Abstract
Although evolutionarily conserved to expel ectoparasites and aid in the clearance of toxins and noxious environmental stimuli from the host, the type 2 immune response can become pathologic in the setting of a variety of allergic disorders. Itch can be a behavioral extension of type 2 immunity by evoking scratching, and in the setting of disease, can become chronic and thus highly pathologic as well. Classically, our understanding of itch mechanisms has centered around the canonical IgE-mast cell-histamine axis. However, therapies aimed at blocking the histaminergic itch pathway have been largely ineffective, suggesting the existence of non-histaminergic pathways of itch. Indeed, recent advances in itch biology have provided critical new insight into a variety of novel therapeutic avenues for chronic itch in the setting of a number of allergic disorders. Herein, we highlight how these new developments will likely inform the problem of pruritus in a variety of well-established and emerging conditions in the field of allergy.
Keywords: Allergic contact dermatitis, atopic dermatitis, cytokine, eczema, histamine, itch, Janus kinase (JAK), mast cell, Mrgpr, neuroimmunology, prurigo nodularis, pruritus
Introduction
Pruritus, or itch, is a highly conserved sensation that evokes the desire to scratch. In its acute form, it aids in the host’s ability to expel ectoparasites, toxins, and noxious environmental substances. However, in its chronic form, itch is generally pathologic and underlies a wide variety of medical disorders.
Chronic pruritus, defined as itch lasting greater than six weeks, is associated with a number of medical conditions including inflammatory skin disorders such as atopic dermatitis (AD), malignancies including various leukemias and lymphomas, chronic kidney and hepatobiliary disease, endocrine disorders such as hyperthyroidism and diabetes, and primary neuropathic disorders such as brachioradial pruritus and postherpetic itch. Despite its association with so many medical disorders and afflicting approximately 15% of the population(1,2), efficacious therapies remain very limited. However, recent advances in our understanding of novel itch-sensory pathways, particularly in AD, have brought new insight into how both new therapies can be developed to specifically target symptoms of itch in patients(3,4).
The purpose of the current review is to highlight recent advances in our understanding of the pathophysiology of itch and broaden our understanding of how chronic pruritic conditions may fall within the family of allergic disorders. First, starting with our classical understanding of histaminergic itch in urticaria, we delve into how new molecular and cellular insights into the basis of non-histaminergic itch in AD broadly inform our understanding of itch in the context of the broader field of allergy. Second, we will discuss new and emerging pathways and therapeutics across a number of chronic itch conditions. Third, and last, throughout the course of this review we will introduce more recently recognized chronic itch disorders that likely share pathophysiology with well-defined allergic disorders (for more comprehensive reviews on chronic itch, see referenced reviews(5–8)). Collectively, we highlight and underscore how a variety of chronic itch conditions, both well-defined and more recently recognized, fall well within the realm of clinical allergy and immunology.
Classical Histaminergic Itch and Beyond
Classically, the most well-defined form of itch is histamine-mediated or histaminergic itch (Figure 1). A defining feature of type I hypersensitivity, histamine is released from mast cells following IgE-mediated activation via its cell surface high-affinity receptor FcεRI. Upon degranulation of mast cells in close proximity to itch-sensory nerve fibers in the skin, a variety of itch-inducing molecules or pruritogens, including histamine, are released and bind their respective receptors on terminal axons. This triggers the opening of nonspecific cation channels such as transient receptor potential A1 (TRPA1) and/or TRPV1, resulting in membrane depolarization and subsequent activation of an action potential by voltage-gated ion channels such as NaV1.7 and NaV1.8. Itch-sensory signals are relayed via unmyelinated and slow conducting C-fibers to their respective cell bodies in the dorsal root ganglia (DRG), and subsequently transmitted to the dorsal horn of the spinal cord in the central nervous system (CNS) and ultimately to the brain where these signals are perceived as itch. Although the IgE-mast cell-histamine axis has been well understood for decades, this pathway has proven to be disappointing as a target for most chronic itch disorders.
Figure 1. Classic histaminergic itch pathway.

Mast cell degranulation via IgE-activation of FcεRI releases histamine to activate itch-sensory neurons.
Pruriceptors, or itch-specific sensory neurons, are now recognized as a distinct class of neurons that transmit itch signals. Recent discoveries of novel itch-mediating molecules such as the gastrin-releasing peptide receptor (GRPR)(9), Mas-related G protein-coupled receptor (Mrgpr) family(10,11), neuropeptide Nppb(12,13), and various itch-mediating cytokine receptors have generated tremendous interest in understanding how new and emerging pathways may be targeted for the treatment of various chronic itch disorders (Figure 2). Although, all of these pathways demonstrate mechanisms independent from other sensations such as pain, further work is needed to assess how these various pathways may be distinct, interdependent, or synergistic in the context of various chronic itch conditions.
Figure 2. The neuroimmune axis in itch biology.

A complex interplay of molecular signals and receptors on immune cells and neurons drive itch. Key immune cells and their associated cytokines include ILC2s (TSLP- and IL-33-responsive), Th2 cells (effector cytokines IL-4/13 and IL-31), basophils (effector molecules IL4/13 and histamine), and mast cells (effector molecules tryptase, serotonin, and histamine); activation of cytokine receptors, ion channels, and GPCRs drive itch-specific signals on sensory neurons. Created with BioRender.
Chronic Spontaneous Urticaria
Urticaria is characterized by pruritic wheals or hives on the skin that typical last for 24 hours or less and often migrate. The causes may be numerous, including allergic reactions (e.g., medications), mechanical stimulation, autoimmunity, or simply idiopathic. In the setting of acute urticaria, it is well understood that activation of IgE by putative antigens results in the activation of mast cells (and basophils) and the release of preformed mediators, the most important being histamine. This process results in the elicitation of hives and associated histaminergic itch that is often responsive to antihistamines. However, when urticaria is chronic and occurring for a period of greater than six weeks, it is described as chronic spontaneous urticaria (CSU) and its associated chronic itch becomes more difficult to manage.
