Abstract
Background:
Conventional diffusion-weighted imaging (DWI) with high b-values may improve lesion conspicuity, but with a low signal intensity and thus a low signal-to-noise ratio (SNR). The voxelwise computed DWI (vcDWI) may generate high-quality images with a strong lesion signal and low background.
Purpose:
To evaluate the feasibility and diagnostic performance of vcDWI.
Study Type:
Retrospective.
Population:
In all, 67 patients with 72 lesions, 33 malignant and 39 benign.
Field Strength/Sequence:
3T, including T2/T1, DWI with two b-values, and dynamic contrast-enhanced MRI (DCE-MRI).
Assessment:
Computed DWI (cDWI) with high b-values of 1500, 2000, 2500 s/mm2 (cDWI1500, cDWI2000, cDWI2500) and vcDWI were generated from measured DWI (mDWI). The mDWI, cDWIs and vcDWI were evaluated by three readers independently to determine lesion conspicuity, background signal suppression, overall image quality using 1–5 rating scales, as well as to give BI-RADS scores. The mean apparent diffusion coefficient (ADC) value for each lesion was measured.
Statistical Tests:
Agreement among the three readers was evaluated by the intraclass correlation coefficient. Receiver operating characteristic (ROC) analysis was performed to compare the diagnostic performance based on reading of mDWI, cDWIs, vcDWI, and the measured ADC values.
Results:
vcDWI provided the best lesion conspicuity compared with mDWI and cDWIs (P < 0.005). For overall image quality, vcDWI was significantly better than cDWI (P < 0.005), but not significantly better compared with mDWI for two readers (P = 0.037 and P = 0.013) and significantly worse for the third reader (P < 0.005). Background signal suppression was the best on cDWI2500, and better on vcDWI than on mDWI, cDWI1500, and cDWI2000. The AUC value for differential diagnosis was 0.868 for mDWI, 0.862 for cDWI1500, 0.781 for cDWI2000, 0.704 for cDWI2500, 0.946 for vcDWI, 0.704 for ADC value, and 0.961 for DCE-MRI.
Data Conclusion:
vcDWI was implemented without increasing scanning time, and it provided excellent lesion conspicuity for detection of breast lesions and assisted in differentiating malignant from benign breast lesions.
MULTIPARAMETRIC MAGNETIC RESONANCE IMAGING (mpMRI) provides comprehensive information, which can be used to characterize lesions for detection and diagnosis of breast cancer as well as for evaluation of response to neoadjuvant chemotherapy.1–4 Diffusion-weighted imaging (DWI) shows information on microscopic motion of water molecules in vivo, and thus can be used to assess the cellular structure of tissue.5 Malignant cancer usually manifests hyperintensity signal because of restricted diffusion in a high cellular density microenviroment.6,7 Contrast between cancerous tissue (more diffusion-restricted) and normal tissue (less diffusion-restricted) is related to diffusion-sensitizing gradients described as the b-value.8 Conventional DWI of breast is obtained with b-value of 600–1000 s/mm2, which is not high enough to optimize contrast between breast lesion and background tissue. DWI with a higher b-value is reported to improve tumor conspicuity by suppressing the signal of normal background tissue.6 Although DWI with higher b-value is practically obtainable, it needs larger diffusion-sensitizing gradients and longer echo time, which may result in susceptibility artifact and severe eddy current distortions, as well as low signal-to-noise ratio (SNR).9,10
Computed DWI (cDWI) is a mathematical computation method that generates higher b-value DWIs from images acquired with lower b-values.10 It was reported that cDWI could obtain good image quality and a high contrast-to-noise ratio (CNR) by suppressing background signal and, thus, might increase lesion conspicuity.11–14 In the detection of breast cancer, it has been shown that cDWI with a higher b-value has the potential to improve diagnostic sensitivity.15 However, as b-value increases, the signal intensity decreases in all tissues. More recently, a new method for enhancing contrast between tissues with low and high apparent diffusion coefficients (ADCs), termed voxelwise-computed diffusion imaging (vcDWI), was proposed.16 This method used different b-value signal intensity according to different ADCs of tissues, so that the signal intensity of background tissue can be suppressed while preserving that of the lesion. Gatidis et al16 performed phantom studies to evaluate the method. The results showed that ADC-dependent vcDWI remarkably increased the contrast, CNR, and SNR of images by effectively reducing T2 shine-through effects. It seemed to provide a feasible method for clinical diagnosis, but the patient dataset was quite small in that study.
