Abstract
Pleuropulmonary blastoma (PPB), the most common primary malignant neoplasm of the lung in childhood, occurs in the same early age group (0–6 years) as the other more common solid tumors such as neuroblastoma and Wilms tumor. The tumor begins as a cystic lung lesion with the potential over a period of 3–5 years to progress to a high grade multipatterned primitive sarcoma in the absence of a malignant epithelial component. Several years after its initial description as a unique clinicopathologic entity, this and other tumors appeared to have a familial predilection which was later confirmed with the discovery of a heterozygous germline mutation in DICER1 whose protein is a member of ribonuclease III family of enzymes. It is estimated that 75%–80% of children with a PPB have the germline mutation. The other notable finding from our studies is the identification of a family of extrapulmonary neoplasms, including cystic nephroma and Sertoli-Leydig cell tumor of the ovary as two examples, also with DICER1 mutations.
Introduction
Between 1988 and 2009, pleuropulmonary blastoma (PPB) fully emerged as a unique clinicopathologic entity to join the other more familiar dysontogenetic or dysembryonic neoplasms of childhood; this group of neoplasms arise in various organs including the eye, liver, kidney, adrenal and soft tissues, and are known respectively as retinoblastoma, hepatoblastoma, Wilms tumor (nephroblastoma), neuroblastoma and rhabdomyosarcoma. All of these tumors have in common a clinical presentation in children 6 years old or less with a peak generally before 5 years of age. One of the unexplained and contradictory problems was that the presumed dysembryonic neoplasm of the lung, pulmonary blastoma, presented in adults, many of whom were cigarette smokers; the latter neoplasm is now regarded as a type of sarcomatoid carcinoma in the World Health Organization classification of neoplasms of the lung.1, 2 It was proposed in 1988 with the report of the first 11 cases of a highly malignant neoplasm of the lung and/or pleura, designated PPB, was the true “pulmonary blastoma” of childhood with its clinical presentation in children 5 years old or less.3 As uncommon as virtually all other malignancies of childhood, PPB is the most common primary pulmonary malignancy in children, just as Wilms tumor and neuroblastoma are in the kidney and adrenal in this same age group.4
Perspective on Childhood Cancer
When we speak of PPB or any other malignancy of childhood, the entire aggregate of malignant tumors in individuals 0–19 years old at the time of diagnosis in the United States is 15,780 cases per year (less than 15 cases per 100,000) in contrast to the 1,688,780 newly diagnosed cancer cases (exclusive of non-melanoma skin cancers) per year in adults (439 cases per 100,000).5 In children the five most common malignancies are acute leukemia (30%), tumors of the central nervous system (~25%), neuroblastoma (6%), Wilms tumor (5%) and lymphoma (8%).6 In adults, non-melanoma skin cancers (5.3 million cases per year), breast (266,000), lung (234,000), prostate (165,000) and colorectum (140,000) are the most commonly diagnosed cancers per year in the United States. Only 13% of all cancers diagnosed in adults are regarded as “rare” (NCI definition <15 cases per 100,000) whereas 100% of all childhood cancers are rare. One of the most dramatic improvements in cancer outcomes over the past 40 years has occurred among the childhood malignancies.7 The 5-year survival for all childhood cancers was 10% in the 1950s, 60% in 1975–79 and almost 80% in the present era; the improved survival over this period is largely influenced by advances in the management of hematopoietic malignancies.8
