Abstract
The recent progress in platinum(II) coordination driven supramolecular polymers has had a substantial effect on the design of functional soft materials. However, the prospect of realizing platinum(II) metallacycle-based host-guest interactions induced polymerization has received little attention until recently. Here we report the realization of supramolecular polymerization driven by platinum(II) metallacycle-based host-guest interactions both in the solid state and in solution. On the basis of the disclosed polymerization mechanism, we present a new strategy for the preparation of platinum(II) metallacycle-based supramolecular polymers.
Graphical Abstract
Natural systems have long inspired chemists in their efforts to produce sophisticated self-assembled supramolecular materials with dynamic and tunable properties.1 One class of these materials is supramolecular polymers assembled from low-molecular-weight monomeric units through non-covalent interactions.2 Supramolecular polymers have demonstrated traditional polymeric properties and become important stimuli-responsive dynamic materials that are attractive for use in drug delivery, tissue engineering, diagnostics, biosensors, and nanotechnology.3 Many small molecules have been used as monomers to prepare supramolecular polymers, but the preparation of monomers usually requires time-consuming multistep synthesis. Therefore, developing efficient and easy synthetic routes to make monomers for the preparation of supramolecular polymers is very important for future applications of supramolecular polymers.
Coordination-driven self-assembly is a well-established method to construct metallacycles and metallacages through the spontaneous formation of metal–ligand bonds drawing inspiration from the design principles of natural systems.4 With a view to functionalize the exterior vertices of metallacycles, Stang and coworkers reported a strategy of using 2-ureido-4-pyrimidinone based externally functionalized platinum(II) metallacycle as monomers that upon self-assembly forms well-defined functional supramolecular polymers in solution.5 This achievement, together with related work reported later,6 represented a new strategy for supramolecular polymerization in the context of precise macromolecular engineering.
Although the functionalization of platinum(II) metallacycles and metallacages to construct supramolecular polymers has advanced, the realization of platinum(II) metallacycle-based host–guest interactions induced polymerization has only recently received attention. The primary challenge facing this strategy is maintaining the dynamic metallacycle structures during host–guest interactions between the platinum(II) metallacycles and guest molecules. To construct supramolecular polymers via platinum(II) metallacycle-based host–guest interactions, three requirements need to be met: (1) Metallacycles should be both simple and stable to reduce the possibility of disassembly; (2) The incorporated functional guest moieties should not interfere with the metal−ligand bonds; (3) Guest molecules should suppress the dynamics of metallacycles and stabilize them through the host–guest interactions. In contrast to previously reported platinum(II) metallacycle-based polymerization strategies,5,6 the approach reported here uses monomers that polymerize spontaneously driven by the host–guest interactions between the metallacycles and guest molecules, allowing efficient preparation of metallacycle-based supramolecular polymers.
Because of the dynamic nature of supramolecular polymers and platinum(II) metallacycles, previously reported platinum(II) metallacycle-based supramolecular polymers were all obtained in solution, no solid-state platinum(II) metallacycle-based supramolecular polymers have been reported.5,6 Suppressing the dynamics of metallacycles and stabilizing platinum(II) metallacycles through host–guest interactions between metallacycles and guest molecules may provide platinum(II) metallacycle-based supramolecular polymers in the solid state. Here, we report a cross-linked supramolecular polymer constructed by platinum(II) metallacycle-based host–guest interactions in the solid state and in solution. Thus, on the basis of the disclosed mechanism, we have demonstrated a new strategy for the platinum(II) metallacycle-based supramolecular polymerization.