In contrast to acute urticaria, antihistamines often demonstrate even less efficacy in CSU(14). These observations provoke the hypothesis that, although histamine is a major component of urticarial itch, there are likely histamine-independent pathways that also mediate chronic itch in this context. Mast cells can also synthesize and release other mediators, including eicosanoids like prostaglandin D2 and leukotriene C4, D4, and E4. Leukotriene receptor antagonists (LTRA) have not been shown to be superior to placebo or antihistamine therapy in the treatment of CSU. However, the combination of LTRA and antihistamines appear to be more effective than antihistamine alone(15).
In the context of CSU, there are also other well recognized immunologic stimuli that can degranulate mast cells through the IgE receptor, including IgG antibodies to IgE (5–10% of patients) or FcεRI (30–40% of patients)(16). Whether alternative modes of IgE-mediated activation result in differential release of pruritogens remains to be explored. Additionally, it is important to note that other cell types have also been implicated, as evidenced by the presence of eosinophils, neutrophils, and lymphocytes in urticarial skin lesions on histology. However, their pathogenic roles in CSU have yet to be fully elucidated. In terms of itch, future studies will be required to fully define the pruritogenic pathways that underlie both acute and chronic urticaria.
Notwithstanding our limited understanding of itch in urticaria, H1 antagonist antihistamines remain a first-line treatment for urticaria. Of the 4 histamine receptors, H1R, and more recently H4R, have been implicated in itch(17). Although H1 antihistamines are used for both acute urticaria and CSU, the presence of other receptors on sensory neurons such as H4R suggests that there may be additional mechanisms by which histamine may mediate itch in urticaria(18). Further, H4R is also expressed on mast cells indicating that there may be additional indirect mechanisms by which mast cells may also be stimulated to release pruritogens. Indeed, a recent clinical trial with an H4 antagonist demonstrated significant reduction in histamine-induced pruritus with no effect on wheal formation (JNJ 39758979)(19). Thus, future studies with newer generation antihistamines (i.e., H4R antagonists) will be required to better determine the role of histamine in urticarial itch.
Although H1 antihistamines are first-line in the treatment of urticaria, many patients do not respond and suffer from persistent hive and itch. In this setting, patients with CSU will often require additional mast cell-targeted therapy, namely omalizumab which blocks IgE and prevents binding onto FcεRI on mast cells and basophils(20–23). Although this likely results in reduced release of histamine, the observation that many patients who are refractory to antihistamines improve on omalizumab suggests that other mast cell-derived factors may also be involved in mediating histamine-independent itch. Additionally, CSU patients given omalizumab show differential response patterns, characterized into “fast” responders (60–70% of pat ients who demonstrate a complete response in 3–7 days), “slow” responders (10–20% of patients who respond in 3–5 months), and “non-responders” (10–20% of patients), suggesting different pathogenic mechanisms in CSU(24). Further, recent seminal work uncovering the role of Mrgprb2 as a key receptor that mediates IgE-independent mast cell activation suggests that other modalities may also be important in mediating urticarial itch(25–27). However future studies will be required to determine the precise role of Mrgprb2 in urticaria and CSU.
Atopic Dermatitis
Atopic dermatitis (AD) is a common chronic and relapsing inflammatory skin disorder characterized by intense pruritus and scaly, red rashes involving classic locations (cheeks and extensor extremities in infants, and flexural sites in children and adults). It typically presents during infancy and can persist into adulthood(28). In addition to localized skin lesions, patients with AD can exhibit systemic immune dysregulation as demonstrated by peripheral eosinophilia and elevated serum IgE(29,30). Further, AD is a clear risk factor for the development of asthma and food allergy. Like these other atopic disorders, AD is associated with elevated expression of epithelial cell-derived cytokines such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) from the epithelial barrier, and enhanced adaptive T helper type 2 (Th2) cell and innate basophil, eosinophil, mast cell, and group 2 innate lymphoid cell (ILC2) responses(31). Collectively, these processes result in elevated production of the effector cytokines IL-4, IL-5, and IL-13 that orchestrate the overall allergic cutaneous immune response in AD.
Beyond skin inflammation, recent studies have begun to unveil how the immune system interacts with the sensory nervous system to evoke symptoms of itch. Although AD is associated with enhanced mast cell activation and histamine release, H1 antihistamines are largely ineffective for the treatment of AD-associated itch. Thus, newer clinical trials are current underway to investigate the effects of H4 antihistamines for the treatment of AD and its resulting itch (). Notwithstanding this, the first cytokine to be identified as a pruritogen was found to be produced by Th2 cells in the context of AD, namely IL-31(32). Indeed, phase 2 clinical trials with nemolizumab, an anti-IL-31 receptor (IL-31RA) blocking monoclonal antibody (mAb) have recently demonstrated anti-pruritic efficacy in AD(33). Additionally, epithelial cell-derived cytokines such as TSLP and IL-33, upstream of type 2 cellular responses, have also been shown to directly stimulate itch-sensory neurons in vivo(34,35) and anti-TSLP (tezepelumab) and anti-IL-33 (etokimab) mAbs are also currently being explored in clinical trials for AD(36). Collectively, these studies will provide novel insight into the anti-pruritic properties of blocking specific signals at the skin neuroimmune interface.
Dupilumab is the first FDA-approved mAb for the treatment of AD and targets the IL-4 receptor alpha subunit (IL-4Rα) to block both IL-4 and IL-13 signaling(37). We recently identified that IL-4Rα is expressed on sensory neurons and critically regulates itch responses in vivo in a murine model of AD-like disease. In contrast to IL-31 and TSLP, rather than acting as pruritogens, it appears that IL-4 and IL-13 regulate itch on neurons by promoting neuronal hypersensitivity to a variety of pruritogens(38). Beyond dupilumab, clinical trials are currently underway for anti-IL-13 mAbs including tralokinumab and lebrikizumab, and tralokinumab significantly improved AD-associated itch(39,40). Ultimately, it remains to be determined which cytokines are the most potent targets for selectively targeting AD-associated itch and will be revealed in these ongoing clinical investigations.
