Abstract
Objective:
Determine the clinical predictors of cardiac diagnosis in children referred for evaluation following a sudden death in the family.
Background:
After sudden death occurs in the young, first-degree family members should undergo clinical screening for occult cardiac disease, but the diagnostic yield from screening is not well-defined in the United States.
Methods:
Patients referred for a family history of sudden death were evaluated in a retrospective review from a tertiary pediatric referral center.
Results:
Among 419 pediatric relatives of 256 decedents, 27% of patients were diagnosed with a disease or had a clinical finding of uncertain significance. Patients were diagnosed with heritable cardiac disease in 39 cases (9.3%). Non-heritable cardiac disease was diagnosed in another 5.5% of patients. Clinical findings of uncertain significance (FUS) were present in 52 patients (12.4%), including abnormal electrophysiological test results (41/52) or imaging test results (11/52). Among patients diagnosed with a heritable cardiac disease, the nearest affected relative was almost always a first-degree relative (37/39, 95%). The strongest predictors for a successful diagnosis in the patient were an abnormal ECG and a first-degree relationship to the nearest affected relative (odds ratio 24.2 and 18.8, respectively).
Conclusions:
Children referred for a family history of sudden death receive cardiac disease diagnoses (14%), but FUS increase the challenge of clinical management. The importance of a diagnosis in first-degree affected relatives supports the clinical practice of testing intervening family members first when patients are second- or higher-degree relatives to the decedent.
Keywords: Familial sudden death, pediatrics, cardiology, arrhythmia
Condensed Abstract:
Among 419 pediatric relatives of 256 decedents, cardiac diseases or findings of uncertain significance were present in 114/419 patients (27%). Patients were diagnosed with heritable cardiac disease in 39 cases (9.3%). Non-heritable cardiac disease was diagnosed in another 5.5% of patients. Among patients diagnosed with a heritable cardiac disease, the nearest affected relative was nearly always a first-degree relative (37/39, 95%).
Introduction
The incidence of sudden death in the young due to potentially heritable causes is 0.5 to 2.3 per 100,000 people under age 40 (1–7). Genetic testing implicates heritable disorders of heart rhythm and function in 30% of post-mortem samples derived from cases of sudden death in the young (8–12). Additional sudden deaths can occur in genotype-negative families, implying that undetected heritable risk factors may exist. The identification of phenotypic abnormalities in relatives of sudden death victims may be a marker of an occult genetic predisposition (13).
Current guidelines recommend that first-degree family members receive a cardiac evaluation when a young relative dies suddenly (14). Studies using large, centralized referral systems in England, France, Australia, New Zealand, and the Netherlands have reported positive clinical screening in 10% to 30% of first-degree family members, depending on the setting and the testing performed (9,15–18). The United States does not operate on a centralized, single-payer healthcare system. Referrals are dictated by insurance patterns, negotiated contracts, geographic considerations and personal or family preference. Referring physicians have discretion to refer relatives for cardiac evaluation. These relatives may have any relationship to the decedent (first-degree, second-degree or higher-degree). Many parents place emphasis on screening the children in the family regardless of relationship to decedent. One consequence of these decisions is that more distantly related children may be screened before more closely related adults.
We used the records of a large, tertiary care pediatric health care system in the United States to review clinical testing and diagnostic success of children referred for evaluation because of a family history of sudden death. Our central goal was to quantify the yield of cardiac testing based on the degree of family separation between the decedent and the referred patient and to determine clinical predictors of a cardiac disease diagnosis.
Methods
Methods of Retrospective Review
We performed a retrospective review of records from 1999 to 2016 in a tertiary pediatric referral center. This included 20 inpatient and outpatient locations, including satellite clinics. We reviewed records for patients with ICD-9 codes related to sudden death (427.1, V17.49, V17.41, V19.8, V61.07, V12.53 or any code beginning with V98), supplemented with queries of clinical cardiology databases. We identified as many family members as possible: relatives mentioned in any patient’s medical record were investigated and included in the retrospective review if they had been evaluated at our center.
We report data from both living and deceased subjects. We have referred to all living subjects as “patients” to distinguish them from the deceased subjects (“decedents”). For inclusion, the patient’s initial cardiac evaluation had to be motivated by the family history of sudden death; we excluded patients with previously known heart disease. In families with more than one sudden death, the most closely-related decedent was chosen as the primary decedent. Several clinic notes referenced the HRS/EHRA/APHRS Expert Consensus Statement after its publication in 2013 and our institution now has a formalized protocol for family evaluation based on this consensus statement (14), but no pre-defined protocol for evaluation of family history of sudden death was in place at our center during the period of this study. All variants were reclassified prior to publication using the 2015 American College of Medical Genetics criteria by a certified genetic counselor and a cardiologist with experience in inherited genetics (19).
Each medical record was reviewed by two separate study members. Study data were collected and managed using REDCap electronic data capture tools hosted at Northwestern University (20). Approval for this study was obtained from our Institutional Review Board.
Nearest Affected Relative
We defined a “nearest affected relative” for each patient. Initially, the decedent was assigned as the patient’s nearest affected relative. If a family member with a heritable cardiac disease was found to have a closer relationship, we updated the patient’s “nearest affected relative” to reflect the closer relationship. This was repeated until all family members had been adjudicated and the nearest affected relative was the most closely related family member with either a heritable cardiac disease phenotype or a history of sudden death.
Pre-Specified Phenotype Categories
We pre-specified five phenotype categories. First, patients could have a diagnosis of hereditary cardiac disease. Second, patients could have cardiac disease that does not typically have a hereditary component nor is associated with sudden death (for example, ventricular septal defect). Third, patients could have one or more “findings of uncertain significance” (FUS). Prior to starting the study, we pre-defined a list of FUS by selecting cardiac test results that are potential early signs of cardiomyopathy or congenital arrhythmia syndromes (Online Table 1). Fourth, patients could have variations of normal on initial testing. These variations of normal were classified separately because these findings might have been dismissed in an otherwise healthy child, but patients typically received further workup in the setting of a family history of sudden death. Fifth, patients could have a normal workup, with no evidence of cardiac disease.
