Abstract
The cornea is a valuable tissue for studying peripheral sensory nerve structure and regeneration due to its avascularity, transparency, and dense innervation. Somatosensory innervation of the cornea serves to identify changes in environmental stimuli at the ocular surface, thereby promoting barrier function to protect the eye against injury or infection. Due to regulatory demands to screen ocular safety of potential chemical exposure, a need remains to develop functional human tissue models to predict ocular damage and pain using in vitro-based systems to increase throughput and minimize animal use. In this review, we summarize the anatomical and functional roles of corneal innervation in propagation of sensory input, corneal neuropathies associated with pain, and the status of current in vivo and in vitro models. Emphasis is placed on tissue engineering approaches to study the human corneal pain response in vitro with integration of proper cell types, controlled microenvironment, and high-throughput readouts to predict pain induction. Further developments in this field will aid in defining molecular signatures to distinguish acute and chronic pain triggers based on the immune response and epithelial, stromal, and neuronal interactions that occur at the ocular surface that lead to functional outcomes in the brain depending on severity and persistence of the stimulus.
Keywords: cornea, pain, nociception, dry eye, neuropeptides, tissue engineering
1. Introduction
Pain serves a physiological role in alerting the central nervous system (CNS) that tissue damage may occur in the absence of further input. As the most densely innervated tissue in the body, the cornea contains intraepithelial nerve fibers that originate from the sub-basal nerves, giving rise to extreme sensitivity of the tissue (Müller et al., 2003; Marfurt et al., 2010). These sub-basal nerves are derived from both the stroma and periphery superficial nerves extending through the epithelium towards the ocular surface. The means by which the cornea is able to retain homeostasis, transparency, structural rigidity, and regeneration throughout a lifetime relies on this interplay between sensory input detected within the peripheral nervous system, resident cells within the tissue, and efferent pathways that are relayed by the brain to regulate ocular surface lubrication and blinking. Injury, infection, or systemic diseases, among others, that affect peripheral nerve functionality, may lead to deleterious effects on corneal surface integrity, including persistent epithelial defects, scarring, and neuropathic corneal pain (NCP) (Cruzat et al., 2010; Stapleton et al., 2013; Cruzat et al., 2017; Dieckmann et al., 2017a).
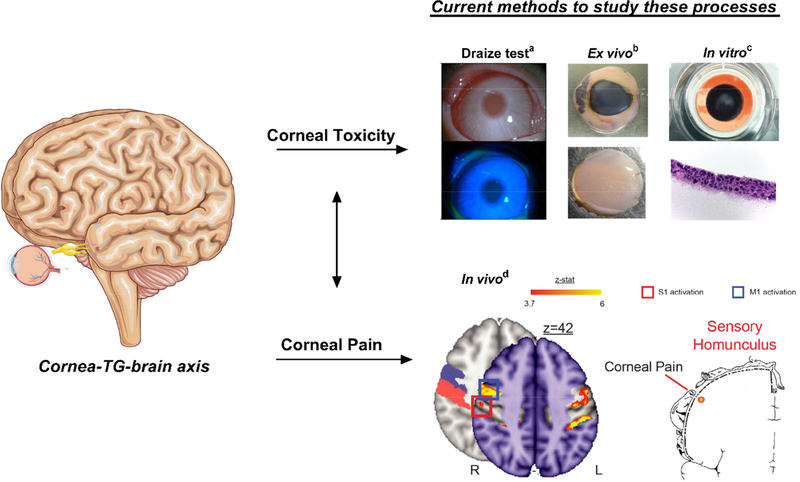
Due to the sensitivity of the eye to chemical damage, federal regulations require assessment of ocular discomfort prior to selling or marketing of select pharmaceutical products in the U.S., thereby highlighting the need for more accurate in vitro models to verify safety prior to the use of current animal models. Distinguishing between chemicals that may cause ocular damage, such as scarring or loss of barrier function, and chemicals that may cause temporary ocular irritation in the absence of permanent defects requires distinct testing metrics that may be lacking in existing approaches (Fig. 1). Current evaluation of ocular irritancy of a chemical relies on macroscopic visualization of the eye by slit-lamp post-chemical application to identify signs of inflammation, surface damage, and haze (Wilhelmus, 2001). Studying pain induction in animal models commonly involves quantifying eye blinking rates (Acosta et al., 2013), eye-wiping frequency (Farazifard et al., 2005), and tear production (Meng and Kurose, 2013). These metrics lend to difficulty in accurately predicting human responses, given inter-species variability in basal rates of tear flow (Chrai et al., 1973), lipid composition of the tear film (Leiske et al., 2010), and corneal sensitivity (Wieser et al., 2013). Studies in human patients rely on brain imaging and subjective pain scoring (Moulton et al., 2012) with ethical considerations limiting these studies to innocuous pain stimulation, such as bright light exposure.
Fig. 1.
Current approaches for studying corneal toxicity and pain. a Albino rabbit eye following application of the standardized Draize test, a common toxicity assay using clinical scoring post-topical irritant application to appropriately label chemicals based on their propensity to cause corneal damage (Wilhelmus, 2001). Image reproduced from (Liu et al., 2015) with permission. b Bright field image of an enucleated porcine eye and isolated cornea proper following chemical application. c Stratified epithelium cultured on curved cellulose filters at an air-liquid interface to assess ocular toxicity. Image reproduced from (Postnikoff et al., 2014) with permission. d Functional magnetic resonance imaging (MRI) image of a human patient exposed to bright light to induce pain sensations. The red box denotes location of pain activation distinct from eye blinking. Sensory homunculus depicts location of corneal pain in the somatosensory cortex. Images reproduced from (Moulton et al., 2012) with permission. Pictorials generated using Servier Medical Art based on a Creative Commons Attribution 3.0 Unported License available at https://creativecommons.org/licenses/by/3.0/.