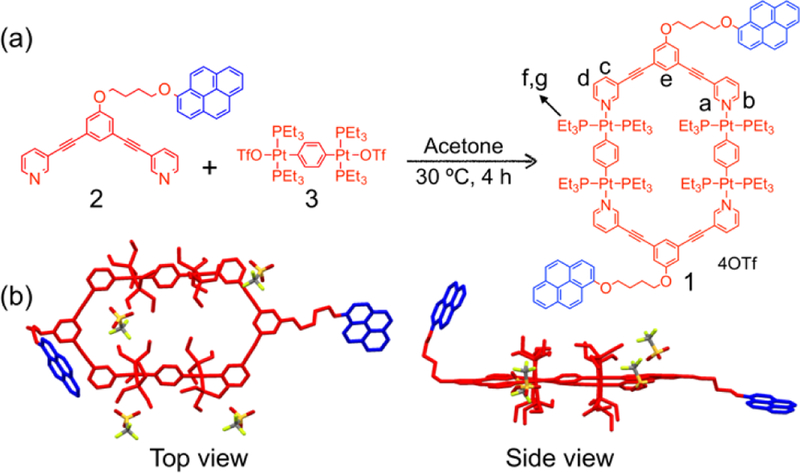
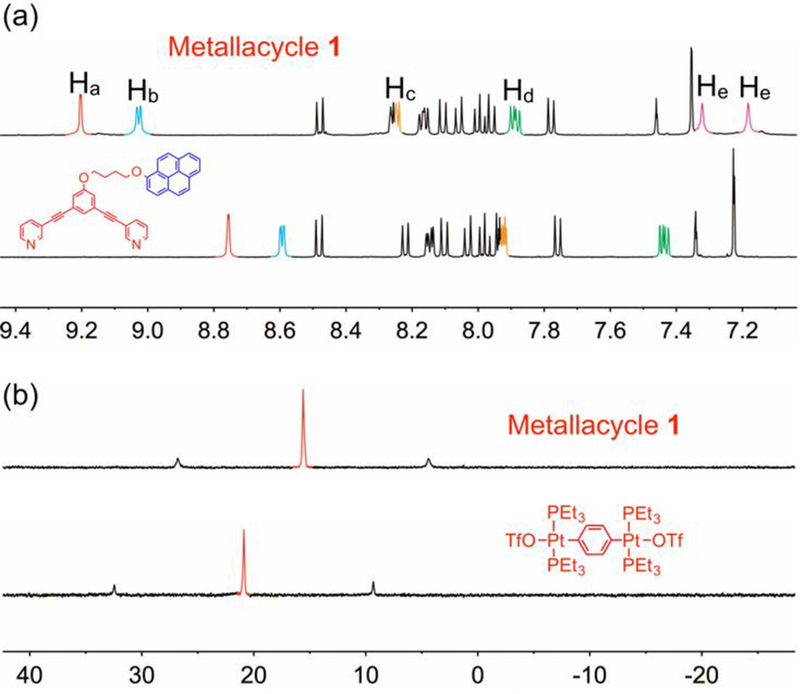
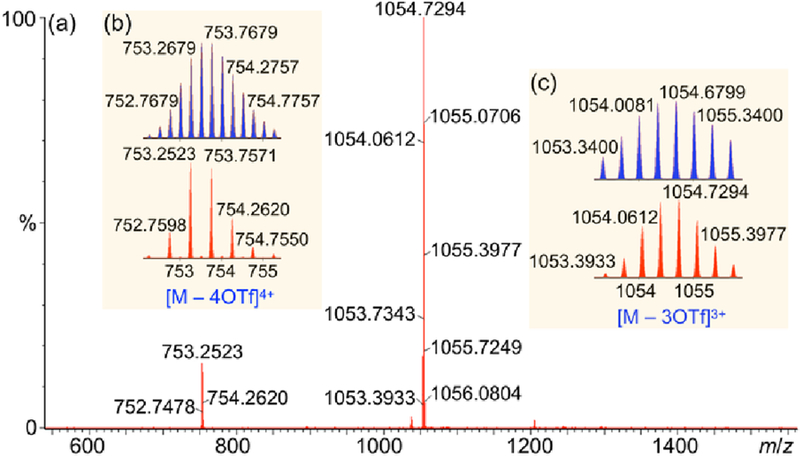
The monomer used here was based on a platinum(II) metallacycle bearing two pyrene moieties, as shown in Scheme 1. Monomer 1 was expected to self-assemble through host–guest interactions between the pyrene groups and metallacycles. Pyrene-functionalized ligand 2 was synthesized by a Pd-catalyzed Sonogashira coupling reaction and subsequent etherification (Scheme S1). Stirring a mixture of ligand 2 and 180° di-Pt(II) acceptor 3 in a 1:1 molar ratio in acetone at room temperature for 4 h resulted in the formation of the self-assembled metallacycle 1 with pendant pyrene moieties at the vertices (Scheme 1). The formation of metallacycle 1 was confirmed by multinuclear (1H and 31P) NMR spectroscopy and electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS). Figure 1 shows that, in the 1H NMR spectrum of 1, the peaks related to protons Ha–Hd on the pyridyl groups showed downfield shifts compared with those of ligand 2, which is consistent with coordination of the N-atoms to the platinum centers resulting in decrease of electron density on the pyridyl groups. The 31P{1H} NMR signal of 1 shifted upfield relative to that of 2, displaying a lone sharp singlet at 15.60 ppm with concomitant 195Pt satellites (JPt−P = 2705.56 Hz), consistent with a single phosphorus environment. ESI-TOF-MS analysis of 1 revealed two main peaks, supporting the formation of a [2 + 2] assembly (Figure 2). The peaks at m/z = 753.2523 and 1054.7294 corresponded to [1 – 4OTf]4+ and [1 – 3OTf]3+, respectively. The two peaks were isotopically resolved and in agreement with their calculated theoretical distributions, indicating the formation of metallacycles with the desired stochiometry.
