Abstract
Increasingly, research suggests that for certain systems, animal models are insufficient for human toxicology testing. The development of robust, in vitro models of human toxicity is required to decrease our dependence on potentially misleading in vivo animal studies. A critical development in human toxicology testing is the use of human primary hepatocytes to model processes that occur in the intact liver. However, in order to serve as an appropriate model, primary hepatocytes must be maintained in such a way that they persist in their differentiated state. While many hepatocyte culture methods exist, the two-dimensional collagen “sandwich” system combined with a serum-free medium, supplemented with physiological glucocorticoid concentrations, appears to robustly maintain hepatocyte character Studies in rat and human hepatocytes have shown that when cultured under these conditions, hepatocytes maintain many markers of differentiation including morphology, expression of plasma proteins, hepatic nuclear factors, phase I and II metabolic enzymes. Functionally, these culture conditions also preserve hepatic stress response pathways, such as the SAPK and MAPK pathways, as well as prototypical xcnobiotic induction responses. This chapter will briefly review culture methodologies but will primarily focus on hallmark hepatocyte structural, expression and functional markers that characterize the differentiation status of the hepatocyte.
Keywords: Primary hepatocytes, hepatocyte differentiation, cell culture, hepatocyte morphology, xenobiotic responsiveness, hepatic nuclear factors, cytochrome P-450, extracellular matrix, dexamethasone, phenobarbital
1. Introduction
The adult liver is the largest glandular organ in mammals and carries out critical life functions involving both endocrine and exocrine pathways. Hepatocytes comprise ~85% of the liver mass (1) and are the predominant contributors to liver physiology. Hepatocyte functions include glycogen storage, lipid and serum protein biosynthesis, biotransformation of a diverse array of dietary substances, and the detoxification of a large variety of xenobiotic compounds. Of the available in vitro hepatic models, primary hepatocytes offer substantial advantages, including conserved uptake and excretion functions, the integration of phase I and phase II metabolic pathways, and the presence of cofactors necessary for enzyme activity. Although in practice since the 1950s, early methods, involving perfusion of rodent livers under pressure, resulted in grossly damaged hepatocytes. Isolation methods were vastly improved by Berry and Friend (2) through the introduction of collagenase as a means to enzymatically disperse cells and by Seglen’s introduction of the two-step method (3). This two-step method, now considered the standard isolation method, consists of an initial perfusion with a calcium-free buffer to disrupt desmosomes that make up the tight junctions between cells followed by a second perfusion with a calcium-rich buffer containing collagenase to further digest cell junctions. Another breakthrough in hepatocyte isolation methods was the modification of the procedure to use only segments of the liver, rather than the entire organ, allowing cost-efficient scale-up of the procedure to use larger livers, such as human (4–6). Despite the improvement in methods, hepatocytes from these early isolation experiments dedifferentiated quickly in culture, within a few hours losing hallmark features of in vivo liver function, such as albumin secretion and biotransformation activity (7–9).
This dediffetentiation phenomenon has sparked investigation both of the culture conditions that preserve the differentiated phenotype and of the mechanisms responsible for differentiation status. In general, an inverse relationship has been described between a well-differentiated, growth-arrested phenotype and a pioliferative one, marked by a G0/G1 transition that is triggered by the isolation process itself as defined by upregulated protooncogenes such as c-fos, c-jun, and c-myc (10, 11). This prolifeiative state in vitro has been further characterized by activation of cell cycle-stimulating and stress-related proteins, such as AP-1 (11–13) and NFκB (12, 14), and by loss of liver-enriched nuclear factors such as C/EBPα and the hepatocyte nuclear factor (HNF) family members (11, 12, 15, 16). While the induction of a proliferative state is advantageous for investigations of liver regeneration mechanisms, studies of xenobiotic metabolism require hepatocytes that respond with the fidelity of the in vivo fiver. Thus, considerable effort has been put forth to identify conditions in which hepatocytes remain well differentiated. Unfortunately, many investigators continue to use sub-optimal culture methodologies.
2. Cell Culture
2.1. Three-Dimensional Bioreactors
Although hepatocyte culture variations are abundant, for and include the culturing of hepatocytes as spheroids (17, 18) and in various co-culture configurations (17, 19), two of the most prevalent culture methodologies, when implemented appropriately, preserve a well-differentiated hepatocyte phenotype, namely the use of three-dimensional bioreactors or two-dimensional sandwich culture configurations. The former methodology embeds hepatocytes within complex three-dimensional chambers, most commonly hollow fiber membrane bioreactors (Fig. 6.1A). The hollow fibers, woven into a three-dimensional scaffold for hepatocyte attachment, act as capillaries through which defined culture medium is perfused, providing a continuous supply of oxygen and nutrients to the cells, efficient removal of waste products, and controlled fluid dynamics designed to mimic in vivo shear stress and interstitial flow (20–24), Under ideal bioreactor conditions, hepatocytes tend to exhibit a differentiated phenotype, over several weeks in culture, with cuboidal morphology, extensive cell-cell contacts (22, 25), and specialized structures such as bile canaliculi (26, 27). Additionally, certain functional hallmarks are preserved, as hepatocytes in bioreactors synthesize both albumin and urea (21, 22, 25–28), excrete galactose (26, 28), and demonstrate various drug biotransformation activities (22, 23, 25).
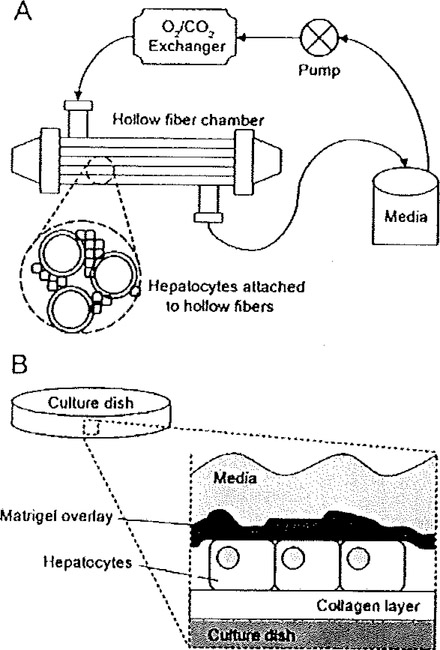
Fig. 6.1.
Illustrations of two primary hepatocyte culturing methodologies that preserve a differentiated phenotype. (A) Hollow fiber membrane bioreactors generally contain the following components: a reservoir containing defined media, a pump, a carbon dioxide/oxygen exchanger, and a chamber containing a complex network of hollow fibers enabling both media perfusion and sites of hepatocyte attachment. (B) In the sandwich culture system, hepatocytes are typically embedded between a collagen substratum and a dilute Matrigel overlay. Other forms of sandwich culture include the direct attachment of cells to either tissue culture plastic or poly-lysine-coated surfaces, followed by Matrigel overlay.
Nonetheless, the continuous perfusion inherent to this model has some associated difficulties, as components derived from cells or present in the media can clog pores on the membranes, subsequently altering the flow and possibly resulting in gradients of nutrients or oxygen through the chamber (20, 26, 29). Additionally, even though the rate of perfusion is controlled, the flow of fluid may introduce excess mechanical stress that may disrupt normal hepatocyte dynamics (30–32).
2.2. Two-Dimensional Sandwich Culture
A relatively simple, but nonetheless, robust methodology is the sandwich culture system, where hepatocytes are embedded between a substratum of collagen and an overlay of either collagen or a commercially available extracellular matrix (ECM), such as Matrigel, a derivative of the Swarm-Engelbreth-Holm carcinoma (Fig. 6.1B). When adopted in the appropriate context, the sandwich culture method is capable of achieving prolonged hepatocyte viability (33, 34) and differentiated morphology, such that hepatocytes remain cuboidal in structure and form closely associated cellular networks (33, 35–37). Functional capacity is also improved, displaying appropriately polarized membrane domains (38–40), enhanced biotransformation activity (33, 41–43), and long-term albumin secretion (34, 36, 40). This configuration mimics the in vivo microenvironment, where, as shown in Fig. 6.2, hepatocytes are anchored to two opposing surfaces, even though the precise signaling pathways that this configuration preserves have not been dearly defined.
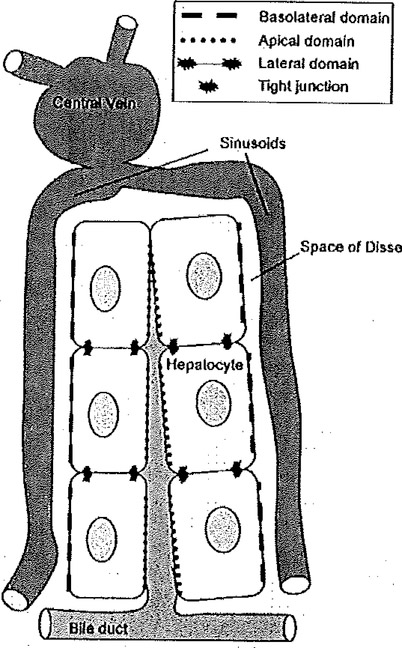
Fig. 6.2.