Bridging this gap between animal-based approaches and human-focused studies requires further developments in delineating the biology underlying peripheral pain responses that occur at the ocular surface and how those relay to functional actions in the brain. We posit that advanced tissue engineering approaches may serve as a useful means to study these processes in a physiologically-relevant system with the inclusion of select cell types or phenotypes (e.g. pathological tissue isolation, gene knockouts, or fluorescent tags) to model human disease and determine how cell-cell interactions are influenced by varying stimuli.
The objective of this review is to describe recent advances in the study of corneal pain that should be considered in the development of a functional model. To lay a foundation to bioengineer more advanced systems to study nociception in vitro, we focus on aspects of corneal tissue biology that define the role of sensory nerves in physiological maintenance of the cornea and the biochemical and electrophysiological responses that are associated with pain. Key features of an ideal corneal tissue model to study nociception and ocular irritancy include:
Utilization of appropriate human cell types present within the cornea, including primary limbal epithelial and stromal stem cells, endothelium, and resident immune cells.
Inclusion of sensory nerves that show responsiveness to mechanical, chemical, or thermal stimuli and promote epithelial stratification.
Maintenance of cultures at an air-liquid interface with tear perfusion to mimic physiological tear flux.
Characterization of known chemical stimulants that evoke pain responses based on nociceptor activation.
Optimizing readouts to assess signaling responses using both biochemical, optical, and electrophysiological approaches.
Stable and sustainable in vitro tissues to accommodate studies of both acute and chronic conditions spanning from days to weeks and months.
Validation of functional responses detected in vitro, such as morphological changes in tissue structure, biochemical responses, and electrophysiological output, to pain responses observed in relevant in vivo animal models and the human patient population.
Thorough understanding of the structural and dynamic features involved in corneal tissue biology will aid in developing accurate models to screen chemicals for potential ocular discomfort and therapeutic application as novel analgesics to treat acute and chronic pain development. This review emphasizes the molecular and structural cues involved in pain propagation with discussion of current tissue engineering approaches to mimic these processes in tissue models based on current in vivo, ex vivo, and in vitro systems.
2. Anatomical and functional characteristics of corneal innervation
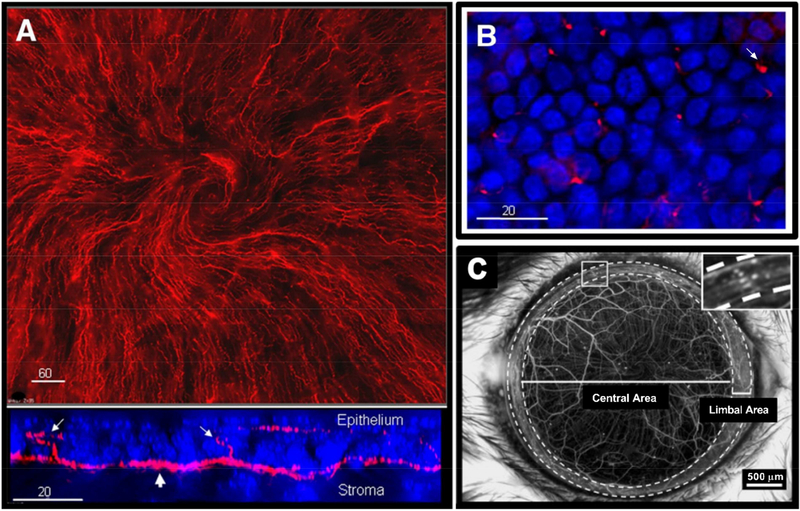
Neurobiology of the cornea has been extensively reviewed (Stapleton et al., 2013; Belmonte et al., 2017; Cruzat et al., 2017). The human cornea has the highest sensory innervation per unit area of any surface epidermal tissue in the body with counts of approximately 50–450 neurons crossing the limbus originating from the ophthalmic region of the trigeminal ganglion (TG) (Müller et al., 2003). Factors that contribute to corneal sensitivity are heavily interconnected with the peripheral nervous system and the multiple cell types (epithelial, stromal, and immune cells) present within the epithelium and stroma that promote neuronal sensitization primarily via secreted factors with recent work highlighting exosomes as potential mediators of epithelial-stromal interactions (Han et al., 2017). The barrier functions of the corneal epithelial and endothelial layers mediated via tight junctional proteins are important for maintaining the microenvironment of the cornea serving to separate the corneal stroma from the outer environment, as well as from the inner aqueous humor (Stiemke et al., 1991; Ban et al., 2003). Structurally, nerves enter the cornea radially from the periphery to form the sub-basal nerve plexus with intraepithelial nerve fibers extending to the epithelium. This sensory presence is prominent at the cornea-scleral rim at 200 μm from the ocular surface with additional bundles distributed from 50–500 μm deep within the stroma (Marfurt et al., 2010) running preferentially in parallel to stromal collagen fibrils (Muller et al., 1996). The average diameter of stromal bundles are 20 μm thick (Marfurt et al., 2010), while corneal nerve fibers range from 2 μm-6 μm with lengths of 200 μm-800 μm from the sub-basal nerve plexus to the mid-stroma (Oliveira-Soto and Efron, 2001). A centripetal orientation of nerve fibers from the limbus to the central cornea gives rise to a whorl-like appearance (Fig. 2). Re-formation of this vortex in the adult mouse by day 28 following superficial trephination (Pajoohesh-Ganji et al., 2015) suggests that this phenomenon is independent of developmental epithelial re-growth. Conformational changes in the sub-basal nerve plexus are associated with pathological conditions that contribute to reduced sensation, such as diabetes (Utsunomiya et al., 2015) and herpes simplex keratitis (Hamrah et al., 2010).
Fig. 2.