The purpose of the present study was to assess the clinical application of vcDWI in the diagnosis of breast cancer. We hypothesized that vcDWI could effectively suppress the signal of normal breast fibroglandular tissue and increase lesion conspicuity, and thus may be used to improve the diagnostic performance in the differentiation of benign and malignant lesions compared with conventional DWI and cDWI.
Materials and Methods
Patients
In a retrospective review of patients receiving breast MRI for diagnosis without prior biopsy or any treatment, a total of 87 patients were identified. Twenty patients were excluded due to artifact on DWI images (n = 15), and undetectable lesion on DWI (n = 5, including one ductal cancerous in situ [DCIS], one fat necrosis with foreign body granulomatous inflammation, one adenomyoepithelial adenosis, and two adenosis). The remaining 67 patients formed the study group. A total of 72 lesions (33 malignant and 39 benign) were con-firmed based on pathological results. Three patients had separately confirmed benign and malignant lesions, and two patients had two confirmed benign lesions. Thirty-three malignant lesions were found in 33 patients (age range, 25–70 years; mean age ± SD,48.6 ± 10.5 years) including: eight DCIS, 21 invasive ductal carcinomas (IDC), two invasive lobular carcinomas (ILC), one mucinous carcinomas, and one medullary carcinoma. Thirty-nine benign lesions were found in 37 patients (age rang, 17–76 years; mean age ± SD, 43.0 ± 10.7 years), including seven fibroadenomas, 19 adenosis (four with fibroadenomas and one with papilloma), eight mastitis (three acute infectious mastitis and five granulomatous mastitis), four papillomas, and one hamartoma. The mean malignant lesion size was 22.3 mm (range, 6.1–121.3 mm; median, 17.0 mm); and the mean benign lesion size was 23.9 mm (range, 4.3–124.2 mm; median, 11.6 mm). This was a retrospective study approved by the hospital Ethics committee and written informed consent was waived.
MRI Protocol
All patients underwent MRI on a 3T scanner (GE SIGNA HDx) using an 8-channel breast coil. For premenopausal women, the examination was performed within the 7th–14th day of the menstrual cycle. The protocol consisted of T2-weighted imaging (T2WI) with fat suppression (repetition time [TR] 8200 msec; echo time [TE] 36 msec; flip angle [FA] 90°; slice thickness/gap 4.0/1.0 mm; field of view [FOV] 32 × 32 cm2; matrix size 128 × 128), T1-weighted imaging (T1WI) with dual-echo chemical-shift imaging (TR 380 msec; TE 2 msec; FA 90°; slice thickness/gap 4.0/1.0 mm; FOV 32 × 32 cm2; matrix size 128 × 128). Axial DWI was performed using a single-shot echo-planar imaging (EPI) sequence of 2 minutes and 57 seconds (TR 5300 msec; TE 60 msec; FA 90°; slice thickness/gap 4.0/1.0 mm; FOV 32 × 32 cm2; matrix size 128 × 128; two b-values: 0/600 (n = 10), 0/800 (n = 2) or 0/1000 (n = 55) s/mm2; spectral adiabatic inversion recovery [SPAIR] fat suppression). The TE was kept at 60 msec for all three pairs of b-values, which was chosen to be as short as possible to improve SNR, and kept the same to minimize its impact affecting the image quality and the reading. The dynamic contrast-enhanced (DCE) scan was acquired by volume imaging for breast assessment (VIBRANT) (TR 5 msec; TE 2 msec; FA 10°; slice thickness 1.2 mm; FOV 34 × 34 cm2; matrix size 416 × 416). The contrast agent, 0.1 mmol/kg gadopantatedimeglumine (Magnevist; Bayer Schering Pharma, Berlin, Germany), was intravenously injected. The DCE series consisted of six phases: one before and five after contrast injection. The total scanning time for one patient was 18 minutes and 32 seconds.
Computed DWI and Voxelwise-Computed DWI
CDWI METHOD.
cDWI is a synthetic image generated by a mathematical computational method. The ADC map and the series of cDWI with different b-values (b = 1500, 2000, and 2500 s/mm2) were generated using a program written in MatLab (2015b, MathWorks, Natick, MA). With a known ADC and signal intensity S0 at b = 0, the cDWI signal intensity can be computed based on the following equation10:
where S0 is the signal intensity without diffusion-weighting (b0 = 0 s/mm2), Sb is the signal intensity with diffusion-weighting, b1 = 1500, 2000, and 2500 s/mm2 for cDWI; and ADC is the apparent diffusion coefficient (mm2/s).
