Abstract
Nonhuman primates provide a human-relevant experimental model system to explore the mechanisms by which oxytocin (OT) regulates social processing and inform its clinical applications. Here, we highlight contributions of the nonhuman primate model to our understanding of OT treatment and address unique challenges in administering OT to awake behaving primates. Prior preclinical research utilizing macaque monkeys has demonstrated that OT can modulate perception of other individuals and their expressions, attention to others, imitation, vigilance to social threats, and prosocial decisions. We further describe ongoing efforts to develop an OT delivery system for use in experimentally naïve juvenile macaque monkeys compatible with naturalistic social behavior outcomes. Finally, we discuss future directions to further develop the rhesus monkey as a preclinical test bed to evaluate the effects of OT exposure and advance efforts to translate basic science OT research into safe and effective OT therapies.
Keywords: delivery, oxytocin, rhesus
1 |. THERAPEUTIC INTEREST IN OXYTOCIN
The prevalence and societal impact of autism spectrum disorder (ASD) creates an urgent need for innovative treatments for affected individuals. Current pharmacological treatments target only peripheral symptoms such as anxiety, aggression, and depression, but not the core impairments in reciprocal social interaction and communication that are hallmark features of ASD (Doyle & McDougle, 2012a, 2012b). Pro-social pharmacological treatments delivered in parallel with early behavioral interventions could play a critical role in modifying social development and greatly improve the quality of life for individuals with autism. The oxytocin (OT) system is emerging as one of the most promising areas of ASD treatment research (Green & Hollander, 2010; Modi & Young, 2012). OT is a neuropeptide that plays a central role in social cognition and behavior across species (Donaldson & Young, 2008; Insel, 2010), including humans (Guastella & Hickie, 2016; Guastella & MacLeod, 2012; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). In neurotypical adults, OT administration enhances a variety of social functions, including social gaze, face processing, emotion recognition, and trust (Guastella, Mitchell, & Dadds, 2008; Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005; Lischke et al., 2012; Schulze et al., 2011). Acute OT administration in adults with ASD is associated with a reduction in repetitive behaviors and improvements in social cognition (Anagnostou et al., 2012; Andari et al., 2010; Hollander et al., 2003, 2007). These results have recently been extended to younger individuals with ASD, where acute (Guastella et al., 2010) and prolonged (Tachibana et al., 2013; Yatawara, Einfeld, Hickie, Davenport, & Guastella, 2016) OT treatments have been associated with improvements in social functioning. Although the data emerging from ASD treatment studies are compelling, many questions remain regarding the therapeutic potential of OT treatment in humans (Bethlehem, van Honk, Auyeung, & Baron-Cohen, 2012; Churchland & Winkielman, 2012; Evans, Dal, Noble, & Averbeck, 2014; Guastella et al., 2013). There is a clear need for systematic evaluation of long-term safety and efficacy, as well as a better understanding of the mechanism of OT interventions in preclinical animal models.
2 |. PRECLINICAL MODEL SYSTEMS
Much of our understanding of the OT system is based on pioneering studies carried out initially in voles (Insel, 2010) and recently extended to ASD-specific mouse models (Bales et al., 2014; Penagarikano et al., 2015; Teng et al., 2013). There are clear advantages to initiating drug discovery efforts in rodent models including the power of genetic manipulations, lower costs, potential for extensive pilot research, and shorter duration of experiments. However, pharmacological interventions targeting the complex social and communication deficits of ASD may ultimately require the use of an animal model more closely related to humans. Nonhuman primates are thus uniquely positioned to bridge the gap between rodent and human OT studies (Freeman & Young, 2016). In the present review, we focus specifically on the rhesus monkey (Mococo mulatto) as a model system to explore the effects of OT administration, though we note that the OT system in New World monkeys supports social traits, such as pair-bonding, not found in macaques and thus provides an alternative preclinical approach (Lee, Cool, & Parker, 2011; Vargas-Pinilla et al., 2015).
Although rodents are separated from humans by more than 70 million years of evolution, rhesus macaques diverged from the human lineage closer to 25 million years ago (Gibbs et al., 2004; Kumar & Hedges, 1998). The resulting similarities between macaques and humans in neurobiology and behavior provide a model system that can be used to evaluate etiologies, identify neurobiological mechanisms, and ultimately develop and test novel pharmacological interventions for human neurodevelopmental disorders (Capitanio & Emborg, 2008; Ruhela, Prakash, & Medhi, 2015). Indeed, rhesus monkeys and humans live in complex social groups, show similar developmental trajectories and activity patterns in brain regions underlying complex social processing (Platt, Seyfarth, & Cheney, 2016), and have evolved a sophisticated social communication system that includes a variety of facial expressions, body postures, and vocalizations not shared with rodents (Chang et al., 2013). Among macaque social signals, the use of facial expressions is one of the most salient features of social behavior and the most similar to our own social communication (Bower, Suomi, & Paukner, 2012; Deaner, Khera, & Platt, 2005; Ferrari et al., 2006; Ferrari, Paukner, lonica, & Suomi, 2009). When viewing faces of conspecifics, macaques show a remarkably human-like pattern of visual attention that focuses heavily on regions of the face that are critical for social processing (i.e., eye and mouth) (Machado, Bliss-Moreau, Platt, & Amaral, 2011). Though not all complex human behaviors can be modeled in any animal (i.e., language, theory of mind), rhesus monkeys are a closer approximation to humans in both brain and behavioral complexity than are rodents. Following this logic, researchers have capitalized on the complex social development of rhesus monkeys to explore genetic and environmental risk factors associated with ASD and are beginning to explore therapeutic interventions (Bauman & Schumann, 2018).
