Abstract
Purpose of review
This review provides an overview of recent findings on new members of the protein disulfide isomerase (PDI) family required for thrombosis.
Recent findings
Twenty years ago PDI was shown to mediate platelet aggregation, and ten years ago PDI was shown to support thrombosis in vivo. Subsequently, other members of this endoplasmic reticulum family of enzymes, ERp57 and ERp5, were demonstrated to support thrombosis. A fourth member, ERp72, was recently shown to be required for platelet accumulation and fibrin deposition in vivo. None of these enzymes can individually support these processes. Moreover, aggregation of platelets deficient in a specific PDI is only recovered by the PDI that is missing. This implies that each PDI has a distinct role in activation of the αIIbβ3 fibrinogen receptor and platelet aggregation. Free thiols can be labeled in both subunits of αIIbβ3 suggesting cysteine-based reactions are involved in relaying conformational changes from the cytoplasmic tails to the integrin headpiece of this integrin.
Summary
Multiple members of the PDI family support platelet function, and hemostasis and thrombosis with distinct roles in these processes. The individual cysteine targets of each enzyme and how these enzymes are integrated into a network that supports hemostasis and thrombosis remain to be elucidated.
Keywords: Platelets, protein disulfide isomerase, ERp57, ERp5, ERp72, αIIbβ3, thrombosis
Introduction
Protein disulfide isomerase (PDI) was known for several decades as an endoplasmic reticulum protein that formed disulfide bond in nascent proteins. The demonstration of a secreted platelet PDI [1] helped open a new field of research on extracellular protein disulfide isomerases (PDIs). PDI was the first member of this family shown to have a role in platelet integrin-mediated platelet aggregation and adhesion [2,3], and to support thrombosis in vivo [4,5]. Subsequently ERp57 [6–9], ERp5 [10], and ERp72 [11**,12**] were shown to mediate platelet accumulation and fibrin generation in vivo.
Recent efforts have used specific antibodies, targeted knockout mice, and in vivo imaging of thrombosis along with in vitro studies to characterize these PDIs. The feasibility of inhibiting PDI to prevent thrombosis in humans has been studied [13*]. Substrates of PDI include adhesive proteins and integrins. However, much is yet unknown about the relevant substrates, the reactions catalyzed, and how these enzymes work together in a coordinated fashion to support platelet function and thrombosis.
PDI, ERp57, ERp5, AND ERp72 ARE REQUIRED FOR PLATELET FUNCTION AND COAGULATION
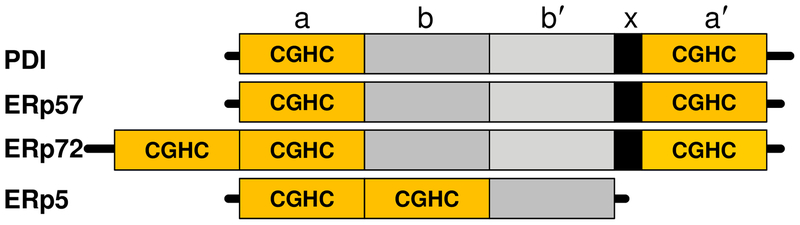
There are now over 20 members of the PDI family of enzymes; seven of these contain the CGHC-active site motif [14, 15]. Four CGHC-active site containing members of the PDI family have important roles in platelet function and thrombosis [4–10,11**,12**,16–18] (Fig 1). The prototypic PDI contains four domains arranged in order of a-b-b′-a′, with a 19 amino acid linker between the b′ and a′ domains termed x (Fig. 1) [19]. The b and b′ domains are non-catalytic, with the hydophobic b′ domain providing the principle substrate-binding domain of PDI. The a and a′ domains of each PDI illustrated contain redox active CGHC motifs with ERp72 having a third a° CGHC motif at the N-terminal region. The cysteine (Cys) in these motifs are in equilibrium between dithiol and disulfide forms; the dithiol form catalyzes cleavage and isomerization of disulfide bonds, while the disulfide form of the motif oxidizes thiols to disulfides [20]. The a′ active site of PDI and ERp57, and the a and a′ active site of ERp72 support thrombosis [8, 11**, 17].
FIGURE 1. CGHC motif-containing PDI family in thrombosis.
The a and a′ domains contain the catalytic CGHC active sites. The non-catalytic b and b′ domains are also shown. PDI, ERp57, and ERp72 contain a flexible x-linker domain.
PDI, ERp57, ERp5 and ERp72 interact with substrates by different mechanisms. Unlike PDI, the b′ domain of ERp72 is not hydrophobic and ERp72 does not bind to small peptides and scrambled RNase [19,21]. Instead hydrophobic patches in the a°, a and a′ active site domains of ERp72 mediate substrate binding [22]. While the two non-catalytic b and b′ domains of ERp72 are similar to ERp57, surface charge differences allow the b′ domain of ERp57, but not ERp72, to bind calnexin [22]. ERp5 lacks a typical b′ domain. Differences in enzyme substrate binding ability contribute to differences in selectivity for substrates, or selectivity for specific Cys residues in a given substrate[23,24].