Illustration of hepatocyte plate structure in the liver. The circulatory blood vessels and polarity features of the hepatocyte are indicated. Hepatocytes in vivo have polarized membranes with specialized function based on location within the liver lobule. The basolateral, or sinusoidal, domain is specialized for exchange with blood, the apical, or canalicular, domain is specialized for bile secretion, and the lateral domain is specialized for intercellular communication. The various domains are separated by tight junctions.
ECM components present in die space of Disse, in particular laminin and collagen, are thought to not only provide anchorage for hepatocytes in vivo, but also to promote differentiation. These matrix components participate in the preservation of normal cytoskeletal organization (35, 44) and regulate the expression of HNF family members (45–47) and albumin (48, 49), highlighting the importance of ECM in the maintenance of hepatocyte differentiation. Since extracellular signals are often communicated to the cytoskeleton via the integrin family of cell surface receptors, it has been suggested that integrin signaling is crucial for maintenance of differentiation (35, 50); αt3βl integrin, in particular, facilitates hepatocyte attachment to collagen (51, 52) and fibronectin (53) and overall preservation of a differentiated morphology (54).
Recently, phosphatidylinositol signaling has been identified as a potential link between integrins and cytoskeletal rearrangement, as ECM/Matrigel attachment causes an increase in phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) phosphatase mRNA, with a subsequent decrease in PI(4,5)P2 levels and actin polymerization (46). Furthermore, integrin-linked kinase (ILK) has recently been shown to play a critical role in matrix-induced hepatocyte differentiation (55). These studies demonstrated that ILK is present in the cell-ECM adhesion sites of cultured hepatocytes. Furthermore, hepatocytes isolated from ILK knockout mice appeared less differentiated in culture than hepatocytes from wild-type mice.
2.3. Defined Media Conditions
In addition to culture configuration, defined media conditions are critical for the maintenance of differentiated hepatocyte phenotype, in particular the presence of physiological, nanomolar levels of glucocorticoids, for example, in the form of the synthetic hormone dexamethasone, when coupled with the absence of serum in the culture medium. Dexamethasone is a potent activator of the glucocorticoid receptor (GR), a member of the nuclear hormone receptor superfamily that, prior to ligand binding, is complexed in the cytosol with HSP90, p23, and one of several tetratricopeptide repeat proteins (56–59). Ligand binding causes a conformational change in GR, revealing nuclear localization signals that stimulate nuclear translocation of the receptor (60, 61). Once in the nucleus, GR binds to specific response elements, acting as an anti-inflammatory and an immunosuppressant, largely through repression of the NFκB and AP-1 pathways (62–64).
In primary hepatocyte culture, dexamethasone additions promote a cuboidal phenotypic architecture, facilitate the expression of liver-enriched transcription factors, such as C/EBPα, HFN-4α, and RXRα (13, 36, 65, 66), and suppress the hepatocyte proliferative state otherwise stimulated by growth factors such as EGF (67). Although high doses of dexamethasone may stimulate proliferation (68), low concentrations are often included in culture media designed to induce hepatic lineage differentiation for embryonic stem cells derived from human (69, 70), monkey (71), and mouse (72). Importantly, inclusion of nanomolar concentrations in the hepatocyte culture media serves to inhibit the induction of stress signaling pathways, such as MAPK and SAPK/JNK (13).
In these respects, for human hepatocyte culture, our laboratory has adopted a highly defined, serum-free, two-dimensional sandwich system that configures hepatocytes with collagen I as the substratum and a dilute overlay of ECM, combined with serum-free medium containing nanomolar levels of dexamethasone (13, 36, 73). This sandwich system is appropriate for rat and human hepatocytes, and our protocol for human hepatocytes is briefly outlined below. In our human studies, primary hepatocytes were obtained from the Liver Tissue Cell Distribution System (reference NIH Contract –#N01-DK-7–0004/HHSN267200700004C). Hepatocytes are isolated according to a three-step collagenase perfusion protocol (74). Preparations enriched for hepatocytes are received plated in collagen-coated, tissue culture plastic flasks, or dishes. The culture media consists of William’s Media E supplemented with 1% penicillin/streptomycin, 10 mM HEPES, 20 μM glutamine, 25 nM dexamethasone, 10 nM insulin, 30 mM linoleic acid, 1 mg/ml BSA, 5 ng/ml selenious acid, and 5 μg/ml transferrin. Within 4–16 h, an ECM overlay is added. A 10 mg/ml stock solution of Matrigei (BD Biosciences, San Jose, CA) is added dropwise to the culture media and evenly distributed by gentle swirling such that the final concentration is 225 μg/ml. Matrigei is a liquid at 4°C temperatures and rapidly gels at room temperature or at 37°C; therefore the additions of Matrigei need to be made rapidly, and typically using pipette tips that are pre-chilled in the freezer. The media is subsequently changed every 48 h until cells are harvested for RNA extraction. The cells are maintained at 37°C under 5% CO2.Under these conditions, the hepatocytes are non-proliferative and are stable in culture for extended periods of culture, e.g., >2 weeks. See also Chapter 3 and 23 of the present volume.
3. Markers of a Differentiated Hepatocyte
3.1. Morphology
An often overlooked aspect of the differentiated hepatocyte is the status of the plasma membrane, namely that the membrane retains polarized domains, forms junctions between cells to facilitate cell-cell communication, and contains specialized structures like bile canaliculi. In vivo, hepatocytes are arranged in plate-like arrays, focing the sinusoids on one side and bile ductules on the other. The plasma membrane is functionally compartmentalized based on these interactions, such that the basolateral, or sinusoidal, membrane is specialized for exchange of metabolites with circulating blood (Fig. 6.2). Similarly, the apical, or canalicular, membrane is specialized for bile secretion, and the lateral membrane, joining adjacent hepatocytes, is specialized for intercellular communication (35, 75). Functional polarity in vitro is demonstrated by marker proteins specific for lateral domains, such as connexins 26 and 32; basolateral domains, like epidermal growth factor receptor; and apical domains, such as dipeptidyl peptidase IV (40, 76–79). Alternatively, hepatobiliary transport, shown by the appropriate accumulation and excretion of bile acids and other organic anions (38, 39, 80–82), and gap junctional intercellular communication between adjacent hepatocytes (78, 79) demonstrate the compartmentalization of these specialized functions. As dedifferentiation occurs, the cuboidal networks of cells often flatten and lose expression of specialized structures such as bile canaliculi, as well as distinct cell–cell contacts (35, 40, 45, 83, 84).
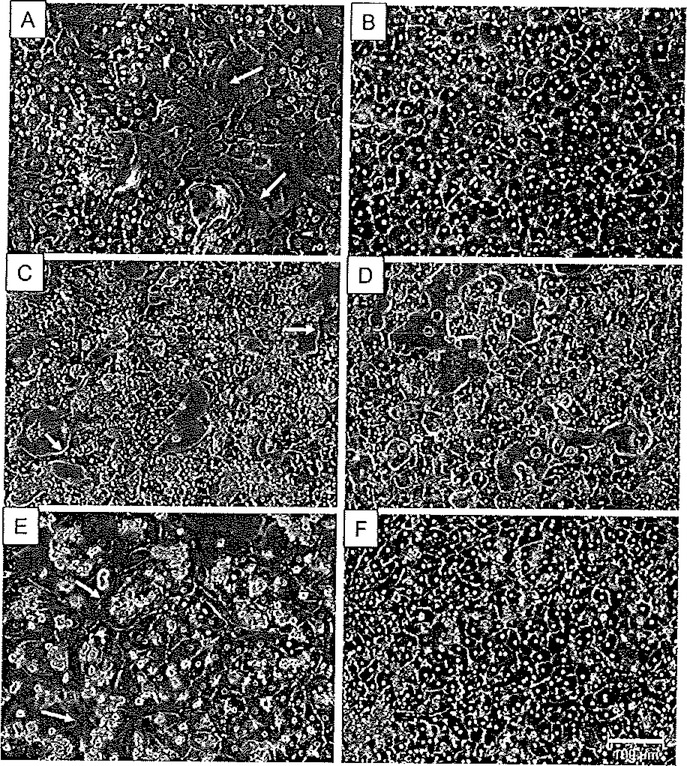
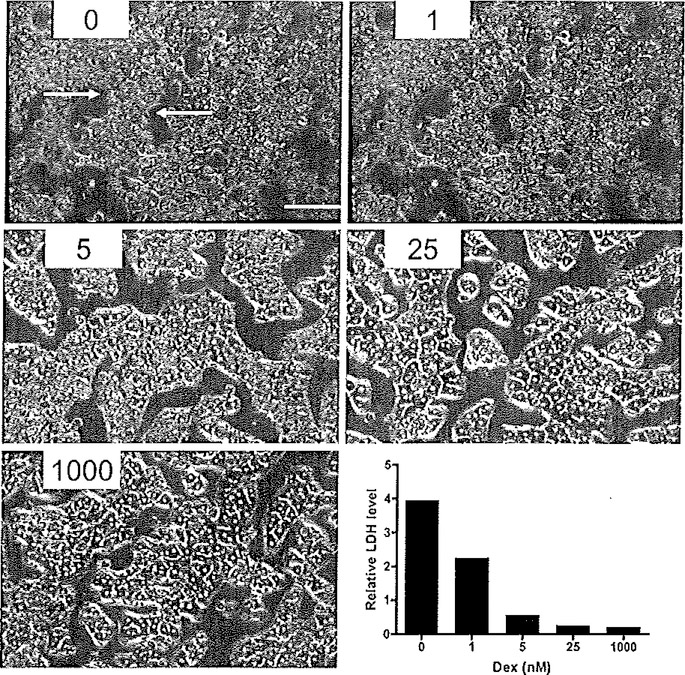
Microscopically, in optimally cultured hepatocyte preparations, many of the morphological features of hepatocytes are visible. Figure 6.3 shows examples of primary human hepatocytes cultured in the absence and presence of Matrigel. The cells cultured in the presence of Matrigel (Fig. 6.3B, D and F) exhibited characteristic cuboidal, three-dimensional structure, and enhanced cell border definition. In contrast, cells cultured without Matrigel (Fig. 6.3A, C and E) exhibit a more flattened appearance, weakly defined borders, and evolve fibroblast-like spinous processes, indicative of dedifferentiation. A further example of the morphological features is illustrated in a previous study of the effect of culture conditions on rat hepatocytes (13), as presented in Fig. 6.4. These rat hepatocyte studies serve to illustrate the importance of low concentrations of glucocorticoid additions. In Fig. 6.4, hepatocytes were cultured in the sandwich configuration as described above along with varying concentrations of dexamethasone. Omission of dexamethasone resulted in perturbation of the cuboidal networks, with cells exhibiting condensed cytoplasm, abnormal rounding of cell structure, and formation of fibroblast-like protrusions. Further, as a measure of hepatocyte toxicity associated with morphological disruption, lactate dehydrogenase (LDH) leakage from the cells was assessed. In addition to protecting morphological integrity, nanomolar additions of dexamethasone protected against cytotoxicity, attenuating LDH leakage (Fig. 6.4).