Diseases with both hereditary and sporadic forms (bicuspid aortic valve and ventricular pre-excitation) and diseases that did not fit our pre-defined taxonomy (tricuspid valve prolapse) were classified on a case-by-case basis. Clinical details are provided in the Online Appendix.
Decedent Cause of Death
The cause of death for each decedent was determined by examining autopsy, histology, toxicology and genetic data. In cases where adjudication was not possible from the decedent’s autopsy data, the clinician’s conclusion was used if it was specific and supported by pre-mortem data. If the cause of death was not explicit and specific, the decedent’s cause of death was classified as “unknown”.
Family Diagnosis
In each family, we assigned a “family diagnosis”. A patient diagnosis with documented phenotype data was the strongest factor in assigning a family diagnosis, followed by data from a family member who was not seen in our institution (but for whom we could review clinical records). The decedent’s diagnosis was given next priority if it was able to be classified. If a family diagnosis could not be determined after reviewing those documents, the family diagnosis was classified as “unknown”.
Statistical Analysis
Descriptive statistics summarized key variables for patients and decedents. Analyses at the family level included univariable associations between decedent level predictors and presence of family diagnosis using unadjusted logistic regression models. Generalized linear mixed effects models for binary outcomes considered associations between each predictor of interest and the presence of a heritable diagnosis in the patient, in turn. A random family effect allowed for clustering of patients related to the same decedent. Adjusted models included variables deemed significant in univariable associations (alpha = 0.05). Positive and negative predictive values evaluated each cardiac test in terms of ability to accurately detect the presence or absence of a diagnosis. All analyses assumed a two-sided type I error rate of 0.05.
Results
We identified 419 pediatric patients who were relatives of 256 decedents. The median age of the patients was 9.8 years (IQR 4.4 – 14.6). The median duration of clinical follow-up was 2.5 years (IQR 1.0 – 5.1) among 240 patients with at least one follow-up visit. No patients died during 833 cumulative years of follow-up. There was no follow-up in 179 of 419 patients.
Diagnoses among decedents
Decedents died of a demonstrable cardiac etiology in 41/256 (16%) families; cardiomyopathy was the most common diagnosis (Table 1). The presence of a heritable cause of death was associated with a heritable diagnosis in the patient (univariable odds ratio 3.1, Table 2). However, the presence of a first-degree nearest affected relative and a personal history of a cardiac phenotype confound this observation. In a multivariable regression model, decedent heritable cause of death was no longer significantly associated with the patient having a heritable diagnosis (p=0.58, Table 3), but a first-degree nearest affected relative and the presence of an abnormal ECG for the patient remain significant.
Table 1.
Demographics among patients and decedents
| Patients | |
|---|---|
| N (%) | |
| Age (mean, standard deviation) | 9.5 ± 6.3 |
| Male | 231 (55) |
| History of any of the following: | |
| Syncope | 30 (7) |
| Seizure | 13 (3) |
| Atrial arrhythmias | 3 (1) |
| Ventricular arrhythmias | 9 (2) |
| On medications | 51 (12) |
| ICD implanted | 8 (2) |
| Decedents | |
| Age (years) | |
| <5 | 29 (11) |
| 5–17 | 42 (16) |
| 18–24 | 37 (14) |
| 25–39 | 87 (34) |
| 40+ | 44 (17) |
| Unknown | 17 (7) |
| Gender | |
| Male | 152 (59) |
| Female | 79 (31) |
| Unknown | 25 (10) |
| Cause of death | |
| Hypertrophic cardiomyopathy | 9 (4) |
| Dilated cardiomyopathy | 8 (3) |
| Long QT syndrome | 8 (3) |
| Adult-onset coronary disease | 5 (2) |
| Arrhythmogenic right ventricular cardiomyopathy | 4 (2) |
| Congenital coronary artery abnormality | 2 (1) |
| Aortic stenosis | 2 (1) |
| Left ventricular non-compaction cardiomyopathy | 1 |
| Catecholaminergic polymorphic VT | 1 |
| Ventricular pre-excitation | 1 |
| Unknown cause of death | 215 (84) |
| Activity at time of death | |
| At rest, sleeping, or light activity | 95 (37) |
| Strenuous activity or emotional trigger | 43 (17) |
| Not documented | 118 (46) |
| History of any of the following: | |
| Syncope prior to the event | 17 (7) |
| Seizure prior to the event | 10 (4) |
| Atrial arrhythmias | 2 (1) |
| Ventricular arrhythmias | 6 (2) |
ICD, implantable cardioverter-defibrillator; MI, myocardial infarction; VT, ventricular tachycardia
Table 2.