Sensory nerve fibers innervating the cornea. (A) Neuronal extensions present between the stroma and epithelial layer in the mouse cornea (top inset: βIII-tubulin: red). z-stack of the corneal epithelium (bottom inset: βIII-tubulin: red, DAPI: blue) from the sub-basal nerve (large arrow) and extending intraepithelial nerve endings reaching into the epithelium (small arrows) in corneas isolated from adult C57/BL6 mice. (B) En face view of spatially dispersed intraepithelial nerve endings (small arrow) innervating the ocular surface. Modified from (Li et al., 2011) and reproduced with permission. (C) Stereofluorescent image of corneal nerves in the transgenic mouse cornea (YFP-labelled immune cells). Inset highlights limbus region containing a high density of YFP+-immune cells. Modified from (Sarkar et al., 2013) and reproduced with permission.
Sensory nociceptor terminals, the stimulation of which results in the sensation of pain and discomfort, are distributed in a manner to detect and allow response to potentially damaging external and internal stimuli, and thereby warn of the risk of injury or long-term tissue damage. Further, nociceptors detect noxious, irritant, and inflammatory stimuli through the expression of transient receptor potential (TRP) channels. Three sensory nerve subgroups have been defined based on the expression of these receptors and functionality within the human cornea: 1) mechano-nociceptors, which comprise 20% of the total density and are responsible for sensing physical perturbations and mechanical distress, 2) polymodal-nociceptors, which make up 70% of the nociceptors and serve to detect temperature flux, endogenous inflammatory mediators, and exogenous chemicals, and 3) thermo-receptors, which form the remaining 10% of nociceptors and function in detecting temperatures induced by tear film evaporation (De Armentia et al., 2000; Belmonte et al., 2004). Variances in receptor expression in the murine cornea have suggested higher distributions of cold-sensitive receptors (49%) and slightly lower polymodal distributions (41%) with the remaining 10% making up mechanical- sensitive receptors (Gonzalez-Gonzalez et al., 2017) suggesting species-differences in nociceptor expression that may contribute to variances in sensitivity to select stimuli. The broad distribution of nociceptors within the cornea allows for rapid monitoring of corneal surface temperature, lubrication, and injury, thus serving a fundamental role in preserving tissue integrity. While the predominant innervation of the cornea is sensory, a small proportion of neuronal input is composed of sympathetic and parasympathetic nerve fibers originating from the superior cervical ganglion (Marfurt, 1988; Marfurt et al., 1998). Notable studies in the field have identified specific features of the electrophysiological responses produced by sensory neurons innervating the cornea depending on nociceptor class and stimulus (Lopez de Armentia et al., 2000; Hirata and Rosenblatt, 2014; Hirata et al., 2015).
The importance of neural innervation in regulating epithelial proliferation has become increasingly clear in the field of Ophthalmology with a growing clinical burden linked to neurotrophic keratitis and diabetic polyneuropathy. As evident in these conditions, loss of sensory nerves may lead to development of structural defects in corneal tissue integrity, resulting in scarring. Physiological conditions, such as adequate tear flow, mucin production, epithelial proliferation, and stromal remodeling, promote retention of transparency of each corneal layer enabling proper visual acuity.
2.1. Interplay between the epithelium, stroma, and nerve fibers
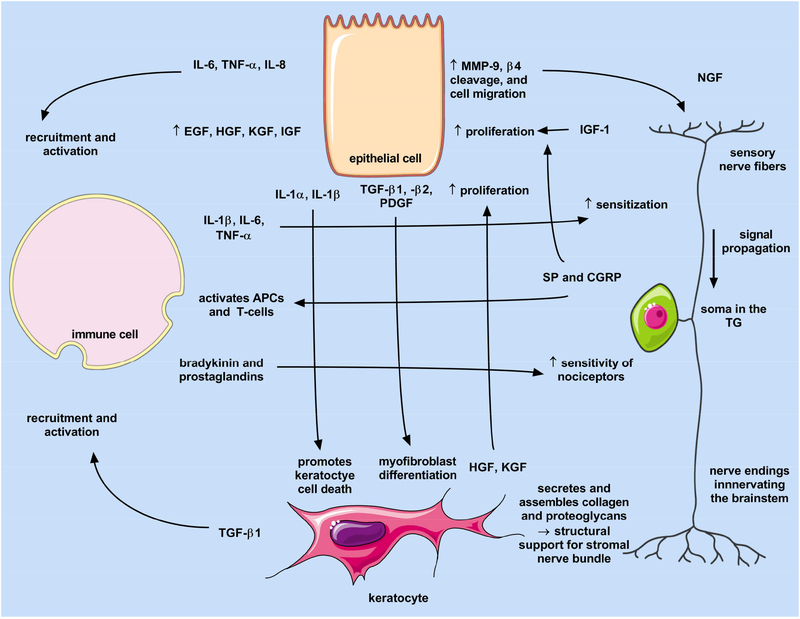
Secreted neurotrophic factors play a role in corneal tissue integrity by promoting nerve growth and survival during steady state, as well as during pathological conditions, such as trauma or infections. Mechanisms involved in the corneal wound-healing cascade have been extensively reviewed (Wilson et al., 2001; Netto et al., 2005) highlighting the multi-factorial biochemical and cellular response that mediates a return to homeostasis post-injury. In terms of neuronal contribution, structural and biochemical functions of sensory nerves within the cornea regulate the collective response to injury, as well as provide pro-survival signals during physiological maintenance of the cornea (Fig. 3).
Fig. 3.
Interplay of the epithelium, stromal, immune cell, and sensory nerves following exposure to mechanical or chemical stimuli that result in a wound healing response. Images generated using Servier Medical Art under a Creative Commons Attribution 3.0 Unported License available at https://creativecommons.org/licenses/by/3.0/.