The disulfide isomerase ERp72 supports arterial thrombosis
Conditional Tie2-Cre/ERp72fl/fl knockout mice with blood and endothelial cells lacking ERp72 were shown to have prolonged tail-bleeding times, and decreased platelet accumulation in laser-induced cremaster arteriole injury and FeCl3-induced mesenteric arterial injury [11**]. Fibrin deposition was decreased in the laser injury model. Both platelet and fibrin accumulation defects were fully rescued by infusion of ERp72 containing functional a and a′ CGHC motifs. ERp72-null platelets had defective aggregation, αIIbβ3 activation, P-selectin expression and ATP secretion. Aggregation and ATP secretion of mouse and human platelets required both the a and a′ active sites of ERp72.
β3-null mice were employed to determine whether ERp72 could directly mediate fibrin formation independently of platelet accumulation. Platelet accumulation was absent and fibrin formation substantially reduced at the site of injury in β3-null mice [11]. Infusion of ERp72 with functional a and a′ active sites recovered fibrin formation, without any recovery of platelet accumulation. Thus, ERp72 plays a critical role in platelet function and coagulation through the a and a′ CGHC motifs.
Using a monoclonal antibody raised against ERp72, Holbrook et al. also found a role for ERp72 in platelet function and thrombosis [12**]. The anti-ERp72 antibody inhibited platelet aggregation, αIIbβ3 activation, P-selectin expression, calcium mobilization, and platelet adhesion to fibrinogen. ERp72 supported clot retraction, suggesting that ERp72 affinity regulation of fibrinogen binding results in outside-in signaling through αIIbβ3. Infusion of anti-ERp72 into mice had a protective effect against thrombosis. Thus, two recent studies highlight an important role for intravascular ERp72 in the regulation of platelet function and thrombosis.
SUBSTRATES OF VASCULAR DISULFIDE ISOMERASES
Substrates of the disulfide isomerases include adhesive proteins, integrins and potentially coagulation factors. The first known extracellular substrate of platelet PDI was the adhesive protein thrombospondin 1 [25]. PDI directly catalyzed thiol-disulfide exchange in thrombospondin 1, resulting in a conformational change affecting its cell adhesive properties.
Interactions of PDI, ERp57, ERp5 and ERp72 with the αIIbβ3 fibrinogen receptor
PDI mediates platelet aggregation [2], a process requiring activation of the αIIbβ3 integrin. Several lines of evidence support a direct interaction of PDI with the αIIbβ3 integrin. First, PDI binds to Mn2+-treated CHO cells expressing αIIbβ3, but not to cells expressing P-selectin [26]. Second, PDI reacts with Mn2+-treated αIIbβ3, or the β3 subunit, by surface plasmon resonance (Kd~1 μM) [26]. Third, PDI binds to thrombin-activated or Mn2+-treated wild-type mouse platelets but not to platelets lacking αIIbβ3 [16,17]. ERp5 immunoprecipitated with the β3 subunit of αIIbβ3 from activated platelets; the β3 subunit also co-precipitated with ERp5 [18]. ERp5 also binds to intact αIIbβ3 or the β3 subunit by surface plasmon resonance (Kd~21 μM) [10]. Both ERp57 and ERp72 bind poorly to thrombin-activated or Mn2+-treated platelets lacking the αIIbβ3 integrin [8,11**] suggesting these enzymes also interact with this integrin.
PDI, ERp57, and ERp72 have distinct roles in platelet aggregation
Deficiency of PDI, ERp57 or ERp72 from platelets results in an ~50–70% decrease in platelet aggregation [8, 11**, 16, 17]. Whether the functions of these enzymes were distinct or redundant was unclear. Aggregation of ERp72, PDI or ERp57-null platelets was only recovered by the PDI that was missing, implying that each enzyme has a different role in activation of αIIbβ3 [11**].
ERp72 generates thiols in αIIbβ3
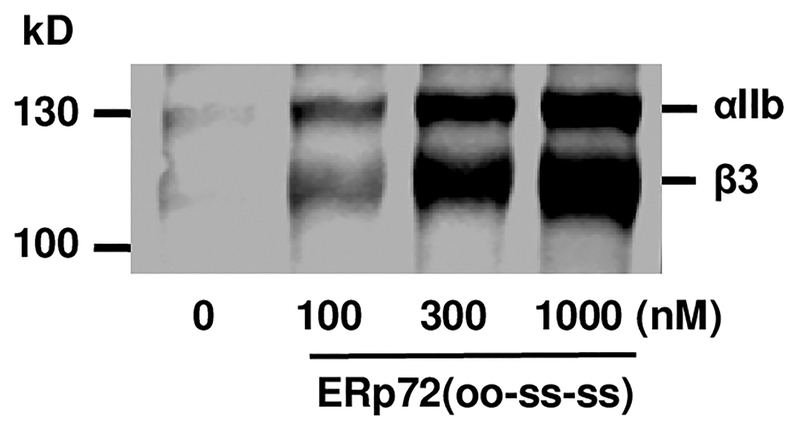
Adding ERp72 to human platelets potentiated thiol-labeling of sulfhydryl’s in αIIb and β3 (Fig. 2), and platelet aggregation [11**]. This suggests ERp72 cleaves disulfide bonds in αIIb and β3 facilitating activation of αIIbβ3. Binding of substrates to ERp72 (or PDI) displaces the x-linker enhancing reductase activity [27*]. This could potentiate the generation of thiols in αIIb and β3 by ERp72.