Univariable associations between patient/decedent predictors and the presence of a heritable cardiac diagnosis in the patient
| Presence of Heritable Disease | OR (95% CI) | P-value1 | ||
|---|---|---|---|---|
| No | Yes | |||
| Patient Predictors | ||||
| Sex | ||||
| Male | 206 (89.2) | 25 (10.8) | Ref | 0.28 |
| Female | 174 (92.6) | 14 (7.5) | 0.7 (0.3, 1.4) | |
| Any history of syncope, seizure, atrial or ventricular tachycardia | Ref | 0.06 | ||
| No | 344 (91.7) | 31 (8.3) | 2.6 (0.97,7.0) | |
| Yes | 36 (81.8) | 8 (18.2) | ||
| Abnormal ECG | ||||
| No | 287 (99.0) | 3 (1.0) | Ref | < 0.0001 |
| Yes | 80 (69.0) | 36 (31.0) | 38.4 (11.0, 134.4) | |
| Missing | 13 (100.0) | 0 (0.0) | -- | |
| Abnormal echocardiogram | ||||
| No | 292 (91.3) | 28 (8.8) | Ref | 0.005 |
| Yes | 24 (72.7) | 9 (27.3) | 4.6 (1.6, 13.3) | |
| Missing | 64 (97.0) | 2 (3.0) | -- | |
| Nearest affected relative is first-degree | ||||
| No | 183 (98.9) | 2 (1.1) | Ref | 0.0004 |
| Yes | 197 (84.2) | 37 (15.8) | 15.7 (3.5,70.8) | |
| Decedent Predictor | ||||
| Sex of decedent | ||||
| Male | 232 (94.3) | 14 (5.7) | Ref | 0.01 |
| Female | 119 (83.8) | 23 (16.2) | 3.0 (1.3,7.1) | |
| Unknown | 23 (93.6) | 2 (6.5) | -- | |
| Heritable diagnosis in the decedent | ||||
| No | 326 (92.6) | 26 (7.4) | Ref | 0.02 |
| Yes | 54 (80.6) | 13 (19.4) | 3.1 (1.2,8.1) | |
P-value from generalized linear mixed effect model with logit link
Table 3.
Multiple regression model for presence of heritable cardiac diagnosis in the patient including patient and decedent predictors1
| Predictor | OR (95% CI) | P-value2 |
|---|---|---|
| Any Abnormal ECG - Patient | ||
| No | Ref | <0.01 |
| Yes | 24.2 (6.1,95.4) | |
| Nearest affected is first-degree | ||
| No | Ref | 0.01 |
| Yes | 18.8 (2.0, 180) | |
| Heritable diagnosis in the decedent | ||
| No heritable diagnosis or unknown | Ref | 0.58 |
| Yes | 1.5 (0.4,5.5) |
Model adjusted for abnormal echocardiogram and sex of decedent.
P-value from generalized linear mixed effect model with logit link
Patients with a Disease
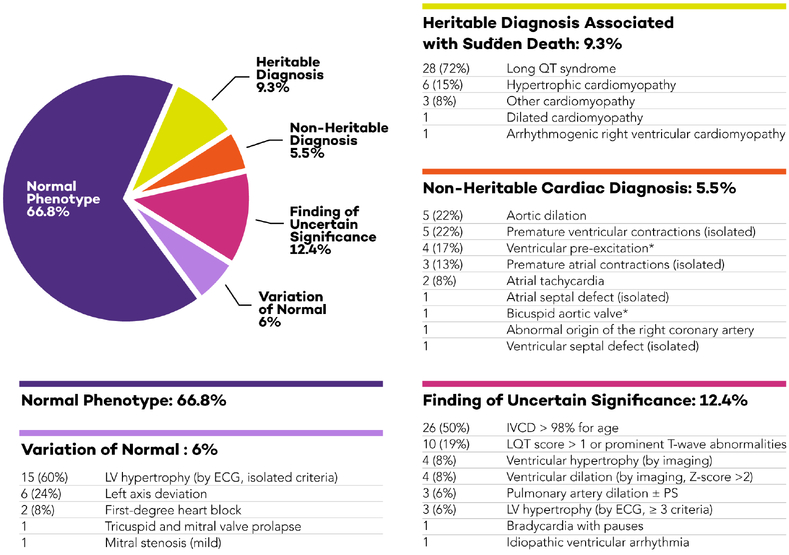
In our most important finding, 114/419 (27%) of patients were diagnosed with a cardiac disease or had a clinical FUS. A heritable cardiac disease was diagnosed in 39/419 patients (9.3%). These included 28/39 with LQTS, six with hypertrophic cardiomyopathy, one with ARVC, and four with other variants of cardiomyopathy. All 39 patients with a heritable cardiac disease received therapy or were prescribed lifestyle modifications. A non-heritable cardiac disease was diagnosed in 23/419 patients (5.5%, Figure 1). Therapy or intervention was begun in five of the 23 patients; the remainder received ongoing cardiology follow-up. Therefore, all 62 patients diagnosed with a disease received modification of their medical plan in the form of direct intervention or the requirement for cardiology follow-up.
Figure 1: Expanded categorization of cardiac diagnoses among children with a relative who suffered sudden death.
Diagnoses were tabulated for all patients and stratified into 5 predetermined categories. While heritable and non-heritable cardiac diagnoses accounted for 15% of all evaluations, an additional 12% of children had findings of uncertain significance.
(*) Ventricular pre-excitation and bicuspid aortic valve have heritable and non-heritable forms; in these patients, first-degree relatives had normal evaluations, suggesting sporadic disease (Clinical Supplement). Abbreviations: ECG, electrocardiogram; IVCD, intraventricular conduction delay; LQT, long QT; LV, left ventricular; PS, pulmonary stenosis.
Symptoms were present in 44/419 patients. Syncope was present in 30 patients, seizure in 13, atrial arrhythmias or SVT in 3, ventricular arrhythmias in 9, and 10 patients had more than one of these symptom classes. The odds of a heritable cardiac diagnosis was not increased in patients with one or more of these symptoms (Table 2).
Patients with Clinical Findings of Uncertain Significance (FUS) or Normal Findings
Not all patients with abnormal cardiac tests were diagnosed with a disease. A FUS was present in 52/419 patients (12.4%). Figure 1 tabulates the identities of the FUS, which primarily included electrical findings (41/52, including intraventricular conduction delay, prolonged repolarization, LV hypertrophy by ECG, bradycardia and arrhythmia) and imaging findings (11/52 including ventricular hypertrophy or dilation and pulmonary artery dilation).
We found mild changes that were variations of normal in 25/419 (6%) patients and 280/419 (66.8%) patients had a normal phenotype.