Complex social behaviors, including dominance status, rearing experience, affiliations, and processing abilities have been associated with variability in endogenous levels and epigenetic regulation of the OT system in rhesus monkeys (Baker et al., 2017; Coplan et al., 2015; Madrid et al., 2017; Weinstein, Bales, Maninger, Hostetler, & Capitanio, 2014; Winslow, Noble, Lyons, Sterk, & Insel, 2003). Moreover, direct manipulation of the OT system by focal infusion into the macaque brain alters social gaze, prosocial decisions (Chang et al., 2015) and behavioral synchrony (Jiang & Platt, 2018), and the release of serotonin in brain regions implicated in social processing (Lefevre, Richard, et al., 2017). OT receptors in the rhesus monkey brain are highly expressed in regions involved with visual processing, including the nucleus basalis of Meynert, the pedunculopontine tegmental nucleus, the superficial gray layer of the superior colliculus, the trapezoid body, and the ventromedial hypothalamus (Freeman, Inoue, Smith, Goodman, & Young, 2014). A recent functional magnetic resonance imaging (fMRI) study carried out in macaques (Liu et al., 2015) reported that treatment with OT diminished BOLD responses to aggressive and fearful faces in the amygdala, as in humans (Domes et al., 2007), and reduced functional coupling between the amygdala and areas in the occipital and inferior temporal cortex. Although a high density of OT receptors has not been found in the regions of interest identified in the fMRI study, activity in these regions may be modulated by anatomically connected areas that do contain OT receptors (e.g., amygdala and nucleus basalis of Meynert) (Jones, Burton, Saper, & Swanson, 1976; Mesulam, Mufson, Levey, & Wainer, 1983). The neuroanatomical and behavioral complexity, paired with the conserved role of OT in humans and nonhuman primates, suggests that the rhesus monkey provides a model system uniquely powered to explore the efficacy and mechanism of OT treatment.
3 |. OT ADMINISTRATION ROUTES AND PHARMACOKINETICS
OT administration studies in rhesus monkeys have utilized a variety of administration routes, including subcutaneous (SC) or intravenous (IV) injections, and intranasal spray delivery (IN), though the majority has used a nebulizer to deliver OT via aerosolized exposure (AE) (Table 1). A small number of nonhuman primate studies have evaluated pharmacokinetic outcomes associated with these different routes of administration, though methodological differences have contributed to inconsistent findings. While rodent studies have demonstrated increases in both brain and plasma OT levels 30–60 min following nasal administration (Neumann, Maloumby, Beiderbeck, Lukas, & Landgraf, 2013), the relationship between central and peripheral levels of OT remains unclear, possibly due to temporal differences in OT bioavailability (Lefevre, Mottolese, et al., 2017). Here, we briefly summarize nonhuman primate studies reporting cerebrospinal fluid (CSF) outcome measures, which provide a direct and relatively noninvasive assessment of central nervous system (CNS) penetration routinely available for nonhuman primates.
TABLE 1.
Summary of studies investigating the effects of OT treatment in rhesus monkeys
| Authors | Dose | Route | Delivery Details | Animal | Test | Timing | Results | PK | Title | Journal | Otar information | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmacokinetic Outcome Measures | ||||||||||||
| Modietal | 24 IU | IN (spray and nebulizer) IV | Under anesthesia (24 IU/2ml for 4 0 5ml/min) |
Rhesus monkey | N=14 (males) 9–14 kg |
PK | 0, 5, 15, 60, 120 minutes | - All routes significantly increased plasma OT, but only the nebulizer route
significantly increased CSF OT - No route affected concentrations of AVP in plasma or CSF |
Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques | Psychoendocrinology, 2014 | - Novartis nasal actuator - Pari Baby Nebulizer |
|
| Dal Monte et al | 48 IU | IN (spray and nebulizer) | Under anesthesia (2 ml for 5–8 minutes) | Rhesus monkey | N=15(males) 8–12 kg 6–10 years of age |
PK | 0, 10, 20, 30, 40 minutes | - IN OT administration increased OT concentration in both CSF and plasma - changes in plasma OT were greater after nasal spray compared to nebulizer |
CSF and blood oxytocin concentration changes following intranasal delivery in macaque | PLOS ONE, 2014 | - Intranasal Mucosal Atomization Device(Wolfe Tory Medical) - Pari Baby Nebulizer |
|
| Freeman el al. | 0.1, 1 and 5 IU/kg | IN (spray) IV |
Awake – chair restraint for IN and IV (multiple doses) | Rhesus monkey | N=4 (females) 5.8–7.3 kg 6–8 years of age |
PK | 0, 5, 15, 30, 60, 120 minutes | 5IU/kg of OT(~32 IU/body) increased OT level in CSF at 15 and 30 min post-treatment | Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin awake macaques | Psychoendocrinology, 2016 | ||
| Pharmacokinetic and Behavior Outcome Measures | ||||||||||||
| Chang et al. | 25 IU | IN | Awake - head restraint (25 lU/ml for 5 minutes) | Rhesus monkey | N=2 | - Reward allocation task - PK |
30 minutes later | - Within the first 2 hours, OT increased selfish choices - Two hours after inhalation, monkeys increased the frequency of prosocial choices |
CSF saline: 20 pg/ml OT: 50 pg/ml |
Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) | PNAS, 2012 | Pari Baby Nebulizer |
| Simpson et al | 25 IU | IN | Awake - hand held (1 25 ml at 20 lU/ml tor 7 minutes) | Rhesus monkey | N=28 (16 males and 12 females) <14 days of age |
- Imitation recognition test - PK (saliva) |
