Abstract
BACKGROUND.
Studies of monogenic gastrointestinal diseases have revealed molecular pathways critical to gut homeostasis and enabled the development of targeted therapies.
METHODS.
We studied 11 patients with abdominal pain and diarrhea caused by early-onset protein-losing enteropathy with primary intestinal lymphangiectasia, edema due to hypoproteinemia, malabsorption, and, less frequently, bowel inflammation, recurrent infections and angiopathic thromboembolic disease following an autosomal recessive pattern of inheritance. Whole-exome sequencing was performed to identify gene variants. We evaluated the function of CD55 in patient cells, which we confirmed through exogenously-induced expression of CD55.
RESULTS.
We identified homozygous loss-of-function mutations in the gene encoding CD55/Decay accelerating factor, leading to loss of protein expression. Patients’ T lymphocytes displayed increased complement activation causing complement surface deposition and the generation of soluble C5a. Co-stimulatory function and cytokine modulation by CD55 were defective. Genetic reconstitution of CD55 or treatment with a complement-inhibitory therapeutic antibody reversed abnormal complement activation.
CONCLUSIONS.
CD55 deficiency with hyperactivation of complement, angiopathic thrombosis, and protein-losing enteropathy (CHAPLE) disease is caused by abnormal complement activation due to biallelic loss-of-function mutations in CD55.
INTRODUCTION
Genetic inquiry has contributed to our understanding of gastrointestinal diseases, associating at least 64 genes with early-onset or very early-onset inflammatory bowel disease (EO-IBD and VEO-IBD, respectively).1 Deleterious gene variants affect the intestinal epithelial barrier, phagocytosis processes, immune regulation, and inflammation. Protein-losing enteropathy, or gastrointestinal protein wasting causing hypoproteinemia, edema, and pleural and pericardial effusions, has also been linked to monogenic disorders.2 A loss-of-function variant in PLVAP (encoding plasmalemma vesicle associated protein) that disrupts endothelial fenestrated diaphragms and compromises barrier integrity, is associated with severe protein-losing enteropathy.3 This condition can develop secondarily from systemic conditions that arrest lymph flow, such as congestive heart failure, or directly from gastrointestinal mucosal damage or impaired lymph drainage from primary intestinal lymphangiectasia (also known as Waldmann’s disease).2,4 Although primary intestinal lymphangiectasia can be a component of multisystemic genetic syndromes, including Hennekam syndrome (caused by bi-allelic loss-of-function variants in CCBE1 and FAT4), the mechanisms of non-syndromic primary intestinal lymphangiectasia and protein-losing enteropathy remain largely unknown.5,6 Here, we define the molecular and clinical features of an autosomal recessive syndrome of early-onset protein-losing enteropathy characterized by primary intestinal lymphangiectasia, bowel inflammation, and thrombotic events.
METHODS
Study Participants
Participants provided written informed consent for approved protocols at respective institutions.
Genetic and Functional Analysis
We performed whole exome sequencing on index patients and CD55 Sanger sequencing in subsequent subjects. Complement assays were performed before and after lentiviral CD55 reconstitution. Details are provided in the Supplementary Appendix.
Statistical analysis
Statistical comparisons were made using Graphpad Prism version 7.0a. We used the Mann-Whitney U test or Student’s t-test to assess the significance between unrelated samples and the Wilcoxon matched pairs signed rank test or a two tailed paired T test for the paired samples, respectively. Two-tailed values of p <0.05 were considered significant.