Impact of family structure
Patients and decedents were most commonly first-degree relatives (172/419, 41%) or second-degree relatives (162/419, 39%). Fewer patients and decedents were higher-degree relatives (85/419, 20%).
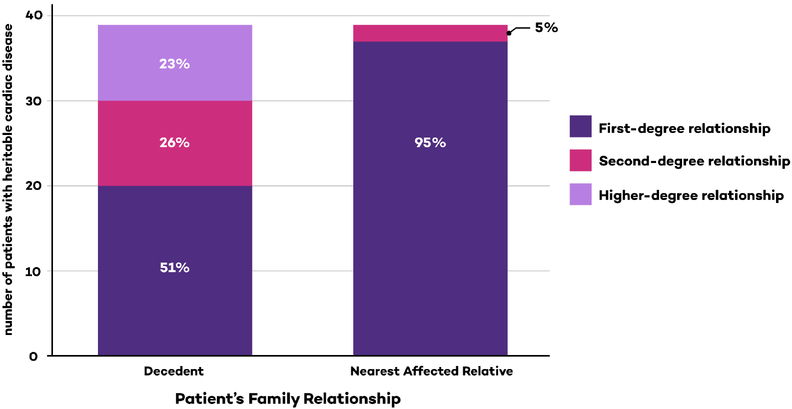
Almost all diagnoses of heritable cardiac disease occurred in patients whose nearest affected relative was a first-degree relationship (35/37 patients with heritable cardiac disease). The presence of a first-degree relationship between the patient and the nearest affected relative pair was associated with an increased odds of having a heritable diagnosis (OR 18.8, Table 3). Only 2/419 patients were diagnosed with a heritable disease without a first-degree nearest affected relative. In each of these two pedigrees, multiple family members had clinical LQTS. In contrast to the information available from having an affected first-degree relative, the relationship between the patient and the decedent was not associated with having a heritable diagnosis (p=0.13).
We considered heritable diagnoses primarily at the patient level, because most cardiologists see patients individually; however, the diagnostic yield can also be considered by family. A heritable cardiac diagnosis was determined in 26/256 families (10%); any cardiac diagnosis (heritable or non-heritable) was found in 47/256 families (18%). A FUS was found in 45/256 families (18%). Families also bring details about a decedent’s death to the clinical evaluation. We considered whether those historical details impact the frequency of a cardiac diagnosis in one or more members of the family. Pre-mortem, 30/256 decedents (12%) had a history of syncope, seizure, atrial or ventricular arrhythmias. The presence of any of these pre-mortem symptoms was associated with an increased odds that a definitive diagnosis would be made in the family in univariable analysis (Table 4). In contrast, the decedent’s age, gender, and his or her activity at death were not associated with determining a diagnosis in the family.
Table 4.
Decedent variables: univariable associations between predictors of interest and the presence of a heritable cardiac diagnosis in the family
| Family Diagnosis | OR (95% CI) | P-value1 | ||
|---|---|---|---|---|
| No (%) | Yes (%) | |||
| Age of Decedent | ||||
| <5 | 26 (14) | 3(4) | 0.2 (0.1,0.8) | 0.11 |
| 5–17 | 27 (15) | 15 (21) | 1.0 (0.4,2.3) | |
| 18–24 | 25 (14) | 12 (16) | 0.8 (0.3,2.1) | |
| 25–39 | 66 (36) | 21 (29) | 0.6 (0.3, 1.2) | |
| 40+ | 28 (15) | 16 (22) | Ref | |
| Unknown | 11 (6) | 6 (8) | --- | |
| Sex | ||||
| Male | 109 (60) | 43 (59) | Ref | 0.47 |
| Female | 53 (29) | 26 (36) | 1.2 (0.7,2.2) | |
| Unknown | 21 (11) | 4 (5) | --- | |
| Activity at death | ||||
| At rest, sleeping, or light activity | 65 (36) | 30 (41) | Ref | 0.15 |
| Strenuous activity or emotional | 24 (13) | 19 (32) | 1.7 (0.8,3.6) | |
| trigger | ||||
| Unknown | 94 (51) | 24 (33) | --- | |
| Any syncope, seizure, atrial or ventricular tachycardia, pre-mortem | ||||
| No | 167 (91) | 59 (81) | Ref | 0.02 |
| Yes | 16 (9) | 14 (19) | 2.5 (1.1, 5.4) | |
Unknown or missing data were excluded from logistic regression models
P-value from simple logistic regression model
Yield of phenotype screening among patients
Nearly all patients received an ECG (406/419, 97%). The positive predictive value of ECG for diagnosing any disease was low at 43% (CI95 34–53%). The negative predictive value for ECG was 96% (CI95 93–98%). For this analysis, FUS were classified as negative or “non-diagnostic” evaluations. However, in a sensitivity analysis, if FUS and cases of non-heritable cardiac disease were classified as positive evaluations because they require long-term follow-up, the test characteristics of ECG were still inadequate (positive predictive value of ECG was 80% and the negative predictive value was 93%).
Echocardiograms were obtained in 353/419 patients (84%) and 33/353 echocardiograms were abnormal. In 9/33 abnormal echocardiograms, the patient had a heritable cardiac diagnosis (five hypertrophic cardiomyopathy, one ARVC, one other cardiomyopathy, and two with LQTS+ASD). There were 10 patients with abnormal echocardiograms who had a non-heritable cardiac diagnosis. The positive predictive value of echocardiogram was 58% and the negative predictive value was 87%.
Exercise stress tests were performed in 106/419 patients. The frequency of exercise stress testing was higher in the years following publication of the 2013 guidelines (14). Abnormal findings were documented in 36/106 exercise stress tests. However, all 36 of the abnormal tests occurred after a diagnosis was made (e.g. LQTS) or were performed for risk stratification (e.g. ventricular pre-excitation). None of the exercise stress tests diagnosed catecholaminergic polymorphic ventricular tachycardia or had ST changes suggestive of ischemia.