Pleuropulmonary Blastoma
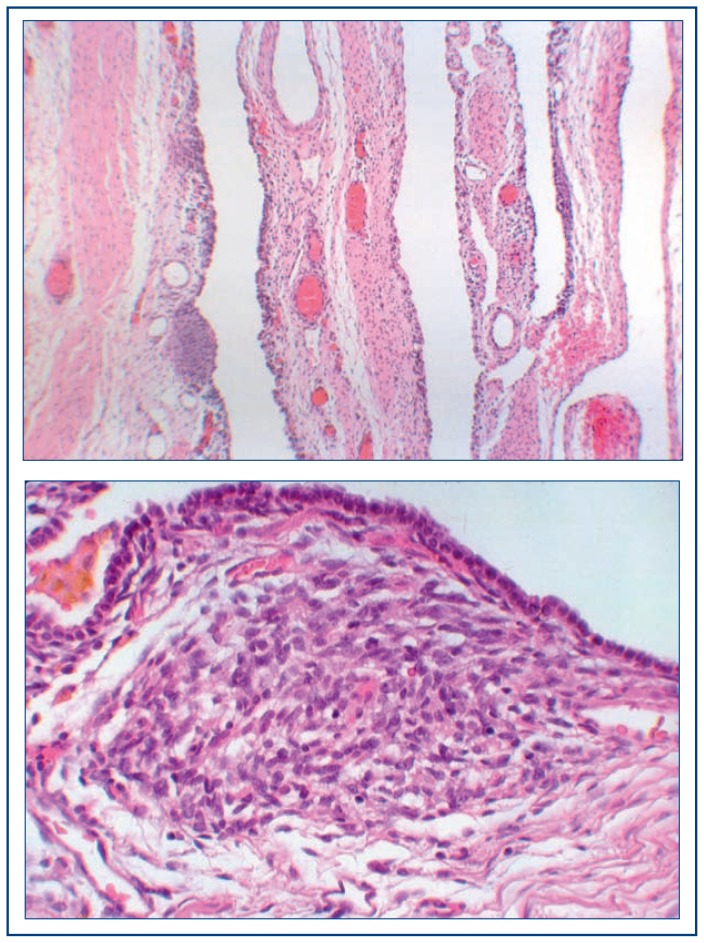
With this digression, let us return to our consideration of PPB in terms of its pathologic progression from an innocuous lung cyst(s) detected in infancy to a high grade multipatterned primitive sarcoma by 3–4 years of age.4 Because the earliest stage of PPB is a lung cyst, it is often the case that the initial impression is a congenital lung cyst with congenital pulmonary airway malformation (CPAM) (congenital cystic adenomatoid malformation) as the favored clinical and imaging impression. If the cystic lesion is asymptomatic, there are some guidelines and recommendations if surgery is not immediately contemplated. When surgery is performed, a multicystic structure is noted at the periphery of the lung on the pleural surface which maintains its appearance when placed in fluid to demonstrate the delicate septated cysts. It is important for the pathologist to thoroughly sample the specimen so that the septal structures can be evaluated for the presence of primitive small cells and/or rhabdomyoblasts and diminutive nodules of fetal appearing cartilage; these features may be localized within the septa rather than as a diffuse finding so that a thorough microscopic examination is necessary (Figure 1A, B). The critical point for the pathologist is to recognize and diagnose the “congenital lung cyst” as a PPB type 1 and not as a CPAM.4
Figure 1.
Pleuropulmonary blastoma, type I.
A. This partially collapsed multicystic architecture displays the rather innocuous features with a small hypercellular nodule of fetal cartilage.
B. A focus of embryonal rhabdomyosarcoma within the stroma beneath the lining cuboidal epithelium is a characteristic microscopic feature.
It was only after the mixed cystic and solid PPB type II and the purely solid tumor stage, PPB type III were described that it was appreciated that the exclusively multicystic lesion PPB type 1 was the earliest stage of tumorigenesis. The latter observation was based upon the presence of the residual multicystic lesion in the PPB type II where the solid tumor, whose pathologic features were very similar to the PPB type III, had overgrown and replaced a substantial portion of the cystic component in its progression to the final stage, PPB type III (Fig. 2A–C). As our experience with PPB expanded through the establishment of the International Pleuropulmonary Blastoma Registry (https://www.ppbregistry.org/), it was observed that there was a correlation between the median age at diagnosis and the pathologic type of PPB: type I, 8 mo; type II, 35 mo; type III, 41 mo. The other significant correlation occurred between the PPB type and survival: type I, 94%; type II, 71%; type III, 53%.9 It is critical to diagnose and manage PPB at the type I stage as evident from these survival figures. Is the eventual fate of every PPB type I that is not diagnosed at this early stage to progress to a PPB type II or type III? We do not have the answer to this question, but we know that PPB type I may present as a multicystic lung lesion without any features of a primitive small cell proliferation or rhabdomyosarcoma; this lesion is designated a PPB type 1r which we have hypothesized represents either the regressive or non-progressive phase of PPB type I. At present, we do not yet know which PPB type I will progress to the more aggressive PPB type II or type III. We have had examples of PPB type Ir with the later development of PPB type II or III.