Scheme 1.
(a) Synthesis and chemical structure of metallacycle 1; (b) Single-crystal structure of metallacycle 1. Hydrogen atoms are omitted for clarity.
Figure 1.
(a) Partial 1H NMR spectra (400 MHz, acetone-d6, 298 K) of ligand 2 and metallacycle 1; (b) 31P{1H} NMR spectra (acetone-d6, 298K, 121.4 MHz) of 180° acceptor 3 and metallacycle 1.
Figure 2.
(a) Full ESI-TOF-MS spectrum of 1; Experimental (red) and calculated (blue) electrospray ionization peaks of the [M – 4OTf]4+ (b) and [M – 3OTf]3+ (c) charge states of 1.
Light yellow single-crystals of the metallacycle were obtained by slow diffusion of ether into an acetone solution of 1 which allowed the structure of monomer 1 to be determined unambiguously by single-crystal X-ray diffraction. X-ray crystallographic analysis revealed the formation of an unusual AA–BB type monomer7 composed of two molecules of monomer 1 connected by C‒H⋯π interactions with lengths of 2.776–2.840 Å (Figure 3a and 3b).8 Moreover, π–π stacking interactions between the benzene rings on 1 with a distance of 3.375 Å were also observed in the crystal structure.8 Each AA–BB type monomer was also stabilized by adjacent trifluoromethanesulfonate anions (−OTf) via C‒H⋯O interactions with H⋯O distances of 2.379–2.535 Å and C‒F⋯H interactions with an F⋯H distance of 2.444 Å, which further stabilizes the assembly. The formation of AA–BB type monomer suppressed the dynamic property of the metallacycle and stabilized the platinum(II) metallacycle.
Figure 3.
(a) Single-crystal structure of the AA–BB monomer of 1; (b) Packing structure of the AA–BB monomer; (c) Assembled single-crystal produced by the host–guest complexation of 1. Hydrogen atoms are omitted for clarity in Figures 3 and 4.
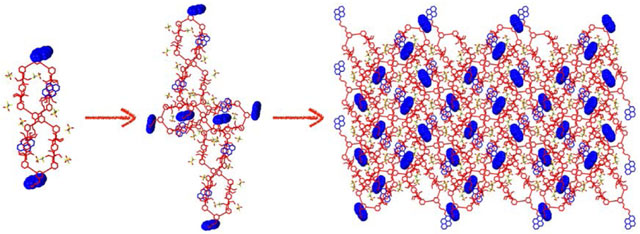
One of the pyrene groups of monomer 1 was encapsulated into the cavity of another molecule of the metallacycle 1 to form a host–guest complex. The crystal structure revealed two hydrogen atoms on the host molecule 1 with C‒H⋯π distances of 2.713–2.864 Å, indicating the existence of C‒H⋯π interactions between the pyrene guest and platinum(II) metallacycle host cavity. The pyrene group in this host–guest system was also stabilized by −OTf via C‒H⋯O interactions with an H⋯O distance of 2.622 Å, which further helped the formation of the assembled structure (Figure 3c and S9). Hence, a cross-linked supramolecular polymer was successfully formed by monomer 1 through host–guest interactions in the solid state (Figure 4). The formation of AA–BB type monomer and host–guest interactions in this system suppressed the dynamic property of the metallacycle and stabilized the platinum(II) metallacycle, thereby leading to the formation of the cross-linked supramolecular polymer in the solid state.
Figure 4.
Two views of the single-crystal structure of the cross-linked supramolecular polymer of monomer 1.