Only 14 patients underwent provocative pharmacologic testing. Procainamide testing was done in 12/14 patients, aged two weeks to 21 years, none of which resulted in a diagnosis of Brugada syndrome. Epinephrine testing was done in 6/14 and yielded two diagnoses of LQTS. Most provocative testing occurred in the last five years as practice patterns have changed at our institution.
Putting these phenotypic results in statistical perspective, in univariable models abnormal ECG and abnormal echocardiogram were associated with higher odds of the patient receiving a heritable diagnosis (Table 2). Multiple regression modeling demonstrated that the presence of an abnormal ECG or an affected first-degree relative remained significantly associated with a higher odds ratio of finding a heritable cardiac diagnosis in models also adjusting for abnormal echocardiogram, sex of decedent, and heritable diagnosis in the decedent (odds ratio 24.2 and 18.8 respectively, Table 3). The presence of a heritable diagnosis in the decedent was not associated with an increased likelihood of the patient receiving a heritable diagnosis.
In our retrospective review, 8/114 patients had dilation of the aorta or pulmonary artery, representing 7% of patients with a cardiac diagnosis or a FUS.
Implantable cardioverter defibrillators (ICDs)
Implantable cardioverter defibrillators were placed in 8 patients (5 with LQTS, one with hypertrophic cardiomyopathy, 2 with cardiomyopathy with depressed systolic function) and no ICD complications occurred. A secondary prevention indication was present in three patients. All three patients with a secondary prevention indication later received one or more appropriate shocks. ICDs were implanted for primary prevention based on family history in four patients. The implant indication could not be determined in one patient. None of these five patients received an appropriate or inappropriate shock.
Genetic testing
Table 5 tabulates the yield of genetic testing within the 46 families. Using this family-centered approach, 12 families (26%) had a likely pathogenic or pathogenic (LP/P) variant, 16 families (33%) had a VUS, and 18 families (39%) had no variant identified. Genetic testing was most frequently performed in families with long QT syndrome or hypertrophic cardiomyopathy (28/46 families).
Table 5:
Genetic results, stratified by Family Diagnosis. The family diagnosis is in bold as the header to each section.
| Family Number | Decedent Diagnosis | Proband | Variant | Interpretation | Genotype Positive Patients (#) | Genotype Negative Patients (#) |
|---|---|---|---|---|---|---|
| Long QT syndrome | ||||||
| 1 | Unknown | Patient | KCNQ1 (–) exon 13–16 | P/LP | 3 | 1 |
| 2 | Unknown | Patient | KCNQl p.Ala344Val | P/LP | 2 | 1 |
| 3 | LQTS | Patient | KCNQl p.Arg591His | P/LP | 2 | 2 |
| 4 | LQTS | Family member | KCNH2 p. Prol034Glyfs*24 | P/LP | 3 | 0 |
| 5 | Unknown | Patient | SCN5A p.Alal326Ser | P/LP | 2 | 0 |
| 6 | Unknown | Patient | SCN5A p.Thrl304Met | P/LP | 1 | 1 |
| 7 | LQTS | Molecular autopsy | CALM1 p.Asn98Ser | P/LP | 0 | 1 |
| 8 | Unknown | Patient | KCNQ1 p.Gln530Pro | VUS | 4 | 3 |
| 9 | Unknown | Patient | KCNQl p.Gly348Asp | VUS | 1 | 2 |
| 10 | Unknown | Molecular autopsy | KCNH2 p.Argl76Trp | VUS | 2 | 0 |
| 11 | Unknown | Family member | KCNH2 p.Ala913Val | VUS | 3 | 0 |
| 12 | LQTS | Molecular autopsy | SCN5A p.Glu462Ala | VUS | 1 | 1 |
| 13 | LQTS | Molecular autopsy | CACNAlC p.R858H | VUS | 0 | 1 |
| 14 | LQTS | Patient | No variants | 3 | 1 | |
| 15 | Unknown | Patient | No variants | 0 | 1 | |
| 16 | Unknown | Patient | No variants | 0 | 1 | |
| Brugada syndrome | ||||||
| 17 | Unknown | Family member | HCN4 p.Ser841Leu | VUS | 2 | 0 |
| Catecholaminergic Polymorphic Ventricular Tachycardia | ||||||
| 18 | Unknown | Family member | No variants | 0 | 2 | |
| Hypertrophic Cardiomyopathy | ||||||
| 19 | HCM | Patient | TPM1 p.Ser215Leu | P/LP | 1 | 0 |
| 20 | Unknown | Patient | MYBPC3 p.Asp75Asn | VUS | 1 | 1 |
| 21 | Unknown | Patient | MYBPC3 p.Gly278Glu | VUS | 1 | 0 |
| 22 | Unknown | Patient | MYBPC3 p.Leu629Phe | VUS | 1 | 0 |
| 23 | HCM | Molecular autopsy | TPM1 p.Lys226Gln | VUS | 1 | 1 |
| 24 | Unknown | Family member | No variants | 0 | 1 | |
| 25 | HCM | Patient | No variants | 0 | 2 | |
| 26 | HCM | Patient | No variants | 0 | 1 | |
| 27 | Unknown | Patient | No variants | 0 | 1 | |
| 28 | Unknown | Patient | No variants | 0 | 2 | |
| 29 | HCM | Molecular autopsy | No variants | 0 | 1 | |
| 30 | Unknown | Molecular autopsy | No variants | 0 | 0 | |
| Arrhythmogenic (Right) Ventricular Cardiomyopathy | ||||||
| 31 | Unknown | Patient | [DSC2 p.L732V and DSG2 p.V392D | VUS | 1 | 1 |
| Dilated Cardiomyopathy | ||||||
| 32 | Unknown | Patient | LMNA S437fs | P/LP | 1 | 0 |
| 33 | DCM | Patient | No variants | 0 | 2 | |
| Other cardiomyopathy | ||||||
| 34 | Unknown | Patient | DMPK 515 repeats | P/LP | 1 | 0 |
| 35 | HCM | Patient | NKX2.5 p.Glnl70* | P/LP | 1 | 0 |
| 36 | Unknown | Family member | [SCN5A p.Q1832Eand DSPp.E1740K] | VUS | 2 | 0 |
| 37 | ARVC | Molecular autopsy | No variants | 0 | 0 | |
| Unknown | ||||||
| 38 | Unknown | Molecular autopsy | TTN p.Phe2814Leufs*12 | P/LP | 1 | 0 |
| 39 | Unknown | Molecular autopsy | RYR2 p.Val2113Met | VUS | 3 | 3 |
| 40 | Unknown | Molecular autopsy | CACNAlC p.Val585Met | VUS | 1 | 1 |
| 41 | Unknown | Patient | [HCN4 p.E30K and COG1 p.T350M] | VUS | 1 | 0 |
| 42 | ARVC | Patient | No variants | 0 | 1 | |