VCDWI METHOD.
Different tissues have different diffusion characteristics with different ADC values. In vcDWI, it calculates signal intensities of voxels with low ADCs at lower β and voxels with high ADC at higher β, which could preserve SNR and enhance the contrast between tissues with different diffusion restrictions. Signal intensity Sβ of voxel position x can be calculated16:
The determination of β(x) on the voxel ADC values is based on a linear function:
With b0 = 0 s/mm2 the signal intensity Sβ of voxel position x is calculated using the following function:
where ADC varies as the corresponding voxel position x, and S0 is the signal intensity with b-value of 0 s/mm2.
QUALITATIVE EVALUATION OF CDWI AND VCDWI.
All DWI image sets were imported to a personal computer for evaluation using ImageJ (NIH, Bethesda, MD). First, a radiologist (L.J.C., with 30 years’ experience) read each DWI image set and recorded suspicious lesions. Then DCE-MRI, surgery record, and pathological report (on hospital system computer) were used as the reference to carefully check the lesion location by the same radiologist. Suspicious lesions shown on DWIs but not on DCE-MRI or pathological result were excluded. Another three radiologists (X.H.Z., W.M.H., and M.H.W., with 15, 18, and 5 years’ experience, respectively), all blinded to histopathological results and clinical diagnosis, reviewed and scored the DWI images, independently, according to the specified lesion location recorded by Reader L.J.C. Sample images with the rating scale from 1–5 for lesion conspicuity, background signal suppression, and overall image quality were prepared and shown to the readers for training before they started to perform the rating. These sample images were not from the datasets that were analyzed in this study. Three readers (X.H.Z., W.M.H., and M.H.W.) reviewed five sets of images (mDWI, cDWI1500, cDWI2000, cDWI2500, and vcDWI) in a random order without labeling, and scored images with respect to lesion conspicuity, background signal suppression, and overall image quality using a 5-point rating scale. The lesion conspicuity ranged from 1 (lesion with faint contrast to background tissue) to 5 (lesion with extremely remarkable contrast to background tissue); background signal suppression ranged from 1–5 according to different signal intensity of normal fibroglandular tissue; overall image quality ranged from 1 (poor) to 5 (excellent). A diagnostic BI-RADS score was given for each lesion based on the signal intensity and morphology of the lesion: 1, negative; 2, benign finding; 3, probably benign; 4, suspicious abnormality; 5, highly suspicious for malignancy. All images were reviewed with the same window level/window width (WL/WW) settings (150/600). In order to ensure totally independent reading, the time interval for reviewing these five sets of DWI was at least 1 week between scoring sessions. At the end of the image evaluations, two radiologists (Readers X.H.Z. and W.M.H.) reviewed the DCE-MRI images together and gave a consensus diagnostic impression of benign vs. malignant for each lesion, and the results were used for evaluation of the DCE-MRI accuracy. Also, they reviewed their respective DWI diagnostic BI-RADS scores, and reached a consensus impression of benign vs. malignant for each lesion.
Statistical Analysis
Data analysis was performed with SPSS 16.0 (Chicago, IL). P < 0.05 was considered statistically significant. Agreement between the three readers was evaluated by intraclass correlation coefficient (ICC)17: ICC < 0.4 = poor agreement; 0.40–0.59 = fair agreement; 0.60–0.74 = good agreement; 0.75–1.00 = excellent agreement. A post-hoc pairwise comparison was performed using the Wilcoxon signed-rank test with Bonferroni correction between five sets of DWIs with an adjusted P < 0.005 as significant. The receiver operating characteristic (ROC) curve was used to evaluate the diagnostic performance based on the BI-RADS scores and the ADC values, and the areas under ROC curves (AUC) were calculated. A post-hoc pairwise comparison was performed using the Wilcoxon signed-rank test with Bonferroni correction among six sets of DWIs, DCE-MRI, and ADC with an adjusted P < 0.003 as significant.