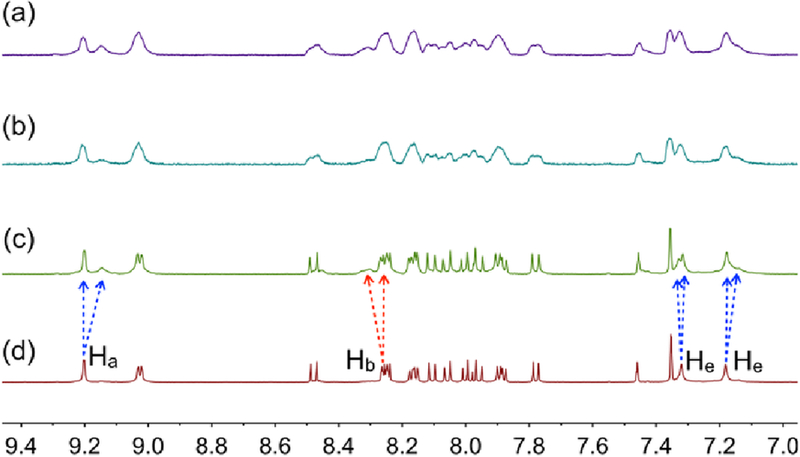
Having established the host–guest interactions between pyrene and metallacycle 1 and obtained the cross-linked supramolecular polymer in the solid state, the polymerization of monomer 1 in solution was also examined. Comparison of the 1H NMR spectra of monomer 1 and organic ligand 2 at a concentration of 0.500 mM in Figure 1 shows that the peak related to proton He of monomer 1 split into two peaks, suggesting host–guest complexation between the pyrene group and metallacycle 1. The peaks related to the protons on the pyrene groups of monomer 1 showed downfield shifts, indicating that C–H⋯π interactions occurred between the pyrene groups and ethyl groups on metallacycle 1. A 2D NOESY NMR study of 1 in acetone was performed. Correlation signals were observed between the protons of the pyrene group of 2 and the protons of the ethyl groups of 1 (Figure S10 and S11). These results indicate that an AA–BB type monomer was formed by 1 driven by multiple non-covalent interactions and the electron rich pyrene group was encapsulated into the cavity of an adjacent cyclic 1 to form a host–guest complex in acetone.
The formation of the cross-linked supramolecular polymer by monomer 1 was investigated by 1H NMR, 2D DOSY NMR, viscosity measurements, dynamic light scattering (DLS), and scanning electron microscopy (SEM). 1H NMR spectra of 1 at concentrations of 1.00–60.0 mM were recorded, and as expected, the proton NMR spectra of 1 was concentration-dependent. Figure 5, S11, and S12 show that the peaks related to protons Ha and Hb shifted downfield, whereas those related to protons He–Hg shifted upfield. All the signals became broad at high concentrations of monomer 1. These results are consistent with the crystal structure of the supramolecular polymer, showing that the electron-rich pyrene group was located in the electron-poor cavity of 1 to form a host–guest complex.
Figure 5.
Partial 1H NMR spectra (400 MHz, 298 K) of 1 in acetone-d6 at various concentrations: (a) 60.0 mM; (b) 50.0 mM; (c) 25.0 mM; (d) 1.00 mM.