FIGURE 2. ERp72 generates thiols in αIIb and β3.
Effect of adding ERp72 (with functional a and a′ active sites) to platelets on MPB labeling of thiols in αIIb and β3.
Reactions involving thiols and disulfides in αIIbβ3
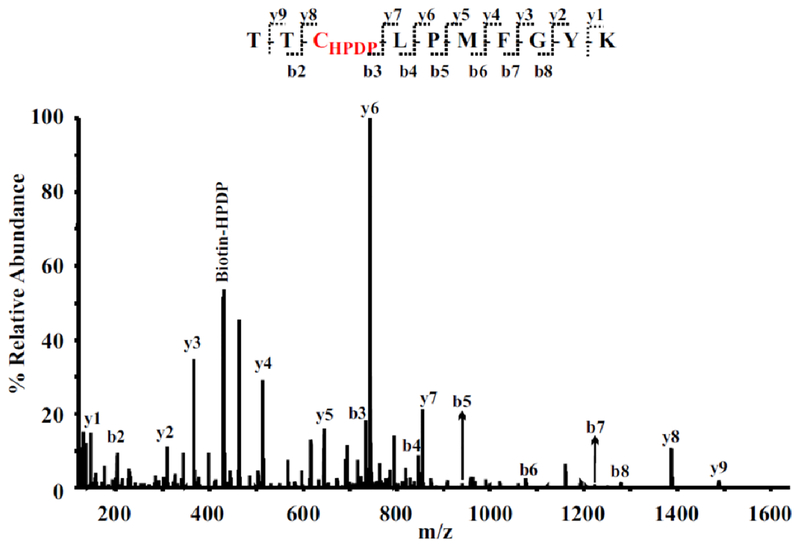
Reactions involving thiols and disulfides in αIIbβ3 were characterized by labeling αIIbβ3 purified from non-activated and activated platelets with the sulfhydryl-reactive biotin-HPDP (Fig. 3) [11**]. Using LC-MS/MS a total of 12 and 14 different labeled cysteine residues were reproducibly identified in non-activated and activated αIIbβ3, respectively [11**]. Since there are only 2.6 thiol/mol of inactivated αIIbβ3 and 4.4 thiol/mol of activated αIIbβ3 [28], these findings imply that each of the labeled Cys is a free thiol in only a fraction of αIIbβ3 molecules, and that the position of the thiol varies through thiol-disulfide exchange. This is consistent with reports showing a role for thiol-disulfide exchange in the activation of αIIbβ3 [28,29].
FIGURE 3.
Representative MS/MS spectrum of a doubly-charged ion (m/z 794.87) corresponding to the peptide sequence of 182-TTCLPMFGYK-191 with a biotin-HPDP modification at Cys184 in the β3 subunit. The observed y- and b-ion series confirmed the peptide sequence, and a biotin-HPDP modified Cys (+428.2 amu) found between b2 and b3 as well as between y7 and y8 ions in the spectrum confirmed the modification of Cys184.
Some of the thiol-containing Cys that were identified in the β3 subunit are functional (allosteric) disulfide bonds [30] predicted by structural studies to be easily broken (Cys184, Cys635 and Cys687) [31,32]. Cys residues in αIIb were also labeled, with Cys484 and Cys490 only labeled in the activated αIIb subunit [11**]. Previous work showed that redox sensitive disulfide bonds in αIIbβ3 were cleaved by low concentrations of reduced glutathione generating thiols in αIIbβ3 and potentiating platelet aggregation [33]; and that vicinal thiols (di-thiols that interconvert with disulfide bonds) have a role in activation of αIIbβ3 [34]. Together these studies indicate a role for reactive thiols and disulfide bonds that may be the targets of ERp72 or other platelet PDIs.
While not all the thiol-containing Cys that were identified [11**] are predicted to be from functional disulfide bonds in the crystal structure [32,35], the protein backbone and disulfide bonds that link it can change shape when in solution or when the protein is in a membrane [31]. Cleavage of the bond may only occur when the disulfide bonds adopts a particular configuration, which could be controlled by inside-out conformation changes induced by platelet activation, ligand binding [36], or mechanical shear in the circulation.