Results
Image Rating and Interobserver Agreement
The average rating scores for lesion conspicuity, background signal suppression, and overall image quality are summarized in Table 1. ICC showed excellent interobserver agreements between three readers for lesion conspicuity (ICC = 0.84, 95% confidence interval [CI] 0.818–0.868), overall image quality (ICC = 0.83, 95% CI 0.797–0.852), and very good interobserver agreements for background signal suppression (ICC = 0.94, 95% CI 0.930–0.950). For lesion conspicuity and overall image quality, three readers gave higher scores for vcDWI compared with the series of cDWI (P < 0.005). Compared with mDWI, vcDWI was scored significantly higher in lesion conspicuity and background signal suppression (P < 0.005) by three readers, but not in overall image quality (P = 0.037 for Reader X.H.Z., P = 0.013 for Reader W.M.H.). Reader M.H.W. gave significantly higher overall image quality scores for mDWI than vcDWI (P < 0.005). Background signal suppression was scored the highest on cDWI2500 followed by vcDWI, cDWI2000, cDWI1500, and mDWI. A case example of cDWIs, vcDWI, mDWI, and DCE-MRI from a malignant lesion is illustrated in Fig. 1, and that of a benign lesion is illustrated in Fig. 2.
TABLE 1.
Mean Reading Scores for Lesion Conspicuity, Background Signal Suppression, and Overall Image Quality
| DWI | mDWI | cDWI1500 | cDWI2000 | cDWI2500 | vcDWI | |
|---|---|---|---|---|---|---|
| Lesion conspicuity | Reader 1 | 3.79 ± 0.60 | 2.97 ± 0.77 | 2.44 ± 0.77 | 1.56 ± 0.58 | 4.36 ± 0.76 |
| Reader 2 | 3.81 ± 0.72 | 3.10 ± 0.67 | 2.28 ± 0.74 | 1.32 ± 0.58 | 4.53 ± 0.80 | |
| Reader 3 | 4.29 ± 0.88 | 3.08 ± 0.44 | 2.03 + 0.24 | 1.06 ± 0.29 | 4.67 ± 0.67 | |
| Background signal suppression | Reader 1 | 1.03 ± 0.17 | 2.08 ± 0.28 | 3.39 ± 0.49 | 5 + 0 | 3.67 + 0.47 |
| Reader 2 | 1 ± 0 | 2.07 ± 0.26 | 3.35 ± 0.48 | 5 ± 0 | 3.65 ± 0.48 | |
| Reader 3 | 1 ± 0 | 2.13 ± 0.33 | 3.32 ± 0.47 | 5 ± 0 | 3.58 ± 0.67 | |
| Overall image quality | Reader 1 | 4.21 ± 0.82 | 3.07 ± 0.68 | 2.28 ± 0.72 | 1.43 ± 0.60 | 3.95 ± 0.59 |
| Reader 2 | 4.26 ± 0.71 | 3.31 ± 0.64 | 2.31 ± 0.68 | 1.51 ± 0.67 | 4.02 ± 0.63 | |
| Reader 3 | 4.74 ± 0.44 | 3.29 ± 0.46 | 2.06 ± 0.26 | 1.08 ± 0.33 | 4.02 ± 0.53 |
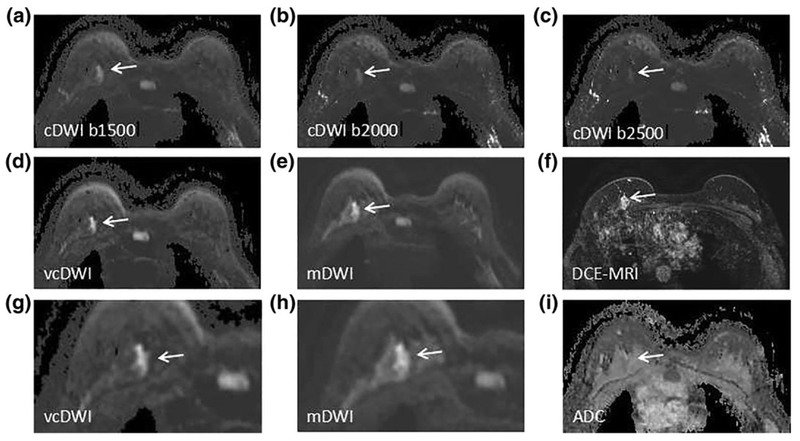
FIGURE 1:
A 34-year-old woman with DCIS in left breast. cDWI (A–C), vcDWI (D,G), mDWI with b-value of 1000 s/mm2 (E,H), DCEMRI (F), and ADC (I) are shown. G,H are magnified views. As b-value increases, the signal intensity of background fibroglandular tissue decreases, as well as that of cancer tissue. The signal intensity of cancer on vcDWI is preserved well with background suppression, and the lesion is more conspicuous and sharper on vcDWI than on cDWIs and mDWI, which allows the lesion to be more easily detected and characterized. The diagnostic BI-RADS scores given by Reader X.H.Z. and Reader W.M.H. were 5, 5, respectively, which correctly diagnose the lesion as malignant.