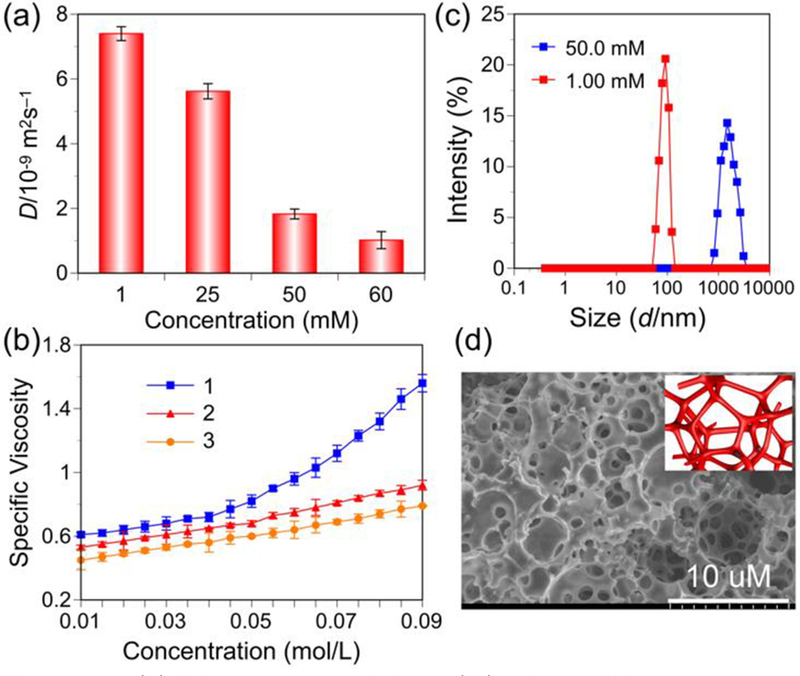
Different aggregates exchange was observed on the DOSY timescale. As the concentration of 1 was increased from 1.00 to 60.0 mM, the measured weight-average diffusion coefficient (D) of 1 in acetone solution decreased from 7.4 × 10−9 m2s−1 to 1.02 × 10−9 m2s−1, suggesting the concentration dependence of the supramolecular polymerization of the monomers (Figure 6a). It is well konwn that a high degree of polymerization for the repeating unit is necessary to observe a sharp decrease in D value.9
Figure 6.
(a) Diffusion coefficient (D) of 1 at different concentrations; (b) Specific viscosity of monomer 1 or organic ligand 2 or Pt(II) acceptor 3 versus its concentration in acetone; (c) Size distribution of assemblies of 1 formed at different concentrations; (d) SEM image of cross-linked supramolecular polymer network drawn from a highly concentrated solution of monomer 1 in acetone. Insert is a cartoon of the cross-linked supramolecular polymer.
As a function of polymerization for monomer 1, the variation of specific viscosity was performed in acetone at 30 °C (Figure 6b).10 The specific viscosity of the individual organic ligand 2 or Pt(II) acceptor 3 was also plotted as a comparison. For the individual compound organic ligand 2 or Pt(II) acceptor 3, specific viscosity changed almost linearly with the concentration, which indicated that no significant entanglements occurred. By contrast, the specific viscosity of monomer 1 changed significantly with the monomer concentration. This observation supports the formation of the cross-linked supramolecular polymer of monomer 1 through host–guest interactions.10b
The average hydrodynamic diameter (d) of 1 at a concentration of 1.00 mM was determined to be 20.6 nm by DLS experiment. As the concentration of 1 increased from 1.00 to 50.0 mM, d increased substantially to 1480 nm, revealing the presence of supramolecular polymers (Figure 6c). These results surpport the formation of an extended, high-molecular-weight polymeric structure through host–guest interactions of monomer 1 in solution.
Figure 6d depicts the morphology and three-dimensional network obtained from a highly concentrated solution of 1 as observed by SEM. Monomer 1 organized into a cross-linked supramolecular polymer in solution. These cross-linked supramolecular polymers entangled together to form a three-dimensional network structure with voids ranging from several hundred nanometers to several micrometers. These results provided direct evidence that monomer 1 self-assembled into a cross-linked supramolecular polymer in solution.
Supramolecular polymers that respond to external stimuli are interesting as they are important in a wide range of applications.11 Gel-like viscous liquids were prepared by dissolving monomer 1 in acetone at a concentration of 100 mM. Figure S14 illustrates the reversible thermo-induced phase transitions of the sample. Moreover, the addition and removal of bromide anions (Br−) led to the reversible gel-like supramolecular polymer and sol transition. Addition of tetrabutylammonium bromide (TBABr) to the sample disrupted the coordination between the N-atoms and platinum centers, causing the gel-like supramolecular polymer to dissociate into a turbid solution within a short time. However, after silver trifluoromethanesulfonate (AgOTf) was added to the mixture, this turbid solution was transformed into the gel-like supramolecular polymer again.12
In summary, we show spontaneous supramolecular polymerization driven by platinum(II) metallacycle-based host–guest interactions. A [2 + 2] platinum(II) metallacycle 1 bearing two pyrene moieties was designed and synthesized. Metallacycle 1 self-assembled into a cross-linked supramolecular polymer both in the solid state and in solution. The host–guest interactions and other non-covalent interactions suppressed the dynamics of the metallacycle, which enabled the formation of the cross-linked supramolecular polymer in the solid state. In solution the cross-linked supramolecular polymer showed thermo and ion responsiveness. This approach may be applied to readily available platinum(II) metallacycle monomers to fabricate functional supramolecular polymers.