| 43 | Unknown | Patient | No variants | 0 | 1 | |
| 44 | Unknown | Patient | No variants | 0 | 1 | |
| 45 | Unknown | Patient | No variants | 0 | 1 | |
| 46 | Unknown | Patient | No variants | 0 | 1 | |
Each row represents one family. “Patient” refers to a living participant in our study. Genotypes are only tabulated for living patients (decedents with molecular autopsy results are not tabulated). “Family member” refers to a family member who was the proband for genetic testing, but did not have clinical care at our center and thus was not eligible for inclusion in the study. Genotypes with more than one variant are enclosed in brackets, e.g. [variant 1 and variant 2]. ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LQTS, Long QT syndrome.
Molecular autopsy was performed in only 11 of 256 decedents, all of which occurred in the last 5 years. Molecular autopsy revealed likely pathogenic or pathogenic (LP/P) variants in two decedents (18%), VUS in six decedents (55%) and no variants in three decedents (27%). Three of the 11 molecular autopsy results occurred in families where neither the decedent nor the patients had an identifiable phenotype, including one truncation variant in titin that was classified by the 2015 guidelines as pathogenic (the surviving child was a 14-year-old girl with a normal ECG, stress test, and echocardiogram).
These genetic tests were distributed among 86 patients (panel tests in 33, family variant tests in 52 patients, and one whole exome test). Likely pathogenic/pathogenic variants were present in 10/33 panel tests (30%). A VUS was present in 5/33 panel tests (15%) and no variants were found in 55% of panel tests. Among patients with a heritable disease phenotype in whom genetic testing was performed, a LP/P variant was present in 19/33 patients (58%).
Family variant testing was performed in 52 patients, including five patients who were genotype-positive, but phenotype-negative. None of these five patients developed clinical disease during the period of follow-up in this retrospective analysis. The one whole exome test that was sent during this study period revealed only a single VUS in a patient with an idiopathic VF arrest.
LP/P variants were more likely to be found in patients with heritable cardiac phenotypes (66% vs. 20%, p<0.001). However, 6 patients were genotype positive for a LP/P variant, but phenotype negative.
Discussion
Diagnoses Affecting Clinical Care
Among children referred for evaluation because of a sudden death in the family, a successful diagnosis was made in 15% (Central Illustration). A heritable cardiac disease was diagnosed in 9.3% of patients and non-heritable cardiac disease was diagnosed in another 5.5% of patients. All of these patients required therapy and/or life-long cardiac follow-up.
We observed a higher rate of non-heritable cardiac findings than reported by international centers with centralized referral networks. This may be attributable to referral patterns. Pediatricians or primary care doctors who were concerned for other reasons may have been more likely to refer a patient for definitive evaluation following a sudden death in the family. While this may bias our study population, referral based on the primary care physician’s recommendation is common in the United States and thus our higher rate of non-heritable disease is likely applicable to other centers with similar referral patterns.
Findings of Uncertain Significance
In addition, our study was unique because we pre-defined a panel of clinical test results that we consider FUS, which were present in 12.4% of our patients. Patients with FUS merit extra clinical consideration for three reasons. First, FUS are – by definition – present in children without a definitive cardiac diagnosis. In some children, the phenotype may eventually blossom into a heritable cardiac disease. Arrhythmogenic right ventricular cardiomyopathy is an example of a disease that may be associated with slight abnormalities in childhood, but can progress to an overt phenotype in adulthood. In our center, we continue to monitor patients with FUS for clinical progression. Second, the presence of a FUS in a child should be a red flag that related adults in the family who have not yet undergone cardiac testing should be screened for a more fully expressed phenotype. The third important consideration about FUS is that they may invoke concern and additional cardiac testing without imparting any additional risk of a heritable cardiac disease. By analogy to genetic VUS, some of these FUS will have no clinical importance. Therefore, families and physicians who embark on a diagnostic odyssey after a family member’s death need to be aware how often they may still confront an ambiguous phenotype at the end of evaluation. Like VUS, clinical FUS should not trigger interventions (especially medications, defibrillators, or exercise restrictions) until time or new clinical information allows for a specific diagnosis to be made.
One strength of this study is that it pre-defined a potential group of FUS and established that a meaningful number of screened patients fall into this group. We do not suggest that our Online Table 1 is a complete compendium of FUS, nor do we suggest that every item on the list would achieve consensus among a clinical committee. A longitudinal study will be required to generate a definitive list of FUS that carry a risk of clinical progression.