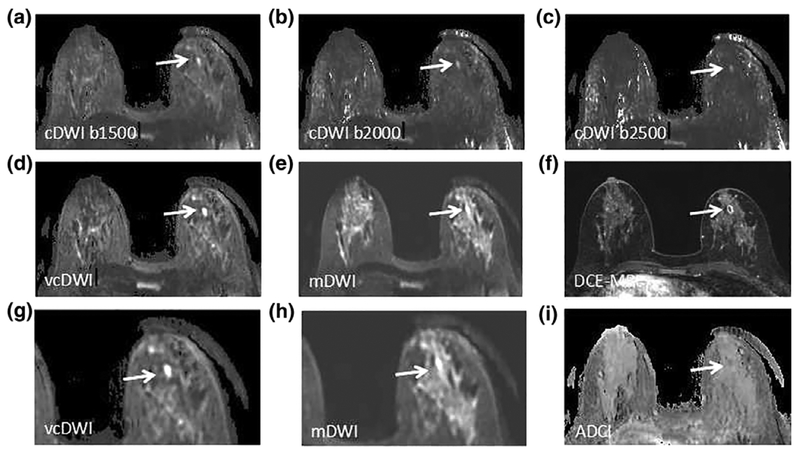
FIGURE 2:
A 43-year-old woman with a microabscess in the right breast. cDWI (A–C), vcDWI (D,G), mDWI with b-value of 1000 s/mm2 (E,H), DCE-MRI (F) and ADC (I) are shown. G,H are magnified views. The microabscess demonstrates extreme hyperintensity on vcDWI, much more conspicuous with more distinct margin than on other DWIs. The diagnostic BI-RADS scores given by Reader X.H.Z. and Reader W.M.H. were 2, 2, respectively, which correctly diagnosed the lesion as benign.
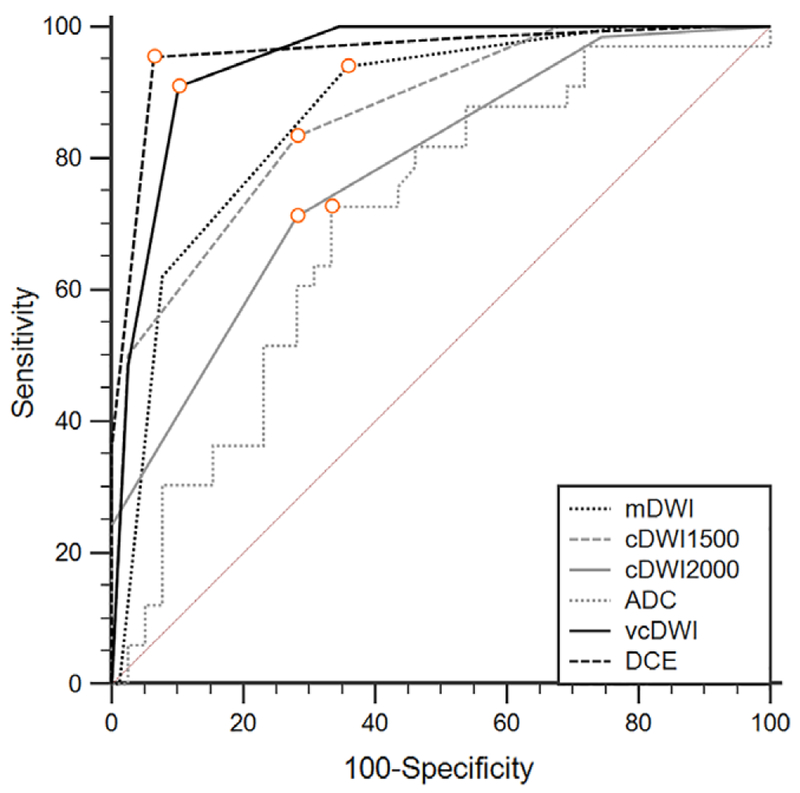
Diagnostic Performance
The mean ADC value for benign lesions and malignant lesions was (1.52 ± 0.23) × 10−3 mm2/s (standard deviation) and (1.13 ± 0.14) × 10−3 mm2/s, respectively. ROC curves based on BI-RADS reading of different sets of DWI, ADC value, and DCE-MRI were generated to evaluate diagnostic performance. The results are summarized in Table 2 and Fig. 3. The AUC value for differential diagnosis was 0.868 for mDWI, 0.862 for cDWI1500, 0.781 for cDWI2000, 0.605 for cDWI2500, 0.946 for vcDWI, 0.704 for ADC value, and 0.961 for DCE-MRI. The DCE-MRI demonstrated the best diagnostic accuracy. By using the optimal cutoff value, vcDWI showed 90.91% sensitivity and 89.74% specificity, and mDWI showed 93.94% sensitivity and 64.10% specificity.