RESULTS
Clinical Phenotype
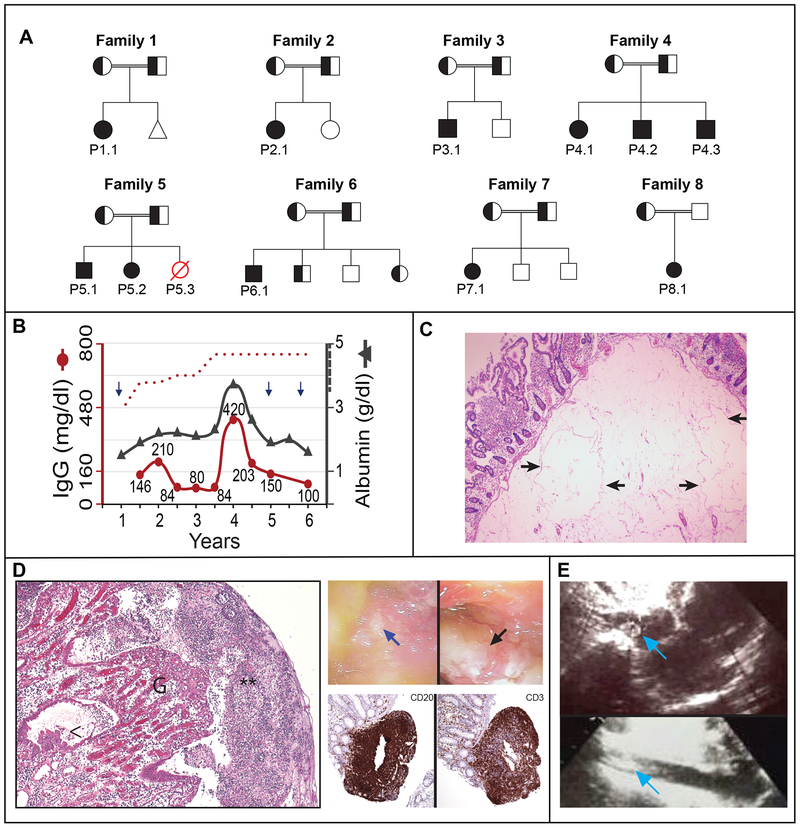
We investigated 11 patients and 2 deceased relatives with a history of protein-losing enteropathy, characterized by early-onset gastrointestinal symptoms, edema, malnutrition, hypoalbuminemia, and hypogammaglobulinemia, from 8 consanguineous families with unaffected parents (Fig. 1A, Table 1; Figure S1 and Table S1 in Supplementary Appendix). Hypoproteinemia was generally stable and together with edema and GI symptoms (abdominal pain, vomiting, and diarrhea) was alleviated by albumin infusion (Table S1, Fig. 1B and S2A in the Supplementary Appendix). Chronic malabsorption caused micronutrient deficiencies of iron, ferritin, calcium, magnesium, folate, and vitamins -D and -B12, together with anemia and growth retardation (Tables 1 and S1, Fig. S2B and CH in the Supplementary Appendix). These were improved by vitamin and micronutrient supplementation, a protein-rich diet with medium-chain triglycerides, albumin, and blood transfusions (Fig. S2B in the Supplementary Appendix).
Figure 1. Clinical presentation of 8 families with familial early-onset protein-losing enteropathy.
Panel A shows pedigrees of eight families with affected individuals homozygous for mutant allele indicated by solid symbols, heterozygous individuals indicated by half solid symbols, and affected individuals with an unknown genotype indicated by red, open symbols. The triangle in Family 1 represents a miscarriage. Panel B shows the serum levels of immunoglobulin G (IgG, left Y axis) in relation to serum albumin (right Y axis) concentrations as a function of age in years for Patient 1.1. Age-specific lower cutoff value for IgG is denoted by the red dotted curve, whereas the reference for albumin level is >3.5 g/dl (indicated by hatched line on right Y axis). Each arrow denotes an episode of pneumonia. Panel C shows H&E sections of resected duodenal tissue with markedly dilated lymphovascular spaces within submucosa in Patient 5.2 (arrows). Panel D (left) shows H&E sections from surgical resection material of small intestine which exhibits ulceration covered by fibrin with dense granulocytic infiltrate (**), granulation tissue (G) with edema in the lamina propria and reactive epithelial changes (<) in Patient 2.1 (left), as well as endoscopy photographs showing a mucosal ulcer (blue arrow) and exudate (black arrow) in the terminal ileum of Patient 1.1 (right, top) and histopathology of a biopsy of the ileum of Patient 2.1 and immunohistochemistry showing presence of B cells (CD20) and T cells (CD3) within lymphoid nodules (right, bottom). Panel E shows an echocardiographic image from Patient 6.1 with thrombus in the right atrium (arrow, top) and inferior vena cava (IVC) (arrow, bottom).
Table 1.
Demographic and clinical characteristics of patients with confirmed CD55 deficiency Demographics of 11 affected individuals and number of patients presenting with characteristic symptoms of CHAPLE disease
| Characteristic | No. of Patients |
|---|---|
| Sex | |
| Female | 6 |
| Male | 5 |
| Age at Presentation < 2 years | 8 |
| GI/IBD Manifestations | |
| Chronic/recurrent diarrhea | 8 |
| Abdominal pain | 4 |
| Vomiting | 6 |
| Features of Protein Losing Enteropathy | |
| Hypoalbuminemia | 10 |
| Hypogammaglobulinemia | 11 |
| Facial and/or extremity edema | 9 |
| Confirmed PIL/Waldmann’s disease§ | 5 |
| Malabsorption features | |
| Growth retardation | 8 |
| Anemia | 9 |
| Vitamin/micronutrient deficiency* | 11 |
| Features of Thrombotic Disease¶ | |
| Thrombocytosis | 2 |
| Thrombosis | 3 |
| Endoscopic findings§ | |
| Mucosal ulcers | 4 |
| Lymphoid infiltrates in mucosa | 6 |
| Recurrent Lung Infections | 5 |
| Additional Features | |
| Hypothyroidism** | 3 |
| Arthritis/Arthralgia | 2 |
| Finger Clubbing | 5 |
Two of the patients were not assessed by endoscopy since they did not have gastrointestinal symptoms.