Fig. 6.3.
Matrigel enhances cellular morphology of primary human hepatocyte cultures. Primary human hepatocytes from Donor A (A and B), Donor B (C and D), and Donor C (E and F) were cultured in the presence (B, D, F) or absence (A, C, E) of a Matrigel overlay. Photomicrographs were taken under ×20 magnification using phase-contrast imaging. Arrows indicate compromised morphology in the absence of a Matrigel overlay. Reproduced from Toxicological Sciences, 2007 (73) with permission from Oxford University Press.
Fig. 6.4.
Effect of dexamethasone concentration dependency on hepatocyte morphology and viability. Primary rat hepatocytes were cultured for 96 h under the stated dexamethasone (Dex) concentrations (nM) in the presence of a Matrigel overlay (×20 magnification). Arrows identify evidence of perturbed morphology: condensed cytoplasm and rounded-up cells, attributed to cytotoxicity. The tower right panel shows the relative level of LDH leakage associated with each Dex concentration. Reproduced from Experimental Cell Research, 2004 (13) with permission from Elsevier
3.2. Immunofluorescence
Expression of cytokeratins 18 and 19 is a widely recognized feature of differentiated hepatocytes, therefore its detection in cells via immunofluorescence is a useful marker of the mature phenotype. For example, investigators assessing the progression of embryonic stem cells down the hepatic lineage often assess these markers (85–87). As indicated previously, expression and localization of connexin 32 is a hallmark feature of hepatocyte gap junctions. Our studies have shown that in the presence of Matrigel, hepatocytes exhibit enhanced gap-junctional formation, as assessed by immunofluorescence detection of connexin 32, when compared with hepatocytes cultured without Matrigel (88). ILK, a key factor in matrix-induced hepatocyte differentiation (55), is another hepatocyte marker that can be assessed using immunofluorescence. This marker is visible at the ECM adhesion sites of hepatocytes in culture.
3.3. Plasma Proteins
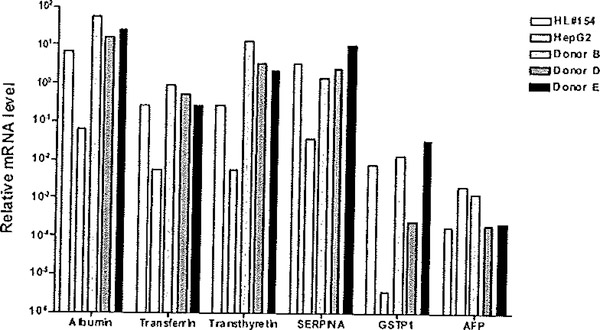
The most frequently assessed markers of hepatocyte differentiation include expression of plasma proteins such albumin, transferrin, transthyretin, and α−1-antitrypsin (45, 80, 84, 89–91), in that this organ is the dominant site of plasma protein synthesis (92, 93). On the other hand, hepatocyte dedifferentiated is reflected typically by the up regulation of alpha-fetoprotein (AFP) and glutathione-S-transferase P1 (GSTP1; GSTπ) (94, 95). AFP is normally silenced in adult livers and therefore an increase in its expression within primary hepatocyte cultures is indicative of a dedifferentiation process toward a fetal lineage (95). Similarly, GSTP1 is expressed selectively in fetal liver and silenced in the mature hepatocyte (94). Therefore, both of these markers are particularly useful indicators of cultured hepatocyte dedifferentiation status, largely repressed in differentiated cells but augmented in hepatocytes undergoing dedifferentiation processes. Quantitative RT-PCR (qRTPCR) analyses are convenient assays to conduct in this regard and assays for literally any human or mouse gene transcript are available commercially from sources such as Applied Biosystems (Carlsbad, CA). Figure 6.5 shows results of qRTPCR analyses for markers of differentiation and dedifferentiation on total RNA isolated from primary human hepatocytes maintained in defined culture media containing dexamethasone at physiological levels, in the absence and presence of ECM/Matrigel. When comparing expression profiles of selected markers between human liver, human hepatocytes cultured with Matrigel, and a commonly used human hepatoma cell line, hepatocytes cultured in the presence of a Matrigel overlay most closely resemble the expression profile of the human liver, while HepG2 cells, although expressing certain markers, differed from the expression levels of the liver by at least 10-fold and as much as 200-fold (Fig. 6.5). In other studies (data not shown), further comparisons to additional human liver tissues, from six different donors, were also conducted, with similar conclusions derived as that for the representative HL#154 liver presented here. Therefore, the cumulative evidence indicated that a Matrigel overlay was a positive regulator of differentiation status of primary human hepatocytes, facilitating the up regulation of differentiation makers, down regulation of de differentiation markers.
Fig. 6.5.
Effects of Matrigel addition on differentiation status of primary human hepatocyte cultures. Total RNA was isolated a section of human liver # 154, from HepG2 cells, as well as three different donor samples of primary human hepatocytes that were cultured for 5 days in the presence of a Matrigel overlay. Relative mRNA transcript expression levels were assessed using TaqMan qRTPCR analyses for a panel of differentiation markers, albumin, transferrin and transthyretin, and alpha-1-antitrypin (SERPINA), and de-differentiation markers GSTP1 and alpha fetoprotein (AFP). The ΔΔCt method was used for quantification (124). The results are graphically depicted, using a fog scale on the ordinate axis. Reproduced from Toxicological Sciences, 2007 (73) with permission from Oxford University Press.
3.4. Cytochromes P450 and Hepatic-Enriched Nuclear Factors
Another hallmark feature of the liver is its biotransformation activity; thus, cytochrome P450 (CYP) monooxygenase and phase II enzyme expression and activity (36, 41, 90, 91, 96) are commonly used markers of hepatocyte differentiation. In addition, a number of liver-enriched nuclear factors, including HNF family members, CAAT/enhancer binding protein α (C/EBPα), and nuclear hormone receptor superfamily members, are prominently expressed in the mature liver and are engaged in critical regulatory roles underlying the maintenance of biotransformation enzyme function as well as many other differentiated features of the hepatocyte. For example, the expression of C/EBPα has been noted to decline both as expression of protooncogenes increase and as normal morphology is altered (11–13,84), whereas the HNF4 family members play a role in liver-specific gene expression; targeted knockdown of this transcription factor results in decreased expression of the plasma proteins albumin and transthyretin (45, 46).
