospitalized at least a week in advance of the surgery. The children came to the hospital or clinic for re-examination every 2 weeks for the first 2 months after discharge, and then were rechecked every month for the first year after transplantation. The MDI was used to assess memory, habituation, problem-solving, early number concepts, classification, vocalizations, early verbal communication, and early abstract thinking ability, and the PDI was used to assess body control such as gross- and fine-motor skills. Each index could be standardized with a mean of 100 and a standard deviation of 15, with a composite score that compared the child’s developmental performance with the norms for typically developing children of the same age. Valid MDI and PDI scores ranged between 50 and 150. A standard score of <85, which was 1 standard deviation below the mean, was considered abnormal [12,13]. The association of neurocognitive development with contributing factors between groups were compared and analyzed.
Statistical analysis
Statistical analyses were performed using SPSS 23.0 software package for Windows (SPSS, Inc., Chicago, IL, USA). Continuous variables were described as the mean ± standard deviation. Development index changes were analyzed by paired-sample t-tests and signed-rank sum tests. Independent sample t-tests and Wilcoxon rank test were used to analyze the difference between groups. Categorical data were described by frequency, and chi-square tests and Fisher’s exact tests were used for the categorical variables. Logistic regression models were used to identify the risk factors associated with cognitive function half a year after transplantation and to determine the impact of post-transplant complications using clinical variables. Data were reported as odds ratios (ORs) with corresponding 95% confidence intervals (CI). The results were evaluated within the 95% reliability index, with P < 0.05 being significant.
Results
Changes in neurocognition before and after liver transplantation
From October 2016 to May 2017, 72 pediatric patients underwent living-donor liver transplantation. Of the 60 patients with BSID-II tests before transplantation in our initial cohort, 12 were excluded because of congenital heart disease, other operations, re-transplantation, and incoordination in the test. Sixty patients (32 males, and 28 females) were included in this study, and all were tested within a week before operation at a mean age of 8.6±2.0 months, with an average weight of 7.4±1.1 kg and an average height of 62.9±5.8 cm. Half a year later, 2 cases were dead, with 1 patient died from graft-versus-host reaction (GVHR), and 1 patient died from multiple organ failure. One patient was excluded because of re-transplantation due to graft failure, and 3 patients had no re-examination half a year after transplantation. In total, 54 cases were included in this trial half a year after transplantation (Figure 1).
Figure 1.
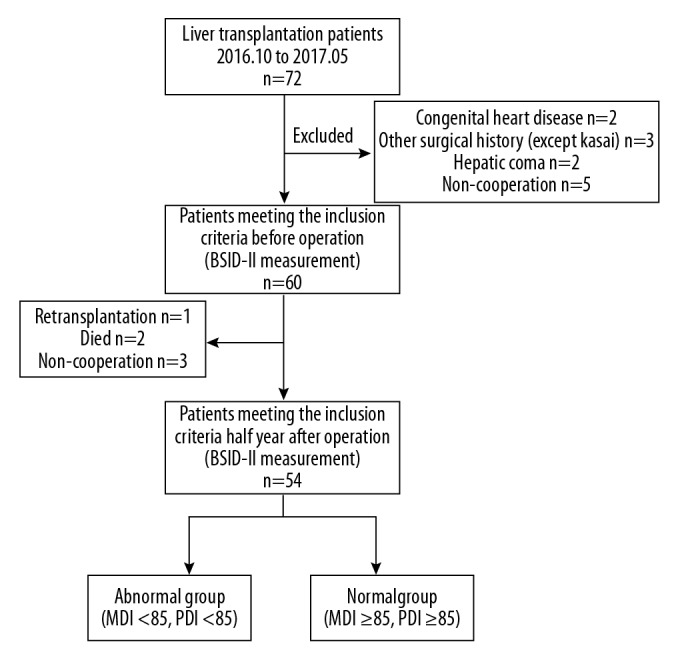
Patient population: From October 2016 to May 2017, 72 pediatric patients underwent living-donor liver transplantation. Of the 60 patients with Bayley Scale of Infant Development-II (BSID-II) tests before transplantation in our initial cohort, 12 were excluded because of congenital heart disease, other operations (except Kasai), re-transplantation, or incoordination in the test. Sixty patients were initially studied in this experiment, and all were tested within a week before their operation. Half a year later, 2 cases were dead, with 1 patient dying from graft-versus-host reaction (GVHR) and 1 patient dying from multiple organ failure. One patient was excluded because of re-transplantation due to graft failure, and 3 patients had no re-examination half a year after transplantation. In total, 54 cases were included in the trial half a year after transplantation.