In lymphocytes, a number of AD-associated cytokines are dependent on JAK-STAT signaling for their effects on cellular transcription and activation(41). Indeed, a number of both topical and systemic JAK inhibitors are currently in development for the treatment of AD and related conditions. Phase 2 clinical trials with topical tofacitinib (JAK1/3 inhibitor), JTE-052 (pan-JAK inhibitor), and ruxolitinib (JAK1/2 inhibitor) have demonstrated efficacy with rapid effects on the resolution of itch(42–45). In recent studies, we have identified that neuronal JAK1 signaling is a critical regulator of AD-associated itch in vivo and that JAK inhibitors likely have neuromodulatory properties(38). In addition to the studies with topical JAK inhibitors, oral JAK1-selective inhibitors (upadacitinib and PF-04965842) are also currently in clinical trials for the treatment of AD and are demonstrating potent anti-itch effects as well ()(46). Baricitinib, a JAK1/2 inhibitor, was also able to improve AD rash in addition to AD-associated pruritus in a phase 2 trial(47). Collectively, these studies are rapidly paving the way for the development of FDA-approved JAK inhibitors for the treatment of AD and already demonstrating the unique anti-pruritic properties of these agents.
In contrast to urticaria, it is well established now that AD-associated itch is largely non-histaminergic in nature. Indeed, a number of cytokines, mostly associated with the type 2 immune response (e.g., IL-4, IL-13, IL-31, IL-33, and TSLP), have now been implicated in mediating both inflammation and neurophysiologic itch in the context of AD. The recent advances in our understanding of the neuroimmune basis of AD-associated itch have emboldened the development of new treatments for AD and also provoke the hypothesis that immunologically related disorders may also be amenable to similar therapeutic interventions.
Prurigo Nodularis
Prurigo nodularis (PN) is classically described as a neurodermatosis, meaning that the underlying itch is believed to drive the development of the rash, which is typically characterized by multiple dome-shaped, hyperkeratotic nodules. These nodules are distributed symmetrically on extensor surfaces of extremities and areas of the torso where one can reach and scratch (hence the mid back is commonly spared); however, flexural surfaces, palms, soles, face and groin are rarely affected (Figure 3). Intense pruritus and chronic repetitive scratching, rubbing, or picking are observed(48). PN presents in the context of a number of systemic diseases including chronic kidney disease and HIV, but can also manifest independently(49–51). Strikingly, 50% of patients with PN exhibit atopy as a comorbid entity. Although exhibiting distinct clinical features even in the context of AD, PN has demonstrated similar pathologic features as AD. First, both conditions are associated with histologic features of hyperkeratosis and a mixed dermal inflammatory infiltrate commonly composed of lymphocytes, neutrophils, mast cells, and occasional eosinophils(52). Second, both conditions have been associated with elevated expression of IL-31 within the lesions(53). Third, as in AD, PN lesions are noted to have increased density of nerve fibers innervating the skin(54,55).
Figure 3. Prurigo nodularis.

Itchy hyperkeratotic nodules distributed on the extensor forearm (right), classically described as a neurodermatosis.
Although a distinct clinical entity, given the common co-presentation with AD and its shared pathologic features, it is likely that PN will respond to a number of therapeutics that are currently being explored or employed in the setting of AD. Indeed, as with AD, a phase 2 clinical trial with nemolizumab was recently completed (), while clinical trials are currently underway with KPL-716 (), an oncostatin M receptor beta (OSMRβ) antagonist, which would also limit IL-31 signaling by disrupting OSMRβ-IL-31RA interactions. A recent case series using dupilumab to treat PN demonstrated a reduction in pruritus and prurigo lesion sizes after 12 weeks in all three patients who underwent treatment(56). Taken together, these developments suggest that a number of AD-associated therapies will likely be directed at PN in the future.
Notwithstanding the shared characteristics between the two conditions, there is empirical evidence to suggest that PN may be more of a neurodermatosis than AD. Indeed, the neurokin-1 receptor (NK-1R) antagonist serlopitant, which blocks the binding of substance P to NK-1R on a variety of neurons, has been found in phase 2 clinical trials to be effective in the context of PN, while both primary and secondary endpoints were not met in AD(57). Taken together, these findings suggest that the neuromodulatory effects of NK-1R antagonism preferentially affect PN rather than AD. Thus, future studies will be required to define what features are truly similar and divergent between PN and AD.
Chronic Pruritus of Unknown Origin
Chronic pruritus of unknown origin (CPUO), also referred to as chronic idiopathic pruritus (CIP) or pruritus of the elderly, presents as generalized pruritus with no clearly defined cause. These patients typically do not exhibit a rash but are widely believed to have a type 2 immune profile(58). Indeed, we have shown that these individuals can harbor eosinophils in the dermis, elevated eosinophils in the blood, and mild elevation of serum IgE(59). Additionally, transcriptional profiling of CPUO skin demonstrated a distinct but similar profile to lesional AD skin(38). However, in contrast to AD, although CPUO patients exhibit a type 2 immune profile, they are believed to have milder systemic inflammation and do not typically have a history of atopic disease. Further, similar to PN, no FDA-approved medications exist for the treatment of CPUO.
It is hypothesized that CPUO patients have sensory neuronal dysfunction that potentiates the effects of type 2 inflammation to drive itch. Based on these findings, we examined whether patients who are refractory to conventional anti-inflammatory or immunosuppressive treatments would respond to the neuromdulatory properties of JAK inhibitors. Indeed, in a non-randomized proof-of-concept study, we found that patients with CPUO responded to systemic tofacitinib(38). Thus, larger randomized studies will be required to fully establish the efficacy of these agents in CPUO. Beyond JAK inhibition, based on the success of serlopitant for chronic pruritus(60), a phase 2 clinical trial is currently underway for the use of serlopitant in CPUO (). Given that patients with CPUO often present with disproportionate itch relative to skin inflammation, it will be interesting to see how such neuromodulators influence itch in this context. Given the highly unmet nature of this condition, additional insight into its pathogenesis is urgently needed to better address the current treatment gaps.