Micronutrient assessment included serum vitamin B12, vitamin D, folate, iron, ferritin, zinc, calcium and magnesium.
Thrombi were located in the deep veins in the abdominal sites, including the mesenteric and hepatic veins, sometimes with extension to inferior vena cava and heart, and leading to pulmonary embolism.
Antithyroglobulin and anti-thyroid peroxidase antibodies were negative.
Histopathology of intestinal biopsies or resections revealed extensive lymphangiectasia, verified by lymphatic endothelial markers, which, together with the patient’s young age, suggested the diagnosis of primary intestinal lymphangiectasia (Fig. 1C and Fig. S3A, Tables 1 and S1 in the Supplementary Appendix).2,4 Transmission electron microscopy (TEM) of the duodenal biopsy of P6.1 showed lymphatic dilatation (Fig. S3B), but, unlike PLVAP deficiency, we found normal capillary architecture (Fig. S3C in the Supplementary Appendix). Surgical removal of the lymphangiectatic segments in P2.1 (who suffered partial bowel obstruction, Fig. S3D in the Supplementary Appendix), P5.1, and P5.2 ameliorated clinical symptoms and protein-losing enteropathy (though P5.2 relapsed), raising the possibility of a causal relationship. Some patients manifested IBD-like bowel inflammation, exudates, and lymphocytic infiltrates without intestinal thromboses (Figs. 1D, Tables 1 and S1, S4A and S4B in the Supplementary Appendix). Radiological exams showed bowel wall edema/thickening in P1.1, P2.1, and P6.1 (Table S1, Fig. S4C, in the Supplementary Appendix). Thus, protein-losing enteropathy and micronutrient deficiencies are likely caused by primary intestinal lymphangiectasia exacerbated by bowel inflammation.
Five patients experienced recurrent respiratory infections associated with hypogammaglobulinemia (Tables 1 and S1, Fig. 1B and S2A, arrows). Major immunological cell subsets and antibody production were normal (Table S2 and CH in the Supplementary Appendix). Notably, P1.1 and P5.1 (but not P5.2) revealed concomitant homozygous gene variants in CD21 and CD27, respectively (Table S3). Consistent with the confirmed CD21 deficiency, P1.1 manifested decreased class-switched IgD−CD27+ memory B cells (Fig. S5, in the Supplementary Appendix).7 P5.1 had subclinical persistent Epstein-Barr Virus (EBV) (420 copies/mL) and is monitored closely, because CD27 deficiency increases the risk of EBV-driven lymphoproliferative disease.8 Intravenous immunoglobulin (IVIG) reduced respiratory infections in P1.1 and P2.1.
Three patients had severe thrombotic vascular occlusion (Tables 1 and S1 and CH in the Supplementary Appendix). P6.1 developed thrombi in the inferior vena cava (IVC), right atrium, and the pulmonary arteries causing arteriovenous malformations (Figs. 1E and S6A in the Supplementary Appendix). TEM of duodenal biopsies revealed malformed erythrocytes binding abnormally to capillary walls and transmural migration (Fig. S6B–D in the Supplementary Appendix). P5.1 had multiple thromboses in the mesenteric and hepatic veins, heart, and cerebral veins associated with an intracranial hemorrhage. Thrombosis was unresolved despite surgical removal of blood clots and anticoagulation. P8.1 developed Budd-Chiari syndrome, presumably due to hepatic vein thrombosis. Extended family histories uncovered two additional patients, P4.4 and P5.3, who died prior to genotyping from thrombotic events with PLE, lymphangiectasia and malnutrition. P6.1 died from pulmonary embolism (Table S1, Fig. S1 and CH in the Supplementary Appendix). Hence, the disease natural history includes early mortality related to severe thrombotic events.