Preoperative neurocognitive development was at a low level with both MDI and PDI below 1 standard deviation (MDI: 83.89±8.36, PDI: 81.23±8.32). Compared with the preoperative index scores, the development index increased significantly half a year after surgery with an MDI of 90.11±7.65 and a PDI of 86.09±6.83. The BSID-II subscales were significantly lower than normal range for the groups of children before and after liver transplantation (Table 1). This showed that although the neurocognition after the operation improved, patients had not yet reached the age-based expected level.
Table 1.
Comparisons in BSID II subscales with normative group pre- and post-operation.
| Subscale | M | SD | t* | P value |
|---|---|---|---|---|
| Pre-MDI | 83.89 | 8.36 | 7.26 | <0.01 |
| Pre-PDI | 81.23 | 8.32 | 8.49 | <0.01 |
| Post-MDI | 90.11 | 7.65 | 4.32 | <0.01 |
| Post-PDI | 86.09 | 6.83 | 6.21 | <0.01 |
Compared with BSID age-based norms.
Predisposing factors for abnormal neurocognitive development after liver transplantation
According to the BSID classification, we defined the neurodevelopmental outcomes as a normal group (MDI ≥85, PDI ≥85) and an abnormal group (MDI <85, PDI <8 5). Half a year after transplantation, 16 patients (29.6%) were in the abnormal MDI group and 38 patients (70.4%) were in the normal group. Height, pre-albumin level, preoperative ammonia, total bilirubin, and creatinine levels, PELD, INR, and incidence of severe pneumonia after transplantation were predictors of abnormal MDI in the univariate analysis (P<0.05) (Table 2). To identify the major independent factors for abnormal MDI after transplantation, multivariate logistic regression models were used (factors with the P value <0.05 in the univariate analysis were included) and they revealed that the preoperative blood ammonia [OR=1.226 (1.012, 1.485), P=0.037] and bilirubin [OR=1.114 (1.003, 1.238), P=0.043] levels were risk factors predicting lower post-operative MDI (Table 3). Half a year after transplantation, 26 patients (48.1%) were in the abnormal PDI group and 28 patients (51.9%) in the normal PDI group. In the abnormal group, the levels of preoperative ammonia, total bilirubin, creatinine, PELD, and INR were higher than those in the normal group. (P<0.05) (Table 4). Higher preoperative albumin [OR=0.795 (0.654, 0.967), P=0.022] and pre-bilirubin [OR=1.019 (1.001, 1.038), P=0.039] levels were independent risk factors forecasting lower PDI (factors with the P value <0.05 in the univariate analysis were included) (Table 5).
Table 2.
The recipient variables predictive of MDI half a year after operation by univariate analysis.