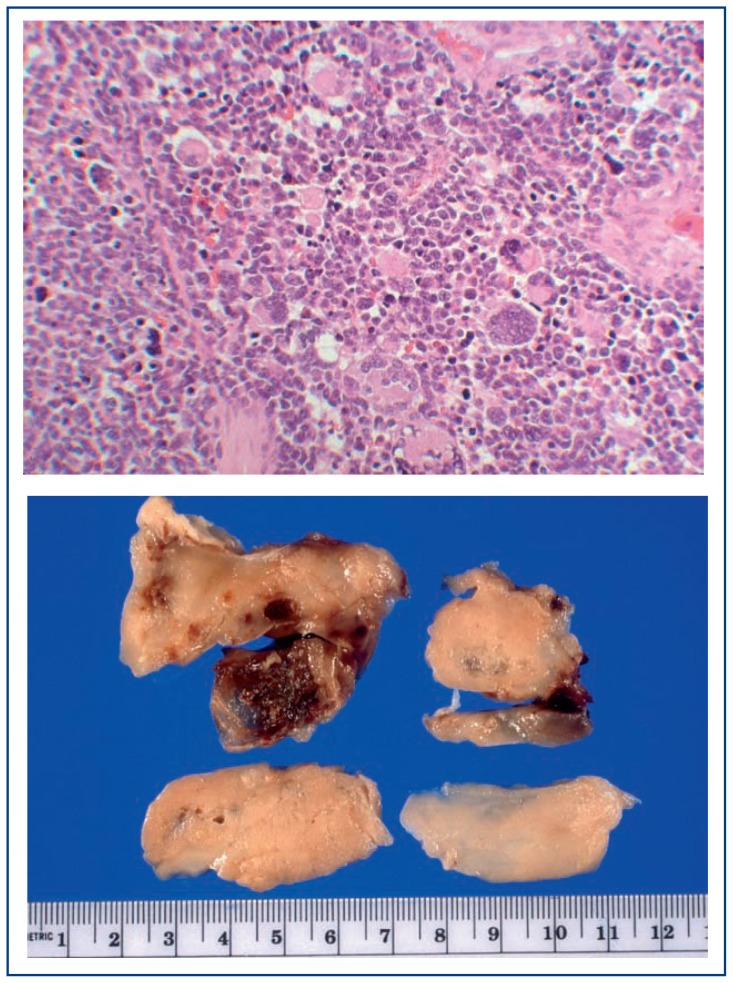
Figure 2.
Pleuropulmonary blastoma, type III
A. The tumor occupies the entire thoracic cavity and extends into the mediastinum of this 4-year-old boy who presented with fever and respiratory failure.
B. Multiple resected fragments of this mass show foci of hemorrhage in this tannish-white, solid tumor. C. A mixture of rhabdomyoblasts, anaplastic tumor cells and small primitive malignant cells is one of several microscopic patterns in this complex sarcoma.
As additional cases of PPB were submitted to the Registry, it became apparent that there were kindreds in whom other family members had a PPB, renal cystic lesion known as cystic nephroma and other childhood neoplasms.10, 11 In the course of a diagnostic evaluation for an ovarian mass, a lung cyst or cysts were identified in some cases; the ovarian mass was a Sertoli-Leydig cell tumor and the lung cysts were PPB type Ir.
Several kindreds agreed to participate and have their blood drawn for the purpose of searching for the suspected germline mutation (appropriate consenting and counseling was obtained from each family member in these kindreds). These samples were tested and in 2009, Hill and associates identified germline mutations in DICER1 as the first and only genetic cause for this familial syndrome which is known as the DICER1-related tumor susceptibility syndrome.12 With phenotype data alone, it was thought originally that 25% of PPB cases were familial, but we now know that approximately 70% of children with a PPB have a heterozygous DICER1 germline mutation, and 84% of those children inherited that mutation from a parent.13 The remainder of PPBs arise from de novo germline mutations, mosaicism or tumor-limited DICER1 mutations.13
Cystic nephroma (CN) of the kidney, a multilocular cystic lesion, occurs in early childhood and is seen in approximately 10% of children with PPB.11 The CN was the first and most commonly recognized extrapulmonary manifestation in children with PPB.14 From that observation, an entire family of DICER1-related neoplasms is now recognized and any one of these various tumors can serve as a conduit into the identification of a patient with a DICER1 germline mutation (Table 1). In other words, the possibility that a child or young adult may have a germline mutation in this autosomal dominant disorder may begin with an enlarged nodular goiter or one or more of the several extrapulmonary tumors. We have encountered multiple examples of children and adolescents who presented with one of the DICER1-related tumors, like a PPB, and a CN is discovered or embryonal rhabdomyosarcoma of the cervix with a PPB type 1r in the lung. Others may develop one in a series of metachronous DICER1-related tumors over a several year period beginning with a PPB in early childhood and a nodular goiter and Sertoli-Leydig cell tumor of the ovary in adolescence.