1 and 2 hours later | OT increased infants’ facial gestures | Saliva saline: 396 pg/ml OT: 26995 pg/ml at 2 hours |
Inhaled oxylocin increases positive social behaviors in new newborn macaques | PNAS. 2014 | Pari Baby Nebulizer |
| Blevins et al | 0 2 and 0 4 mg/kg |
SC | Awake – injections(twice a day for 2 weeks for each dose) | Rhesus monkey | N=5 (males) 17.5 +/− 1.1 kg 10 18 years of age |
- Body weight, food intake, energy, expenditure, blood chemistry - PK |
chronic administration | - OT reduced body weight, food intake, glucose, TG and total cholesterol - OT increased energy expenditure, FFA and glycerol |
Plasma OT level 0 4 mg/kg for 4 weeks: 4250 pg/ml (basal 2942 pg/ml) |
Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed ohese rhesus monkeys | American Journal of Physiology - Regulatory, Integrate and Comparative Physiology, 3014 | |
| Behavior Outcome Measure | ||||||||||||
| Parr et al | 24 IU | IN | Awake - hand held (<4mos), Awake – dosing box(>4mos) delivered once or 3 times per week for 4 months | Rhesus monkey | N=24 (males) 2 months of age |
Eye tracking test using facial expressions | chronic administration | - OT increased mean total looking time - OT reduced proportion of lookmg duration towards eyes in neutral expression - OT increased mean fixation duration (once a week dosing group) |
No data | Effects of chronic oxytocin on attention to dynamic facial expression in infat macaques | Psych0neuroendocrinology. 2016 | <16wks, nebulizer >16wks, dosing box |
| Parr et al | 48 IU | IN | Awake - trained on nebula izer mask (1.71 ug/IU/2ml) 4 cumulative minutes within 5 minutes | Rhesus monkey | N=6 (4 males and 2 females) 3 (N=2) and 12 (N=4) years of age |
Dot-probe task | 60 minules later | OT reduced monkeys’ attention selectively to negative facial expression | No data | Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions | Psychoendocrinology, 2013 | Pari Baby Nebulizer |
| Ebitz et al. | 10, 25 IU | IN | Awake head restraint (25 lU/ml for % minutes) |
Rhesus monkey | N=7 (males) 4–11 years of age |
- Unconstrained viewing task - Image choice task - Social interference task |
10–30 minutes | OT suppressed vigilance toward potential social threats | No data | Oxylocin blunts social vigilance in the rhesus macaque | PNAS, 2013 | Pari Baby Nebulizer |
| Liu et al. | 24 IU | IN (spray) | Awake head restraint (60 lU/ml) | Rhesus monkey | N=3 (males) 6.5–7.5 kg 9 years of age |
fMRI responses evoked by the perception of facial expression | 40 minules later | - OT reduced responses to both fearful and aggressive faces in face-responsive
regions. - OT reduced functional coupling between the amygdala and areas in the occipital and inferior temporal cortex during viewing of fearful |
No data | Oxytocin modulates fMRI responses to facial expression in macaques | PNAS, 2015 | Intranasal Mucosal Atmizalion Device (Wolfe tory Medical) |
| Dal Monte et al. | 24 IU | IN (spray) | Awake - head restraint (24 lU/ml) | Rhesus monkey | N=4 (males) 7–11 kg 6–10 years of age |
Free viewing task of conspecifics displaying one of three facial expression | 45 minutes later | OT increased fixations to the eye region relative to the mouth region | No data | Oxytocin enhances attention to the eye region in rhesus monkeys | Frontiers in Neuroscience, 2014 | |
| Simpson et al. | 18.4 IU | IN | Hand held (20 lU/ml, 7 min) | Rhesus monkey | N=24(11 males 13 females) 1 month of age | - Working memory task - Gaze following task |
60 minutes later | - OT improved working memory and gaze following only in males | No data | Acute oxytocin improves memory and gaze following in male but not female nursery-reared infat macaques | Psychopharmacology (Berl), 2017 | Pari Baby Nebulizer |
| Hess et al. | 5 IU (1.2 IU/kg) |
IN (spray) | Awake - cage restraint (5 lU/ml, 8.5ug/ml) | Rhesus monkey | N=20 (males and female) 2.6–5 4 kg 1–3 years of age |
- Aggressive behavior classification - blood pressure, pulse rate and oxygen saturation of hemoglobin |
Right after dosing | - OT caused a dose-dependent loss of agressiveness and/or anxiety - OT reduced pulse rate |
No data | Sedative effects of intranasal oxytocin in rabbits and rhesus monkeys | Physiological Research. 2016 | LMA MAD Nasal lntranasal Mucosal Atomization Device (Teleflex) |
| Putnam et al. | 50 IU | IN | Awake - head restraint (25 lU/ml) | Rhesus monkey | N=4 (males) 6–12 years of age |
Gaze following test | 10 minutes later | OT increased gaze following saccades | No data | Oxytocin enhances gaze-following responses to videos of natural social behavior in adult male rhesus monkeys | Psychoneuroendocrinology. 2016 | Pari Baby Nebulizer |
| Landman et al. | 25 IU | IN | Awake - head restnanl (Nebulization tor 5–15 minutes) | Rhesus monkey | N=3 | Covert attention task | 60 minutes later | OT ameliorated the interfering effects of faces | No data | Effect of distracting faces on visual selective attention in the monkey | PNAS. 2014 | Pari Baby Nebulizer |
| Jiang et al. | 25 IU | IN | Awake - head restraint (25 lU/ml for 5 minutes) | Rhesus monkey | N=14(10 males,4 females), 6–18 years of age | Quantification of species typical social behaviors in chair restrained animals | 30 minutes later | OT relaxed social interactions flattened the social hierarchy, and enhanced social communication | No data | Oxytocin and vasopressin flatten dominance hierarchy and enhance behavioral synchrony in part via anterior cingulate cortex | Sci Rep, 2018 | Pan Baby Nebulizer |