| Variables | Normal group (n=38) | Abnormal group (n=16) |
|---|---|---|
| Age (month) | 14.26±3.84 | 14.50±4.86 |
| Gender [boy/girl, (n)] | 20/18 | 10/6 |
| Weight (kg) | 9.16±0.88 | 8.79±0.57 |
| Height (cm) | 69.63±4.72 | 66.00±3.52* |
| Pre-bilirubin (μmol/L) | 327.17±60.84 | 444.14±32.66* |
| Pre-creatinine (μmol/L) | 14.33±5.74 | 18.70±5.01* |
| Pre-PELD | 16.86±3.05 | 19.63±1.96* |
| Pre-albumin (g/L) | 31.51±3.96 | 27.18±3.83* |
| Pre-WBC (×109/L) | 20.58±5.2 | 23.6±8.2 |
| Pre-ammonia (μmol/L) | 70.39±14.50 | 100.5±11.00* |
| Pre-INR (IU) | 1.51±0.31 | 1.86±0.14* |
| Anhepatic time (min) | 60.87±7.97 | 62.63±5.15 |
| Urine volume (ml) | 455.53±60.26 | 488.75±60.10 |
| Operation time (h) | 9.20±1.27 | 9.28±1.56 |
| Anesthesia duration (h) | 10.71±1.19 | 10.58± 1.58 |
| Infusion volume (ml) | 789.74±76.17 | 801.25±96.88 |
| Post- bilirubin (μmol/L) | 33.51±9.42 | 39.34±12.23 |
| Post-creatinine (μmol/L) | 12.35±5.83 | 13.43±5.20 |
| Post-ammonia (μmol/L) | 45.34±5.57 | 40.23±9.34 |
| Acute pneumonia [no/yes, (n)] | 35/3 | 10/6* |
| Re-operation [no/yes, (n)] | 34/4 | 13/3 |
| Postoperative biliary complications [no/yes, (n)] | 33/5 | 12/4 |
All P values for the variables presented as “n” were obtained with the chi-square test. The variables presented as mean ±SD were obtained with the Student t test.
P<0.05.
Table 3.
Multivariate analysis of variables predictive of postoperative MDI.
| B | S.E. | Wald | Sig. | Exp(B) | OR 95% C.I | |
|---|---|---|---|---|---|---|
| Pre-ammonia | 0.204 | 0.098 | 4.353 | 0.037 | 1.226 | (1.012, 1.485) |
| Pre-bilirubin | 0.108 | 0.054 | 4.097 | 0.043 | 1.114 | 1.003, 1.238) |
| Constant | −65.786 | 30.816 | 4.557 | 0.033 | 0.000 |
Factors were included according to the results of univariate analysis with the P value <0.05 (preoperative ammonia, total bilirubin, creatinine, albumin, PELD, INR, acute pneumonia after transplantation, and present height).
P<0.05.
Table 4.
The recipient variables predictive of MDI half year after operation by univariate analysis.
| Variables | Normal group (n=28) | Abnormal group (n=26) |
|---|---|---|
| Age (month) | 14.04±4.34 | 13.4±3.45 |
| Gender [boy/girl, (n)] | 16/12 | 14/12 |
| Weight (kg) | 9.09±0.92 | 9.02±0.71 |
| Height (cm) | 69.39±4.75 | 67.65±4.52 |
| Pre-bilirubin (μmol/L) | 323.39±69.60 | 403.22±60.10* |
| Pre-creatinine (μmol/L) | 13.59±5.81 | 17.82±5.14* |
| Pre-PELD | 17.01±3.57 | 18.42±2.17* |
| Pre-albumin (g/L) | 32.28±3.26 | 28.02±4.38 |
| Pre-WBC (×109/L) | 20.01±4.58 | 23.05±7.52 |
| Pre-ammonia (μmol/L) | 71.32±17.84 | 87.77±17.21* |
| Pre-INR (IU) | 1.49±0.35 | 1.35±0.20* |
| Anhepatic time (min) | 60.64±8.43 | 62.19±5.43 |
| Urine volume (ml) | 474.64±56.80 | 455.38±65.98 |
| Operation time (h) | 8.89±1.23 | 9.59±1.39 |
| Anesthesia duration (h) | 10.37±1.38 | 11.00±1.37 |
| Infusion volume (ml) | 780.36±83.51 | 806.92±79.79 |
| Post-bilirubin (μmol/L) | 56.7±12.33 | 62.12±11.35 |
| Post-critinine (μmol/L) | 12.36±5.22 | 14.1±5.43 |
| Post-ammonia (μmol/L) | 35.00±22.80 | 46.0±20.16 |
| Acute pneumonia [no/yes, (n)] | 26/2 | 21/5 |
| Re-operation[no/yes, (n)] | 27/1 | 23/3 |
| Postoperative biliary complications [no/yes, (n)] | 23/5 | 21/5 |
All P values for the variables presented as “n” were obtained with the chi-square test. The variables presented as mean ±SD were obtained with the Student t test.