Allergic Contact Dermatitis
Allergic contact dermatitis is an itchy eczematous reaction of the skin that occurs hours to days after coming into contact with an allergen. Unlike irritant contact dermatitis (ICD), which results from an irritant chemical causing a direct toxic effect on the skin, ACD is a delayed type IV hypersensitivity reaction to a hapten or antigen(61). Unlike other hypersensitivity reactions, ACD is not dependent on antibodies but relies on a Th1 response to an antigen presented by an antigen-presenting cell (APC). It is dependent on 2 phases – the induction phase whereby the immune system is sensitized to an antigen, and the elicitation phase whereby the immune response is triggered upon re-challenge of the allergen(62). Common allergens include metals (e.g. nickel), plants (e.g. poison ivy), topical medications (e.g. neomycin), and household products (e.g. formaldehyde, fragrance mix). Although ICD and ACD are often difficult to distinguish from each other, patch testing is the gold standard test to determine culprit allergens that favor a diagnosis of ACD(62,63).
The mainstay of ACD treatment is through allergen avoidance, which is often challenging given that minute amounts of allergen exposure can be enough to trigger a reaction. After complete avoidance of an allergen, resolution may take six weeks or longer as the allergen may be present in the skin up to three weeks(64). Furthermore, the culprit allergen is often not identified and frequently remains elusive. Systemic and topical steroids are effective in the short-term for symptomatic relief, but they are not appropriate long-term use. In the event that longer term therapy is required, other agents employed include topical calcineurin inhibitors and/or systemic cyclosporine, mycophenolate mofetil, and light therapy. Similar to many other chronic itch disorders, antihistamines are largely ineffective for ACD(65). Strikingly, the precise mechanisms that broadly underlie the development of itch in ACD remain poorly defined.
Although classically defined as a type IV hypersensitivity reaction mediated by Th1 and/or cytotoxic T cells, recent studies are demonstrating novel T cell-independent mechanisms as key players in ACD-associated itch. In a murine model of poison ivy dermatitis, neuronal IL-33R signaling was found to be a key mediator of itch, indicating that epithelial cell-derived IL-33 may directly stimulate itch-sensory neurons in this context(34). Although somewhat controversial, mast cells have been implicated in modulating ACD-associated inflammation(66–69). Along these lines, we sought to investigate the role of novel mast cell-associated Mrgprb2 (MRGPRX2 in humans) in the setting of ACD itch and identified that, proadrenomedullin peptide (PAMP), an endogenous activator of Mrgprb2/X2 was critically upregulated in lesional human ACD skin. In addition to eliciting itch in an Mrgprb2- and mast cell-dependent manner, its activity was entirely independent of histamine. In contrast to IgE-mediated activation of mast cells which resulted in large amounts of histamine release, Mrgprb2-elicited activation resulted in smaller amounts of histamine release but greater release of tryptase (Figure 4). Strikingly, ACD-associated itch was dependent on Mrgprb2 across multiple models of ACD in mice(26). Along these lines, Soliniski et al. recently showed that chemogenetic activation of mast cells results in the release of serotonin, leukotriene C4 (LTC4), and sphingosine-1-phosphate (S1p). Notably, Nppb neurons were found to express receptors for these molecules which activate itch along the gastrin-releasing peptide (GRP) spinal cord pathway(70). Collectively, these studies demonstrate unique itch mechanisms in the context of ACD and heterogeneous pathways by which mast cells can be activated to mediate itch beyond histamine.
Figure 4. Histamine-dependent and histamine-independent itch pathways of mast cells.
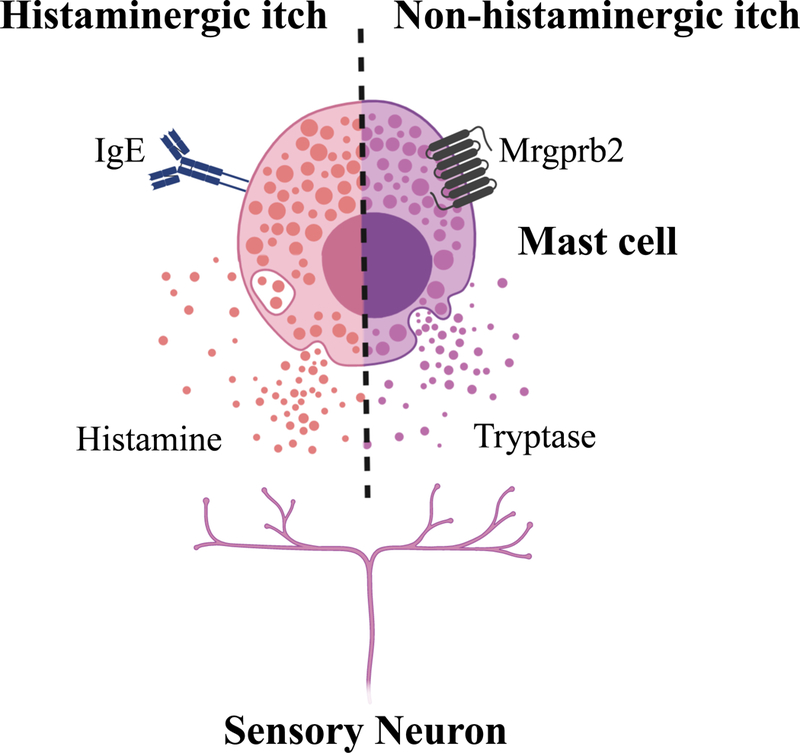
Mast cells can be activated by classic IgE-mediated release of histamine (left), or by Mrgprb2 activation and subsequent release of distinct effector molecules (e.g., tryptase) (right). Created with BioRender.