While nerve fibers in the limbus and peripheral cornea are protected by myelin, sensory nerves within the central corneal stroma are thought to be supported by non-myelinating Schwann cells, and terminal fibers extending into the epithelium supported by corneal epithelial cells (Stepp et al., 2017). Likewise, the role of heparan sulfate glycosaminoglycans in sensory nerve guidance has been reported in the cornea with null deletions of the proteoglycan syndecan-1 showing a reduction in developmental growth of intraepithelial nerve endings and lower epithelial wound healing post-debridement in mice (Pal-Ghosh et al., 2017). Severity of the corneal wound has notable effects on recovery of nerve structure with stromal nerve injury and sub-basal debridement leading to reduced recovery up to 28 days post-injury with associated epithelial cell death and loss of neuronal extensions occurring at the corneal apex independent of overt inflammation (Pajoohesh-Ganji et al., 2015). Moreover, studies in the avian cornea have identified synaptic-like interactions between intraepithelial nerve endings and apical corneal epithelial cells expressing neuron-specific class III β-tubulin (TuJ-1+) and synaptic vesicle component (SV2) (Kubilus and Linsenmayer, 2010a). Of interest, a number of these select TuJ-1 positive epithelial cells show the presence of mitotic spindles indicative of a dividing cell suggesting that at least in the developing cornea, apical epithelial cells may contribute to the superficial corneal layers in addition to basal cells. Furthermore, co-culture studies in vitro have shown increased sensory nerve outgrowth in the presence of corneal epithelial cells (Kowtharapu et al., 2014) further supporting the likely contribution of the epithelium in generating a favorable microenvironment to support nerve regeneration.
Stromal contributions to sensory nerve health are less understood but have been investigated in vitro using chick dorsal root ganglion and corneal stromal keratocytes and fibroblasts. Conditioned media transfer from corneal fibroblast cultures improve neurite outgrowth in a dorsal root ganglion model suggesting that neurotrophic factors secreted by activated keratocytes post-injury may contribute to nerve recovery (Yam et al., 2017).
2.2. Immune cells in the cornea
The immune system, both resident and invading leukocytes, play a fundamental role in influencing corneal sensitivity to pain during infection or injury. Pain sensation is heavily influenced by the inflammatory responses that occur in conjunction with mechanical or chemical injuries. Inflammatory processes caused by wounding or infection originating at the corneal surface can also lead to inflammatory processes detected in the TG (Ferrari et al., 2014; Matundan et al., 2016).
As the precise microanatomy of the cornea is crucial for vision, an overactive inflammatory response to injury, noxious stimuli, or the invasion of opportunistic bacterial and viral pathogens can result in collateral structural damage to the cornea and lead to corneal opacity and ultimately vision impairment. The high success rate of corneal transplantation (Medawar, 1948, 1961) indicated the cornea is immune privileged, an evolutionary adaptation enabling the protection of vital tissues, some incapable of regeneration. Historically, with the exception of a population of intraepithelial dendritic cells (DCs) in the limbal region, the cornea was considered to be a tissue devoid of bone marrow (BM)-derived cells (Streilein et al., 1979; Gillette et al., 1982; Streilein, 1999). Several active and passive mechanisms were thought to contribute to corneal immune privilege, including lack of blood and lymphatic vessels (Streilein et al., 2002), anti-inflammatory mediators, such as transforming growth factor (TGF)-β and Fas Ligand (Streilein, 1999; Streilein et al., 2002; Niederkorn, 2003), and the absence of major histocompatibility complex (MHC)-II antigens (Streilein et al., 2002). However, the discovery of resident BM-derived cells in the cornea (Brissette-Storkus et al., 2002; Hamrah et al., 2002; Hamrah et al., 2003c; Nakamura et al., 2004) altered the dogma that the central cornea is devoid of immune cells and resulted in a paradigm shift in corneal immunology.
Distinct populations of resident BM-derived cells distributed throughout the steady state corneal epithelium, and between the collagen lamellae and keratocytes of the stroma, include antigen-presenting cells (APCs), such as conventional DCs (cDCs) and macrophages, and decrease in density centripetally (Hamrah and Dana, 2010). The presence of central corneal cDCs in the cornea was first described in 2002 (Hamrah et al., 2002). Further phenotypic differences between resident cDCs were then noted, showing that cDCs in the central cornea were immature (negative for MHCII, CD80 and CD86), whereas peripheral cDCs include subpopulations of both immature and mature (positive for MHCII, CD80 and CD86) (Hamrah et al., 2002; Hamrah et al., 2003a). The immature phenotype of central corneal cDCs is unique in that they are unable to sensitize T cells in draining lymph nodes (Hamrah and Dana, 2007). Further, Langerin-positive stromal are localized throughout the peripheral and central corneal and epithelial DCs only to the periphery and limbus in the naïve murine cornea (Hattori et al., 2011).
Further, the corneal stroma contains resident macrophages, localized in the posterior stroma (Brissette-Storkus et al., 2002; Hamrah et al., 2002; Nakamura et al., 2004; Chinnery et al., 2007; Takayama et al., 2009; Gautier et al., 2012; Seyed-Razavi et al., 2014; Chinnery et al., 2015). Interestingly, loss of either CCR2-positive or -negative macrophage subsets was recently seen to affect corneal wound healing post-epithelial debridement (Liu et al., 2017). Another recent study reported Thy-1 YFP-positive myeloid derived suppressor cells (MDSC) in an established neurofluorescent trangenic murine model infiltrating the cornea following topical benzalkonium chloride (Sarkar et al., 2012). MDSCs infiltrate the cornea following annular keratectomy and are capable of secreting nerve growth factor (NGF) (Sarkar et al., 2013).
Another subtype of leukocyte also identified to be resident in the naïve cornea are plasmacytoid dendritic cells (pDCs) located in the anterior stroma (Zheng et al., 2010). Phenotypically distinct from cDCs (Asselin-Paturel et al., 2001; Bjorck, 2001; Nakano et al., 2001), pDCs are known to be potent producers of type I Interferons (IFN-γ) (Lund et al., 2006; Smit et al., 2006; Wang et al., 2006; Cervantes-Barragan et al., 2007; Reizis et al.) and are able to function both as regulators of T cell immunity as well as regulators of tolerance (Colonna, 2006; Ochando et al., 2006; Gautreau et al., 2011).