P<0.05.
Table 5.
Multivariate analysis of variables predictive of postoperative PDI.
| B | S.E. | Wald | Sig. | Exp(B) | OR 95% C.I | |
|---|---|---|---|---|---|---|
| Pre-ammonia | −0.29 | 0.100 | 5.276 | 0.022 | 0.795 | (0.654, 0.967) |
| Pre-bilirubin | 0.019 | 0.009 | 4.255 | 0.039 | 1.019 | (1.001, 1.038) |
| Constant | 2.857 | 4.331 | 0.435 | 0.509 | 17.414 |
Factors were included according to the results of univariate analysis of PDI with the P value <0.05 (preoperative ammonia, total bilirubin, creatinine, PELD, INR).
P<0.05.
Discussion
This pilot study accessing the mental and psychomotor development of patients who underwent liver transplantation demonstrated that those who suffered end-stage liver diseases had a higher prevalence of neurocognitive deficit and challenges affecting motor skills, communication, and executive functioning skills before and after liver transplantation. Liver transplantation clearly improved the children’s neurocognitive function. Preoperative blood ammonia and bilirubin levels were the independent risk factors for low MDI half a year after transplantation, and preoperative albumin and bilirubin levels were the risk factors for low PDI. Preoperative intervention and liver transplantation were essential to the neurocognitive development of children with end-stage liver disease.
Pediatric liver transplantation is an important part of clinical liver transplantation [14]. Liver transplantation can improve cholestasis symptoms in children with the long-term survival rate exceeding 90% [6]. Currently, the main cause of liver transplantation in children is biliary atresia, which influences the growth and development of children [15]. Pediatric liver transplantation clinical guidelines recommend to reasonably assess and correct malnutrition and the neurocognitive development of children before and after transplantation [16]. Children with biliary atresia before liver transplantation have poor nutrition, which seriously affects their growth and development and can even affect their neurocognitive function [17]. Growth parameters, such as height and weight, have been indicated to lead to nearly 37% of cognitive function at early age [4,18,19]. Our study also found that patients in the normal MDI group had higher height. This experiment proved that before liver transplantation, young children with biliary atresia demonstrated a distinct series of weakness of neurocognitive function that were remarkable for both gross and fine motor ability, language expression, communication development, and problem-solving. The score for the total groups was below a standard deviation of 100 (MDI: 83.89±8.36, PDI: 81.23±8.32) before transplantation. After liver transplantation, the bilirubin level decreased significantly to a normal level within 1 week in children with liver disease, and mental reaction improved, according to parental input. While the mean intelligence scores did not reach the mean of the normative population of 100, the score for the total group was within a standard deviation of 100, suggesting no clinically significant difference from population norms (MDI: 90.11±7.65, PDI: 86.09±6.83).
Children are vulnerable to stimuli during stages of growth and development. The trauma, long time, and complicated procedure combined with intraoperative ischemia and reperfusion injury during liver transplantation may cause severe stroke in children after surgery [20–22]. Some complications within 1 month after operation, such as severe pneumonia, leakage of bile, and obstruction of the portal vein, may affect the recovery and growth of children after operation in the short-term [8,23]. In the postoperative liver recovery period of an infant, part of the content of the BSID scale, especially the motor scale, were not suitable for children in the short-term. Therefore, the new assessment was carried out 6 months after surgery. On the other hand, many studies have found that long-term neurocognitive development in children after liver transplantation is not ideal, with lower preschool IQ scores [2,24]. The behavioral scoring, such as verbal writing, Wechsler intelligence scores, and even adaptability to society, are not satisfactory [25]. In the same way, anesthetic drugs may influence neurocognitive function in children due to long operation time [26]. It was a weakness of our study that we could not define the respective effects between the liver transplantation surgery and the long-term anesthesia, for both could affect the neurodevelopment of children after the operation. Previous studies did not consider the neurocognitive development before transplantation. In this study, we fortunately found that half a year after transplantation, both the MDI and PDI were higher than before, which demonstrated that liver transplantation may effectively alleviate the poor pre-neurocognitive status.