TABLE 2.
Diagnostic Performance for Different DWIs, ADC, and DCE-MRI
| Image set | AUC | 95% CI | Youden index J | Sensitivity | Specificity | P value |
|---|---|---|---|---|---|---|
| vcDWI | 0.946 | 0.896 to 0.977 | 0.8065 | 90.91 | 89.74 | — |
| mDWI | 0.868 | 0.802 to 0.919 | 0.5804 | 93.94 | 64.10 | 0.0036 |
| cDWI1500 | 0.862 | 0.795 to 0.914 | 0.5513 | 83.33 | 71.79 | 0.0024 |
| cDWI2000 | 0.781 | 0.704 to 0.845 | 0.4301 | 71.21 | 71.79 | <0.0001 |
| ADC | 0.704 | 0.584 to 0.805 | 0.3939 | 72.73 | 66.67 | <0.0001 |
| DCE-MRI | 0.961 | 0.915 to 0.986 | 0.8904 | 95.45 | 93.59 | 0.5903 |
P values indicate comparison between vcDWI and mDWI, cDWI, ADC, DCE-MRI.
P < 0.003 is considered statistically significant.
FIGURE 3:
Diagnostic ROC curves based on different DWIs, ADC, and DCE-MRI to differentiate between benign and malignant lesions.
In pairwise comparison of ROC curves, vcDWI showed significantly higher diagnostic efficacy compared with cDWI1500, cDWI2000, and ADC (P < 0.003), but no significant difference was found between vcDWI and mDWI (P = 0.0036).
Discussion
Our results demonstrated that vcDWI could provide prominent lesion conspicuity by effectively suppressing background fibroglandular tissue; thus, it might assist in detection of the suspicious lesion. Although DCE-MRI is known to be the most accurate method for detecting and characterizing breast lesions,18,19 vcDWI may provide an effective noncontrast method and has the diagnostic potential for patients having a contraindication for injection of gadolinium contrast agents.
Normal breast fibroglandular tissue shows high signal intensity on DWI with a low b-value, and low signal intensity on DWI with a high b-value.6 In conventional DWI with b-values of 600–1000 s/mm2, the background fibroglandular tissue could not be suppressed completely and resulted in indistinctive contrast between lesion and normal tissues. On the other hand, acquiring DWI with higher b-values also has various problems, particularly SNR, and is not able to detect lesions reliably for further characterization. In order to resolve these problems, the artificially synthesized higher b-value DWI has been studied recently.10–12,20 O’Flynn et al investigated 27 patients with breast cancer and 34 normal cases, showing cDWI with higher b-values resulted in higher sensitivity than mDWI with lower b-value.15
For vcDWI, Gatidis et al performed phantom studies and clinical data analysis of patients with metastatic malignancies and showed that vcDWI could effectively reduce T2 shine-through effects, and thus improve CNR and SNR of image and improve lesion detection.16 Our results also supported that vcDWI enhanced the visual contrast between lesion and background tissue by effectively suppressing background fibroglandular tissue, making it easier to detect lesions. Another prominent improvement in vcDWI was to provide a better lesion conspicuity, which could be used to evaluate the lesion morphology and assess its probability of malignancy. As in the case example illustrated in the figures, the contrast between malignant and benign tissue was highlighted and the outline of a lesion was sharper on vcDWI. In turn, vcDWI could achieve both high diagnostic sensitivity (90.91%) and specificity (87.84%). In contrast, although mDWI has a better overall image quality and could achieve a high sensitivity of 93.94%, the specificity was only64.10%. One possible explanation was that it is difficult for conventional DWI to provide precise morphological features of lesions.21 Compared with cDWI with high b-values, vcDWI also had superior diagnostic performance to differentiate benign from malignant lesions.