Supplementary Material
ACKNOWLEDGMENT
P.J.S. thanks the U.S. National Institutes of Health (Grant R01 CA215157) for financial support. F.H thanks the National Natural Science Foundation of China 21620102006 for financial support.
Footnotes
Supporting Information
Experimental details, NMR spectra, crystal data, and other materials. These material are available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Zimmerman SC; Zeng F; Reichert DEC; Kolotuchin SV Self-Assembling Dendrimers. Science 1996, 271, 1095–1098. [DOI] [PubMed] [Google Scholar]; (b) Breslow R Biomimetic Chemistry: Biology as an Inspiration. J. Biol. Chem 2009, 284, 1337–1342. [DOI] [PubMed] [Google Scholar]
- 2.(a) Greef T. F. A. de; Meijer EW Supramolecular Polymers. Nature 2008, 453, 171–173. [DOI] [PubMed] [Google Scholar]; (b) Aida T; Meijer EW; Stupp SI Functional Supramolecular Polymers. Science 2012, 335, 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Price TL Jr.; Gibson HW Supramolecular Pseudorotaxane Polymers from Biscryptands and Bisparaquats. J. Am. Chem. Soc 2018, 140, 4455–4465. [DOI] [PubMed] [Google Scholar]
- 3.(a) Brunsveld L; Folmer BJB; Meijer EW; Sijbesma RP Supramolecular Polymers. Chem. Rev 2001, 101, 4071–4097. [DOI] [PubMed] [Google Scholar]; (b) Cordier P; Tournilhac F; Soulie-Ziakovic C; Leibler L Self-Healing and Thermoreversible Rubber from Supramolecular Assembly. Nature 2008, 51, 977–980. [DOI] [PubMed] [Google Scholar]; (c) Stuart MAC; Huck WTS; Genzer J; Müller M; Ober C; Stamm M; Sukhorukov GB; Szleifer M; Tsukruk VV; Urban M; Winnik F; Zauscher S; Luzinov I; Minko S, Emerging Applications of Stimuli-Responsive Polymer Materials. Nature Mater 2010, 9, 101–113. [DOI] [PubMed] [Google Scholar]; (c) Ogi S; Stepanenko V; Sugiyasu K; Takeuchi M; Würthner F Mechanism of Self-Assembly Process and Seeded Supramolecular Polymerization of Perylene Bisimide Organogelator. J. Am. Chem. Soc 2015, 137, 3300– 3307. [DOI] [PubMed] [Google Scholar]
- 4.(a) Fujita M; Tominaga M; Hori A; Therrien B Coordination Assemblies from a Pd(II)-Cornered Square Complex. Acc. Chem. Res 2005, 38, 369–378. [DOI] [PubMed] [Google Scholar]; (b) Schmitt F; Freudenreich J; Barry NPE; Juillerat-Jeanneret L; Süss-Fink G; Therrien B Organometallic Cages as Vehicles for Intracellular Release of Photosensitizers. J. Am. Chem. Soc 2012, 134, 754–757. [DOI] [PubMed] [Google Scholar]; (c) Cook TR; Zheng Y-R; Stang PJ Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev 2013, 113, 734–777. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Clever GH; Punt P Cation−Anion Arrangement Patterns in Self-Assembled Pd2L4 and Pd4L8 Coordination Cages. Acc. Chem. Res 2017, 50, 2233–2243. [DOI] [PubMed] [Google Scholar]; (e) Sun Y; Li S; Zhou Z; Saha ML; Datta S; Zhang M; Yan X; Tian D; Wang H; Wang L; Li X; Liu M; Li H; Stang PJ Alanine-Based Chiral Metallogels via Supramolecular Coordination Complex Platforms: Metallogelation Induced Chirality Transfer. J. Am. Chem. Soc 2018, 140, 3257–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Roberts DA; Pilgrim BS; Sirvinskaite G; Ronson TK; Nitschke JR Covalent Post-Assembly Modification Triggers Multiple Structural Transformations of a Tetrazine-Edged FeII₄L₆ Tetrahedron. J. Am. Chem. Soc 2018, 140, 9616–9623. [DOI] [PubMed] [Google Scholar]