An immune response in the cornea occurs in a process similar to that in other tissues. Inflammatory stimulus by way of trauma or tissue injury, including damage-associated molecular patterns (DAMPS, endogenous danger signals released by dying cells during stress or tissue injury that are able to activate innate immune cells to produce a non-infectious inflammatory response), pathogen-associated molecular patterns (PAMPs, not found in host cells and recognized by Toll-Like Receptors (TLRs) (Bianchi, 2007)), and other antigens initiate local inflammation by stimulating production and release of inflammatory cytokines including interleukin (IL)-1, tumor necrosis factor (TNF)-α, and IL-6 by epithelial cells. Inflammatory cytokines, in turn, result in the activation of resident immature APCs and an increase in vascular adhesion molecules within limbal vessels culminating in recruitment of circulating inflammatory cells including neutrophils and monocytes, where differentiation into macrophages and DCs occurs, contributing to host defense, tissue remodelling, and repair (Van Furth et al., 1973). Further, epithelial cells have been shown to secrete pro-inflammatory cytokines IL-1α, TNF-α, IL-6 and IL-8, whereas stromal keratocytes may produce IL-1α, TNF-α, IL-6 and IL-8, both of which contribute to the chemotaxis and activation of leukocytes in the cornea (Cumberbatch et al., 1997; Lambiase et al., 2011). Immune cells recruited to the ocular surface following acute inflammation due to epithelial injury that do not reside in the steady state cornea include neutrophils (Li et al., 2006) and γδ-T cells localizing at the limbal epithelium (Li et al., 2007). A recent study has also revealed a subset of classic natural killer (NK) cells that migrate into the corneal limbus in response to locally-generated chemokines following central epithelial abrasion and limit the innate acute inflammatory reaction to corneal wounding through regulating neutrophil influx (Liu et al., 2012).
Chronic inflammation, as is the case with dry eye disease (DED), involves the adaptive immune system. The inflammatory microenvironment of DED facilitates both maturation and migration of resident APC populations from the cornea to the limbus/conjunctiva region where they are able to travel to draining lymph nodes, including the submandibular draining lymph node via lymphatics (afferent arm of the alloimmune response), where they present antigen and activate T cells towards a T helper (Th)1 effector and Th17 (autoreactive) subtype (Tsubota et al., 1999; Shen et al., 2007; Barabino et al., 2012; Gandhi et al., 2013; Pflugfelder et al., 2013; Yagci and Gurdal, 2014). CD4+ effector T cells in turn migrate to the limbus/conjunctiva via the vasculature (efferent arm of the alloimmune response) and enter the tissue through diapedesis. Further, desiccating stress-induced autoreactive T cells can selectively cause inflammation similar to Sjögren’s disease in the cornea, conjunctiva, and lacrimal gland (Niederkorn et al., 2006). Damaged ocular surface cells and infiltrating lymphocytes and leukocytes continue the release of DAMPS (Matzinger, 1998), as well as further pro-inflammatory cytokines and chemokines, which exacerbate and perpetuate ocular surface inflammation. Preliminary data from a recent study of DED subjects suggests an increase in the ocular surface levels of a DAMP, high mobility group box-1 (HMGB-1), in the damaged ocular surface (Alven A., 2015). Severe DED is, therefore, caused by the increasing cycle of inflammation with ocular surface injury due to collateral damage (Johnson and Murphy, 2004; 2007; Baudouin et al., 2013).
Laser in vivo confocal microscopy (IVCM), a non-invasive high-resolution real-time imaging device allowing layer-by-layer analysis of the corneal ultrastructure, has been utilized by clinicians and researchers to assess and monitor corneal and conjunctival immune cells (Cruzat et al., 2010; Hamrah et al., 2010; Qazi et al., 2014; Hamrah et al., 2016; Cruzat et al., 2017). IVCM has also been utilized to confirm the presence of dendritiform cells at the basal epithelial cell and the sub-basal nerve plexus layer of the cornea (Zhivov et al., 2005; Cruzat et al., 2011; Mayer et al., 2012). Several studies have also confirmed the distribution of corneal immune cells found within the murine cornea with immunofluorescence staining of human corneal tissues (Hamrah et al., 2003b; Yamagami et al., 2005; Yamagami et al., 2006; Knickelbein et al., 2014), including the identification of dendritiform cells localizing in the basal epithelium of the cornea to be CD11c+ DCs (Mayer et al., 2007; Knickelbein et al., 2014). Interestingly, IVCM investigations have revealed correlations between nerve density and immune cells in various types of keratitis. Analysis of bacterial, fungal, and Acanthamoeba keratitis IVCM images highlighted the increase in epithelial dendritiform cell density, compared with normal controls, to inversely correlate with sub-basal corneal nerve density (Cruzat et al., 2011). A similar result was noted in other studies where the increase in dendritiform cell density and size in the affected and contralateral eyes of unilateral infectious keratitis patients, including herpes simplex, herpes zoster, Acanthamoeba, and fungal and bacterial keratitis, was also inversely correlated with a bilateral decrease in the sub-basal nerve plexus (Cruzat et al., 2015; Cavalcanti et al., 2018). Taken together, these studies suggest an interplay between the nervous and immune systems (Hamrah et al., 2016).