Potential mechanisms of activation of αIIbβ3 by disulfide isomerases
It is possible that αIIbβ3 is a direct target of several disulfide isomerases. In the endoplasmic reticulum some proteins are substrates of multiple members of the PDI family [23]. Laminin and thyroglobulin are large heavily disulfide-linked proteins that form mixed disulfides with four members of the PDI family, with each PDI targeting different Cys to contribute to oxidative folding [23, 24]. Similarly, multiple members of the PDI family act distinctly and coordinately on the polyomavirus [37], simian virus, [38], and bovine pancreatic trypsin inhibitor [39]. αIIbβ3 is a large disulfide-linked complex and the complimentary enzymatic activities of multiple members of PDI family are required for optimal activation of the receptor.
While the stoichiometry of interactions of PDI, ERp57, ERp5, and ERp72 with αIIbβ3 is unknown, a single PDI molecule could interact with multiple molecules of αIIbβ3. Alternatively, a subpopulation of αIIbβ3 that controls platelet aggregation could be targeted. For example, a subpopulation of αIIbβ3 that interacts with the platelet cytoskeleton is required for activation of αIIbβ3 [40], and activation of individual αIIbβ3 molecules rapidly initiates αIIbβ3 clustering of intracellular signal-generating complexes [41]. Thus, a limited number of disulfide isomerase-catalyzed reactions targeting a subpopulation of αIIbβ3 molecules could amplify platelet responses required for aggregation.
The PDIs could catalyze sequential or simultaneous reactions in αIIbβ3. Release of client-substrates from PDI after the reaction cycle is completed [42], would allow access for another member of the PDI family to catalyze a subsequent reaction [37]. It is also possible could act in series, rather than in parallel, with one member of the PDI family affect the activity of second PDI. Such a redox communication network among PDIs facilitates efficient disulfide bond formation in the mammalian endoplasmic reticulum[43]. Thus, the PDIs could act in sequential fashion in one of two ways: one PDI may generate thiols in αIIbβ3 while another PDI subsequently catalyzes a thiol-disulfide exchange reaction; or one PDI acting on another PDI that in turn acts on αIIbβ3.
Working Model of the role of disulfide isomerases and thiols in activation of the αIIbβ3 integrin
Working Model.
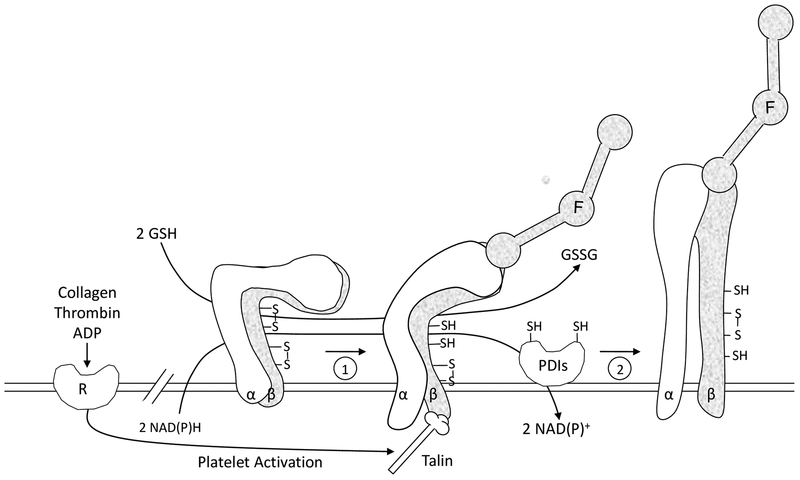
The working model (Fig. 4) focuses on inter-relations of the PDIs and the integrin αIIbβ3 in platelet aggregation. The major points are: Agonist stimulation causes platelet activation resulting in talin binding to the β3 cytoplasmic domain of αIIbβ3 [36]. This initiates inside-out signaling resulting in low affinity binding of fibrinogen to αIIbβ3 (1); this is followed by further conformational changes in αIIbβ3 that are facilitated by disulfide isomerase catalyzed events (2). These isomerase-catalyzed events include thiol-disulfide exchange, although reduction of disulfide bonds or oxidation of thiols are also possible. Thiol-disulfide rearrangement in αIIbβ3 facilitates formation of the high affinity/avidity state. The interaction of PDIs with αIIbβ3 may be direct [44], at least for a population of αIIbβ3. External GSH (or low molecular weight thiol/disulfide pairs) or a transplasma membrane NAD(P)H dependent reductase generate sulfhydryls in PDI or αIIbβ3 potentiating the reactions [33,45,46].
FIGURE 4.