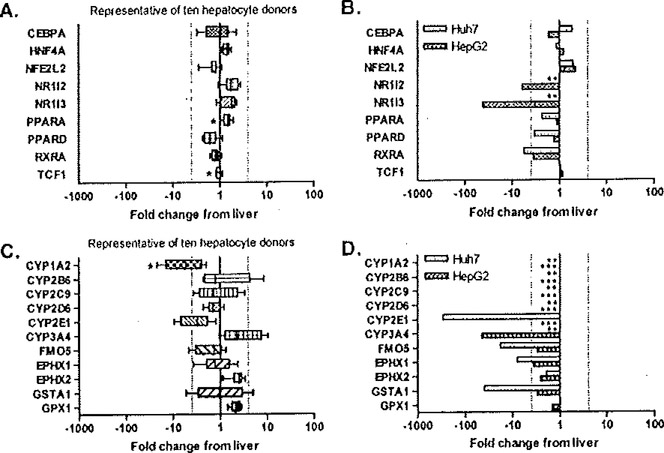
Studies from our laboratory have also used whole genome expression profiling in human liver samples and in the commonly used HepG2 and Huh7 human hepatoma cell lines to determine mRNA expression levels coding for biotransformation enzymes and hepatic nuclear factors. When cultured in a two-dimensional Matrigel sandwich configuration, the transcription factors were tightly regulated in hepatocytes obtained from various human donors, as expression of the genes was maintained at levels less than 4-fold changed from liver (Fig. 6.6A). Among the two hepatoma cell lines studied, the expression profiles of the various transcription factors varied considerably compared to that of liver or primary hepatocytes, and there were notable differences in expression character even between the two cell lines. For example, mRNAs for NR1I2 (pregnane X receptor (PXR)) and NR1I3 (constitutive androstane receptor (CAR)) were undetectable in Huh7 cells and were >6- and 42-fold decreased in HepG2 cells, respectively (Fig. 6.6B). The expression levels for the retinoid X receptor-α (RXRoα) were reduced ~5-fold in both of the respective cell lines, compared to liver. Generally, mRNAs for CϒP450 family members were expressed in hepatocytes at levels comparable to those detected directly in liver, with the exception of CϒP1A2 and CϒP2E1, which were decreased (Fig. 6.6C). In contrast, in the hepatoma lines expression of CϒP450 isoforms is dramatically decreased or non-existent (Fig. 6.6D). These studies demonstrated that in vitro hepatocytes, in a sandwich culture with defined medium, are reasonably representative of in vivo liver, while the HepG2 and Huh7 ceils exhibited markedly deviant, dedifferentiated phenotype. When considering these comparative studies, one should also keep in mind that liver itself is comprised of ~80% hepatocytes, with the remainder of the tissue consisting of other types of cells, such as endothelial, biliary, and stellate cells. In tills regard, the measured comparisons refered to here between primary hepatocyte cultures and actual liver are likely even closer then otherwise indicated in these studies (88).
Fig. 6.6.
Gene-level expression analysis of selected liver-specific categories in human hepatocyte donors and hepatoma-derived cell lines using microarray profiling. Distribution of fold change from the liver in 10 hepatocyte donors is shown for genes encoding select transcription factors (A) and drug-metabolizing enzymes (C). For comparison, the fold change for the same genes in HepG2 and Huh7 hepatoma cells are presented in panels B and D. Differential expression is defined as greater than 4-fold change from the human liver (dotted lines). * indicates the measured probe set is detected as absent in at least one human hepatocyte donor (PPARA: absent in two donors; TCF1: absent two donors; CYP1A2: absent in one donor). ** indicates the probe set is detected as absent in Huh7 cells (NR1I2, NR1I3, CYP1A2, CYP2B6, CYP2C9, CYP2D6, CYP3A4). *** indicates the probe set is detected as absent in HepG2 (CYP1A2, CYP286, CYP2C9, CYP2D6, CYP2E1). Reproduced from Toxicology and Applied Pharmacology (88) 2007, with permission from Elsevier.
4. Stress Pathways and Hepatocyte Integrity
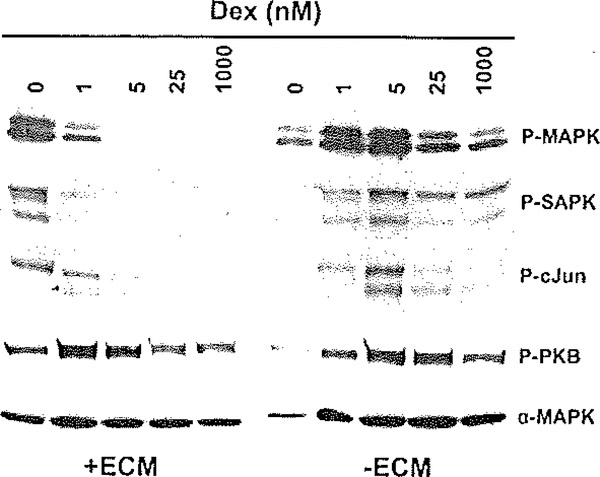
The importance of appropriate culture conditions on hepatocyte differentiation has been outlined above, but to further illustrate this point, previous studies from our laboratory demonstrating the interaction of culture conditions and stress pathways are presented. A compromised differentiation status is associated with the activation of stress-associated pathways in cultured hepatocytes, including the MAPK, SAPK/JNK, and c-Jun signaling pathways. For these studies, rat hepatocytes were cultured in a serum-free, highly defined medium in the absence and presence of Matrigel/ECM and with varying concentrations of dexamethasone. Cells cultured in the absence of dexamethasone exhibited a marked stimulation of p42/44 MAPIC, SAPK/JNK, and c-Jun phosphorylation (Fig. 6.7). The presence of Ma trigel served to attenuate the activation of these pathways, even at the 1 nM dexamethasone dose. The stress activation responses were blunted completely with 5 nM dexamethasone. In contrast, cells cultured in the absence of a Matrigel overlay exhibited stress pathway activation responses that could only be attenuated modestly, even at the highest concentrations of dexamethasone tested. Thus, there is an apparent synergy between the effects of Matrigel and dexamethasone in providing attenuation of the stress cascades. It is interesting to note that omission of dexamethasone or Matrigel only had minimal impact on the phosphorylation status of PKB, a critical and positive effector of cell survival and death (Fig. 6.7). This latter result suggests that the cell survival stimulus associated with dexamethasone is independent of a PI3 kinase pathway. Consistent with the activation of the MAPK, SAPK/JNK, and c-Jun signaling pathways, limiting dexamethasone concentration also resulted in increased nuclear accumulation of the AP-1 complex ((13); data not shown). These results are consistent with a loss of control of the signaling machinery regulating cell cycle progression and mitogen-activated growth. Thus, it appears that dexamethasone and Matrigel prevent proliferative signals at the level of AP-1 activation and cell cycle progression, thus preserving the differentiated hepatocyte phenotype.
Fig. 6.7.
Effect of ECM overlay and dexamethasone concentration on the activation of stress signaling pathways in primary rat hepatocytes. Primary rat hepatocytes were cultured for 96 h under the variable concentrations of dexamethasone (Dex), as indicated, and in the presence (+ECM) or absence (−ECM) of an ECM/Matriget overlay. Total cell extracts were prepared and analyzed by Western blot analysis, Phospho-specific antibodies were used to discern the phosphorylation status of p42/44 MAPK (Thr202/Tyr204), SAPK/JNK (Thr183/Tyr185), c-Jun (Ser63), and Akt (Ser473). The levels of each targeted immunoreactive protein were assessed in parallel with phosphorylation-independent antibodies, as shown for αMAPK, Reproduced from Experimental Cell Research, 2004 (13), with permission from Elsevier.
5. Functional Assessment of Hepatic Phenotype
An array of additional functional end points can offer insight into the degree of differentiation, due to the wealth of physiological functions in which the in vivo liver plays a role, including the synthesis of urea, clotting factors, and acute phase proteins (25, 26, 28, 91), synthesis of glucose and subsequent glycogen storage (26, 28, 80), excretion of bilirubin (39), and lipid and cholesterol transport (84). Use of the periodic acid Schiffs staining technique (American Master Tech Scientific Inc., Lodi, CA) is a useful method for detection of intracellular glycogen (85). Hepatic glutamine metabolism in connection with urea synthesis is required for systemic ammonia detoxication and pH regulation. Due to the important role of the liver in maintaining ammonia and bicarbonate homeostasis under physiologic and pathologic conditions, ammonia metabolism is often used as a functional marker of hepatic phenotype (97, 98).