Conclusions
Although it was long recognized that chronic pruritus was not solely driven by histaminergic processes, treatments have been historically limited to antihistamines and non-specific immunosuppressive therapies such as corticosteroids. Recent advances in neuroimmunology and itch biology have yielded critical new insight into how the skin transmits itch signals through a complex interplay of molecular signals mediated by monoamines, cytokines, ion channels, and various receptors expressed by keratinocytes, immune cells, and neurons. These studies have emboldened the development of new targeted therapeutics like dupilumab, tralokinumab, lebrikizumab, neurokinin receptor antagonists, Mrgpr antagonists, and JAK inhibitors that will likely have major impact on chronic itch disorders within allergy and immunology in the next decade (Table 1).
Table 1. Emerging therapeutics for chronic pruritus in allergic skin disorders.
MC, mast cell; KC, keratinocyte; AD, atopic dermatitis; PN, prurigo nodularis; CSU, chronic spontaneous urticaria; CPUO, chronic pruritus of unknown origin; ACD, allergic contact dermatitis. Disorders are in bold when therapeutic is FDA-approved.
| Target | Source | Therapeutics (target) | Disorder |
|---|---|---|---|
| IL4 | Th2, basophil, MC, ILC2 | Dupilumab (IL-4RA) | AD |
| IL-13 | Th2, ILC2 | Dupilumab (IL-4RA) Tralokinumab (IL-13) Lebrikizumab (IL-13) |
AD |
| IL-33 | KC | Etokimab (IL-33) | AD ACD |
| TSLP | KC | Tezepelumab (TSLP) | AD |
| IL-31 | Th2 | Nemolizumab (IL-31RA) | AD PN |
| IgE | Plasma cells | Omalizumab (IgE) | CSU |
| JAK | Lymphocytes | Tofacitinib (JAK1/3) Ruxolitinib (JAK1/2) Baricitinib (JAK1/2) Upadacitinib (JAK1) PF-04965842 (JAK1) JTE-052 (pan-JAK) |
AD |
| N1K-R | Neurons | Serlopitant (NK1R) | PN CPUO |
| Histamine | Basophil, MC, KC | JNJ 39758979 (H4R) ZPL-389 (H4R) |
AD CSU |
| MrgprX2 | MC | N/A | CSU |
What we know:
Chronic pruritus is highly debilitating and a major component of many allergic diseases.
The classical IgE-mast cell-histamine axis is a poor therapeutic target in many chronic itch disorders.
A number of non-histaminergic itch-sensory pathways have been uncovered with the discovery of the GRP, Mrgpr, and Nppb circuits.
Atopic dermatitis-associated itch is histamine-independent.
Recent studies indicate that neuronal IL-4, IL-13, IL-31, TSLP, and JAK1 signaling as critical targets for itch in this context.
Mrgprb2 is a novel target for mast cell-associated inflammation.
What is unknown?
Which newly identified itch-specific pathways underlie various forms of allergic itch?
How conserved are various itch targets across multiple allergic conditions?
Does activation of the Mrgprb2/MRGPRX2 pathway underlie refractory forms of urticaria?
What are the key IgE-independent mast cell-derived pruritogens?
Acknowledgements
Work in the Kim laboratory is supported by K08AR065577 and R01AR070116 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) at the National Institutes of Health (NIH), the American Skin Association (ASA), the Doris Duke Charitable Foundation (DDCF) Clinical Scientist Development Award, Celgene Corporation, and LEO Pharma. We also thank members of the Kim laboratory for insightful discussions.
Abbreviations/Acronyms:
- ACD
allergic contact dermatitis
- AD
atopic dermatitis
- CIP
chronic idiopathic pruritus
- CNS
central nervous system
- CPUO
chronic pruritus of unknown origin
- CSU
chronic spontaneous urticaria
- DRG
dorsal root ganglia
- FcεRI
high-affinity IgE receptor, or Fc epsilon receptor I
- FDA
U.S. Food and Drug Administration
- GRPR
gastrin-releasing peptide receptor
- H1R
histamine receptor 1
- H4R
histamine receptor 4
- ICD
irritant contact dermatitis
- IgE
immunoglobulin E
- IgG
immunoglobulin G
- IL-4
interleukin 4
- IL-5
interleukin 5
- IL-13
interleukin 13
- IL-25
interleukin 25
- IL-31
interleukin 31
- IL-33
interleukin 33
- IL-4Rα
interleukin 4 receptor alpha
- IL-31RA
interleukin 31 receptor A
- IL-33R
interleukin 33 receptor
- ILC2
group 2 innate lymphoid cell
- JAK
Janus kinase
- LTRA
leukotriene receptor antagonist
- Mrgpr
Mas-related G protein-coupled receptor
- NaV
voltage-gated sodium channel
- NK-1R
neurokin-1 receptor
- Nppb
natriuretic peptide B
- PAMP
proadrenomedullin peptide
- PN
prurigo nodularis
- STAT
signal transducer and activator of transcription
- Th1
T helper type 1
- Th2
T helper type 2
- TRPA1
transient receptor potential A1
- TRPV1
transient receptor potential V1
- TSLP
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
B.S.K. has served as a consultant for AbbVie, Inc., Cara Therapeutics, Concert Pharmaceuticals, Incyte Corporation, Menlo Therapeutics, and Pfizer, Inc. He has served on advisory boards for Celgene Corporation, Kiniksa Pharmaceuticals, Menlo Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi, and Theravance Biopharma. He is founder, chief scientific officer, and stockholder of Nuogen Pharma.