These data had some similar conclusion with other authors who have studied pediatrics with end-stage liver disease [4]. It was not surprising to find evidence in other studies of cognitive disorders in patients suffering from cholestasis liver diseases [11,27]. Postoperative neurocognitive development is closely related to preoperative development in children. The growth and development of patients with liver disease often lags behind that of normal children before transplantation. For the abnormal MDI, the levels of preoperative ammonia, total bilirubin, creatinine, PELD, INR, and the incidence of severe pneumonia after transplantation were higher than those in the normal group, as was shorter height and lower pre-albumin. Especially preoperative ammonia and total bilirubin levels were important risk factors. For PDI, the levels of preoperative ammonia, total bilirubin, and creatinine, and PELD and INR in the abnormal group were higher than in the normal group. The result of the multivariate analysis showed that pre-albumin and pre-bilirubin levels were risk factors impacting postoperative PDI. Given the critical stages and vulnerabilities of child brain development, it is not hard to imagine this outcome, that the decline neurocognition could be traced back to the poor preoperative nutritional status of the whole body. Children suffering from end-stage liver disease generally also have severe malnutrition such as hyperbilirubinemia, hyperammonemia, and hypoproteinemia. Our experience also showed that preoperative blood ammonia and bilirubin levels had some influence on neurocognitive development in children. Hyperammonemia-related neurologic injury ranges among individuals from lethal cerebral edema to mild or subclinical cognitive impairment [28]. Liver cirrhosis in patients with biliary atresia before transplantation is accompanied with ascites and esophageal varicose venous congestion, which increases blood ammonia by causing the concentrated ammonia to permeate from the intestinal tract into the blood because of gastrointestinal bleeding, intestinal congestion, and intestinal protein catabolism [29]. During the period of growth in children with weak blood brain barrier (BBB) function, the increased permeability of the BBB to serum bilirubin may result in bilirubin reducing cell surface tension, leading to toxic effects on brain capillary endothelial cell walls [30]. Higher blood ammonia and unconjugated bilirubin easily passing through the BBB into the brain could damage the balance of nervous system excitatory amino acid and inhibitory amino acid quantities [31], which could make the brain mitochondrial oxidative phosphorylation uncoupling effect the energy metabolism of brain cells, leading to central nervous system disorders and even severe central nervous system disease such as hepatic encephalopathy and nuclear jaundice [32,33]. Patients in the abnormal PDI group had lower serum albumin level before transplantation which was related to height and weight stunting. Liver is the important organ to synthesize albumin. The ability of albumin synthesis is decreased in patients with end-stage liver disease. Hypoproteinemia leads to ascites, edema, and even effects the growth and development of children. Also, some previous studies have indicated that compared with albumin, prealbumin was more sensitive and more representative in liver diseases because of its shorter half-life and the obvious changes occurring in earlier liver disease. Therefore, prealbumin is also an important basic indicator for the diagnosis of infant nutritional diseases [34,35].
According to this experiment, liver transplantation may clearly improve children’s neurocognitive function. Poor nutritional status in children before operation will directly affect postoperative recovery and neurocognitive development. Thus, it is necessary to evaluate and correct the nutritional status of children before and after liver transplantation. While we must admit that on the one hand, since the sample size is small, there may be errors in the interpretation of effect of postoperative complications on the development index. This experiment only investigated the small sample population. On the other hand, in this study, neurocognitive development was observed only for half a year after surgery rather than for a long time. It must be acknowledged that longer neurocognitive follow-up after surgery is more meaningful. In addition, only one neurodevelopmental scale was used in this study, and only a portion of the indicators were included in this study. Further studies are needed.
Conclusions
In this study we have provided novel evidence that there is some neurocognitive impairment in children with biliary atresia before liver transplantation and that liver transplantation may improve the neurocognitive functions of these children. The development of postoperative neurocognition is closely correlated to the preoperative condition.
Abbreviation
- BSID
Bayley Scale of Infant Development
- MDI
Mental Development Index
- PDI
Psychomotor Development Index
- INR
international normalized ratio
- WBC
white blood cell
- PELD
pediatric end-stage liver disease
- GVHR
graft versus host reaction
- OR
odds ratio
- BBB
blood–brain barrier
Footnotes
Conflict of interest
None.