Impact of Family Structure
The next finding in our study is that the relationship between the patient and the nearest affected relatives is an important predictor of diagnostic success. In 95% of our cases with a heritable diagnosis, the nearest affected relative was a first-degree relative. Having a first-degree nearest affected relative was associated with an increased odds of finding a heritable diagnosis in the patient and this result was durable in multivariable analysis. In 419 cases, we only twice observed a child with a heritable diagnosis in whom no first-degree relative was affected. In contrast, a first-degree relationship between the patient and the decedent was not significantly associated with an increase the odds of a heritable diagnosis for the patient. This occurs because many of the patients with second and higher-degree relationships with the decedent also had more closely affected relatives. When the patient and decedent are not first-degree relatives, the first priority should be to determine if any intervening members of the family tree have a positive phenotype.
In idealized referral patterns, all first-degree relatives would be screened after a sudden death event. If disease is found in the first-degree family, the workup “cascades” outward. In our center, and likely in other U.S. centers, medical evaluations can be requested because parents and/or physicians are eager for grandchildren, nieces, and nephews to be screened, even if the intervening adult relatives had not been screened. Our data show that the yield in children without an affected first-degree relative is low and incidental findings are common. These data provide quantitative support for prioritizing the screening of the most closely related family members.
Decedent Factors
The decedent’s history is important. A history of the decedent having pre-mortem syncope, seizure, or atrial/ventricular tachycardia was associated with a higher odds ratio of making a diagnosis in the family. In contrast, the decedent’s age, gender and what activity he or she was doing at the time of death were not associated with a higher likelihood of making a diagnosis. These data will be useful in giving families guidance at the initial visit about the likelihood of discovering cardiac disease in the family.
Genetic Testing
Genetic tests were ordered in 18% of our families. While LP/P variants were more likely to be found in patients with heritable cardiac phenotypes, six patients without a heritable cardiac phenotype were heterozygous for LP/P variants, underscoring that isolated genotype cascade screening is not sufficient to quantify family risk.
Table 5 reveals that the overall yield of a LP/P variant using a family-based approach to genetic testing was 26%. The current literature on molecular autopsy demonstrates a LP/P variant in 10–30% of cases (8–12). In our practice, we tell families that we expect to find a LP/P result after molecular autopsy or family screening in approximately a quarter of families. This number may continue to increase as more sophisticated data from molecular autopsy efforts are available.
Limitations
Neither ECG nor echocardiography is a perfect screening tool in children with a relative who died suddenly, although our data may be skewed by the retrospective nature and the referral bias mentioned above. We provided all ECG and echocardiogram PPV/NPV values with and without FUS included to establish bounds of sensitivity and specificity. Genetic tests were ordered most often in families with long QT syndrome and hypertrophic cardiomyopathy where the relationship between strongly positive phenotype and a monogenetic cause of disease is high. However, use of genetic tests from 1999 to 2016 was more idiosyncratic that current evidence-based approaches. Additionally, our relatively small number of heritable diseases affects the precision of estimates in regression models; and thus, odds ratios with large confidence intervals should be interpreted with caution. Finally, our medical record system did not retain detailed referral records over this period so we did not tabulate specific referral patterns.
Conclusions
In our center, one quarter of children who underwent a cardiac evaluation for a family history of sudden death had a cardiac diagnosis or a finding of uncertain significance. Ultimately, 9% of children were diagnosed with a heritable cardiac disease, and a non-heritable cardiac disease was found in another 5% of children. Findings of uncertain significance accounted for an additional 14% of the patients. In addition to demonstrating the frequency of non-heritable cardiac diagnoses and FUS, our study provides quantitative justification for the current policy of screening first-degree relatives before screening higher-degree relatives.
Supplementary Material
Online Table 1. Pre-specified criteria for “findings of uncertain significance” in the absence of other diagnostic criteria.
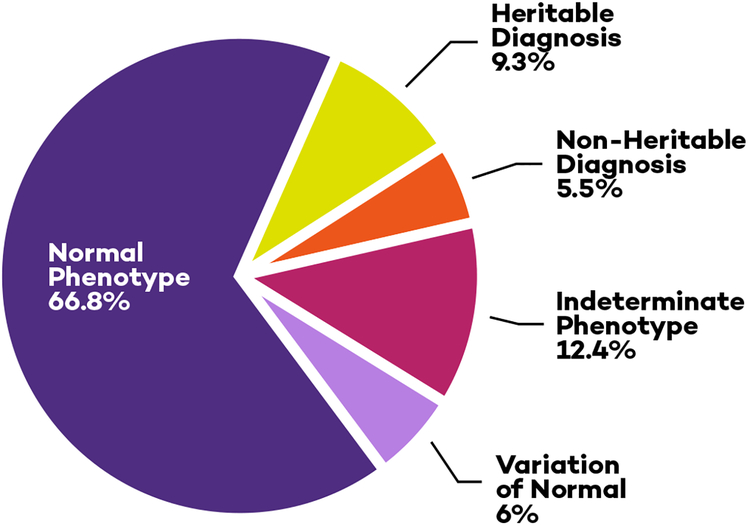
Central Illustration. Evaluations of Children after Familial Sudden Death.
Pie chart categorizing the cardiac evaluations of children referred for evaluation after a sudden death in the family. One quarter of children evaluated for a family history of sudden death had a cardiac disease or a finding of uncertain significance, but only 9% were found to have a heritable disease.
Figure 2: Comparison between two methods of identifying family relationships in patients diagnosed with a heritable disease.
The same 39 patients are tabulated in both columns. The relationships between the patients affected with a heritable disease and the decedent are shown in the left column. The relationships between the patients affected with a heritable disease and their nearest affected relative are shown in the right column. This paired bar graph illustrates that in patients diagnosed with a heritable disease, the relationship between the patient and the decedent was not the most reliable clinical marker. Instead, it was more important to establish whether intervening members of the family pedigree are affected. Presence of an affected first-degree relative was associated with a 19-fold increase in odds of determining a diagnosis in the patient in multivariable analysis.