References
- 1.Ständer S, Schäfer I, Phan NQ, Blome C, Herberg K, Heigel H, et al. Prevalence of Chronic Pruritus in Germany: Results of a Cross-Sectional Study in a Sample Working Population of 11,730. Dermatology [Internet] 2010. [cited 2019 May 5];221(3):229–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20924157 [DOI] [PubMed] [Google Scholar]
- 2.Matterne U, Apfelbacher C, Loerbroks A, Schwarzer T, Büttner M, Ofenloch R, et al. Prevalence, correlates and characteristics of chronic pruritus: a population-based cross-sectional study. Acta Derm Venereol [Internet] 2011. [cited 2019 May 5];91(6):674–9. Available from: http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1159 [DOI] [PubMed] [Google Scholar]
- 3.Werfel T, Allam J-P, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol [Internet] 2016. August [cited 2019 Jun 23];138(2):336–49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27497276 [DOI] [PubMed] [Google Scholar]
- 4.Leonard A, Guttman-Yassky E. The Unique Molecular Signatures of Contact Dermatitis and Implications for Treatment. Clin Rev Allergy Immunol [Internet] 2019. February 12 [cited 2019 Jun 23];56(1):1–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29754191 [DOI] [PubMed] [Google Scholar]
- 5.Trier AM, Kim BS. Cytokine modulation of atopic itch. Curr Opin Immunol [Internet] 2018. October [cited 2019 May 5];54:7–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0952791518300141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack MR, Kim BS. The Itch–Scratch Cycle: A Neuroimmune Perspective. Trends Immunol [Internet] 2018. December [cited 2019 May 5];39(12):980–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30471983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oetjen LK, Kim BS. Interactions of the immune and sensory nervous systems in atopy. FEBS J [Internet] 2018. September [cited 2019 May 5];285(17):3138–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29637705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson S, Nahmias Z, Rosman IS, Kim BS. Immunomodulating Agents as Antipruritics. Dermatol Clin [Internet] 2018. July [cited 2019 May 5];36(3):325–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29929604 [DOI] [PubMed] [Google Scholar]
- 9.Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature [Internet] 2007. August 25 [cited 2019 May 5];448(7154):700–3. Available from: http://www.nature.com/articles/nature06029 [DOI] [PubMed] [Google Scholar]
- 10.McNeil B, Treatment XD-IM and, 2014. undefined 12 Mrgprs as Itch Receptors.books.google.com [Internet]. [cited 2019 May 5]; Available from: https://books.google.com/books?hl=en&lr=&id=tTXSBQAAQBAJ&oi=fnd&pg=PA213&ots=wEvLqUuC9g&sig=ib5MV7NKXpnZ7ePqFDGN5Bvgxec
- 11.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus. Cell [Internet] 2009. December 24 [cited 2019 May 5];139(7):1353–65. Available from: https://www.sciencedirect.com/science/article/pii/S0092867409014925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X-Y, Wan L, Huo F-Q, Barry DM, Li H, Zhao Z-Q, et al. B-Type Natriuretic Peptide is Neither Itch-Specific Nor Functions Upstream of the GRP-GRPR Signaling Pathway. Mol Pain [Internet] 2014. January 18 [cited 2019 May 5];10:1744-8069-10-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24438367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science [Internet] 2013. May 24 [cited 2019 May 5];340(6135):968–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23704570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan AP. Chronic Spontaneous Urticaria: Pathogenesis and Treatment Considerations. Allergy Asthma Immunol Res [Internet] 2017. November [cited 2019 May 5];9(6):477–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28913986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Silva NL, Damayanthi H, Rajapakse AC, Rodrigo C, Rajapakse S. Leukotriene receptor antagonists for chronic urticaria: a systematic review. Allergy Asthma Clin Immunol [Internet] 2014. [cited 2019 Apr 30];10(1):24 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24817895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil Phenotypes in Chronic Idiopathic Urticaria in Relation to Disease Activity and Autoantibodies. J Invest Dermatol [Internet] 2008. August [cited 2019 May 5];128(8):1956–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18356810 [DOI] [PubMed] [Google Scholar]
- 17.Thurmond RL, Kazerouni K, Chaplan SR, Greenspan AJ. Antihistamines and Itch In Springer, Berlin, Heidelberg; 2015. [cited 2019 May 5]. p. 257–90. Available from: http://link.springer.com/10.1007/978-3-662-44605-8_15 [DOI] [PubMed] [Google Scholar]
- 18.Schneider EH, Seifert R. The histamine H4-receptor and the central and peripheral nervous system: A critical analysis of the literature. Neuropharmacology [Internet] 2016. July 1 [cited 2019 May 5];106:116–28. Available from: https://www.sciencedirect.com/science/article/pii/S0028390815001859 [DOI] [PubMed] [Google Scholar]
- 19.Kollmeier A, Francke K, Chen B, Dunford PJ, Greenspan AJ, Xia Y, et al. The histamine H4 receptor antagonist, JNJ 39758979, is effective in reducing histamine-induced pruritus in a randomized clinical study in healthy subjects. J Pharmacol Exp Ther [Internet] 2014. July 1 [cited 2019 May 5];350(1):181–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24817035 [DOI] [PubMed] [Google Scholar]
- 20.CHANG T, SHIUNG Y Anti-IgE as a mast cell–stabilizing therapeutic agent. J Allergy Clin Immunol [Internet] 2006. June [cited 2019 Mar 12];117(6):1203–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16750976 [DOI] [PubMed] [Google Scholar]
- 21.Maurer M, Rosén K, Hsieh H-J, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the Treatment of Chronic Idiopathic or Spontaneous Urticaria. N Engl J Med [Internet] 2013. March 7 [cited 2019 May 5];368(10):924–35. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1215372 [DOI] [PubMed] [Google Scholar]
- 22.Saini SS, Bindslev-Jensen C, Maurer M, Grob J-J, Bülbül Baskan E, Bradley MS, et al. Efficacy and Safety of Omalizumab in Patients with Chronic Idiopathic/Spontaneous Urticaria Who Remain Symptomatic on H1 Antihistamines: A Randomized, Placebo-Controlled Study. J Invest Dermatol [Internet] 2015. January 1 [cited 2019 May 5];135(1):67–75. Available from: https://www.sciencedirect.com/science/article/pii/S0022202X15370652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck LA, Marcotte GV., MacGlashan D, Togias A, Saini S Omalizumab-induced reductions in mast cell FcεRI expression and function. J Allergy Clin Immunol [Internet] 2004. September 1 [cited 2019 May 5];114(3):527–30. Available from: https://www.sciencedirect.com/science/article/pii/S0091674904017348 [DOI] [PubMed] [Google Scholar]