5.1. Xenobiotic/Drug Induction Responses
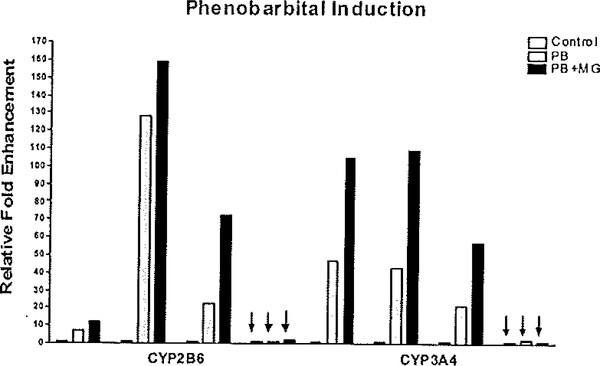
A primary function of the liver is to conduct the metabolism of endogenous, dietary, and xenobiotic substances. Typically, the xenobiotic biotransformation process is typified by both phase I monooxygenation reactions, followed by phase II synthetic processes. The phase I process trends toward detoxification, with the resulting metabolites being more water soluble and exhibiting increased likelihood to undergo further reactions via phase II conjugation pathways. However, a large number of procarcinogens and other environmental toxins are bioactivated by the xenobiotic metabolizing CYPs. Several classes of environmental and therapeutic substances are recognized for their capacity to markedly modulate the transcriptional status of mammalian biotransformation enzymes. There are several prototypical inducing agents, including the polyaromatic and polychlorinated hydrocarbons, ethanol and organic solvents, peroxisome proliferator compounds such as the phthalate esters, dexamethasone, and several sedative-hypnotic medications. These substances tend to regulate their corresponding biotransformation enzyme pathways via the interplay of an array of soluble and nuclear receptors (99). Therefore, based on the complex series of events leading to xenobiotic induction of hepatic gene function, the ability of cultured hepatocytes to respond to xenobiotic inducers is insightful and potentially a uniquely specific indicator of their differentiated state. Studies from our laboratory (13, 36, 73, 88) and others (83, 100–102) have shown that under proper maintenance conditions, hepatocytes will respond appropriately and robustly to a given xenobiotic-inducing agents. Several of the induction pathways are rather robust and are maintained in both established cell lines and even in hepatocytes that are maintained sub-optimally in culture. An exception is phenobarbital (PB). Although used in humans as a sedative and anti-seizure agent without serious long-term adverse effects (103), PB promotes rodent tumorigenesis through mechanisms including inhibition of apoptosis (104), activation of β-catenin (105), selective promotion of cells with low TGFβ receptor expression (106), reduction in G1 checkpoint efficiency (107), and alteration of DNA methylation (108). Mechanistically, PB mediates these effects through activation of the constitutive androstane receptor (NR1I3, or CAR), a member of the nuclear hormone receptor super family of transcription factors (reviewed in (109–112)), In vivo, CAR is retained in the cytoplasm complexed with HSP90 and die tetratricopeptide repeat-containing protein cytoplasmic CAR retention protein (CCRP), until activation by xenobiotics such as PB induces nuclear translocation (113–116). Once in die nucleus, CAR forms a dimer with RXRα (117) and recruits coactivator proteins, such as steroid receptor coactivator 1 (SRC-1) (118), GR-interacting protein 1 (GRIP-1) (119), and peroxisomal proliferator-activated receptor-γ coactivator 1α (PGC1α) (120), to drive transcription of genes, notably CϒP2B and CϒP3A family members, containing PB-responsive enhancer modules (PBREMs) within their promoter regions (121, 122). The PB induction response is typically lost in hepatoma-derived cells or in primary hepatocytes cultured in sub-optimal conditions. An example of the PB induction response that is obtainable in primary cultures of human hepatocytes, and not apparent in most human hepatoma cell lines, is shown in Fig. 6.8. The, authors contend that assessment of the PB induction response in particular appears to serve as a uniquely sensitive and important marker of hepatocyte differentiation status (13).
Fig. 6.8.
Effects of Matrigel addition on the phenobarbital induction activity primary human hepatocyte cultures. Primary human hepatocytes were cultured in the absence (control) or the presence of Matrigel (MG). Cultures of primary human hepatocytes and HepG2 hepatoma cells (indicated by arrows) were treated on day 4 with 0.5 mM phenobarbital (PB alone: PB; or PB in combination with MG, PB+MG) or DMSO (control, leftmost bars in each section of the graph) for 24 h prior to RNA isolation. Relative fold changes in transcript levels for the PB-inducible marker genes, CYP2B6 and CYP3A4, are indicated, normalized to OMSO control levels set (= 1). Reproduced from Toxicological Sciences, 2007 (73) with permission from Oxford University Press.
6. Species-Specific Considerations
Even though there are noted differences across species, the vast majority of validation studies have been carried out in hepatocytes of rodent origin due to limitations in the availability of human hepatocytes. Although further experiments with human hepatocytes may only confirm current culture methodologies, past experience has shown that there are inherent species-specific phenotypic differences in hepatocytes. For instance, early isolation studies reported significantly lower viability in rat and hamster hepatocytes vs. those from mouse and rabbit under the same conditions, as well as a steep decline in cytochrome P450 content in mouse and rat hepatocytes vs. nearly unchanged concentrations in those from rabbit (9). Time-course discrepancies have also been noted for membrane repolarization, in that co-localization of canalicular transport proteins with canalicular markers occurs faster in hepatocytes from rats compared to those from humans (76). Fur titer, while a sandwich culture configuration was demonstrated as critical for the induction of biotransformation enzymes in rat hepatocytes (36, 83), some studies have concluded that a collagen or Matrigel overlay is not vital for enzyme induction in primary human hepatocytes, despite improved morphology and cytoarchitecture in sandwich culture (123). Considering these species-specific responses to in vitro conditions, thorough evaluation of any primary hepatocyte culture systems is warranted in order to secure confidence in its use as a model for liver biology or as predictive tool for in risk assessment.
7. Conclusion
This chapter summarizes an otherwise large body of available information relating to hepatocyte function and provides the reader with an overview of appropriate experimental methodology that can be applied to assess the biological character of primary hepatocytes in culture. It is not intended to be a complete compilation of these issues; rather, this chapter strives to delineate and discuss several important considerations of hepatocyte biology that should be considered in the evaluation of a given primary culture system. Careful attention to criteria such as morphology, functional end points, and expression of appropriate differentiation/dedifferentiation markers are required in any in vitro hepatocyte model system in order to validate its use and robustness as accurate model of hepatocyte phenotype as it exists in vivo.
References
- 1.Perkins EJ, Bao W, Guan X, Ang CY, Wolfinger RD, Chu TM, Meyer SA, and Inouye LS (2006) Comparison of transcriptional responses in liver tissue and primary hepatocyte cell cultures after exposure to hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine. BMC Bioinform. 7 Suppl 4, S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry MN and Friend DS (1969) High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J. Cell Biol, 43, 506–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seglen PO (1976) Preparation of isolated rat liver cells. Methods Cell Biol. 13, 29–83. [DOI] [PubMed] [Google Scholar]
- 4.Guguen-Guillouzo C, Campion JP, Brissot P, Glaise D, Launois B, Bourel M, and Guillouzo A (1982) High yield preparation of isolated human adult hepatocytes by enzymatic perfusion of the liver. Cell Biol. Int. Rep 6, 625–628. [DOI] [PubMed] [Google Scholar]
- 5.Reese JA and Byard JL (1981) Isolation and culture of adult hepatocytes from liver biopsies, hi Vitro 17, 935–940. [DOI] [PubMed] [Google Scholar]
- 6.Strom SC, Jirtle RL, Joncs RS, Novicki DL, Rosenberg MR, Novotny A, Irons G, McLain JR, and Michalopoulos G (1982) Isolation, culture, and transplantation of human hepatocytes. J. Natl. Cancer Inst 68, 771–778. [PubMed] [Google Scholar]
- 7.Clayton DF, and Darnell JE Jr. (1983) Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol. Cell Biol 3, 1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzelian PS, Bissell DM, and Meyer UA (1977) Drug metabolism in adult rat hepatocytes in primary monolayer culture. Gastroenterology 72, 1232–1239. [PubMed] [Google Scholar]
- 9.Maslansky CJ and Williams GM (1982) Primary cultures and the levels of cytochrome P450 in hepatocytes from mouse, rat, hamster, and rabbit liver. In Vitro 18, 683–693. [DOI] [PubMed] [Google Scholar]
- 10.Loyer P, Cariou S, Glaise D, Bilodeau M, Baffet G, and Guguen-Guillouzo C (1996) Growth factor dependence of progression through G1 and S phases of adult rat hepatocytes in vitro. Evidence of a mitogen restriction point in mid-late Gl. J. Biol. Chem 271, 11484–11492. [DOI] [PubMed] [Google Scholar]