Working model of the role of sulfhydryls and PDI in platelet function. The platelet fibrinogen receptor αIIbβ3 (α, β) is shown in three different activation states. The nonactivated state is on the left side. Redox sensitive disulfide bonds in αIIbβ3 are depicted. Agonist induced stimulation leads to cytoplasmic events and talin binding resulting in inside-out signaling and an initial ligand binding interaction of fibrinogen (F) with the receptor (1). A PDI catalyzed event then converts αIIbβ3 to the high affinity conformation (2) represented by secondary platelet aggregation. During platelet activation, sulfhydryls are generated in αIIbβ3 as well as in the active site of PDI from cytoplasmic reducing equivalents supplied by NAD(P)H. GSH or other low molecular weight thiols in the external redox environment also generate sulfhydryls in both αIIbβ3 and PDI facilitating the reactions shown.
There are likely multiple substrates of the PDIs on the platelet surface. The secreted platelet proteins thrombospondin 1 and vitronectin are PDI substrates with roles in platelet function [25,47,48*]. PDI, ERp57, ERp5 and ERp72 have roles in ATP release from dense granules and P-selectin expression from α-granules of platelets [8,10,11**, 12**,17], raising the possibility of targets in platelet secretion pathways. The differential regulation of some platelet functions by disulfide isomerases also suggests different functions. For example, ERp72 and ERp57 have roles in GPVI-stimulated Ca2+-mobilization in platelets [6,12**], while PDI and ERp5 do not [16,18].
PDI trapping mutants containing intervening sequence variants, CGPC and CGRC (instead of CGHC) captured PDI substrates from platelet lysate and releasate [49*]. Proteins identified included cathepsin G, glutaredoxin-1, thioredoxin, GP1b, fibrinogen, annexin V, heparinase, ERp57, kallekrein-14, serpin B6, tetranectin, and collagen VI. Using a different PDI active site-variant, and platelet-rich plasma, additional putative substrates of PDI were identified [48*]. These included vitronectin, complement factor 3, complement factor 5, C4b-binding protein, α2-macroglobulin, protein S, and CD5 antigen-like protein. These findings raise important possibilities requiring further study on the roles of these proteins in platelet function and thrombosis.
Vitronectin as a PDI substrate
Vitronectin has emerged as a potentially important PDI substrate. Using an antibody to vitronectin with fluorescent imaging, in vivo deposition of vitronectin was decreased when anti-PDI antibodies were infused into mice [48*]. Mice deficient in vitronectin had decreased platelet accumulation and fibrin deposition. PDI cleaved disulfides (Cys137-Cys161 and Cys274-Cys453) in vitronectin, and reduction of vitronectin by PDI enabled binding to β3 integrins. ERp57 did not catalyze these reactions.
The role of PDI in coagulation reactions
PDI, ERp57, ERp5 and ERp72 have roles in fibrin deposition in vivo [4,5,9,10,11**,17]. The prototypical PDI modulates tissue factor [50], and factor XI activation [51]. PDI also has a role in activation of platelet factor V, although it is not known if this is due to a direct effect of PDI on factor V, or to release of platelet factor V from multimerin, which binds factor V in the α-granules of platelets [13]. PDI also has an effect on platelet and endothelial cell-dependent coagulant activity [52,53].
SECRETION OF DISULFIDE ISOMERASES AT THE SITE OF VASCULAR INJURY
Platelets and endothelial cells are the principal sources of the extracellular disulfide Isomerases at the side of vessel injury [4,5,8–10,11**,16,54]. This is not unexpected, considering the immediate proximity of platelets and endothelial cells to the site of injury, and the ability of these cells to secrete disulfide isomerases [7,8,10,16,54,55]. PDI, ERp5, ERp57 and ERp72 are present on the resting platelet surface, and the levels increase following platelet activation [7,8,18,56]. PDI and ERp57 are found in the dense tubular system (DTS) of platelets and are released on the surface of activated platelets in an actin-dependent process [57,58]. While thrombin-activated platelets only secrete ~10% of total platelet PDI [55], this is sufficient to support platelet accumulation in vivo [4,5,16,17]. PDI activity is detected at the core of the platelet thrombus that contains the most highly activated platelets [59]. Endothelial cells provide another source of these enzymes at the site of vascular injury, and physiological levels of arterial laminar shear have been shown to greatly enhance externalization of endothelial-cell PDI, predominantly by a golgi-independent route [60].
DISULFIDE ISOMERASE INHIBITORS AS ANTI-THROMBOTIC THERAPY
Administration of an oral PDI inhibitor, isoquercitin, inhibits platelet-dependent thrombin generation in healthy humans and patients with anti-phospholipid antibodies by 50–60%[13*]. PDI inhibitors targeting extracellular PDI [61], the substrate binding domains of PDI [27,62], or the C-terminal active site involved in thrombosis [8,11**,17,63] have all been proposed [64]. It is unknown whether targeting one of these PDI-family members has benefits over targeting the traditional PDI, or whether targeting several PDIs simultaneously would provide optimal anti-thrombotic therapy. Dual inhibition of platelets and coagulation with both antiplatelet agents and anticoagulants may become the standard of care for cardiovascular diseases [65,66]. Inhibiting the appropriate member(s) of PDI family is appealing, as this could provide dual inhibition with a single agent.