DCE-MRI is the standard method to detect and diagnose breast cancer on MRI, which has been shown to have a high sensitivity but with variable specificity, ranging from 37% to 96%.22–24 There are substantial overlaps in enhancement characteristics of malignant and benign lesions. The enhancement kinetic curve measured by DCE-MRI reveals vascular volume and permeability of lesions; on the other hand, DWI reveals the cellular density, membrane integrity, and microstructure of lesions; thus, they provide distinct and complementary information for characterization of breast lesions. The information derived from mpMRI from all sequences is more comprehensive than that from any sequence alone. In recent years, with the concern of toxicity associated with the injection of gadolinium contrast agents in the brain and kidney, DWI may provide an alternative non-contrast method, particularly for patients who cannot undergo contrast-enhanced MRI. It is worth mentioning that vcDWI is a method that can be implemented without increasing scanning time. It could be a practical functional MRI tool that allows quick lesion detection and qualitative evaluation. In addition to diagnosis of suspicious lesions, the higher b-value DWI was also recommended to be added for the screening population, to heighten lesion visibility and lower the false-positive rate.6
In the present study the fat suppression for DWI was done by using SPAIR, and the suppression quality was pretty good, as shown in the figures. The comparison between two commonly used techniques for breast DWI, SPAIR, and STIR, on fat-suppression uniformity, SNR, CNR, image quality, lesion visibility, and ADC values, have been reported before.25,26 It was found that STIR could achieve a better uniformity compared with SPAIR, but SPAIR had slightly improved lesion visibility. Their SNR and CNR, and also the overall diagnostic performance, were comparable; thus, it was concluded that both techniques were suitable for use in clinical practice.
There were several limitations in our study. First, it was a retrospective study, and the number of patients was quite small. A larger study is needed to confirm the diagnostic value of vcDWI. Second, mDWIs with high b-values of 1500, 2000, and 2500 mm2/s were not acquired; thus, the comparison on image quality and lesion conspicuity could only be done by using synthetic images. Third, image quality of vcDWI was dependent on ADC values, which was in turn influenced by b-values that were used in the DWI acquisition. During the study period we were tuning the sequence and used different b-values, including 0/600, 0/800, and 0/1000 s/mm2, which might affect the measured ADC values and the diagnostic evaluation based on mDWI. Fourth, we excluded five cases with isointensity or hypointensity lesions on measured DWI, which would affect the evaluated diagnostic performance. Fifth, quantitative evaluation used in the present study was a subjective method, which was dependent on the individual experiences of radiologists. To minimize the variation, all three radiologists participating in the reading study went through the same training, which helped to reach a good interobserver agreement.
In conclusion, we have shown that ADC-dependent vcDWI is a feasible technique for diagnosis of breast cancer on MRI. It could provide high lesion conspicuity through effective reduction of the T2 shine-through effect and background tissue suppression, and could improve the diagnostic performance compared with mDWI and cDWI. Although DCE-MRI is the most important and accurate method for breast cancer diagnosis, for patients having a contraindication or concern about the side effects of contrast agent injection, vcDWI could provide an alternative method to assist in detection and characterization of breast lesions.
Acknowledgments
The authors thank Haiwei Miao for the scoring of images and Jingliang Cheng for statistical analyses.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Méndez CA, Pizzorni Ferrarese F, Summers P, Petralia G, Menegaz G. DCE-MRI and DWI integration for breast lesions assessment and heterogeneity quantification. Int J Biomed Imaging 2012; 2012:676808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato F, Kudo K, Yamashita H, et al. Differences in morphological features and minimum apparent diffusion coefficient values among breast cancer subtypes using 3-Tesla MRI. Eur J Radiol 2016;85:96–102. [DOI] [PubMed] [Google Scholar]
- 3.Park SH, Moon WK, Cho N, et al. Diffusion-weighted MR imaging: pre-treatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology 2010;257:56–63. [DOI] [PubMed] [Google Scholar]