2.3. Inflammation and neuronal sensitization
The International Association for the Study of Pain (IASP) defines neuropathic pain as pain caused by a lesion or disease state of the somatosensory nervous system (Jensen et al., 2011). This definition includes direct damage to the cell body of nociceptors in the central nervous system, severing of the peripheral nerve terminals, and/or damage resulting from the local inflammatory component following the insult (von Hehn et al., 2012). Pain can be broadly divided into sub-categories based on the stimulus from which they are activated: 1) nociceptive pain, where an acute noxious agent (thermal stimulus and chemical irritants) stimulates nociceptors to activate the body’s protective and avoidance response, 2) inflammatory pain, which is driven by the inflammatory response resulting in the sensitization of nociceptor terminals and lower thresholds to noxious stimulation, and 3) neuropathic pain, which is caused by neural damage or lesions (Hucho and Levine, 2007). These pain responses are heavily influenced by inflammatory processes, both by tissue inflammation, which is mediated via secretion of pro-inflammatory molecules and immune cell activation, and neurogenic inflammation, which is characterized by secretion of Substance P (SP) and calcitonin gene related peptide (CGRP), thereby activating resident and recruiting invading immune cells, respectively (Chiu et al., 2012).
Corneal nerve dysfunction is the pathophysiologic basis of many ocular surface diseases, such as arising from surgery (Linna et al., 2000), diabetic neuropathy (Rosenberg et al., 2000; Efron, 2011; Chen et al., 2013; Leppin et al., 2014), DED (Benitez del Castillo et al., 2004), contact lens wear, post-surgical (Theophanous et al., 2015a), herpetic keratitis (Pavan-Langston, 2008; Hamrah et al., 2010; Hamrah et al., 2013) and systemic small fiber polyneuropathy (Bucher et al., 2015). The release of inflammatory mediators, from the inflammatory component of the aforementioned cases, results in the dysfunction of corneal nerve terminals and modification of the normal nociceptor responses with irregular impulse firing–ectopic discharge, decreased nociceptor activation thresholds and increased discharge of impulses evoked by supra-threshold stimulation (Belmonte et al., 2015). Chronic and persistent ectopic activity of injured or dysfunctional corneal nerves in turn results in discomfort in response to innocuous stimuli (allodynia) and to enhanced discomfort to noxious stimuli (hyperalgesia). Nociceptor sensitization can be specifically localized to the periphery (Aggarwal et al., 2015; Hamrah et al., 2017), possess both a peripheral and central component, or be specifically centralized within second order neurons (Dieckmann et al., 2017a).
Immune cell infiltration and local inflammation following peripheral nerve injury are critical for the initiation and development of neuropathic pain (Austin and Moalem-Taylor, 2010; Stein and Machelska, 2011). Inflammatory mediators released following local injury, including bradykinin, cytokines, histamine, nerve growth factor, prostaglandin E2, 5-hydroxytryptamine (5-HT), ATP and nitric oxide (Kidd and Urban, 2001; Chiu et al., 2012), are capable of sensitizing nociceptors by decreasing their activation threshold and/or increasing suprathreshold responses through transient receptor potential vanilloid receptor-1 (TRPV1) activation (Immke and Gavva, 2006). ATP, for example, is recognized by ligate-gated cation channel purinergic receptors P2X3, present on both nociceptor neurons and immune cells (Cockayne et al., 2000; Souslova et al., 2000), while P2Y2 receptors function to sensitize TRP and voltage-gated sodium channels (Yousuf et al., 2011; Hockley et al., 2016). Furthermore, inflammatory cytokines such as IL-1β and TNF-α can be directly sensed by nociceptors that express the cognate receptors, which in turn induce the activation of p38 mitogen-associated protein (MAP) kinases and leading to increased membrane excitability (Binshtok et al., 2008; Zhang et al., 2011). Once activated, the action potential that is sent centrally towards the soma, and disseminated into other terminal branches mediating the local release of pro-inflammatory neuropeptides (including SP and CGRP). The release of pro-inflammatory neuropeptides, chemokines and cytokines from activated nociceptors, in addition to the pro- and anti-inflammatory mediators released by infiltrating and resident immune cells, result in a positive feedback loop, contributing to further inflammation and leading to the alteration of nociceptors (Mantelli et al., 2010).
Typically, resolution of the initial inflammation, and the normalization of the ocular surface, may result in the reversal of peripheral sensitization (Launay et al., 2016). The prolonged sensory hypersensitivity, after the original etiological cause is resolved, can progress if the primary disease continues to damage the nervous system (e.g. chronic tissue inflammation or neurogenic inflammation). Ongoing insults and chronic changes to the peripheral and central nervous system can lead to permanent sensitization, e.g. chronic DED. Cytokines released in the pro-inflammatory environment, such as in DED, by infiltrating T cells, macrophages including, but not limited to, IL-2, IL-6, IL-8, IL-10, IL-17, macrophage inflammatory protein (MIP)-1α and TNF-α (Kiguchi et al., 2010; Gandhi et al., 2013; Lee et al., 2013) are able to lower the activation thresholds of local nociceptors to noxious stimuli (Sommer and Kress, 2004; Gold and Gebhart, 2010; Ren and Dubner, 2010). Blocking pro-inflammatory cytokines such as IL-1β, which has been shown to act directly on sensory neurons to increase their susceptibility for noxious heat via an IL-1RI/TyrK/PKC-dependent mechanism (Obreja et al., 2002), IL-6, as well as TNF-α, leads to reduced hyperalgesia in animal models of painful neuropathy (Sommer et al., 1998; Wagner et al., 1998; Sommer and Kress, 2004). Th1 cells secrete IFN-γ that activates macrophages (Mills et al., 2000), while Th17 cells express IL-17 (Stockinger and Veldhoen, 2007; Weaver et al., 2007) that induce local production of pro-inflammatory cytokines (IL-6, IL-8, and granulocyte-colony-stimulating factor) and matrix metalloproteinases (MMPs) (Fossiez et al., 1996; De Paiva et al., 2009). Furthermore, IL-17A has direct and widespread effects on neurons, but can also impact neuronal function via signaling to immune cells. Further, infiltrating T cells also contribute to neuropathic pain following peripheral and/or central sensitization (Zhang et al., 2014). Neuro-immune signaling with microglia is critical for initiation and development of the central component of neuropathic pain (Tsuda et al., 2003). In this instance, nerve injury induces gliosis, where microglia (and astrocytes) surrounding the affected primary afferent terminals functionally altered towards a “pain-related enhanced response states” (McMahon and Malcangio, 2009), which contributes to sensitization and results in enhanced neuro-immune communication (Clark and Malcangio, 2014).