- 24.Gericke J, Metz M, Ohanyan T, Weller K, Altrichter S, Skov PS, et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol [Internet] 2017. March [cited 2019 May 5];139(3):1059–1061.e1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674916312829 [DOI] [PubMed] [Google Scholar]
- 25.Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest [Internet] 2016. [cited 2019 May 5];126(10):3981–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27643442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity [Internet] 2019. April 23 [cited 2019 May 5]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/31027996 [DOI] [PMC free article] [PubMed]
- 27.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature [Internet] 2015. March 17 [cited 2019 May 5];519(7542):237–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25517090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollenberg A, Oranje A, Deleuran M, Simon D, Szalai Z, Kunz B, et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatology Venereol [Internet] 2016. May 1 [cited 2019 May 5];30(5):729–47. Available from: http://doi.wiley.com/10.1111/jdv.13599 [DOI] [PubMed] [Google Scholar]
- 29.Jenerowicz D, Czarnecka-Operacz M, Silny W. Peripheral blood eosinophilia in atopic dermatitis. Acta dermatovenerologica Alpina, Pannonica, Adriat [Internet] 2007. June [cited 2019 May 5];16(2):47–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17992457 [PubMed] [Google Scholar]
- 30.Liu F-T, Goodarzi H, Chen H-Y. IgE, Mast Cells, and Eosinophils in Atopic Dermatitis. Clin Rev Allergy Immunol [Internet] 2011. December 20 [cited 2019 May 5];41(3):298–310. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21249468 [DOI] [PubMed] [Google Scholar]
- 31.Kim BS. Innate Lymphoid Cells in the Skin. J Invest Dermatol [Internet] 2015. March 1 [cited 2019 May 5];135(3):673–8. Available from: https://www.sciencedirect.com/science/article/pii/S0022202X15371566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol [Internet] 2014. February [cited 2019 Mar 9];133(2):448–460.e7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24373353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti– Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N Engl J Med [Internet] 2017. March 2 [cited 2019 Mar 10];376(9):826–35. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1606490 [DOI] [PubMed] [Google Scholar]
- 34.Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci [Internet] 2016. November 22 [cited 2019 May 16];113(47):E7572–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27821781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell [Internet] 2013. October 10 [cited 2019 Mar 17];155(2):285–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24094650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parnes JR, Sullivan JT, Chen L, Dias C. Pharmacokinetics, Safety, and Tolerability of Tezepelumab ( AMG 157) in Healthy and Atopic Dermatitis Adult Subjects. Clin Pharmacol Ther [Internet] 2019. March 23 [cited 2019 Apr 14];cpt.1401. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/cpt.1401 [DOI] [PMC free article] [PubMed]
- 37.Guttman-Yassky E, Bissonnette R, Ungar B, Suár ez-Fariñas M, Ardeleanu M, Esaki H, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol [Internet] 2019. January [cited 2019 Jun 23];143(1):155–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30194992 [DOI] [PubMed] [Google Scholar]
- 38.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell [Internet] 2017. September 21 [cited 2019 May 5];171(1):217–228.e13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28890086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J Allergy Clin Immunol [Internet] 2019. January [cited 2019 May 5];143(1):135–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29906525 [DOI] [PubMed] [Google Scholar]
- 40.Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol [Internet] 2018. May [cited 2019 May 5];78(5):863–871.e11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29353026 [DOI] [PubMed] [Google Scholar]
- 41.Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAK-STAT [Internet] 2013. July 1 [cited 2019 May 6];2(3):e24137 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24069552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bissonnette R, Papp KA, Poulin Y, Gooderham M, Raman M, Mallbris L, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol [Internet] 2016. November [cited 2019 Mar 18];175(5):902–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27423107 [DOI] [PubMed] [Google Scholar]
- 43.Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol [Internet] 2015. September [cited 2019 Mar 18];73(3):395–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26194706 [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa H, Nemoto O, Igarashi A, Nagata T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase II, multicentre, randomized, vehicle-controlled clinical study. Br J Dermatol [Internet] 2018. February [cited 2019 May 5];178(2):424–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28960254 [DOI] [PubMed] [Google Scholar]
- 45.Owens S, Howell MD. Ruxolitinib Cream Suppresses Th2 Inflammation in Adult Patients With Atopic Dermatitis. J Allergy Clin Immunol [Internet] 2019. February 1 [cited 2019 May 5];143(2):AB128 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674918321298 [Google Scholar]
- 46.Beck L, Hong C, Hu X, Chen S, Calimlim B, Teixeira H, et al. UPADACITINIB EFFECT ON PRURITUS IN MODERATE-TO-SEVERE ATOPIC DERMATITIS; FROM A PHASE 2B RANDOMIZED, PLACEBO-CONTROLLED TRIAL. Ann Allergy, Asthma Immunol [Internet] 2018. November 1 [cited 2019 May 5];121(5):S21 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1081120618308093 [Google Scholar]
- 47.Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol [Internet] 2019. April [cited 2019 Apr 14];80(4):913–921.e9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29410014 [DOI] [PubMed] [Google Scholar]