CONCLUSION
Substantial progress has been made recently in the discovery of new disulfide isomerases required for thrombosis. However, we are only beginning to understand how this extracellular network of enzymes controls thrombosis. Two important issues that need to be addressed are: what is the full complement of disuflde isomerases required for platelet function and thrombosis, and how do all these disulfide isomerases act together to achieve thrombosis. Rearrangement of disulfide bonds may be part of a cascade of events that couples platelet stimulation to the various responses including aggregation and secretion. Dissecting out the key target Cys/disulfide bonds for each PDI and/or the reactions each one catalyzes will provide a novel concept of how major functional receptors are regulated by multiple PDIs. The knowledge gained should enable a more in-depth understanding of diseases in which platelets are involved, and lead to interventions that alleviate these diseases.
Key Points.
ERp72 is the latest member of the PDI family found to be required for platelet function and thrombosis.
PDI, ERp57, ERp5 and ERp72 are required for platelet accumulation and fibrin deposition in vivo.
PDI, ERp57, ERp5 and ERp72 function by different mechanisms in platelet aggregation.
The C-terminal or a′ active sites in PDI and ERp57, and the a and a′ active sites in ERp72, are required critical for platelet aggregation and secretion, and thrombosis.
Integrins and adhesive proteins represent substrates of disulfide isomerases.
Acknowledgements
Because of the focus on recent work and space limitations, we regret we could not include many other important contributions in this field.
Financial support
This work was supported by a grant from the NIH (HL118526).
Funding: NIH grants R01HL118526 (DWE)
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
REFERENCES AND RECOMMENDED READINGS
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Chen K, Lin Y, Detwiler TC. Protein disulfide isomerase activity is released by activated platelets. Blood. 1992;79:2226–8. [PubMed] [Google Scholar]
- 2.Essex DW, Li M. Protein disulphide isomerase mediates platelet aggregation and secretion. Br J Haematol. 1999;104:448–54. [DOI] [PubMed] [Google Scholar]
- 3.Lahav J, Gofer-Dadosh N, Luboshitz J, et al. Protein disulfide isomerase mediates integrin-dependent adhesion. FEBS Lett. 2000;475:89–92. [DOI] [PubMed] [Google Scholar]
- 4.Cho J, Furie BC, Coughlin SR, et al. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhardt C, von Bruhl ML, Manukyan D, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holbrook LM, Sasikumar P, Stanley RG, et al. The platelet-surface thiol isomerase enzyme ERp57 modulates platelet function. J Thromb Haemost. 2012;10:278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Ahmad SS, Zhou J, et al. The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood. 2012;119:1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Wu Y, Zhou J, et al. Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the alphaIIbbeta3 integrin. Blood. 2013;122:3642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Wu Y, Wang L, et al. The disulfide isomerase ERp57 is required for fibrin deposition in vivo. J Thromb Haemost. 2014;12:1890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passam FH, Lin L, Gopal S, et al. Both platelet- and endothelial cell-derived ERp5 support thrombus formation in a laser-induced mouse model of thrombosis. Blood. 2015;125:2276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.**.Zhou J, Wu Y, Chen F, et al. The disulfide isomerase ERp72 supports arterial thrombosis in mice. Blood. 2017;130:817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using conditional Tie2-Cre and Pf4-Cre knockout mice this study showed a role for endothelial cell and platelet-derived ERp72 in platelet accumulation and fibrin generation in vivo. The a and a′ active site motifs of ERp72 were critical for both of these processes. Distinct roles for PDI, ERp57 and ERp72 in activation of the αIIbβ3 integrin were demonstrated.
- 12.**.Holbrook LM, Sandhar GK, Sasikumar P, et al. A humanized monoclonal antibody that inhibits platelet-surface ERp72 reveals a role for ERp72 in thrombosis. J Thromb Haemost. 2018;16:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used an antibody raised to ERp72 that inhibited ERp72 activity to document a role for ERp72 in platelet function. The anti-ERp72 antibody inhibited integrin activation, platelet aggregation, α-granule secretion, calcium mobilization, clot retraction and platelet adhesion to fibrinogen.
- 13.*.Stopa JD, Neuberg D, Puligandla M, et al. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI insight. 2017;2:e89373. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed inhibition of platelet-dependent thrombin generation in healthy volunteers and patients with anti-phospholipid syndrome following ingestion of an oral PDI inhibitor, isoquercetin.