- 11.Rana B, Mischoulon D, Xie Y, Bucher NL, and Farmer SR (1994) Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Mol. Cell Biol 14, 5858–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, and Michalopoulos GK (1996) Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J. Cell Biol 132, 1133–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidhu JS, Liu F, and Omiecinski CJ (2004) Phenobarbital responsiveness as a uniquely sensitive indicator of hepatocyte differentiation status: requirement of dexamethasone and extracellular matrix in establishing the functional integrity of cultured primary rat hepatocytes. Exp. Cell Res 292, 252–264. [DOI] [PubMed] [Google Scholar]
- 14.Paine AJ and Andreakos E (2004) Activation of signalling pathways during hepatocyte isolation: relevance to toxicology in vitro. Toxicol. In Vitro 18, 187–193. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki A, Iwama A, Miyashita H, Nakauchi H, and Taniguchi H (2003) Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development 130, 2513–2524. [DOI] [PubMed] [Google Scholar]
- 16.Range D, Range DM, Bowen WC, Locker J, and Michalopoulos GK (1997) Matrix induced re-differentiation of cultured rat hepatocytes and changes of CCAAT/enhancer binding proteins. Biol. Chem 378, 873–881. [DOI] [PubMed] [Google Scholar]
- 17.Thomas RJ, Bhandari R, Barrett DA, Bennett AJ, Fry JR, Powe D, Thomson BJ, and Shakesheff KM (2005) The effect of three-dimensional co-culture of hepatocytes and hepatic stellate cells on key hepatocyte functions in vitro. Cells Tissues Organs 181, 67–79. [DOI] [PubMed] [Google Scholar]
- 18.Tzanakakis ES, Waxman DJ, Hansen LK, Remmel RP, and Hu WS (2002) Long-term enhancement of cytochrome P450 2B1/2 expression in rat hepatocyte spheroids through adenovirus-mediated gene transfer. Cell Biol. Toxicol 18, 13–27. [DOI] [PubMed] [Google Scholar]
- 19.Nahmias Y, Casali M, Barbe L, Berthiaume F, and Yarmush ML (2006) Liver endothelial cells promote LDL-R expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology 43, 257–265. [DOI] [PubMed] [Google Scholar]
- 20.Williams SN, Callies RM, and Brindle KM (1997) Mapping of oxygen tension and cell distribution in a hollow-fiber bioreactor using magnetic resonance imaging. Biotechnol. Bioeng 56, 56–61. [DOI] [PubMed] [Google Scholar]
- 21.Nyberg SL, Shatford RA, Peshwa MV, White JG, Cerra FB, and Hu WS (1993) Evaluation of a hepatocyte-entrapment hollow fiber bioreactor: a potential bioartificial liver. Biotechnol. Bioeng 41, 194–203. [DOI] [PubMed] [Google Scholar]
- 22.Li AP, Barker G, Beck D, Colburn S, Monsell R, and Pellegrin C (1993) Culturing of primary hepatocytes as entrapped aggregates in a packed bed bioreactor: a potential bioartificial liver. In Vitro Cell Dev. Biol 29A, 249–254. [DOI] [PubMed] [Google Scholar]
- 23.De Bartolo L, Morelli S, Rende M, Campana C, Salerno S,, e N, and Drioli E (2007) Human hepatocyte morphology and functions in a multibore fiber bioreactor. Macromol. Biosci 7, 671–680. [DOI] [PubMed] [Google Scholar]
- 24.Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, Kurzawski P, Wack KE, Stolz DB, Kamm R, and Griffith LG (2002) A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol. Bioeng 78, 257–269. [DOI] [PubMed] [Google Scholar]
- 25.De Bartolo L, Salerno S, Morelli S, Giorno L, Rende M, Memoli B, Procino A, Andreucci VE, Bader A, and Drioli E (2006) Long-term maintenance of human hepatocytes in oxygen-permeable membrane bioreactor. Biomaterials 27, 4794–4803. [DOI] [PubMed] [Google Scholar]
- 26.Flendrig LM, la Soe JW, Joining GG, Steenbeek A, Karlsen OT, Bovce WM, Ladiges NC, te Velde AA, and Chamuleau RA (1997) In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound non-woven polyester matrix for hepatocyte culture as small aggregates. J. Hepatol 26, 1379–1392. [DOI] [PubMed] [Google Scholar]
- 27.Lu HF, Lim WS, Zhang PC, Chia SM, Yu H, Mao HQ, and Leong KW (2005) Galactosylated poly(vinylidene difluoride) hollow fiber bioreactor for hepatocyte culture. Tissue Eng. 11, 1667–1677. [DOI] [PubMed] [Google Scholar]
- 28.Pless G, Steffen I, Zeilinger K, Sauer IM, Katenz E, Kehr DC, Roth S, Mieder T, Schwartlander R, Muller C, Wegner B, Hout MS, and Gerlach JC (2006) Evaluation of primary human liver cells in bioreactor cultures for extracorporeal liver support on the basis of urea production. Artif. Organs 30, 686–694. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald JM, Wolfe SP, Roy-Chowdhury I, Kubota H, and Reid LM (2001) Effect of flow configuration and membrane characteristics on membrane fouling in a novel multicoaxial hollow-fiber bioartificial liver. Ann. Nϒ Acad. Sci 944, 334–343. [DOI] [PubMed] [Google Scholar]
- 30.Kan P, Miyoshi H, Yanagi K, and Ohshima N (1998) Effects of shear stress on metabolic function of the co-culture system of hepatocvte/nonparenchymal cells for a bioartificial liver. ASAIO J. 44, M441–M444. [DOI] [PubMed] [Google Scholar]
- 31.Mufti NA, Bleckwenn NA, Babish JG, and Shuler ML (1995) Possible involvement of the Ah receptor in the induction of cytochrome P-450IA1 under conditions of hydrodynamic shear in microcarrier-attached hepatoma cell lines. Biochem. Biophys. Res. Commun 208, 144–152. [DOI] [PubMed] [Google Scholar]
- 32.Miyazawa M, Torii T, Toshimitsu Y, Okada K, and Koyama I (2007) Hepatocyte dynamics in a three-dimensional rotating bioreactor. J. Gastroenterol. Hepatol 22, 1959–1964. [DOI] [PubMed] [Google Scholar]
- 33.Kono Y, Yang S, and Roberts EA (1997) Extended primary culture of human hepatocytes in a collagen gel sandwich system. In Vitro Cell Dev. Biol. Anim 33, 467–472. [DOI] [PubMed] [Google Scholar]
- 34.Nussler AK, Wang A, Neuhaus P, Fischer J, Yuan J, Liu L, Zeilinger K, Gerlach J, Arnold PJ, and Albrecht W (2001) The suitability of hepatocyte culture models to study various aspects of drug metabolism. ALTEX 18, 91–101. [PubMed] [Google Scholar]
- 35.Ezzell RM, Toner M, Hendricks K, Dunn JC, Tompkins RG, and Yarmush ML (1993) Effect of collagen gel configuration on the cytoskeleton in cultured rat hepatocytes. Exp. Cell Res 208, 442–452. [DOI] [PubMed] [Google Scholar]
- 36.Sidhu JS, Parin FM, and Omiecinski CJ (1993) Influence of extracellular matrix overlay on phenobarbital-mediated induction of CYP2B1, 2B2, and 3A1 genes in primary adult rat hepatocyte culture. Arch. Biochem. Biophys 301, 103–113. [DOI] [PubMed] [Google Scholar]
- 37.Tuschl G and Mueller SO (2006) Effects of cell culture conditions on primary rat hepatocytes-cell morphology and differential gene expression. Toxicology 218, 205–215. [DOI] [PubMed] [Google Scholar]
- 38.Annaert PP, Brouwer KL (2005) Assessment of drug interactions in hepatobiliary transport using rhodamine 123 in sandwich-cultured rat hepatocytes. Drug Metab. Dispos 33, 388–394. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, and Brouwer KL (1999) Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am. J. Physiol 277, G12–G21. [DOI] [PubMed] [Google Scholar]
- 40.Moghe PV, Berthiaume F, Ezzell RM, Toner M, Tompkins RG, and Yarmush ML (1996) Culture matrix configuration and composition in the maintenance of hepatocyte polarity and function, Biomaterials 17, 373–385. [DOI] [PubMed] [Google Scholar]
- 41.Kern A, Bader A, Pichlmayr R, and Sewing KF (1997) Drug metabolism in hepatocyte sandwich cultures of rats and humans. Biochem. Pharmacol 54, 761–772. [DOI] [PubMed] [Google Scholar]
- 42.Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, Downey AD, Czerwinski M, Forster J, Ribadeneira MD, Gan LS, LeCluyse EL, Zech K, Robertson P Jr., Koch P, Antonian L, Wagner G, Yu L, and Parkinson A (2003) Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab. Dispos 31, 421–431. [DOI] [PubMed] [Google Scholar]
- 43.Mingoia RT, Nabb DL, Yang CH, and Han X (2007) Primary culture of rat hepatocytes in 96-well plates: effects of extracellular matrix configuration on cytochrome P450 enzyme activity and inducibility, and its application in in vitro cytotoxicity screening. Toxicol. In Vitro 21,165–173. [DOI] [PubMed] [Google Scholar]
- 44.Ben Ze’ev A, Robinson GS, Bucher NL, and Farmer SR (1988) Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeietal genes in primary cultures of rat hepatocytes. Proc. Natl. Acad. Sci. USA 85, 2161–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagaki M, Shidoji Y, Yamada Y, Sugiyama A, Tanaka M, Akaike T, Ohnishi H, Moriwaki H, and Muto Y (1995) Regulation of hepatic genes and liver transcription factors in rat hepatocytes by extracellular matrix. Biochem. Biophys. Res. Commun 210, 38–43. [DOI] [PubMed] [Google Scholar]
- 46.Kimata T, Nagaki M, Tsukada Y, Ogiso T, and Moriwaki H (2006) Hepatocyte nuclear factor4alpha and −1 small interfering RNA inhibits hepatocyte differentiation induced by extracellular matrix. Hepatol. Res 35, 3–9. [DOI] [PubMed] [Google Scholar]