Centrally, resident satellite glial cells have been shown to release a variety of molecules that modulate the excitability of TG neurons in different states, including steady state, following noxious stimuli, as well as following persistent activation by noxious stimuli (Goto et al., 2016). The precise role that satellite glial cells play in modifying the excitability of TG neurons supplying the ocular surface is not fully known. In the dorsal horn, microglial-neuronal communication is initiated through activation of the purinergic receptor, P2X4 receptor, resulting in the release of brain-derived neurotrophic factor (BDNF) leading to activation of TrkB receptor and down-regulation of the neuronal potassium/chloride co-transporter KCC2 (Ulmann et al., 2008; Trang et al., 2009), or activation of the low affinity P2X7 receptor, resulting in the release of the lysosomal protease Cathepsin S (CatS) and CX3CL1 (Clark et al., 2010). Similar responses, including release of microglial TNF-α following activation of P2X7, can also occur in the TG following peripheral nerve damage (Ito et al., 2013; Murasaki et al., 2013).
CX3CL1 signaling is ideally placed to mediate neuron-microglial communication, during both steady state and following peripheral nerve damage, and therefore is a potential candidate to investigate its role in the modulation of nociceptor signaling and sensitization (Harrison et al., 1998; Zhuang et al., 2007; Clark and Malcangio, 2014). CX3CL1, also known as fractalkine (humans) or neurotactin (mice), is a structurally unique chemokine principally expressed by neurons that plays a role in neuropathic pain (Harrison et al., 1998; Verge et al., 2004). CX3CR1, the sole receptor for fractalkine, mediates both the adhesive (when membrane bound) and chemokine properties (soluble form) of fractalkine, and is expressed on several immune cell sub-types including monocytes, CD16-positive NK cells, T cells, DCs and microglia (Imai et al., 1997; Nishiyori et al., 1998; Papadopoulos et al., 1999; Jung et al., 2000). Further, fractalkine signaling has been shown to mediate the migration of CX3CR1 bearing cells during neuronal injury (Lu et al., 2009; Gao and Ji, 2010; Sun et al., 2013). In the cornea, the dissociation of macrophages intimately associating with nerves in the corneal stroma following epithelial injury has been shown to be partly CX3CR1 dependent (Seyed-Razavi et al., 2014), revealing another role for fractalkine in mediating the migration of resident immune cells in the cornea (Chinnery et al., 2007). Investigations in the CNS have revealed that CX3CR1 may play an important role in the genesis of neuropathic pain via regulating neuronal-glial interactions (Gao and Ji, 2010). How fractalkine is involved in NCP progression, however, is currently not known.
3. Corneal microenvironment and the pain response
The nociceptive properties of the cornea are dependent on the chemical nature and persistency of the stimulus. When noxious stimuli reach above a threshold, the nociceptors induce ion-influx via voltage-gated receptors resulting in the production of an action potential, which is transmitted to the TG cell bodies and eventually to higher-order neurons within the brain. Functional responses to painful stimuli are mandated by structural determinants (e.g. lid closure, tear flow), biochemical responses (e.g. expression and distribution of nociceptive receptors, signal propagation to the TG), and cellular responses (e.g. secretion of pro-inflammatory factors, influx of inflammatory cells, higher-order brain processes that contribute to pain registration).
3.1. Voltage-gated receptors and pain triggers
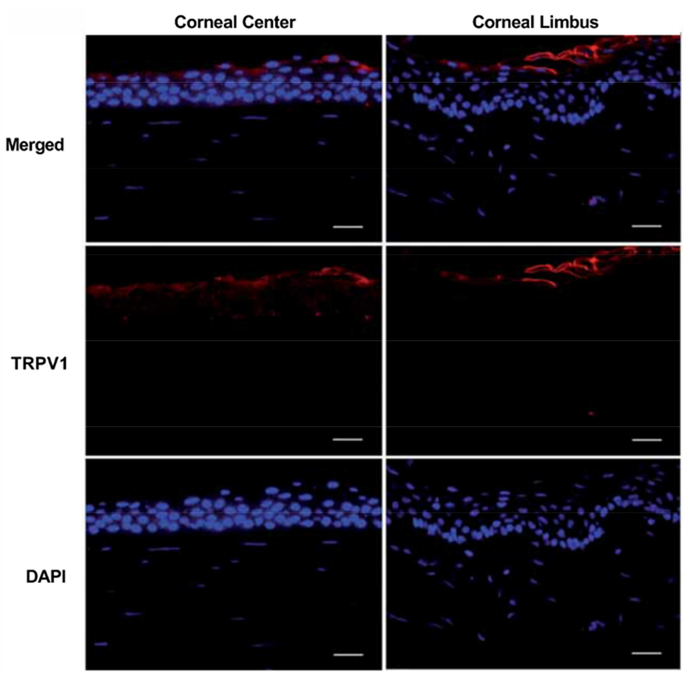
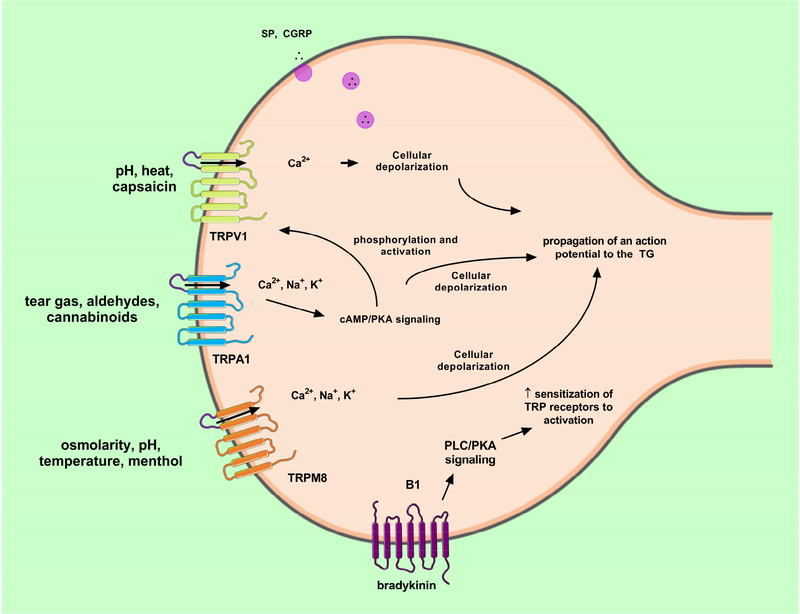