- 5.Yan X; Li S; Pollock JB; Cook TR; Chen J; Zhang Y; Ji X; Yu Y; Huang F; Stang PJ Supramolecular Polymers with Tunable Topologies via Hierarchical Coordination-Driven Self-Assembly and Hydrogen Bonding Interfaces. Proc. Natl. Acad. Sci. U.S.A 2013, 110, 15585–15590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Zhang M; Li S; Yan X; Zhou Z; Saha ML; Wang Y-C; Stang Peter J. Fluorescent Metallacycle-Cored Polymers via Covalent Linkage and Their Use as Contrast Agents for Cell Imaging. Proc. Natl. Acad. Sci. U.S.A 2016, 113, 11100–11105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zheng W; Chen L-J; Yang G; Sun B; Wang X; Jiang B; Yin G-Q; Zhang L; Li X; Liu M; Chen G; Yang H-B Construction of Smart Supramolecular Polymeric Hydrogels Cross-Linked by Discrete Organoplatinum(II) Metallacycles via Post-Assembly Polymerization. J. Am. Chem. Soc 2016, 138, 4927–4937. [DOI] [PubMed] [Google Scholar]; (c) Zhou Z; Yan X; Cook TR; Saha ML; Stang PJ Engineering Functionalization in a Supramolecular Polymer: Hierarchical Self-Organization of Triply Orthogonal Non-covalent Interactions on a Supramolecular Coordination Complex Platform. J. Am. Chem. Soc 2016, 138, 806–809. [DOI] [PubMed] [Google Scholar]; (d) Chen L-J; Yang H-B Construction of Stimuli-Responsive Functional Materials via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res 2018, 51, 2699–2710. [DOI] [PubMed] [Google Scholar]
- 7.(a) Binder WH; Bernstoff C; Kluger L; Petraru L; Kunz MJ Tunable Materials from Hydrogen-Bonded Pseudo Block Copolymers. Adv. Mater 2005, 17, 2824–2828. [Google Scholar]; (b) Castellano RK; Clark R; Craig SL; Nuckolls C; Rebek J Jr. Emergent Mechanical Properties of Self-Assembled Polymeric Capsules. Proc. Natl. Acad. Sci. U.S.A 2000, 97, 12418–12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bondi A van der Waals Volumes and Radii. J. Phys. Chem 1964, 68, 441–451. [Google Scholar]
- 9.Greef T. F. A. de; Ercolani G; Ligthart GBWL; Meijer EW; Sijbesma RP; Influence of Selectivity on the Supramolecular Polymerization of AB-Type Polymers Capable of Both AA and AB Interactions. J. Am. Chem. Soc 2008, 130, 13755–13764. [DOI] [PubMed] [Google Scholar]
- 10.(a) Gibson HW; Yamaguchi N; Jones JW Supramolecular Pseudorotaxane Polymers from Complementary Pairs of Homoditopic Molecules. J. Am. Chem. Soc 2003, 125, 3522–3533. [DOI] [PubMed] [Google Scholar]; (b) Chen L; Tian Y-K; Ding Y; Tian Y-J; Wang F Multistimuli Responsive Supramolecular Cross-Linked Networks On the Basis of the Benzo-21-Crown-7/Secondary Ammonium Salt Recognition Motif. Macromolecules 2012, 45, 8412–8419. [Google Scholar]
- 11.(a) Liu F; Urban MW Recent Advances and Challenges in Designing Stimuli-Responsive Polymers. Prog Polym Sci 2010, 35, 3–23. [Google Scholar]; (b) Wojtecki RJ; Meador MA; Rowan SJ Using the Dynamic Bond to Access Macroscopically Responsive Structurally Dynamic Polymers. Nature Mater 2011, 10, 14–27. [DOI] [PubMed] [Google Scholar]; (c) Yan X; Wang F; Zheng B; Huang F Stimuli-Responsive Supramolecular Polymeric Materials. Chem. Soc. Rev 2012, 41, 6042–6065. [DOI] [PubMed] [Google Scholar]
- 12.Li Z-Y; Zhang Y; Zhang C-W; Chen L-J; Wang C; Tan H; Yu Y; Li X; Yang H-B Cross-Linked Supramolecular Polymer Gels Constructed from Discrete Multi-pillar[5]arene Metallacycles and Their Multiple Stimuli-Responsive Behavior. J. Am. Chem. Soc 2014, 136, 8577–8589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.