- 48.Zeidler C, Yosipovitch G, Ständer S. Prurigo Nodularis and Its Management. Dermatol Clin [Internet] 2018. July [cited 2019 Mar 10];36(3):189–97. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0733863518300147 [DOI] [PubMed] [Google Scholar]
- 49.Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Ständer S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatology Venereol [Internet] 2013. May 1 [cited 2019 Mar 10];27(5):550–7. Available from: http://doi.wiley.com/10.1111/j.1468-3083.2012.04481.x [DOI] [PubMed] [Google Scholar]
- 50.Matthews SN, Cockerell CJ. Prurigo nodularis in HIV-infected individuals. Int J Dermatol [Internet] 1998. June [cited 2019 Mar 10];37(6):401–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9646121 [DOI] [PubMed] [Google Scholar]
- 51.Lotti T, Buggiani G, Prignano F. Prurigo nodularis and lichen simplex chronicus. Dermatol Ther [Internet] 2008. January [cited 2019 Mar 10];21(1):42–6. Available from: http://doi.wiley.com/10.1111/j.1529-8019.2008.00168.x [DOI] [PubMed] [Google Scholar]
- 52.Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol [Internet] 2010. May 1 [cited 2019 May 5];37(5):578–86. Available from: http://doi.wiley.com/10.1111/j.1600-0560.2009.01484.x [DOI] [PubMed] [Google Scholar]
- 53.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol [Internet] 2006. February 1 [cited 2019 Mar 9];117(2):411–7. Available from: https://www-sciencedirect-com.beckerproxy.wustl.edu/science/article/pii/S0091674905023274?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 54.Liang Y, Jacobi HH, Reimert CM, Haak-Frendscho M, Marcusson JA, Johansson O. CGRP-immunoreactive nerves in prurigo nodularis - an exploration of neurogenic inflammation. J Cutan Pathol [Internet] 2000. August 1 [cited 2019 Mar 9];27(7):359–66. Available from: http://doi.wiley.com/10.1034/j.1600-0560.2000.027007359.x [DOI] [PubMed] [Google Scholar]
- 55.Haas S, Capellino S, Phan NQ, Böhm M, Luger TA, Straub RH, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci [Internet] 2010. June 1 [cited 2019 Mar 9];58(3):193–7. Available from: https://www.sciencedirect.com/science/article/pii/S0923181110000988 [DOI] [PubMed] [Google Scholar]
- 56.Beck KM, Yang EJ, Sekhon S, Bhutani T, Liao W. Dupilumab Treatment for Generalized Prurigo Nodularis. JAMA Dermatology [Internet] 2019. January 1 [cited 2019 Mar 10];155(1):118 Available from: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/jamadermatol.2018.3912 [DOI] [PubMed] [Google Scholar]
- 57.Ständer S, Kwon P, Hirman J, Perlman AJ, Weisshaar E, Metz M, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol [Internet] 2019. May [cited 2019 Jun 22];80(5):1395–402. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30894279 [DOI] [PubMed] [Google Scholar]
- 58.Berger TG, Steinhoff M. Pruritus in Elderly Patients-Eruptions of Senescence. Semin Cutan Med Surg [Internet] 2011. [cited 2019 May 5];30:113–7. Available from: https://scmsjournal.com/wp-content/uploads/2016/01/Vol30_i2-Berger-Elderly-Patients.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu AZ, Tripathi SV., Kau AL, Schaffer A, Kim BS Immune dysregulation underlies a subset of patients with chronic idiopathic pruritus. J Am Acad Dermatol [Internet] 2016. May [cited 2019 May 5];74(5):1017–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27085236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yosipovitch G, Ständer S, Kerby MB, Larrick JW, Perlman AJ, Schnipper EF, et al. Serlopitant for the treatment of chronic pruritus: Results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. J Am Acad Dermatol [Internet] 2018. May [cited 2019 May 5];78(5):882–891.e10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29462657 [DOI] [PubMed] [Google Scholar]
- 61.Kostner L, Anzengruber F, Guillod C, Recher M, Schmid-Grendelmeier P, Navarini AA. Allergic Contact Dermatitis. Immunol Allergy Clin North Am [Internet] 2017. February 1 [cited 2019 Apr 16];37(1):141–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27886903 [DOI] [PubMed] [Google Scholar]
- 62.Kimber I, Basketter DA, Gerberick GF, Dearman RJ. Allergic contact dermatitis. Int Immunopharmacol [Internet] 2002. February [cited 2019 May 6];2(2–3):201–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11811925 [DOI] [PubMed] [Google Scholar]
- 63.Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician [Internet] 2010. August 1 [cited 2019 May 6];82(3):249–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20672788 [PubMed] [Google Scholar]
- 64.Mowad C, Marks J. Allergic Contact Dermatitis. In: Bolognia J, Jorizzo J, Schaffer J, editors. Dermatology 3rd ed. Philadelphia: Elsevier Saunders; 2012. [Google Scholar]
- 65.Kamo A, Negi O, Tengara S, Kamata Y, Noguchi A, Ogawa H, et al. Histamine H 4 Receptor Antagonists Ineffective against Itch and Skin Inflammation in Atopic Dermatitis Mouse Model. J Invest Dermatol [Internet] 2014. February [cited 2019 May 6];134(2):546–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23962807 [DOI] [PubMed] [Google Scholar]
- 66.Askenase PW, Van Loverent H. Delayed-type hypersensitivity: activation of mast cells by antigen-specific T-cell factors initiates the cascade of cellular interactions. Immunol Today [Internet] 1983. September [cited 2019 Apr 15];4(9):259–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25290305 [DOI] [PubMed] [Google Scholar]
- 67.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Köhler A, et al. Mast Cells Are Key Promoters of Contact Allergy that Mediate the Adjuvant Effects of Haptens. Immunity [Internet] 2011. June 24 [cited 2019 Apr 16];34(6):973–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21703544 [DOI] [PubMed] [Google Scholar]
- 68.Gimenez-Rivera V-A, Siebenhaar F, Zimmermann C, Siiskonen H, Metz M, Maurer M. Mast Cells Limit the Exacerbation of Chronic Allergic Contact Dermatitis in Response to Repeated Allergen Exposure. J Immunol [Internet] 2016. November 1 [cited 2019 Apr 16];197(11):4240–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27807191 [DOI] [PubMed] [Google Scholar]
- 69.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell–derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol [Internet] 2007. October 2 [cited 2019 Apr 16];8(10):1095–104. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17767162 [DOI] [PubMed] [Google Scholar]
- 70.Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, et al. Nppb Neurons Are Sensors of Mast Cell-Induced Itch. Cell Rep 2019; [DOI] [PMC free article] [PubMed]