Prior to evaluating the effects of OT administration on the behavior of awake, behaving monkeys Chang et al. assayed CSF OT levels 0.5 h after awake AE and reported ~2.5-fold increase in CSF OT levels compared with saline (Chang, Barter, Ebitz, Watson, & Platt, 2012). Modi, Connor-Stroud, Landgraf, Young, and Parr (2014) later compared effects of IN, IV, and AE routes of OT administration under anesthesia on concentrations of OT and vasopressin (AVP) in plasma and CSF, and found that all three administration routes significantly increased plasma OT concentrations, but only the AE route significantly increased concentrations of CSF OT. In contrast, Dal Monte, Noble, Turchi, Cummins, and Averbeck (2014) found that IN and AE routes of OT exposure under anesthesia produced similar elevations of OT concentration in CSF, while the changes in plasma OT concentration were greater after IN compared to AE. It is important to note that variations in AE nebulizer delivery protocols (i.e., mask placement, fit, and cooperation of the animal) could influence the amount of OT actually inhaled by an individual. More recently, Freeman et al. (2016) compared IV and IN spray administration in awake adult female macaques with chronic intrathecal catheters to investigate the pharmacokinetic profile of OT in the central nervous system and the peripheral vasculature. Following IV administration, they documented a dose-dependent effect of OT treatment on plasma OT levels, though a change in CSF OT was only observed at the highest IV dose. In contrast, there was no significant change in plasma OT at any of the three doses following IN administration, though an increase in CSF OT was detected at the highest dose. Collectively, the studies highlighted above provide evidence that OT exposure results in increased CSF OT in rhesus macaques, though variability in dose, sample collection timing, and route of administration have yielded inconsistent findings and made it challenging to compare across studies. Importantly, CSF samples are not compatible with all experimental paradigms, including chronic administration studies (Blevins et al., 2015).
4 |. BEHAVIORAL OUTCOMES AND EXPERIMENTAL DESIGN CONSIDERATIONS
Although several routes of administration have been associated with increased OT levels in the CSF, AE delivered via nebulizer is most compatible with measuring outcomes in awake behaving animals. Initial studies in adult nonhuman primates found that OT delivered to awake monkeys via nebulizer (25 lU/ml; Agrilabs) into the nose and mouth continuously for 5 min (5 IU/min) resulted in increased prosocial behaviors and social gaze in a reward allocation test (Chang et al., 2012). It is important to note that the animals used in this study were equipped with head-restraint prostheses to facilitate single-neuron recordings and monitor eye position, which also allowed for OT nebulizer delivery. Indeed, studies utilizing similar acute OT delivery approaches in adult male monkeys have found that OT suppresses vigilance toward potential social threats (Ebitz, Watson, & Platt, 2013), increases the frequency of gaze following saccades in response to naturalistic social stimuli (Putnam, Roman, Zimmerman, & Gothard, 2016), increases fixations to the eye region relative to the mouth (Dal Monte, Noble, Costa, & Averbeck, 2014), and alters attention to emotional distractors (Landman, Sharma, Sur, & Desimone, 2014). Most recently, Jiang and Platt (2018) showed that nebulized OT flattens the social hierarchy and increases synchrony of spontaneous social behaviors in pairs of male rhesus macaques, and that these effects are reproduced by injecting OT into the medial prefrontal cortex, a brain region associated with social processing. Taken together, these studies in adult macaques suggest homologies between rhesus monkeys and humans in the effects of OT on social behavior and underlying neural circuits (Chang & Platt, 2013; Ebitz & Platt, 2013).
Given that ASD is a neurodevelopmental disorder, it is also essential to evaluate the effects of OT exposure in young animals. The nebulizer delivery approach described above is also compatible with infant monkeys that can be hand-held during delivery and thus do not require head-restraint. Nursery-reared macaques treated with intranasal OT demonstrate increased facial gesturing to human care givers and a positive correlation between salivary OT levels and time spent in proximity to the care giver (Simpson et al., 2014). In this acute exposure study, early imitation skills predicted OT-associated increases in affiliative behaviors, suggesting that infant macaques who demonstrate a high propensity for social interactions early in life may be more sensitive to OT manipulation. A subsequent acute exposure study from this same group found that OT improved working memory and gaze following in nursery-reared macaques, but only for males (Simpson et al., 2017). While acute exposure of OT in young monkeys has yielded intriguing pro-social outcomes, initial evaluations of long-term OT exposure have raised concerns. Chronic administration of OT from 2 to 6 months of age increased the time spent viewing videos of dynamic facial expression, but selectively reduced attention to the eye region of neutral faces in a dose-dependent manner (Parr et al., 2016). The authors suggest that repeated administration of OT may homeostatically down-regulate OT receptors in regions of the brain that regulate social attention—which bears important implications for treating children with OT. Additional preclinical research is clearly needed to explore the differences between the effects of acute and chronic OT treatments given that studies in patient populations are more likely to use repeated OT treatments.