Table 1.
DICER1-Associated Neoplasms
|
Genetics of Pleuropulmonary Blastoma
The DICER1 tumor susceptibility syndrome appears to represent another example of Knudson’s two-hit hypothesis to explain several tumor syndromes with an inherited germline mutation followed by a post-zygotic somatic mutation in the same gene.15 Some hereditary predisposition syndromes affect one cell type though all of the various somatic cells have the particular mutation. In the case of the DICER1 syndrome, an entire range of cell types are involved in the tumor development from neuroectoderm, bronchoenteric endoderm and urogenital ridge mesoderm.
DICER1 encodes the DICER1 enzyme which is a member of the ribonuclease III family whose function is to cleave long double-stranded RNA into small RNAs (microRNA and small interfering RNA); these small RNAs have critical regulatory roles.16 The vast majority of DICER1 cancers have biallelic mutations composed of one allele with a complete loss of function, i.e. nonsense codon or small indel leading to premature truncation of the mRNA and protein, and the second allele with missense base substitutions affecting one of five amino acids in the critical RNase IIIb domain of the protein. The combination of these two mutations leads to lack of an entire class of miRNAs; those generated from the 5p end of the precursor miRNA hairpin. The DICER1 protein has a central role in development as a switch so that its function is ubiquitous in organogenesis so that all organs are potential targets in the presence of this critical faulty protein. As a developmental switch in the lung and kidney, dysembrogenesis results in the formation of cysts which in the lung become PPB type I and CN in the kidney. These cysts remain as such or undergo malignant progression to PPB type II or III or anaplastic sarcoma from CN. In the PPB type I, there may be scattered individual cells with aberrant TP53 nuclear staining indicating mutation, but consistently identified to a lesser degree in PPB type II or greater degree in PPB type III with the implication that mutant TP53 has an important role in malignant progression. Table 1.
Future Directions
The understanding of pathogenesis and technical advances in delivery of genes, miRNAs and drugs to tumor cells specifically, opens the door to developing safer and more effective treatments in the future. Our translational research program has developed in vivo models of human PPBs using immunocompromised mice hosts and viable tumor fragments from children with PPB shipped overnight to our laboratory. These mice provide the testing ground for preclinical studies on newly developed drugs. Leveraging the unique genetics of PPB we are also developing a circulating tumor DNA biomarker assay both to improve early detection and also to provide objective evidence of therapeutic effect (or lack thereof). We hope that these advancements will help us as we work to solve the most important problems for PPB drug development. What is the best way to restore homeostasis in PPB cells? Should we replace the missing miRNAs predicted by in vitro studies to halt cell proliferation? Should we attempt to fix the TP53 or DICER1 mutations using advances in gene delivery or CRISPR technology, respectively? And if one or more of those methods are effective, can we solve the problem of delivering to PPB tumor cells specifically?
Conclusions
PPB is an established, unique pathologic entity of childhood which serves to validate the continued role of the light microscope in the identification of new diseases in this era of molecular genetics and pathology. When one of us (LPD) saw the first case in 1977 at the University of Minnesota Hospital, histopathologic features were an unfamiliar mixed spindle cell, small blastemal cell, rhabdomyoblast and cartilaginous neoplasm arising as a pulmonary-mediastinal mass in a 4-year-old boy. It took almost 10 years to collect an additional 10 cases and report them as PPB as the bona fide pulmonary blastoma of childhood.3 We have made remarkable progress in the 30 years since this initial report. Through the extraordinary cooperation of the affected families, the combined efforts of numerous collaborators and the generous financial support of private and public sources, we continue our efforts in the investigation of this challenging familial tumor syndrome with its polymorphous manifestations.