- 47.DiPersio CM, Jackson DA, and Zaret KS (1991) The extracellular matrix coordinately modulates liver transcription factors and hepatocyte morphology. Mol. Cell Biol 11, 4405–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caron JM (1990) Induction of albumin gene transcription in hepatocytes by extracellular matrix proteins. Mol, Cell Biol 10, 1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiPersio CM, Jackson DA, and Zaret KS (1991) The extracellular matrix coordinately modulates liver transcription factors and hepatocyte morphology. Mol. Cell Biol 11, 4405–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juliano RX. and Haskitl S (1993) Signal transduction from the extracellular matrix. J. Cell Biol 120, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gullberg D, Turner DC, Borg TK, Terracio L, and Rubin K (1990) Different beta 1-integrin collagen receptors on rat hepatocytes and cardiac fibroblasts. Exp. Cell Res 190, 254–264. [DOI] [PubMed] [Google Scholar]
- 52.Gullberg D, Gehlsen KR, Turner DC, Ahlen K, Zijenah LS, Barnes MJ, and Rubin K (1992) Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell – collagen interactions: identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. EMBO J. 11, 3865–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson S, Forsberg E, and Lundgren B (1987) Comparison of fibronectin receptors from rat hepatocytes and fibroblasts. J. Biol. Chem 262, 7819–7824. [PubMed] [Google Scholar]
- 54.Lora JM, Rowader KE, Soares L, Giancotti F, and Zaret KS (1998) AJpha3betal-integrin as a critical mediator of the hepatic differentiation response to the extracellular matrix. Hepatology 28, 1095–1104. [DOI] [PubMed] [Google Scholar]
- 55.Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, Orr A, St Arnaud R, Dedhar S, Kaestner KH, Wu C, and Michalopoulos GK (2008) Liver-specific ablation of integrin- linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 48,1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bresnick EH, Dalman FC, Sanchez ER, and Pratt WB (1989) Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J. Biol. Chem 264, 4992–4997. [PubMed] [Google Scholar]
- 57.Johnson JX. and Toft DO (1995) Binding of p23 and hsp90 during assembly with die progesterone receptor. Mol. Endocrinol 9,670–678. [DOI] [PubMed] [Google Scholar]
- 58.Davies TH, Ning YM, and Sanchez ER (2002) A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol. Chem 277, 4597–4600. [DOI] [PubMed] [Google Scholar]
- 59.Smith DF (2004) Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones 9, 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glass CK and Rosenfeld MG (2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14, 121–141. [PubMed] [Google Scholar]
- 61.Freedman ND and Yamamoto KR (2004) Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor. Mol. Biol. Cell 15, 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garside H, Stevens A, Farrow S, Normand C, Houle B, Berry A, Maschera B, and Ray D (2004) Glucocorticoid ligands specify different interactions with NF-kappaB by allosteric effects on the glucocorticoid receptor DNA binding domain. J. Biol. Cbem 279, 50050–50059. [DOI] [PubMed] [Google Scholar]
- 63.Widen C, Gustafsson JA, and Wikstrom AC (2003) Cytosolic glucocorticoid receptor interaction with nuclear factor-kappa B proteins in rat liver cells. Biochem. J 373, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson G, Wilde GJ, Spiller DG, Kennedy SM, Ray DW, Sullivan E, Unitt JF, and White MR (2003) NF-kappaB signalling is inhibited by glucocorticoid receptor and STAT6 via disdnct mechanisms. J. Cell Sci 116, 2495–2503. [DOI] [PubMed] [Google Scholar]
- 65.Li WC, Ralphs KL, Slack JM, and Tosh D (2007) Keratinocyte serum-free medium maintains long-term liver gene expression and function in cultured rat hepatocytes by preventing the loss of liver-enriched transcription factors. Int. J. Biocbem. Cell Biol 39,541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dasgupta A, Hughey R, Lancin P, Larue L, and Moglie PV, (2005) E-cadherm synergistically induces hepatospecific phenotype and maturation of embryonic stem cells in conjunction with hepatotrophic factors. Biotechnol Bioeng. 92, 257–266. [DOI] [PubMed] [Google Scholar]
- 67.Scheving LA, Stevenson MC, Zhang X, and Russell WE (2008) Cultured rat hepatocytes upregulate Akt and ERK in an ErbB-2-dependent manner. Am. J. Physiol Gastrointest. Liver Physiol 295, G322–G331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheving LA, Buchanan R, Krause MA, Zhang X, Stevenson MC, and Russell WE (2007) Dexamediasone modulates ErbB tyrosine kinase expression and signaling through multiple and redundant mechanisms in cultured rat hepatocytes. Am. J Physiol Gastrointest. Liver Physiol 293, G55 2–G559. [DOI] [PubMed] [Google Scholar]
- 69.Baharvand H, Hashemi SM, and Shahsavani M (2008) Differentiation of human embryonic stem cells into functional hepatocyte-like cells in a serum-free adherent culture condition. Differentiation 76, 465–477. [DOI] [PubMed] [Google Scholar]
- 70.Miki R, Tatsumi N, Matsumoto K, and Yokouchi Y (2008) New primary culture systems to study the differentiation and proliferation of mouse fetal hepatoblasts. Am. J. Physiol Gastrointest. Liver Physiol 294, G529–G539. [DOI] [PubMed] [Google Scholar]
- 71.Saito K, Yoshikawa M, Ouji Y, Moriya K, Nishiofoku M, Ueda S, Hayashi N, Ishizaka S, and Fukui H (2006) Promoted differentiation of cynomolgus monkey ES cells into hepatocyte-like cells by co-culture with mouse fetal liver-derived cells. World J Gastroenterol. 12, 6818–6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirahashi H, Wu J, Yamamoto N, Catana A, Wege H, Wager B, Okita K, and Zern MA (2004) Differentiation of human and mouse embryonic stem cells along a hepatocyte lineage. Cell Transplant. 13,197–211. [DOI] [PubMed] [Google Scholar]
- 73.Page JL, Johnson MC, Olsavsky KM, Strom SC, Zarbi H, and Omiecinski CJ (2007) Gene expression profiling of extracellular matrix as an effector of human hepatocyte phenotype in primary cell culture. Toxicol Sci. 97, 384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strom SC, Pisarov LA, Dorko K, Thompson MT, Schuetz JD, and Schuetz EG (1996) Use of human hepatocytes to study P450 gene induction. Methods Enzymol. 272, 388–401. [DOI] [PubMed] [Google Scholar]
- 75.Zegers MM and Hoekstra D (1998) Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells. Biochem. J 336 (Pt 2), 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoffmaster KA, Turncliff RZ, LeCluyse EL, Kim RB, Meier PJ, and Brouwer KX (2004) P-glycoprotein expression, localization, and function in sandwich-cultured primary rat and human hepatocytes: relevance to the hepatobiliary disposition of a model opioid peptide. Pharm. Res, 21, 1294–1302. [DOI] [PubMed] [Google Scholar]
- 77.Fougere-Deschatrette C, Imaizumi-Scherrer T, Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, Faust DM, and Weiss MC (2006) Plasticity of hepatic cell differentiation: bipotential adult mouse liver clonal cell lines competent to differentiate in vitro and in vivo. Stem Cells 24, 2098–2109. [DOI] [PubMed] [Google Scholar]
- 78.Kojima T, Mitaka T, Paul DL, Mori M, and Mochizuki Y (1995) Reappearance and long-term maintenance of connexin32 in proliferated adult rat hepatocytes: use of serum-free L-15 medium supplemented with EGF and DMSO. J. Cell Sci 108 (Pt 4), 1347–1357. [DOI] [PubMed] [Google Scholar]
- 79.Stoehr SA and Isom HC (2003) Gap junction-mediated intercellular communication in a long-term primary mouse hepatocyte culture system. Hepatology 38, 1125–1135. [DOI] [PubMed] [Google Scholar]
- 80.Agarwal S, Holton KL, and Lanza R (2008) Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells 26, 1117–1127. [DOI] [PubMed] [Google Scholar]
- 81.Lilt X, Brouwer KL, Gan LS, Brouwer KR, Stieger B, Meier PJ, Audits KL, and LeCluyse EL (1998) Partial maintenance of taurocholate uptake by adult rat hepatocytes cultured in a collagen sandwich configuration. Pharm. Res 15, 1533–1539. [DOI] [PubMed] [Google Scholar]
- 82.Chandra P, LeCluyse EL, and Brouwer KL (2001) Optimization of culture conditions for determining hepatobiliary disposition of taurocholate in sandwich-cultured rat hepatocytes. In Vitro Cell Dev. Biol. Anim, 37, 380–385. [DOI] [PubMed] [Google Scholar]
- 83.LeCluyse E, Bullock P, Madan A, Carroll K, and Parkinson A (1999) Influence of extracellular matrix overlay and medium formulation on the induction of cytochrome P-450 2B enzymes in primary cultures of rat hepatocytes. Drug Metab. Dispos 27, 909–915. [PubMed] [Google Scholar]
- 84.Runge D, Runge DM, Jager D, Lubecki KA, Beer SD, Karathanasis S, Kietzmann T, Strom SC, Jungermann K, Fleig WE, and Michalopoulos GK (2000) Serum-free, long-term cultures of human hepatocytes: maintenance of cell morphology, transcription factors, and liver-specific functions. Biochem. Biopbys. Res. Commun 269, 46–53. [DOI] [PubMed] [Google Scholar]