- 4.Rahbar H, Partridge SC. Multiparametric MR imaging of breast cancer. Magn Reson Imaging Clin N Am 2016;24:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: Applications and challenges in oncology. Am J Roentgenol 2007;188:1622–1635. [DOI] [PubMed] [Google Scholar]
- 6.Pereira FP, Martins G, Carvalhaes de Oliveira Rde V. Diffusion magnetic resonance imaging of the breast. Magn Reson Imaging Clin N Am 2011; 19:95–110. [DOI] [PubMed] [Google Scholar]
- 7.Partridge SC, McDonald ES. Diffusion weighted MRI of the breast: protocol optimization, guidelines for interpretation, and potential clinical applications. Magn Reson Imaging Clin N Am 2013;21:601–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura T, Usui S, Murakami S, et al. Comparisons of multi b-value DWI signal analysis with pathological specimen of breast cancer. Magn Reson Med 2012;68:890–897. [DOI] [PubMed] [Google Scholar]
- 9.Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging 2006;24:478–488. [DOI] [PubMed] [Google Scholar]
- 10.Blackledge MD, Leach MO, Collins DJ, Koh DM. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology 2011;261:573–581. [DOI] [PubMed] [Google Scholar]
- 11.Rosenkrantz AB, Chandarana H, Hindman N, et al. Computed diffusion-weighted imaging of the prostate at 3 T: impact on image quality and tumour detection. Eur Radiol 2013;23:3170–3177. [DOI] [PubMed] [Google Scholar]
- 12.Feuerlein S, Davenport MS, Krishnaraj A, Merkle EM, Gupta RT. Computed high b-value diffusion-weighted imaging improves lesion contrast and conspicuity in prostate cancer. Prostate Cancer Prostatic Dis 2015; 18:155–160. [DOI] [PubMed] [Google Scholar]
- 13.Maas MC, Fütterer JJ, Scheenen TW. Quantitative evaluation of computed high b value diffusion-weighted magnetic resonance imaging of the prostate. Invest Radiol 2013;48:779–786. [DOI] [PubMed] [Google Scholar]
- 14.Moribata Y, Kido A, Fujimoto K, et al. Feasibility of computed diffusion weighted imaging and optimization of b-value in cervical cancer. Magn Reson Med Sci 2017;16:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Flynn EA, Blackledge M, Collins D, et al. Evaluating the diagnostic sensitivity of computed diffusion-weighted MR imaging in the detection of breast cancer. J Magn Reson Imaging 2016;44:130–137. [DOI] [PubMed] [Google Scholar]
- 16.Gatidis S, Schmidt H, Martirosian P, Nikolaou K, Schwenzer NF. Apparent diffusion coefficient-dependent voxelwise computed diffusion-weighted imaging: An approach for improving SNR and reducing T2 shine-through effects. J Magn Reson Imaging 2016;43:824–832. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Yun B, Jang M, et al. Comparison of the diagnostic performance of synthetic versus acquired high b-Value (1500 s/mm2) diffusion-weighted MRI in women with breast cancers. J Magn Reson Imaging. 2018. doi: 10.1002/jmri.26259 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Yu J, Peng Y. Association between dynamic contrast enhanced MRI imaging features and WHO histopathological grade in patients with invasive ductal breast cancer. Oncol Lett 2016;11:3522–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baltzer PAT, Bennani-Baiti B, StÖttinger A, Bumberger A, Kapetas P, Clauser P. Is breast MRI a helpful additional diagnostic test in suspicious mammographic microcalcifications?. Magn Reson Imaging 2018;46:70–74. [DOI] [PubMed] [Google Scholar]
- 20.Ueno Y, Takahashi S, Kitajima K, et al. Computed diffusion-weighted imaging using 3-T magnetic resonance imaging for prostate cancer diagnosis. Eur Radiol 2013;23:3509–3516. [DOI] [PubMed] [Google Scholar]
- 21.Baltzer PA, Benndorf M, Dietzel M, Gajda M, Gamara O, Kaiser WA. Sensitivity and specificity of unenhanced MR mammography (DWI combined with T2-weighted TSE imaging, ueMRM) for the differentiation of mass lesions. Eur Radiol 2010;20:1101–1110. [DOI] [PubMed] [Google Scholar]
- 22.Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. Am J Roentgenol 2009;193:1716–1722. [DOI] [PubMed] [Google Scholar]
- 23.Peters NH, Borel Rinkes IH, Zuithoff NP, Mail WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 2008;246:116–124. [DOI] [PubMed] [Google Scholar]
- 24.Kinkel K, Helbich TH, Esserman LJ, et al. Dynamic high-spatial-resolution MR imaging of suspicious breast lesions: diagnostic criteria and interob-server variability. Am J Roentgenol 2000;175:35–43. [DOI] [PubMed] [Google Scholar]
- 25.Noqueiral L, Brandäo S, Nunes RG, Ferreira HA, Loureiro J, Ramos I. Breast DWI at 3T: influence of the fat-suppression technique on image quality and diagnostic performance. Clin Radiol 2015;70:286–294. [DOI] [PubMed] [Google Scholar]
- 26.Brandäo S, Noqueiral L, Matos E, et al. Fat suppression techniques (STIR vs. SPAIR) on diffusion-weighted imaging of breast lesions at 3.0T: preliminary experience. Radiol Med 2015;120(8):705–713. [DOI] [PubMed] [Google Scholar]