5 |. OT DELIVERY CHALLENGES
OT delivered by nebulizer has been successfully used for (i) adult animals fitted with a head-restraint prosthesis, (ii) infant monkeys less than 6 months of age that can be hand held, or (iii) 4–6 months old infant monkeys exposed in a small chamber. Given that monkeys mature approximately four times as fast as humans, the ideal age range to explore OT treatments relevant to treating human children would be between 6 months and 3 years of age. In this age range, the monkeys are too large for physical restraint techniques used with infant monkeys, and the head restraint approaches used in adult animals are not often compatible with more naturalistic social interactions, which may be more relevant to ASD targeted treatments. For example, the infants in the Parr et al. (2016) chronic administration study were hand held for OT nebulizer administration until 4 months of age and then exposed to OT using a small chamber equipped with several port openings for the nebulizers until reaching 6 months of age. The chamber delivery approach may be compatible with younger animals, but would likely become cost prohibitive due to the amount of OT required to expose juvenile monkeys in a larger chamber. To address this challenge, Parr, Modi, Siebert, and Young (2013) developed a specially designed cage equipped with a nebulizer that does not require restraint for OT delivery. Monkeys (four adults and two 3 year olds) were trained to maintain their face in a position over the nebulizer and breathe through their nose by sipping from a drink tube administering fluid reward (diluted yogurt) for 4 cumulative minutes, within a 5 min window. For these studies, a dose of 48 IU was used because much of the aerosol evaporates. One hour after OT administration, the monkeys performed a computerized task to measure their attentional bias to social, emotional, and nonsocial images. OT exposure significantly reduced monkeys’ attention to negative facial expressions, but not neutral social or nonsocial images. Although this study yielded intriguing behavioral changes associated with a novel route of OT administration, pharmacokinetic evaluation of OT penetrance was not included.
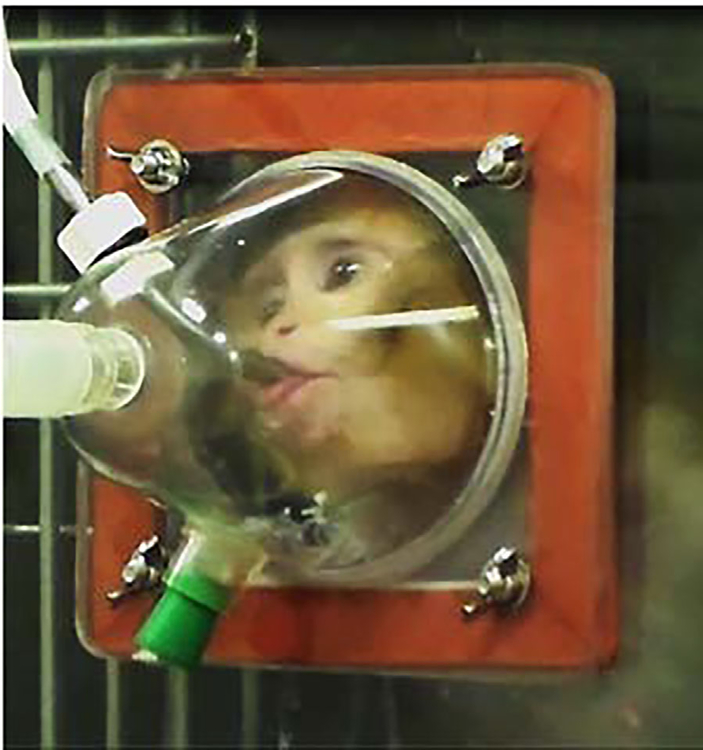
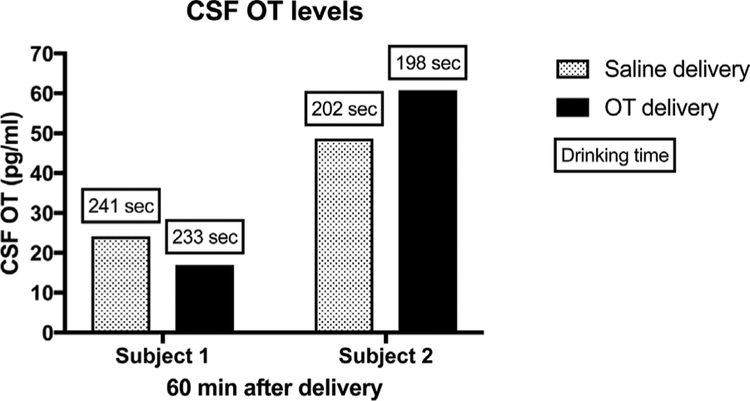
We therefore recently carried out a pilot study to modify the Parr nebulizer mask described above for use in juvenile (2 years old) macaques raised in a naturalistic social environment with no previous laboratory experience (Figure 1). Experimental procedures were developed in consultation with the veterinary staff at the California National Primate Research Center and performed in accordance with the University of California, Davis Institutional Animal Care and Use Committee. Four juvenile monkeys (2 male and 2 female) were trained to drink a fluid reward (i.e., yogurt, baby food, etc.) from a small tube embedded adjacent to a Pari Baby Nebulizer that delivered OT (Sigma-Aldrich, St. Louis, MO) or saline placebo. We adapted the mask design and guidelines established by Parr (4 cumulative minutes, within a 5 min window) (Parr et al., 2013) to be more compatible with a population of young, experimentally naïve animals (4 cumulative minutes, within a 10 min window). A series of positive reinforcement training techniques were utilized over several months to reach a target mask exposure time of approximately 4–5 min for each 10-min session. OT doses ranging from 11.5 to 46 IU were evaluated in pilot studies and cumulative drinking time was quantified for each experiment as an index of OT exposure within the 10 min window. Levels of OT in CSF were measured using commercially available OT ELISA kits (Enzo Life Sciences, Farmingdale, NY) following previously established methods (Freeman et al., 2016). Briefly, assays were performed following the manufacturer’s protocols. CSF samples were not diluted prior to assay and no samples went through extraction prior to assay. Limits of detection were 15.6–1,000 pg/ml. Values falling below or above the limits of detection were set to 15.6–1,000 pg/ml, respectively. Sample OT CSF data from animals exposed for 3–4 cumulative minutes at the highest dose (46IU) are shown in Figure 2. Although our pilot study of OT exposure in experimentally naïve juvenile animals is ongoing, our initial pharmacokinetic analysis has yielded inconsistent results across multiple animals (both male and female), doses and seasons. To illustrate these inconsistencies, we have selected CSF samples from two juveniles (1 male, 1 female) with comparable mask exposure times (Figure 2). It is plausible that 3–4 min cumulative mask time was not sufficient or that respiration parameters and kinetics into the sinuses may be different in juvenile macaques or that sex differences may influence OT responses. Individual variability in CSF oxytocin levels has also been reported in adult female macaque monkeys following intranasal and intravenous OT exposure, though the functional significance of this variability is not well understood (Freeman et al., 2016). At this time, the majority of OT exposure studies in macaque monkeys is not adequately powered to explore sex effects (Table 1), and will require additional research. Given these inconsistencies experienced in our pilot dosing studies we adapted our project to include younger monkeys (<6 mos) that can be hand held for OT delivery (Figure 3), but present our juvenile pilot data to alert other investigators to the challenges in delivering OT to experimentally naïve juveniles without restraint.