- 14.Kozlov G, Määttänen P, Thomas DY, et al. A structural overview of the PDI family of proteins. Febs J. 2010;277:3924–36. [DOI] [PubMed] [Google Scholar]
- 15.Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim Biophys Acta. 2008;1783:535–48. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Hahm E, Li J, et al. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122:1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Wu Y, Wang L, et al. The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis. J Clin Invest. 2015;125:4391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan PA, Stevens JM, Hubbard GP, et al. A role for the thiol isomerase protein ERP5 in platelet function. Blood. 2005;105:1500–7. [DOI] [PubMed] [Google Scholar]
- 19.Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11:2807–50. [DOI] [PubMed] [Google Scholar]
- 20.Essex DW. Redox control of platelet function. Antioxid Redox Signal. 2009;11:1191–225. [DOI] [PubMed] [Google Scholar]
- 21.Kramer B, Ferrari DM, Klappa P, et al. Functional roles and efficiencies of the thioredoxin boxes of calcium-binding proteins 1 and 2 in protein folding. Biochem J. 2001;357:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozlov G, Maattanen P, Schrag JD, et al. Structure of the noncatalytic domains and global fold of the protein disulfide isomerase ERp72. Structure. 2009;17:651–9. [DOI] [PubMed] [Google Scholar]
- 23.Jessop CE, Watkins RH, Simmons JJ, et al. Protein disulphide isomerase family members show distinct substrate specificity: P5 is targeted to BiP client proteins. Journal of cell science. 2009;122:4287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Jeso B, Morishita Y, Treglia AS, et al. Transient covalent interactions of newly synthesized thyroglobulin with oxidoreductases of the endoplasmic reticulum. J Biol Chem. 2014;289:11488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang EM, Detwiler TC, Milev Y, et al. Thiol-disulfide isomerization in thrombospondin: effects of conformation and protein disulfide isomerase. Blood. 1997;89:3205–12. [PubMed] [Google Scholar]
- 26.Cho J, Kennedy DR, Lin L, et al. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of beta3 integrins. Blood. 2012;120:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.*.Bekendam RH, Bendapudi PK, Lin L, et al. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nature communications. 2016;7:12579. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that PDI inhibitors called Bepristats bind to the b′ domain of PDI resulting in displacement of the x-linker and allosteric enhancement of reductase activity. This activity was reproduced with physiologic substrates.
- 28.Yan B, Smith JW. A redox site involved in integrin activation. J Biol Chem. 2000;275:39964–72. [DOI] [PubMed] [Google Scholar]
- 29.Essex DW, Li M, Miller A, et al. Protein disulfide isomerase and sulfhydryl-dependent pathways in platelet activation. Biochemistry. 2001;40:6070–5. [DOI] [PubMed] [Google Scholar]
- 30.Cook KM, Hogg PJ. Post-translational control of protein function by disulfide bond cleavage. Antioxid Redox Signal. 2013;18:1987–2015. [DOI] [PubMed] [Google Scholar]
- 31.Butera D, Cook KM, Chiu J, et al. Control of blood proteins by functional disulfide bonds. Blood. 2014;123:2000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mor-Cohen R. Disulfide Bonds as Regulators of Integrin Function in Thrombosis and Hemostasis. Antioxid Redox Signal. 2016;24:16–31. [DOI] [PubMed] [Google Scholar]
- 33.Essex DW, Li M. Redox control of platelet aggregation. Biochemistry. 2003;42:129–36. [DOI] [PubMed] [Google Scholar]
- 34.Manickam N, Ahmad SS, Essex DW. Vicinal thiols are required for activation of the alphaIIbbeta3 platelet integrin. J Thromb Haemost. 2011;9:1207–15. [DOI] [PubMed] [Google Scholar]
- 35.Xiao T, Takagi J, Coller BS, et al. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walczak CP, Tsai B. A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J Virol. 2011;85:2386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue T, Dosey A, Herbstman JF, et al. ERdj5 Reductase Cooperates with Protein Disulfide Isomerase To Promote Simian Virus 40 Endoplasmic Reticulum Membrane Translocation. J Virol. 2015;89:8897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satoh M, Shimada A, Kashiwai A, et al. Differential cooperative enzymatic activities of protein disulfide isomerase family in protein folding. Cell stress & chaperones. 2005;10:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett JS, Zigmond S, Vilaire G, et al. The platelet cytoskeleton regulates the affinity of the integrin alpha(IIb)beta(3) for fibrinogen. J Biol Chem. 1999;274:25301–7. [DOI] [PubMed] [Google Scholar]
- 41.Fong KP, Zhu H, Span LM, et al. Directly Activating the Integrin alphaIIbbeta3 Initiates Outside-In Signaling by Causing alphaIIbbeta3 Clustering. J Biol Chem. 2016;291:11706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Wang X, Wang CC. Protein disulfide-isomerase, a folding catalyst and a redox-regulated chaperone. Free Radic Biol Med. 2015;83:305–13. [DOI] [PubMed] [Google Scholar]
- 43.Okumura M, Kadokura H, Inaba K. Structures and functions of protein disulfide isomerase family members involved in proteostasis in the endoplasmic reticulum. Free Radic Biol Med. 2015;83:314–22. [DOI] [PubMed] [Google Scholar]
- 44.Manickam N, Sun X, Li M, et al. Protein disulphide isomerase in platelet function. Br J Haematol. 2008;140:223–9. [DOI] [PubMed] [Google Scholar]
- 45.Essex DW, Li M, Feinman RD, et al. Platelet surface glutathione reductase-like activity. Blood. 2004;104:1383–5. [DOI] [PubMed] [Google Scholar]
- 46.Raturi A, Mutus B. Characterization of redox state and reductase activity of protein disulfide isomerase under different redox environments using a sensitive fluorescent assay. Free Radic Biol Med. 2007;43:62–70. [DOI] [PubMed] [Google Scholar]
- 47.Essex DW, Miller A, Swiatkowska M, et al. Protein disulfide isomerase catalyzes the formation of disulfide-linked complexes of vitronectin with thrombin-antithrombin. Biochemistry. 1999;38:10398–405. [DOI] [PubMed] [Google Scholar]
- 48.*.Bowley SR, Fang C, Merrill-Skoloff G, et al. Protein disulfide isomerase secretion following vascular injury initiates a regulatory pathway for thrombus formation. Nature communications. 2017;8:14151. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated a role for PDI-catalyzed reactions in deposition of the adhesive protein vitronection in vivo. Decreased thrombus formation was found in mice lacking vitronectin.