Loss-of-function Mutations in CD55
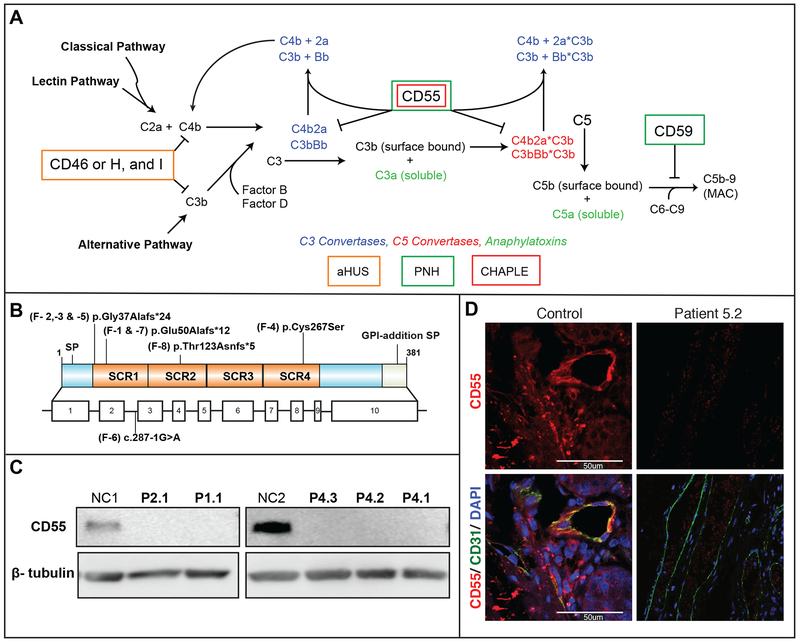
Whole-exome sequence analysis of P1.1, P2.1, P3.1 and P5.1 revealed novel homozygous variants in the gene encoding the complement regulatory protein CD55/decay accelerating factor (DAF)(Fig. 2A and Figs. S1, S7A, and Table S3 in the Supplementary Appendix).9 These variants segregated recessively with disease and heterozygous individuals were unaffected. These variants were not present in the ExAC database and predicted by bioinformatics to be deleterious (Table S4 in the Supplementary Appendix). Further screening of 640 (V)EO-IBD and IBD cases and a cohort of 239 Turkish samples revealed only one person with a heterozygous CD55 variant that was predicted to be only moderately deleterious (c.107delTGCCCGCGGCGC, CADD score: 13.23) indicating CD55 loss-of-function variants are rare. Screening of the ExAC database of 60,000 unrelated individuals revealed 53 individuals with heterozygous loss-of-function CD55 variants and one homozygote, possibly from an IBD cohort although lack of informed consent prevented us from identifying and contacting this person. CD55 had a “probability of loss-of-function intolerant” (pLI) score of 0.0, indicating that heterozygous loss-of-function variants are likely benign.10 Screening of additional early-onset protein-losing enteropathy patients uncovered six with homozygous CD55 loss-of-function variants in Families 4, 6, 7, and 8. Specifically, P1.1 and P7.1 were homozygous for a dinucleotide deletion and a 4-nucleotide insertion at position c.149–150. P2.1, P3.1, P5.1, and P5.2 were homozygous for a single nucleotide deletion in CD55 at position c.109. P8.1 was homozygous for a single nucleotide insertion (c.367). All three variants resulted in a frameshift in codon usage and were predicted to cause premature termination of CD55 mRNA translation. The variant common to families 1 and 7, and the variant common to families 2,3, and 5, led to mRNA nonsense-mediated decay (Fig. S7C in the Supplementary Appendix). In Family 4, a novel homozygous missense mutation in CD55 encodes a cysteine to serine substitution in the fourth short consensus repeat domain (c.800G>C., p.Cys267Ser); the wildtype Cys267 disulfide bond with Cys225 is presumably disrupted by the “substituted” serine at residue 267 (Fig. S7E and S7F in the Supplementary Appendix).11 In P6.1, a variant disrupting an exon 3 splice acceptor site probably caused alternative splicing. In all cases, CD55 protein expression was lost, with only P6.1 showing minor residual expression (Fig. 2B and Fig. S7D in the Supplementary Appendix). We observed that CD55 was normally expressed on capillary endothelial cells in the basal submucosa and lamina propria, the brush border columnar epithelium, and in infiltrating lymphocytes and was absent in patient tissues (Fig. 2C and S6E in the Supplementary Appendix). These variants have very strong or strong (P6.1) evidence of pathogenicity according to the ACMG guidelines.12 Altogether, we identified 5 distinct homozygous, novel, loss-of-function CD55 variants in 9 Turkish, 1 Syrian, and 1 Moroccan patients (Fig. 2A and Fig. S1 and S7 in the Supplementary Appendix).
Figure 2. Variants in CD55 Lead to Loss of Protein Expression.
Panel A shows the identified mutations relative to the CD55 protein structure depicting the four short consensus repeat (SCR1 – 4) domains (top) and exon structure (bottom) SP: signal peptide. Panel B shows a protein immunoblot for CD55 in activated CD4+ T cells. Panel C shows indirect immunofluorescence staining of duodenal biopsy taken from Patient 5.2 and healthy control staining for CD55 (red), the endothelial marker, CD31, (green) and DAPI (blue).