FIGURE 1.
Juvenile macaques trained to drink from a masknebulizer apparatus
FIGURE 2.
CSF OT levels from animals exposed for 3–4 cumulative minutes at the highest dose (46 IU). These juveniles were trained to reach a cumulative mask exposure of 4 min (240 s) over a 10 min exposure period. CSF samples were collected 60 min post-exposure. Mask exposure time is noted above each bar. Note that Subject #1 (male) reached 233 s OT exposure, yet OT CSF levels decreased from saline while Subject #2 (female) reached only 198 s and demonstrated an increase in OT CSF compared to saline. Similar inconsistencies were noted across multiple subjects, doses, and seasons
FIGURE 3.
Infant macaques hand held during OT exposure (<6 mos of age)
6 |. CONCLUSION AND FUTURE DIRECTIONS
Assuming that pharmacological agents targeted at improving social behavior ultimately act upon brain regions that underlie species-typical social behavior, it will be essential to evaluate these compounds in a species with neural and behavioral social functions homologous to humans. The nonhuman primate model system is experimentally poised to provide insights into the anatomy, physiology, and behavioral effects of the OT system. However, there are challenges in delivering OT to experimentally naïve juveniles. It is plausible that OT administration that utilizes some form of restraint may provide the most experimental control and therefore the best penetrance of the CNS. However, restraint may not be compatible with experimental designs that seek to study the effects of OT on more naturalistic interactions with conspecifics, especially in juveniles 6–24 months of age. We propose the following considerations for OT delivery: (i) physiological differences in respiration between adult and juvenile monkeys may contribute to inconsistencies; (ii) training juvenile animals reared in naturalistic social environments will require a significant time commitment (i.e., months) and may not be compatible with experimental constraints; (iii) once juveniles reach target cumulative drinking time, additional training may be required to maintain a constant exposure; (iv) it may be necessary to employ training techniques that prepare laboratory animals to cooperate with restraint (Bliss-Moreau & Moadab, 2016) to obtain adequate OT exposure.
ACKNOWLEDGMENTS
Experimental procedures were developed in consultation with the veterinary staff at the California National Primate Research Center and performed in accordance with the University of California, Davis Institutional Animal Care and Use Committee. This work was supported by NICHD R21HD080498 to MDB. and the California National Primate Center (CNPRC) base grant OD011107.
Funding information
National Institute of Child Health and Human Development, Grant number: R21HD080498
REFERENCES
- Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, … Hollander E. (2012). Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Molecular Autism, 3(1), 16 10.1186/2040-2392-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, & Sirigu A (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America, 107(9), 4389–4394. 10.1073/pnas.0910249107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Lindeil SG, Driscoll CA, Zhou Z, Yuan Q, Schwandt ML, … Barr CS. (2017). Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America, 114(44), 11769–11774. 10.1073/pnas.1706206114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, … Mendoza SP. (2014). Long-term exposure to intranasal oxytocin in a mouse autism model. Translational Psychiatry, 4, e480 10.1038/tp.2014.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, & Schumann CM (2018). Advances in nonhuman primate models of autism: Integrating neuroscience and behavior. Experimental Neurology, 299(Pt A), 252–265. 10.1016/j.expneurol.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, & Baron-Cohen S (2012). Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology, 38(7), 962–974. 10.1016/j.psyneuen.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG, & Havel PJ (2015). Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 308(5), R431–R438. 10.1152/ajpregu.00441.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, & Moadab G (2016). Variation in behavioral reactivity is associated with cooperative restraint training efficiency. Journal of the American Association for Laboratory Animal Science, 55(1), 41–49. [PMC free article] [PubMed] [Google Scholar]
- Bower S, Suomi SJ, & Paukner A (2012). Evidence for kinship information contained in the rhesus macaque (Macaca mulatto) face. Journal of Comparative Psychology, 126(3), 318–323. 10.1037/a0025081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, & Emborg ME (2008). Contributions of non-human primates to neuroscience research. Lancet, 371(9618), 1126–1135. https://doi.org/S0140-6736(08)60489-4[pii] [DOI] [PubMed] [Google Scholar]
- Chang SW, Barter JW, Ebitz RB, Watson KK, & Platt ML (2012). Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proceedings of the National Academy of Sciences of the United States of America, 109(3), 959–964. 10.1073/pnas.1114621109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Brent LJ, Adams GK, Klein JT, Pearson JM, Watson KK, & Platt ML (2013). Neuroethology of primate social behavior. Proceedings of the National Academy of Sciences of the United States of America, 110(Suppl 2), 10387–10394. 10.1073/pnas.1301213110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Fagan NA, Toda K, Utevsky AV, Pearson JM, & Platt ML (2015). Neural mechanisms of social decision-making in the primate amygdala. Proceedings of the National Academy of Sciences of the United States of America, 112(52), 16012–16017. 10.1073/pnas.l514761112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, & Platt ML (2013). Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Research, 1580, 57–68. https://doi.org/lO.lOl6/j.brainres.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland PS, & Winkielman P (2012). Modulating social behavior with oxytocin: How does it work? What does it mean? Hormones and Behavior, 61(3), 392–399. 10.1016/j.yhbeh.