Source of support: This work was financially supported by the Key Projects of Health Industry in Tianjin (protocolno. 13KG105, 16KG101) and Natural Science Foundation of Tianjin City in China (protocol no. 17JCYBJC28000)
References
- 1.Dennis M, Spiegler BJ, Juranek JJ, et al. Age, plasticity, and homeostasis in childhood brain disorders. Neurosci Biobehav Rev. 2013;37:2760–73. doi: 10.1016/j.neubiorev.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilmour SM, Sorensen LG, Anand R, et al. School outcomes in children registered in the studies for pediatric liver transplant (SPLIT) consortium. Liver Transpl. 2010;16:1041–48. doi: 10.1002/lt.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh PS, Hupertz V, Ghosh D. Neurological complications following pediatric liver transplant. J Pediatr Gastroenterol Nutr. 2012;54:540–46. doi: 10.1097/MPG.0b013e3182407de3. [DOI] [PubMed] [Google Scholar]
- 4.Caudle SE, Katzenstein JM, Karpen SJ, et al. Language and motor skills are impaired in infants with biliary atresia before transplantation. J Pediatr. 2010;156:936–40.e1. doi: 10.1016/j.jpeds.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram SS, Mack CL, Feldman AG, et al. Biliary atresia: indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl. 2017;23:96–109. doi: 10.1002/lt.24640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazigi NA. Long term outcomes after pediatric liver transplantation. Pediatr Gastroenterol Hepatol Nutr. 2013;16:207–18. doi: 10.5223/pghn.2013.16.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JY, Lim LT, Quak SH, et al. Cholangitis in children with biliary atresia: Health-care resource utilisation. J Paediatr Child Health. 2014;50:196–201. doi: 10.1111/jpc.12463. [DOI] [PubMed] [Google Scholar]
- 8.Sheng M, Lin Y, Weng Y, et al. Predictive value of intraoperative troponin I elevation in pediatric living donor liver transplant recipients with biliary atresia. Transplantation. 2017;101(10):2385–90. doi: 10.1097/TP.0000000000001732. [DOI] [PubMed] [Google Scholar]
- 9.Fine RN, Alonso EM, Fischel JE, et al. Pediatric transplantation of the kidney, liver and heart: Summary report. Pediatr Transplant. 2004;8:75–86. doi: 10.1111/j.1399-3046.2004.2s050.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Yum MS, Kim EH, et al. Risk factors for neurological complications and their correlation with survival following pediatric liver transplantation. Pediatric Transplantation. 2014;18:177–84. doi: 10.1111/petr.12218. [DOI] [PubMed] [Google Scholar]
- 11.Robertson CM, Dinu IA, Joffe AR, et al. Neurocognitive outcomes at kindergarten entry after liver transplantation at <3 years of age. Pediatr Transplant. 2013;17:621–30. doi: 10.1111/petr.12134. [DOI] [PubMed] [Google Scholar]
- 12.Bayley N. Bayley scales of infant development. 2nd ed. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 13.Cahill RK, Rose J. Toddle temporal-spatial deviation index: Assessment of pediatric gait. Gait Posture. 2016;49:226–31. doi: 10.1016/j.gaitpost.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Otte JB. Pediatric liver transplantation: Personal perspectives on historical achievements and future challenges. Liver Transpl. 2016;22:1284–94. doi: 10.1002/lt.24470. [DOI] [PubMed] [Google Scholar]
- 15.Feldman AG, Mack CL. Biliary atresia: Clinical lessons learned. J Pediatr Gastroenterol Nutr. 2015;61:167–75. doi: 10.1097/MPG.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 16.Squires RH, Ng V, Romero R, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology. 2014;60:362–98. doi: 10.1002/hep.27191. [DOI] [PubMed] [Google Scholar]
- 17.Stewart SM, Uauy R, Waller DA, et al. Mental and motor development correlates in patients with end-stage biliary atresia awaiting liver transplantation. Pediatrics. 1987;79:882–88. [PubMed] [Google Scholar]