Complement Activation on CD55-deficient Cells
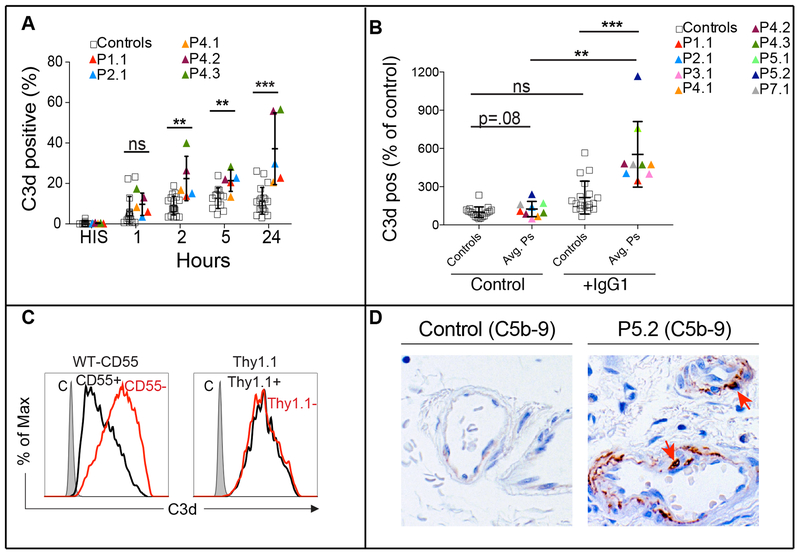
CD55 is attached to the surface by a glycosylphosphatidylinositol moiety and inhibits complement activation by destabilizing and preventing the formation of C3 and C5 convertases, which prevents complement damage (Fig. S7A and S7B in the Supplementary Appendix).9 We therefore tested whether CD55 deficiency accelerated complement activation.13,14 Following incubation with human serum, we observed increased C3 fragment deposition on patient CD4+ T-cell blasts by staining for an epitope common to C3, C3b, and C3d, which was increased through stimulation of the classical pathway by coating the cells with mouse IgG1 (Fig. 3A, 3B, and S7B in the Supplementary Appendix). We failed to detect the C3b fragment (not shown), suggesting rapid degradation, possibly by Factor I and cofactor activity (Fig. S7A).15 Importantly, CD55 reconstitution reduced complement deposition on patient T cells (Fig. 3C). Immunohistochemistry of duodenal biopsies revealed in-vivo terminal complement activation (membrane attack complex/C5b-9) in submucosal arterioles (Fig. 3D).
Figure 3. Loss of CD55 and Increased Complement Deposition.
Panel A shows pooled analyses of C3d staining on T cells of five CD55-deficient patients after incubation with media (pH 7.4) containing pooled normal human serum (nHS) for the times indicated (right) HIS: heat inactivated serum. Panel B shows the pooled analysis of C3d staining with (+IgG1) or without (-IgG1) precoating with an anti-CD28 antibody to activate the classical pathway. Each color coded patient (P) data point represents the average (avg) of at least three repeats for a single patient. Panel C shows C3d staining in Patient 1.1 CD4+ T cells reconstituted with wild-type CD55 (left) or the control marker Thy1.1 (right) and then incubated with serum for 24 hours. Panel D shows C5b-9 staining of duodenal biopsies from a healthy donor and P5.2. In figure 3A the t-test was used to assess the significance between the two groups for the 5 hour incubation, while the nonparametric Mann–Whitney U-test was performed to assess the significance for the 1-hr, 2-hr and 24-hr conditions. In figure 3B the Mann–Whitney U-test was performed for comparisons between the control and patient groups and the Wilcoxon matched pairs signed rank test was applied to comparisons of the same group with or without treatment. (Isotype control (C), not significant (n.s.), *p<.05, **p<.01, ***p<.001).
Excessive Production of Inflammatory Cytokines by CD55-deficient T cells
Complement proteins can provide costimulatory and differentiation signals to T cells through either CD46-mediated C3b sensing or anaphylatoxin receptors.16–20 Knockout mice have confirmed a role for Cd55 in adaptive immune regulation, with Cd55−/− mice producing more interferon (IFN)-γ and less interleukin-10 (IL-10) in auto-immune models.21,22 Patient CD4+ T cells produced increased tumor necrosis factor (TNF), reduced IL-10, with normal IFNγ, and normal proliferation after T-cell receptor engagement (Fig. S8A–C and S8H–I, in the Supplementary Appendix). C3aR and C5aR1 dual inhibition, primarily due to C5aR1, decreased TNF overproduction to control levels (Fig. S8A and S8D in the Supplementary Appendix). Anaphylatoxin inhibition did not increase IL-10, suggesting this is independently regulated (Fig. S8B in the Supplementary Appendix).21 Inflammatory cytokines, including TNF, could instigate the severe thrombophilia in CD55-deficient patients by reducing thrombomodulin (TM) and augmenting tissue factor (TF) expression on endothelial cells.23 Indeed, we found that TNF and IFNγ induced procoagulatory decreases in TM and increases in TF (Fig. S8E in the Supplementary Appendix). Interestingly, CD55 expression increased in human umbilical vein endothelial cells after TNF treatment, suggesting that CD55 limits complement mediated damage during inflammation (Fig. S8F in the Supplementary Appendix).