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Karim A, Chandra P, St Germain G, Abdallah CG, & Altemus M (2015). Neurobiology of maternal stress: Role of social rank and central oxytocin in hypothalamic-pituitary adrenal axis modulation. Frontiers in Psychiatry, 6,100 10.3389/fpsyt.2015.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Costa VD, &Averbeck BB. (2014). Oxytocin enhances attention to the eye region in rhesus monkeys. Frontiers in Neuroscience, 8, 41. 10.3389/fnins.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Turchi J, Cummins A, &Averbeck BB. (2014). CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS ONE, 9(8), el03677 10.1371/journal.pone.0103677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, & Platt ML (2005). Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Current Biology, 15(6), 543–548. 10.1016/j.cub.2005.01.044 [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchei C, Braus DF, & Herpertz SC (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62(10), 1187–1190. 10.1016/j.biopsych.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, & Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science, 322(5903), 900–904. 10.1126/science.1158668 [DOI] [PubMed] [Google Scholar]
- Doyle CA, & McDougle CJ (2012a). Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialogues in Clinical Neuroscience, 14(3), 263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle CA, & McDougle CJ (2012b). Pharmacotherapy to control behavioral symptoms in children with autism. Expert Opinion on Pharmacotherapy, 13(11),1615–1629. 10.1517/14656566.2012.674110 [DOI] [PubMed] [Google Scholar]
- Ebitz RB, & Platt ML (2013). An evolutionary perspective on the behavioral consequences of exogenous oxytocin application. Frontiers in Behavioral Neuroscience, 7, 225 10.3389/fnbeh.2013.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Watson KK, & Platt ML (2013). Oxytocin blunts social vigilance in the rhesus macaque. Proceedings of the National Academy of Sciences of the United States of America, 110(28), 11630–11635. 10.1073/pnas.1305230110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SL, Dal Monte O, Noble P, & Averbeck BB (2014). Intranasal oxytocin effects on social cognition: A critique. Brain Research, 1580, 69–77. 10.1016/j.brainres.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Paukner A, lonica C, & Suomi SJ (2009). Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Current Biology, 19(20), 1768–1772. 10.1016/j.cub.2009.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, & Suomi SJ (2006). Neonatal imitation in rhesus macaques. PLoS Biology, 4(9), e302 10.1371/journal.pbio.0040302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, & Young LJ (2014). The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatto). Psychoneuroendocrinology, 45,128–141. 10.1016/j.psyneuen.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GG, & Roberts JA (2016). Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology, 66, 185–194. 10.1016/j.psyneuen.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Freeman SM, & Young LJ (2016). Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. Journal of Neuroendocrinology, 28(4), 1–12. https://doi.org/10.llll/jne.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S,… Collins F. (2004). Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature, 428-(6982), 493–521. 10.1038/nature02426 [DOI] [PubMed] [Google Scholar]
- Green JJ, & Hollander E (2010). Autism and oxytocin: New developments in translational approaches to therapeutics. Neurotherapeutics, 7(3), 250–257. 10.1016/j.nurt.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, & Hickie IB (2010). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry, 67(7), 692–694. 10.1016/j.biopsych.2009.09.020 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, & Hickie IB (2016). Oxytocin treatment, circuitry, and autism: A critical review of the literature placing oxytocin into the autism context. Biological Psychiatry, 79(3), 234–242. 10.1016/j.biopsych.2015.06.028 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, … Banati RB. (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology, 38(5), 612–625. 10.1016/j.psyneuen.2012.ll.019 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, & MacLeod C (2012). A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Hormones and Behavior, 61(3), 410–418. 10.1016/j.yhbeh.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Dadds MR (2008). Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry, 63(1), 3–5. 10.1016/j.biopsych.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, … Wasserman S. (2007). Oxytocin increases retention of social cognition in autism. Biological Psychiatry, 61(4), 498–503. 10.1016/j.biopsych.2006.05.030 [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, & Mosovich S (2003). Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology, 28(1), 193–198. 10.1038/sj.npp.1300021 [DOI] [PubMed] [Google Scholar]
- Insel TR (2010). The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron, 65(6), 768–779. 10.1016/j.neurnn.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, & Platt ML (2018). Oxytocin and vasopressin flatten dominance hierarchy and enhance behavioral synchrony in part via anterior cingulate cortex. Scientific Reports, 8(1), 8201 10.1038/s41598-018-25607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Burton H, Saper CB, & Swanson LW (1976). Midbrain, diencephalic and cortical relationships of the basal nucleus of Meynert and associated structures in primates. The Journal of Comparative Neurology, 167(4), 385–419. 10.1002/cne.901670402 [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, & Fehr E (2005). Oxytocin increases trust in humans. Nature, 435(7042), 673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Kumar S, & Hedges SB (1998). A molecular timescale for vertebrate evolution. Nature, 392(6679), 917–920. 10.1038/31927 [DOI] [PubMed] [Google Scholar]