- 49.*.Stopa JD, Baker KM, Grover SP, et al. Kinetic-based trapping by intervening sequence variants of the active sites of protein-disulfide isomerase identifies platelet protein substrates. J Biol Chem. 2017;292:9063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using active site mutants of PDI many disulfide-linked intermediates of PDI with platelet substrates were captured. This demonstrates the feasibility of this approach and opens up new avenues to pursue.
- 50.Langer F, Ruf W. Synergies of phosphatidylserine and protein disulfide isomerase in tissue factor activation. Thromb Haemost. 2014;111:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zucker M, Seligsohn U, Yeheskel A, et al. An allosteric disulfide bond is involved in enhanced activation of factor XI by protein disulfide isomerase. J Thromb Haemost. 2016;14:2202–11. [DOI] [PubMed] [Google Scholar]
- 52.Jurk K, Lahav J, VANA H, et al. Extracellular protein disulfide isomerase regulates feedback activation of platelet thrombin generation via modulation of coagulation factor binding. J Thromb Haemost. 2011;9:2278–90. [DOI] [PubMed] [Google Scholar]
- 53.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jasuja R, Furie B, Furie BC. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood. 2010;116:4665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen K, Detwiler TC, Essex DW. Characterization of protein disulphide isomerase released from activated platelets. Br J Haematol. 1995;90:425–31. [DOI] [PubMed] [Google Scholar]
- 56.Holbrook LM, Watkins NA, Simmonds AD, et al. Platelets release novel thiol isomerase enzymes which are recruited to the cell surface following activation. Br J Haematol. 2010;148:627–37. [DOI] [PubMed] [Google Scholar]
- 57.van Nispen Tot Pannerden HE, van Dijk SM, Du V, et al. Platelet protein disulfide isomerase is localized in the dense tubular system and does not become surface expressed after activation. Blood. 2009;114:4738–40. [DOI] [PubMed] [Google Scholar]
- 58.Crescente M, Pluthero FG, Li L, et al. Intracellular Trafficking, Localization, and Mobilization of Platelet-Borne Thiol Isomerases. Arterioscler Thromb Vasc Biol. 2016;36:1164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu S, Welsh JD, Brass LF, et al. Platelet-targeting thiol reduction sensor detects thiol isomerase activity on activated platelets in mouse and human blood under flow. J Thromb Haemost. 2016;14:1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Araujo TL, Zeidler JD, Oliveira PV, et al. Protein disulfide isomerase externalization in endothelial cells follows classical and unconventional routes. Free Radic Biol Med. 2017;103:199–208. [DOI] [PubMed] [Google Scholar]
- 61.Jasuja R, Passam FH, Kennedy DR, et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest. 2012;122:2104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin L, Gopal S, Sharda A, et al. Quercetin-3-rutinoside Inhibits Protein Disulfide Isomerase by Binding to Its b’x Domain. J Biol Chem. 2015;290:23543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sousa HR, Gaspar RS, Lena EML, et al. Novel antiplatelet role for a protein disulfide isomerase-targeted peptide: Evidence of covalent binding to C-terminal CGHC redox motif. J Thromb Haemost. 2017. [DOI] [PubMed] [Google Scholar]
- 64.Flaumenhaft R. Advances in vascular thiol isomerase function. Curr Opin Hematol. 2017;24:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319–30. [DOI] [PubMed] [Google Scholar]
- 66.Braunwald E. An Important Step for Thrombocardiology. N Engl J Med. 2017;377:1387–8. [DOI] [PubMed] [Google Scholar]