CD55 can convey a co-stimulatory signal for T-cell activation and production of IL-10, a cytokine inhibitory to intestinal inflammation.24,25 We found that patient cells showed impaired proliferation and IL-10 production in response to an agonistic anti-CD55 antibody or recombinant CD97 together with TCR stimulation (Fig. 8H and I in the Supplementary Appendix).
In Vitro Inhibition of Complement by Eculizumab Formulation
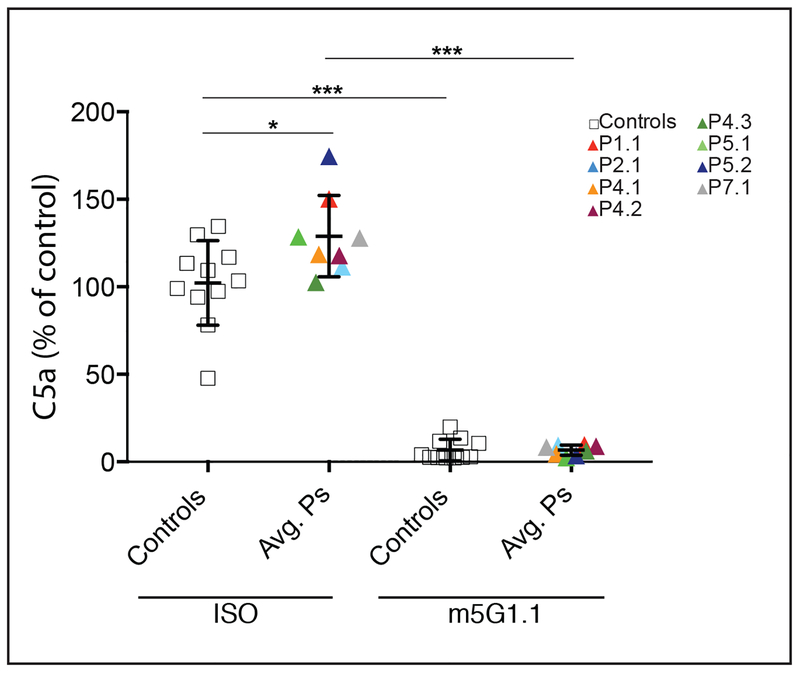
Finally, we investigated whether clinically available complement inhibitors could prevent the enhanced activation in patient samples. We observed that C5a production, which was elevated upon incubation with patient cells, was abrogated by co-incubation with an experimental formulation of eculizumab, a complement inhibitory therapeutic used to treat paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) (Fig. 4).
Figure 4. Effect of Eculizumab on C5a production on Patient T Cells.
Graph shows the levels C5a in supernatants of CD4+ T cell cultures, color coded by patient, after 2 hours of incubation with 10% normal human plasma (nHP) and 10 μg/mL of either isotype control (ISO) or C5 inhibitory (m5G1.1) antibodies. Each triangle represents the average of at least three repeat measurements for a single donor or patient. A two-tailed unpaired T test with Welch’s correction was performed for comparisons between the control and patient groups and the two tailed paired T test was applied to comparisons of the same group with or without treatment (not significant (n.s.), *p<.05, **p<.01, ***p<.001).
DISCUSSION
We define a genetic syndrome comprising CD55/DAF deficiency with hyperactivation of complement, angiopathic thrombosis, and protein-losing enteropathy (CHAPLE syndrome). Protein-losing enteropathy is probably secondary to primary intestinal lymphangiectasia, intestinal inflammation, and possibly thromboses (Fig. S9 in the Supplementary Appendix).2,4
Complement is a system of interacting proteins that provides host defense by destroying microbes and modulating immunity through soluble anaphylatoxins governed by multiple regulators, including CD55/DAF.9 Genetic variants that increase complement activation cause PNH, aHUS, C3 glomerulopathy, and age-related macular degeneration (Fig. S7A).26–28 PNH results from somatic mutations that disable the glycosylphosphatidylinositol anchor that tethers CD55 and CD59 to the cell surface, leading to complement-mediated hemolysis and thrombosis.29–31 Heterozygous germline loss-of-function variants affecting C3, Factor H, Factor I, or CD46 trigger aHUS by complement-mediated damage to glomerular microvascular endothelial cells, hemolysis, and kidney failure.32 These genetic defects also cause complement-mediated retinal damage and age-related macular degeneration.33 Unlike PNH or aHUS, isolated CD55 deficiency causes early-onset protein-losing enteropathy due to primary intestinal lymphangiectasia and bowel inflammation. We found that CD55 is upregulated by retinoic acid, which is highly concentrated in the gut from the diet (Fig. S8G in the Supplementary Appendix). Persons with CHAPLE and PNH have an increased risk of thrombosis and aHUS patients develop thrombotic microangiopathy, indicating cross-regulation of the complement and coagulation cascades.