Footnotes
Louis P. Dehner, MD, (above), MSMA member since 1990 and Missouri Medicine Editorial Board member for Pathology, is Professor of Pathology at Washington University School of Medicine, St. Louis, Missouri. Kris Ann Schultz, MD, Director, International Pleuropulmonary Blastoma and DICER1 Registry, and pediatric hematologist-oncologist of Children’s Minnesota-Children’s - Children’s Hospital and Clinics, Minneapolis, MN. D. Ashley Hill, MD, Professor of Pathology, George Washington University School of Medicine and Health Sciences, and Children’s National Medical Center, Washington, D.C.
Contact: dehner@wustl.edu
Disclosure
None reported.
References
- 1.Koss MN. Pulmonary blastomas. Cancer Treat Res. 1995;72:349–62. doi: 10.1007/978-1-4615-2630-8_16. [DOI] [PubMed] [Google Scholar]
- 2.Nakatani Y, Koss KM, Kerr MN, et al. Pulmonary Blastoma. In: Travis WD, Brambila E, Burke AP, et al., editors. WHO Classification of Tumours of Lung, Pleura, Thymus and Heart. 4th ed. IARC; Lyon: 2015. p. 93. [Google Scholar]
- 3.Manivel JC, Priest JR, Watterson J, Steiner M, Woods WG, Wick MR, et al. Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer. 1988;62(8):1516–26. doi: 10.1002/1097-0142(19881015)62:8<1516::aid-cncr2820620812>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Dehner LP, Messinger YH, Schultz KA, Williams GM, Wikenheiser-Brokamp K, Hill DA. Pleuropulmonary blastoma: evolution of an entity as an entry into a familial tumor predisposition syndrome. Pediatr Dev Pathol. 2015;18(6):504–11. doi: 10.2350/15-10-1732-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 6.Radhi M, Fulbright JM, Ginn KF, Guest EM. Childhood cancer for the primary care physician. Prim Care. 2015;42(1):43–55. doi: 10.1016/j.pop.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Saletta F, Seng MS, Lau LM. Advances in paediatric cancer treatment. Transl Pediatr. 2014;3(2):156–82. doi: 10.3978/j.issn.2224-4336.2014.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Leary M, Krailo M, Anderson JR, Reaman GH Children’s Oncology G. Progress in childhood cancer: 50 years of research collaboration, a report from the Children’s Oncology Group. Semin Oncol. 2008;35(5):484–93. doi: 10.1053/j.seminoncol.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messinger YH, Stewart DR, Priest JR, Williams GM, Harris AK, Schultz KA, et al. Pleuropulmonary blastoma: a report on 350 central pathology-confirmed pleuropulmonary blastoma cases by the International Pleuropulmonary Blastoma Registry. Cancer. 2015;121(2):276–85. doi: 10.1002/cncr.29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priest JR, Watterson J, Strong L, Huff V, Woods WG, Byrd RL, et al. Pleuropulmonary blastoma: a marker for familial disease. J Pediatr. 1996;128(2):220–4. doi: 10.1016/s0022-3476(96)70393-1. [DOI] [PubMed] [Google Scholar]
- 11.Boman F, Hill DA, Williams GM, Chauvenet A, Fournet JC, Soglio DB, et al. Familial association of pleuropulmonary blastoma with cystic nephroma and other renal tumors: a report from the International Pleuropulmonary Blastoma Registry. J Pediatr. 2006;149(6):850–4. doi: 10.1016/j.jpeds.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 12.Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325(5943):965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenneman M, Field A, Yang J, Williams G, Doros L, Rossi C, et al. Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in DICER1 syndrome: a unique variant of the two-hit tumor suppression model. F1000 Res. 2015;4:214. doi: 10.12688/f1000research.6746.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delahunt B, Thomson KJ, Ferguson AF, Neale TJ, Meffan PJ, Nacey JN. Familial cystic nephroma and pleuropulmonary blastoma. Cancer. 1993;71(4):1338–42. doi: 10.1002/1097-0142(19930215)71:4<1338::aid-cncr2820710427>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Berger AH, Knudson AG, Pandolfi PP. A continuum model for tumour suppression. Nature. 2011;476(7359):163–9. doi: 10.1038/nature10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song MS, Rossi JJ. Molecular mechanisms of Dicer: endonuclease and enzymatic activity. Biochem J. 2017;474(10):1603–18. doi: 10.1042/BCJ20160759. [DOI] [PMC free article] [PubMed] [Google Scholar]