- Landman R, Sharma J, Sur M, & Desimone R (2014). Effect of distracting faces on visual selective attention in the monkey. Proceedings of the National Academy of Sciences of the United States of America, 111(50), 18037–18042. 10.1073/pnas.1420167111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC Jr., Neal DE, Buckmaster CL, Cheng MY,… Parker KJ. (2011). A novel form of oxytocin in New World monkeys. Biology Letters, 7(4), 584–587. 10.1098/rsbl.2011.0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Mottolese R, Dirheimer M, Mottolese C, Duhamel JR, & Sirigu A (2017). A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Scientific Reports, 7(1), 17222 10.1038/s41598-017-17674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Richard N, Jazayeri M, Beuriat PA, Fieux S, Zimmer L,… Sirigu A. (2017). Oxytocin and serotonin brain mechanisms in the nonhuman primate. Journal of Neuroscience, 37(28),6741–6750. 10.1523/JNEUROSCI.0659-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, & Domes G(2012). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology, 37(4), 475–481. 10.1016/j.psyneuen.2011.07.015 [DOI] [PubMed] [Google Scholar]
- Liu N, Hadj-Bouziane F, Jones KB, Turchi JN, Averbeck BB, & Ungerleider LG (2015). Oxytocin modulates fMRI responses to facial expression in macaques. Proceedings of the National Academy of Sciences of the United States of America, 112(24), E3123–E3130. 10.1073/pnas.l508097112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bliss-Moreau E, Platt ML, & Amaral DG (2011). Social and nonsocial content differentially modulates visual attention and autonomic arousal in Rhesus macaques. PLoS ONE, 6(10), e26598 10.1371/journal.pone.0026598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid JE, Oztan O, Sclafani V, Del Rosso LA, Calonder LA, Chun K, … Parker KJ. (2017). Preference for novel faces in male infant monkeys predicts cerebrospinal fluid oxytocin concentrations later in life. Scientific Reports, 7(1), 12935 10.1038/s41598-017-13109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, & Wainer BH (1983). Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. The Journal of Comparative Neurology, 214(2), 170–197. 10.1002/cne.902140206 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M (2011). Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience, 12(9), 524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Modi ME, Connor-Stroud F, Landgraf R, Young LJ, & Parr LA (2014). Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology, 45, 49–57. 10.1016/j.psyneuen.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, & Young LJ (2012). The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Hormones and Behavior, 61(3), 340–350. 10.1016/j.yhbeh.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, & Landgraf R (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology, 38(10), 1985–1993. 10.1016/j.psyneuen.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Parr LA, Brooks JM, Jonesteller T, Moss S, Jordano JO, & Heitz TR (2016). Effects of chronic oxytocin on attention to dynamic facial expressions in infant macaques. Psychoneuroendocrinology, 74, 149–157. 10.1016/j.psyneuen.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Modi M, Siebert E, & Young LJ (2013). Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology, 38(9), 1748–1756. 10.1016/j.psyneuen.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, … Geschwind DH. (2015). Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Science Translational Medicine, 7(271), 271ra278 10.1126/scitranslmed.3010257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Seyfarth RM, & Cheney DL (2016). Adaptations for social cognition in the primate brain. Philosophical Transactions of the Royal Society of London, 371(1687), 20150096 10.1098/rstb.2015.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam PT, Roman JM, Zimmerman PE, & Gothard KM (2016). Oxytocin enhances gaze-following responses to videos of natural social behavior in adult male rhesus monkeys. Psychoneuroendocrinology, 72, 47–53. 10.1016/j.psyneuen.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhela RK, Prakash A, & Medhi B (2015). An urgent need for experimental animal model of autism in drug development. Annals of Neurosciences, 22(1), 44–49. 10.5214/ans.0972.7531.220210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, & Domes G(2011). Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology, 36(9), 1378–1382. 10.1016/j.psyneuen.2011.03.011 [DOI] [PubMed] [Google Scholar]
- Simpson EA, Paukner A, Sclafani V, Kaburu SS, Suomi SJ, & Ferrari PF (2017). Acute oxytocin improves memory and gaze following in male but not female nursery-reared infant macaques. Psychopharmacology, 234(3), 497–506. 10.1007/s00213-016-4480-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Sclafani V, Paukner A, Hamel AF, Novak MA, Meyer JS, … Ferrari PF. (2014). Inhaled oxytocin increases positive social behaviors in newborn macaques. Proceedings of the National Academy of Sciences of the United States of America, 111(19), 6922–6927. 10.1073/pnas.1402471111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, … Taniike M. (2013). Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology, 23(2), 123–127. 10.1089/cap.2012.0048 [DOI] [PubMed] [Google Scholar]
- Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV,… Moy SS. (2013). Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology, 72, 187–196. 10.1016/j.neumpharm.2013.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Pinilla P, Paixao-Cortes VR, Pare P, Tovo-Rodrigues L, Vieira CM, Xavier A,… Bortolini MC. (2015). Evolutionary pattern in the OXT-OXTR system in primates: Coevolution and positive selection footprints. Proceedings of the National Academy of Sciences of the United States of America, 112(1), 88–93. 10.1073/pnas.1419399112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein TA, Bales KL, Maninger N, Hostetler CM, & Capitanio JP (2014). Early involvement in friendships predicts later plasma concentrations of oxytocin and vasopressin in juvenile rhesus macaques (Macaca mulatto). Frontiers in Behavioral Neuroscience, 8, 295 10.3389/fnbeh.2014.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, & Insel TR (2003). Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology, 28(5), 910–918. 10.1038/sj.npp.1300128 [DOI] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, & Guastella AJ (2016). The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Molecular Psychiatry, 21(9), 1225–1231. 10.1038/mp.2015.162 [DOI] [PMC free article] [PubMed] [Google Scholar]