CD55 deficiency has been previously found in persons with sporadic gastrointestinal abnormalities and lacking Cromer Blood Group red blood cell antigens (the Inab phenotype).34–37 The Inab phenotype can be transient (3 cases) or persistent (9 cases), and sometimes associated with GI disease variously diagnosed as Crohn’s, capillary angioma, protein-losing enteropathy with intestinal tumor, and food intolerances. Three loss-of-function variants were identified (Fig. S7G), though no definitive disease correlation was made. Also, Cd55-deficient mice develop exacerbated dextran sulfate sodium-induced colitis, consistent with our patients’ intestinal disease, and T-cell mediated autoimmunity in autoimmune models, likely due to immunoregulatory abnormalities similar to our patients.21,22,38 Only one CHAPLE patient presented with autoimmunity in the form of polyarthritis, and none had inflammatory markers or elevated levels of cytokine in the blood.
The disease in our patients showed variable expressivity, potentially attributable to background genetics, diet, microbiome composition, or other influences. Conventional treatments were only transiently effective, although more sustained benefit followed resection of lymphangiectatic intestinal segments. Recurrent infections responded to IVIG. Eculizumab, which suppressed C5a production on patient cells, warrants further investigation as a potential treatment of CHAPLE.39
Supplementary Material
ACKNOWLEDGEMENTS, FUNDING, CONFLICT of INTEREST
We thank our clinical collaborators, patients and their families. We thank Brigitte Langer and Helga Schachner for technical support. We thank Gus Dunn, Andrew Oler, and Celine Hong for help with genomic analysis. We thank Ian Lamborn, Bernice Lo, and Qian Zhang for assistance and a critical reading of the manuscript and Evan Masutani for the molecular rendering of CD55. We thank Alexion Pharmaceuticals for generously providing reagents. This project was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, the European Research Council (ERC StG 310857 to K.B.), ClinicalTrials.gov number (), the Scientific and Technological Research Council of Turkey (1059B191400660, to A.O.), a DOC Fellowship of the Austrian Academy of Sciences at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences to R.C.A., a fellowship Grant from the American Diabetes Association (Grant # 1–16-PDF-025), and a F12 Post-doctoral fellowship from NIGMS (1FI2GM119979–01) to W. A. C. The authors have no conflicts of interest to report.
REFERENCES
- 1.McGovern DP, Kugathasan S, Cho JH. Genetics of Inflammatory Bowel Diseases. Gastroenterology 2015;149:1163–76 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umar SB, DiBaise JK. Protein-losing enteropathy: case illustrations and clinical review. Am J Gastroenterol 2010;105:43–9; quiz 50. [DOI] [PubMed] [Google Scholar]
- 3.Elkadri A, Thoeni C, Deharvengt SJ, et al. Mutations in Plasmalemma Vesicle Associated Protein Result in Sieving Protein-Losing Enteropathy Characterized by Hypoproteinemia, Hypoalbuminemia, and Hypertriglyceridemia. Cell Mol Gastroenterol Hepatol 2015;1:381–94 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldmann TA, Steinfeld JL, Dutcher TF, Davidson JD, Gordon RS Jr., The role of the gastrointestinal system in “idiopathic hypoproteinemia”. Gastroenterology 1961;41:197–207. [PubMed] [Google Scholar]
- 5.Alders M, Hogan BM, Gjini E, et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nature genetics 2009;41:1272–4. [DOI] [PubMed] [Google Scholar]
- 6.Alders M, Al-Gazali L, Cordeiro I, et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum Genet 2014;133:1161–7. [DOI] [PubMed] [Google Scholar]
- 7.Thiel J, Kimmig L, Salzer U, et al. Genetic CD21 deficiency is associated with hypogammaglobulinemia. The Journal of allergy and clinical immunology 2012;129:801–10 e6. [DOI] [PubMed] [Google Scholar]
- 8.Salzer E, Daschkey S, Choo S, et al. Combined immunodeficiency with life-threatening EBV-associated lymphoproliferative disorder in patients lacking functional CD27. Haematologica 2013;98:473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annual review of immunology 1989;7:35–58. [DOI] [PubMed] [Google Scholar]
- 10.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano Y, Sumida K, Kikuta N, Miura NH, Tobe T, Tomita M. Complete determination of disulfide bonds localized within the short consensus repeat units of decay accelerating factor (CD55 antigen). Biochim Biophys Acta 1992;1116:235–40. [DOI] [PubMed] [Google Scholar]