- 18.Skuse D, Pickles A, Wolke D, et al. Postnatal growth and mental development: Evidence for a “sensitive period”. J Child Psychol Psychiatry. 1994;35:521–45. doi: 10.1111/j.1469-7610.1994.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 19.Grantham-McGregor S. A review of studies of the effect of severe malnutrition on mental development. J Nutr. 1995;125(8 Suppl):2233S–38S. doi: 10.1093/jn/125.suppl_8.2233S. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Cheng Y, Shen W, et al. The short-term effect of liver transplantation on the low-frequency fluctuation of brain activity in cirrhotic patients with and without overt hepatic encephalopathy. Brain Imaging Behav. 2017;11(6):1849–61. doi: 10.1007/s11682-016-9659-6. [DOI] [PubMed] [Google Scholar]
- 21.Weiss S, Kotsch K, Francuski M, et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7:1584–93. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 22.Bundzikova J, Pirnik Z, Lackovicova L, et al. Brain-liver interactions during liver ischemia reperfusion injury: A minireview. Endocr Regul. 2011;45:163–72. doi: 10.4149/endo_2011_03_163. [DOI] [PubMed] [Google Scholar]
- 23.Enrol I, Alehan F, Ozcay F, et al. Neurologic complications of liver transplantation in pediatric patients with the hepatic form of Wilson’s disease. J Child Neurol. 2008;23:293–300. doi: 10.1177/0883073807309233. [DOI] [PubMed] [Google Scholar]
- 24.Ee LC, Lloyd O, Beale K, et al. Academic potential and cognitive functioning of long-term survivors after childhood liver transplantation. Pediatr Transplant. 2014;18:272–79. doi: 10.1111/petr.12246. [DOI] [PubMed] [Google Scholar]
- 25.Schulz KH, Wein C, Boeck A, et al. Cognitive performance of children who have undergone liver transplantation. Transplantation. 2003;75:1236–40. doi: 10.1097/01.TP.0000062843.10397.32. [DOI] [PubMed] [Google Scholar]
- 26.Geng YJ, Wu QH, Zhang RQ. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: A randomized controlled trial. J Clin Anesth. 2017;38:165–71. doi: 10.1016/j.jclinane.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Sultan MI, Leon CD, Biank VF. Role of nutrition in pediatric chronic liver disease. Nutr Clin Pract. 2011;26:401–8. doi: 10.1177/0884533611405535. [DOI] [PubMed] [Google Scholar]
- 28.Diaz GA, Krivitzky LS, Mokhtarani M, et al. Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology. 2013;57:2171–79. doi: 10.1002/hep.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis C, Tesar GE, Dale R. Valproate-induced hyperammonemic encephalopathy in general hospital patients with one or more psychiatric disorders. Psychosomatics. 2017;58:415–20. doi: 10.1016/j.psym.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara R, Nguyen N, Chen S, et al. Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci USA. 2010;107:5024–29. doi: 10.1073/pnas.0913290107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark JF, Loftspring M, Wurster WL, et al. Bilirubin oxidation products, oxidative stress, and intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:7–12. doi: 10.1007/978-3-211-09469-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tranah TH, Vijay GK, Ryan JM, et al. Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis. 2013;28:1–5. doi: 10.1007/s11011-012-9370-2. [DOI] [PubMed] [Google Scholar]
- 33.Dias Costa F, Moinho R, Ferreira S, et al. Acute liver failure related to inherited metabolic diseases in young children. An Pediatr (Barc) 2018;88(2):69–74. doi: 10.1016/j.anpedi.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Yuksekkaya HA, Cakir M, Tumgor G, et al. Nutritional status of infants with neonatal cholestasis. Dig Dis Sci. 2008;53:803–8. doi: 10.1007/s10620-007-9917-y. [DOI] [PubMed] [Google Scholar]
- 35.Beck FK, Rosenthal TC. Pre-albumin: A marker for nutritional evaluation. Am Fam Physician. 2002;65:1575–78. [PubMed] [Google Scholar]