- 85.Rambhada L, Chiu CP, Kundu P, Peng Y, and Carpenter MK (2003) Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 12, 1–11. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto Y, Teratani T, Yamamoto H, Quinn G, Murata S, Ikeda R, Kinoshita K, Matsubara K, Kato T, and Ochiya T (2005) Recapitulation of in vivo gene expression during hepatic differentiation from murine embryonic stem cells. Hepatology 42, 558–567. [DOI] [PubMed] [Google Scholar]
- 87.Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, Wolf R, and Cui W (2008) Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 26, 894–902. [DOI] [PubMed] [Google Scholar]
- 88.Olsavsky KM, Page JL, Johnson MC, Zarbl H, Strom SC, and Omiecinski CJ (2007) Gene expression profiling and differentiation assessment in primary human hepatocyte cultures, established hepatoma cell lines, and human liver tissues. Toxicol. Appl. Pharmacol 222, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farkas D and Tannenbaum SR (2005) Characterization of chemically induced hcpatotoxicity in collagen sandwiches of rat hepatocytes. Toxicol. Sci 85, 927–934. [DOI] [PubMed] [Google Scholar]
- 90.Hino H, Tateno C, Sato H, Yamasaki C, Katayama S, Kohashi T, Aratani A, Asahara T, Dohi K, and Yoshizato K (1999) A long-term culture of human hepatocytes which show a high growth potential and express their differentiated phenotypes. Biochem. Biopbys. Res. Com mu n 256, 184–191. [DOI] [PubMed] [Google Scholar]
- 91.Fcrrini JB, Pichard L, Domergue J, and Maurel P (1997) Long-term primary cultures of adult human hepatocytes. Chem. Biol. Interact 107, 31–45. [DOI] [PubMed] [Google Scholar]
- 92.Miller LL, Bly CG, Watson ML, and Bale YV.F. (1951) The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J. Exp. Med 94, 431–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feldmann G, Scoazec JY, Racine L, and Bernuau D (1992) Functional hepatocellular heterogeneity for the production of plasma proteins. Enzyme 46,139–154. [DOI] [PubMed] [Google Scholar]
- 94.Ali-Osman F, Brunner JM, Kutluk TM, and Hess K (1997) Prognostic significance of glutathione S-transferase pi expression and subcellular localization in human gliomas. Clin. Cancer Res 3, 2253–2261. [PubMed] [Google Scholar]
- 95.Lavon N and Benvenisty N (2005) Study of hepatocyte differentiation using embryonic stem cells. J. Cell Biochem 96, 1193–1202. [DOI] [PubMed] [Google Scholar]
- 96.Kim HM, Han SB, Hyun BH, Ahn CJ, Cha YN, Jeong KS, and Oh GT (1995) Functional human hepatocytes: isolation from small liver biopsy samples and primary cultivation with liver-specific functions. J. Toxicol. Sci 20, 565–578. [DOI] [PubMed] [Google Scholar]
- 97.Takagi M, Kojima N, and Yoshida T (2000) Analysis of the ammonia metabolism of rat primary hepatocytes and a human hepatocyte cell line Huh 7. Cytotechnology 32, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haussinger D and Schliess F (2007) Glutamine metabolism and signaling in the liver. Front. Biosci 12, 371–391. [DOI] [PubMed] [Google Scholar]
- 99.Handsehin C and Meyer UA (2003) Induction of drug metabolism: the role of nuclear receptors. Pharmacol. Rev 55, 649–673. [DOI] [PubMed] [Google Scholar]
- 100.LeCluyse EL (2001) Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur. J. Pharm. Sci 13, 343–368. [DOI] [PubMed] [Google Scholar]
- 101.Gerbal-Chaloin S, Daujat M, Pascttssi JM, Pichard-Garcia L, Vilarem MJ, and Maurel P (2002) Transcriptional regulation of CYP2C9 gene. Role of glucocorticoid receptor and constitutive androstane receptor. J. Biol. Chem 277, 209–217. [DOI] [PubMed] [Google Scholar]
- 102.Pascttssi JM, Gerbal-Chaloin S, Fabre JM, Maurel P, and Vila rem MJ (2000) Dexamethasone enhances constitutive androstane receptor expression in human hepatocytes: consequences on cytochrome P450 gene regulation. Mol. Pharmacol 58, 1441–1450. [DOI] [PubMed] [Google Scholar]
- 103.Whysner J, Ross PM, and Williams GM (1996) Phenobarbital mechanistic data and risk assessment: enzyme induction, enhanced cell proliferation, and tumor promotion. Pharmacol. Ther 71, 153–191. [DOI] [PubMed] [Google Scholar]
- 104.Sanders S and Thorgeirsson SS (1999) Phenobarbital promotes liver growth in c-myc/TGF-alpha transgenic mice by inducing hypertrophy and inhibiting apoptosis. Carcinogenesis 20 ^ 41–49. [DOI] [PubMed] [Google Scholar]
- 105.Calvisi DF, Ladu S, Factor VM, and Thorgeirsson SS (2004) Activation of beta-catcnin provides proliferative and invasive advantages in c-myc/TGF-alpha hepatocarcinogenesis promoted by phenobarbital, Carcinogcncsis 25, 901–908. [DOI] [PubMed] [Google Scholar]
- 106.Mansbach JM, Mills JJ, Boyer IJ, De Souza AT, Hankins GR, and Jirtle RL (1996) Phenobarbital selectively promotes initiated cells with reduced TGF beta receptor levels. Carcinogencsis 17, 171–174. [DOI] [PubMed] [Google Scholar]
- 107.Gonzales A, Christensen JG, Preston RJ, Goldsworthy TL, Tlsty TD, and Fox TR (1998) Attenuation of G1 checkpoint function by the non-genotoxic carcinogen phenobarbital. Canin agenesis 19, 1173–1183. [DOI] [PubMed] [Google Scholar]
- 108.Phillips JM, Yamamoto Y, Negishi M, Maronpot RR, and Goodman JI (2007) Orphan nuclear receptor constitutive active/androstane receptor-mediated alterations in DNA methylation during phenobarbital promotion of liver tumorigencsis. Toxicol. Sci 96, 72–S2. [DOI] [PubMed] [Google Scholar]
- 109.Kakizaki S, Yamamoto Y, Ueda A, Moore R, Sueyoshi T, and Negishi M (2003) Phenobarbital induction of drng/steroid-metabolizing enzymes and nuclear receptor CAR. Biochim. Biophys. Acta 1619, 239–242. [DOI] [PubMed] [Google Scholar]
- 110.Kodama S and Negishi M (2006) Phenobarbital confers its diverse effects by activating the orphan nuclear receptor car. Drug Metab. Rev 38, 75–87. [DOI] [PubMed] [Google Scholar]
- 111.Qatanani M and Moore DD (2005) CAR, the continuously advancing receptor, in drug metabolism and disease. Cnrv. Drug Metab 6, 329–339. [DOI] [PubMed] [Google Scholar]
- 112.Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, and Maronpot RR (2004) The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 64, 7197–7200. [DOI] [PubMed] [Google Scholar]
- 113.Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, and Negishi M (1999) Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 19, 6318–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kobayashi K, Sueyoshi T, Inoue K, Moore R, and Negishi M (2003) Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol. Pharmacol. 64, 1069–1075. [DOI] [PubMed] [Google Scholar]
- 115.Yoshinari K, Kobayashi K, Moore R, Kawamoto T, and Negishi M (2003) Identification of the nuclear receptor CAR: HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEES Lett. 548, 17–20. [DOI] [PubMed] [Google Scholar]
- 116.Zelko I, Sueyoshi T, Kawamoto T, Moore R, and Negishi M, (2001) The peptide near the C terminus regulates receptor CAR nuclear translocation induced by xenochemicals in mouse liver. Mol. Cell Biol 21, 2838–2846, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frank C, Gonzalez MM, Oinoncn C, Dunlop TW, and Carlberg C (2003) Characterization of DNA complexes formed by the nuclear receptor constitutive androstane receptor. J. Biol. Chem 278, 43299–43310. [DOI] [PubMed] [Google Scholar]
- 118.Muangmoonchai R, Smirlis D, Wong SC, Edwards M, Phillips IR, and Shephard EA (2001) Xenobiotic induction of cytochrome P450 2B1 (CYP2B1) is mediated by the orphan nuclear receptor constitutive androstane receptor (CAR) and requires steroid co-activator 1 (SRC-1) and the transcripdon lac tor Spl, Biochem. J 355, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Min G, Kemper JK, and Kemper B (2002) Glucocorticoid receptor-interacting protein 1 mediates ligand-independent nuclear translocation and activation of constitutive androstane receptor in vivo. J. Biol. Chem 277, 26356–26363. [DOI] [PubMed] [Google Scholar]
- 120.Shiraki T, Sakai N, Kanaya E, and Jingami H (2003) Activation of orphan nuclear constitutive androstane receptor requires subnuclear targeting by peroxisome proliferator-activated receptor gamma coactivator-1 alpha. A possible fink between xenobiotic response and nutritional state. J. Biol. Chem 278, 11344–11350. [DOI] [PubMed] [Google Scholar]
- 121.Honkakoski P and Negishi M (1997) Characterization of a phenobarbital- responsive enhancer module in mouse P450 Cyp2bl0 gene. J. Biol. Chem 272, 14943–14949. [DOI] [PubMed] [Google Scholar]
- 122.Ramsden R, Beck NB, Sommer KM, and Omiecinski CJ (1999) Phenobarbital responsiveness conferred by the 5’-flanking region of the rat CYP2B2 gene in transgenic mice. Gene 228,169–179. [DOI] [PubMed] [Google Scholar]
- 123.LeCluyse E, Madan A, Hamilton G, Carroll K, DeHaan R, and Parkinson A (2000) Expression and regulation of cytochrome P450 enzymes in primary cultures of human hepatocytes. J. Biochem. Mol. Toxicol 14,177–188. [DOI] [PubMed] [Google Scholar]
- 124.Livak KJ and Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and die 2(-Delta Delta C(T)) Method. Methods 25, 402–08. [DOI] [PubMed] [Google Scholar]