- 12.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajic G, Degn SE, Thiel S, Andersen GR. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J 2015;34:2735–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ham TH, Dingle JH. Studies on Destruction of Red Blood Cells. Ii. Chronic Hemolytic Anemia with Paroxysmal Nocturnal Hemoglobinuria: Certain Immunological Aspects of the Hemolytic Mechanism with Special Reference to Serum Complement. J Clin Invest 1939;18:657–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barilla-LaBarca ML, Liszewski MK, Lambris JD, Hourcade D, Atkinson JP. Role of membrane cofactor protein (CD46) in regulation of C4b and C3b deposited on cells. J Immunol 2002;168:6298–304. [DOI] [PubMed] [Google Scholar]
- 16.Kolev M, Le Friec G, Kemper C. Complement--tapping into new sites and effector systems. Nat Rev Immunol 2014;14:811–20. [DOI] [PubMed] [Google Scholar]
- 17.Cardone J, Le Friec G, Vantourout P, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nature immunology 2010;11:862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolev M, Dimeloe S, Le Friec G, et al. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity 2015;42:1033–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strainic MG, Liu J, Huang D, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 2008;28:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghannam A, Fauquert JL, Thomas C, Kemper C, Drouet C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Molecular immunology 2014;58:98–107. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Miwa T, Hilliard B, et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med 2005;201:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miwa T, Maldonado MA, Zhou L, et al. Decay-accelerating factor ameliorates systemic autoimmune disease in MRL/lpr mice via both complement-dependent and -independent mechanisms. Am J Pathol 2007;170:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hot A, Lenief V, Miossec P. Combination of IL-17 and TNFalpha induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Annals of the rheumatic diseases 2012;71:768–76. [DOI] [PubMed] [Google Scholar]
- 24.Spendlove I, Sutavani R. The role of CD97 in regulating adaptive T-cell responses. Adv Exp Med Biol 2010;706:138–48. [DOI] [PubMed] [Google Scholar]
- 25.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine 2009;361:2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol 2009;9:729–40. [DOI] [PubMed] [Google Scholar]
- 27.Noris M, Remuzzi G. Glomerular Diseases Dependent on Complement Activation, Including Atypical Hemolytic Uremic Syndrome, Membranoproliferative Glomerulonephritis, and C3 Glomerulopathy: Core Curriculum 2015. Am J Kidney Dis 2015;66:359–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liszewski MK, Java A, Schramm EC, Atkinson JP. Complement Dysregulation and Disease: Insights from Contemporary Genetics. Annu Rev Pathol 2017;12:25–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson-Weller A, March JP, Rosenfeld SI, Austen KF. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A 1983;80:5066–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson-Weller A, Spicer DB, Austen KF. Deficiency of the complement regulatory protein, “decay-accelerating factor,” on membranes of granulocytes, monocytes, and platelets in paroxysmal nocturnal hemoglobinuria. The New England journal of medicine 1985;312:1091–7. [DOI] [PubMed] [Google Scholar]
- 31.Risitano AM. Paroxysmal nocturnal hemoglobinuria and the complement system: recent insights and novel anticomplement strategies. Adv Exp Med Biol 2013;735:155–72. [DOI] [PubMed] [Google Scholar]
- 32.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. The New England journal of medicine 2009;361:1676–87. [DOI] [PubMed] [Google Scholar]
- 33.Montes T, Goicoechea de Jorge E, Ramos R, et al. Genetic deficiency of complement factor H in a patient with age-related macular degeneration and membranoproliferative glomerulonephritis. Molecular immunology 2008;45:2897–904. [DOI] [PubMed] [Google Scholar]
- 34.Yazer MH, Judd WJ, Davenport RD, et al. Case report and literature review: transient Inab phenotype and an agglutinating anti-IFC in a patient with a gastrointestinal problem. Transfusion 2006;46:1537–42. [DOI] [PubMed] [Google Scholar]
- 35.Daniels GL, Green CA, Mallinson G, et al. Decay-accelerating factor (CD55) deficiency phenotypes in Japanese. Transfus Med 1998;8:141–7. [DOI] [PubMed] [Google Scholar]
- 36.Daniels GL, Tohyama H, Uchikawa M. A possible null phenotype in the Cromer blood group complex. Transfusion 1982;22:362–3. [DOI] [PubMed] [Google Scholar]
- 37.Lin RC, Herman J, Henry L, Daniels GL. A family showing inheritance of the Inab phenotype. Transfusion 1988;28:427–9. [DOI] [PubMed] [Google Scholar]
- 38.Lin F, Spencer D, Hatala DA, Levine AD, Medof ME. Decay-accelerating factor deficiency increases susceptibility to dextran sulfate sodium-induced colitis: role for complement in inflammatory bowel disease. J Immunol 2004;172:3836–41. [DOI] [PubMed] [Google Scholar]
- 39.Cofiell R, Kukreja A, Bedard K, et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 2015;125:3253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.