Funding:
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences (Bethesda, MD), grant number KL2TR001424 and the National Institutes of Health, National Heart, Lung and Blood Institute, grant numbers K23HL130554 and U01HL131914, the American Heart Association Mentored Clinical and Population Research Award (Dallas, TX) and the Smeds Family Foundation (Chicago, IL). REDCap access was provided by Northwestern University Clinical and Translational Sciences Institute, funded in part by NIH UL1TR001422.
Abbreviations:
- ARVC
Arrhythmogenic right ventricular cardiomyopathy
- ASD
Atrial septal defect
- HCM
Hypertrophic cardiomyopathy
- ICD
Implantable cardioverter-defibrillator
- IVCD
Intraventricular conduction delay
- LQTS
Long QT syndrome
- LV
Left ventricle
- MRI
Magnetic resonance imaging
- MI
Myocardial infarction
- PS
Pulmonary stenosis
- QTc
QT interval, corrected with Bazett’s formula
- RV
Right ventricle
- SAECG
Signal averaged electrocardiogram
- VT
Ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Perspectives
Competency in Medical Knowledge: Evaluation of children with a family history of sudden death identified cardiac disease in 15% of cases. Nearly all children in whom a heritable cardiac disease was identified had an affected first-degree relative.
Translational Outlook: More sophisticated tools are needed to identify presently occult genetic factors that may contribute to heritable causes of sudden death.
Disclosures: Dr. George is a member of the Amgen Scientific Advisory Board (Thousand Oaks, CA). The authors have no other conflicts of interest to report.
Tweet for @JACCJournals: One quarter of children evaluated for a family history of sudden death in the young had clinical cardiac findings.
References
- 1.Bureau USC. National Population by Characteristics: 2010–2017. U.S. Department of Commerce, 2017. [Google Scholar]
- 2.Pilmer CM, Kirsh JA, Hildebrandt D, Krahn AD, Gow RM. Sudden Cardiac Death in Children and Adolescents Between 1 and 19 Years of Age. Heart Rhythm 2014;11:239–45. [DOI] [PubMed] [Google Scholar]
- 3.Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of Sudden Cardiac Death in National Collegiate Athletic Association Athletes. Circulation 2011;123:1594–600. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden Deaths in Young Competitive Athletes: Analysis of 1866 Deaths in the United States. 1980–2006. Circulation 2009;119:1085–92. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Reinier K, Balaji S et al. Population-Based Analysis of Sudden Death in Children: The Oregon Sudden Unexpected Death Study. Heart Rhythm 2009;6:1618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maron BJ, Gohman TE, Aeppli D. Prevalence of Sudden Cardiac Death During Competitive Sports Activities in Minnesota High School Athletes. J Am Coll Cardiol 1998;32:1881–4. [DOI] [PubMed] [Google Scholar]
- 7.Driscoll DJ, Edwards WD. Sudden Unexpected Death in Children and Adolescents. J Am Coll Cardiol 1985;5:118b–121b. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JH, Tester DJ, Will ML, Ackerman MJ. Whole-Exome Molecular Autopsy After Exertion-Related Sudden Unexplained Death in the Young. Circ Cardiovasc Genet 2016. 2016;9(3):259–65 [DOI] [PubMed] [Google Scholar]
- 9.Bagnall RD, Weintraub RG, Ingles J et al. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. New Engl J Med. 2016;374:2441–52. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen SL, Hertz CL, Ferrero-Miliani L et al. Genetic Investigation of 100 Heart Genes in Sudden Unexplained Death Victims in a Forensic Setting. Eur J Hum Genet 2016;24(12):1797–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner JR, Crawford J, Smith W et al. Prospective, Population-Based Long QT Molecular Autopsy Study of Postmortem Negative Sudden Death in 1 to 40 Year Olds. Heart Rhythm 2011;8:412–9. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Shah KR, Um SY et al. Cardiac Channelopathy Testing in 274 Ethnically Diverse Sudden Unexplained Deaths. Forensic Sci Int 2014;237:90–9. [DOI] [PubMed] [Google Scholar]
- 13.van der Werf C, Stiekema L, Tan HL et al. Low Rate of Cardiac Events in First-Degree Relatives of Diagnosis-Negative Young Sudden Unexplained Death Syndrome Victims During Follow-Up. Heart Rhythm 2014;11:1728–32. [DOI] [PubMed] [Google Scholar]
- 14.Priori SG, Wilde AA, Horie M et al. HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes: Document Endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 15.Hofman N, Tan HL, Alders M et al. Yield Of Molecular and Clinical Testing for Arrhythmia Syndromes: Report of 15 Years’ Experience. Circulation 2013;128:1513–21. [DOI] [PubMed] [Google Scholar]
- 16.Quenin P, Kyndt F, Mabo P et al. Clinical Yield of Familial Screening After Sudden Death in Young Subjects: The French Experience. Circ Arrhythm Electrophysiol 2017;10(9). [DOI] [PubMed] [Google Scholar]
- 17.Steinberg C, Padfield GJ, Champagne J et al. Cardiac Abnormalities in First-Degree Relatives of Unexplained Cardiac Arrest Victims: A Report From the Cardiac Arrest Survivors With Preserved Ejection Fraction Registry. Circ Arrhythm Electrophysiol 2016;9(9). [DOI] [PubMed] [Google Scholar]
- 18.Wong LC, Roses-Noguer F, Till JA, Behr ER. Cardiac Evaluation of Pediatric Relatives in Sudden Arrhythmic Death Syndrome: A 2-Center Experience. Circ Arrhythm Electrophysiol 2014;7:800–6. [DOI] [PubMed] [Google Scholar]
- 19.Richards S, Aziz N, Bale S et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (Redcap)--A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table 1. Pre-specified criteria for “findings of uncertain significance” in the absence of other diagnostic criteria.