Induction of an action potential is mediated by voltage-gated receptors present on sensory nerve endings, which upon ligand binding promote ion flux leading to cell depolarization. The major receptors within the cornea are TRPV1–4, TRPM8, and TRPA1, as well as a number of other receptors (Table 1). Expression of these receptors is not restricted to the sensory neurons but has also been identified on corneal epithelium, keratocytes, endothelium, and resident immune cells suggesting a multifactorial response to stimuli involving multiple cell types (Stephan Mergler, 2011). The highly-expressed TRPV1 receptor is dominant in the epithelium of the human likely correlating to the location of intraepithelial nerve fibers of innervating sensory nerves (Zhang, 2007) (Fig. 4).
Table 1.
Ocular surface receptors implicated in pain-response mechanisms.
| Family | Receptor | Cell type | Comment | References |
|---|---|---|---|---|
| transient receptor potential channels | TRPV1 | epithelium, stroma, sensory nerves, macrophages | Involved in heat sensation, nociception, receptor for capsaicin; influences cytokine production in leukocytes | (Weil, 2005; Zhang, 2007; Fernandes et al., 2012) |
| TRPV3 | epithelium, corneal endothelium | Involved in heat sensation and epithelial proliferation | (Takahiro Yamada, 2010) | |
| TRPV4 | corneal endothelium | Involved in heat sensation | (Stephan Mergler, 2011) | |
| TRPM2 | macrophages, monocytes, neutrophils | Involved in cytokine production in response to intracellular bacterial infection | (Heiner et al., 2003; Yamamoto et al., 2008; Knowles et al., 2011) | |
| TRPM4 | macrophages, neutrophils | Involved in proliferative and phagocytic activity of macrophages | (Serafini et al., 2012) | |
| TRPM8 | epithelium, stroma, sensory nerves | Involved in cold sensation, nociception, responsive to osmolarity changes, menthol receptor | (Quallo et al., 2015) | |
| TRPA1 | epithelium, stroma, sensory nerves | Sensitive to pH changes, receptor for mustard oil | (Okada et al., 2014) | |
| neurotrophic factor receptors | TrkA | limbal epithelial cells, sensory nerves, monocytes | High-affinity receptor for NGF, involved in innervation during development, nociception, nerve repair, and inflammation | (Qi et al., 2008; Prencipe et al., 2014) |
| p75NTR | limbal epithelial cells, sensory nerves, monocytes | Low-affinity receptor for NGF; influences peripheral inflammatory response | (Qi et al., 2008; Lee et al., 2016) | |
| TrkB | limbal epithelial and stromal cells, epithelium, lymphocytes | Receptor for BDNF, NT-3, and NT-4; promotes neuronal differentiation; influences lymphocyte survival | (Garcia-Suarez et al., 2002; Qi H, 2007) | |
| GFRa1 | limbal epithelium, immature T cells | Receptor for GDNF; promotes neuronal differentiation | (Qi H, 2007; Almeida et al., 2012) | |
| opioid | MOR, DOR | nerve fibers, leukocytes (monocytes, macrophages) | Involved in analgesia | (Zollner C, 2008; Sauer et al., 2014; Celik et al., 2016) |
| opioid growth factor | OGFr | epithelium, leukocytes (monocytes, macrophages) | Involved in wound healing in response to nociception; analgesia | (Ian Zagon, 2003; Zagon et al., 2011; Schreiter et al., 2012; Sauer et al., 2014) |
| purinergic | P2X7, P2Y2 | epithelium, thymocytes, monocytes | Involved in corneal wound healing and epithelial migration following injury; influences differentiation patterns | (Mayo et al., 2008; Caragnano et al., 2012; Frascoli et al., 2012; Martin Minns, 2016) |
| K+ channel | Kv1.1, Kv3.4 | epithelium, sensory nerves, thymocytes (Kv1.1 only) | Involved in mediating stress-induced responses to cold-induction; influences thymocyte maturation | (Freedman et al., 1995; Lu, 2006; Madrid et al., 2009) |
| Ca2+ channel | Cav1.3 | corneal endothelium, sensory nerves, lymphocytes | Induce intracellular calcium influx in response to stimulation; influence lymphocyte development | (Mergler, 2003; Jha et al., 2015) |
Fig. 4.
TRPV1 expression in the apical layer of the central and limbal corneal epithelium of the human cornea. (red- TRPV1, blue- DAPI). Reproduced from (Zhang, 2007) with permission. Scale bar = 25 μm.
Through activation of TRP receptors on nociceptors distributed throughout the cornea, two parallel means of detecting changes in sensation/ pain and discomfort on the ocular surface have been investigated: 1) irritant-induced tearing: proceeds via activation of TRPV1 and TRPA1 receptors, which then translates this information to an electrical signal characterized by Ca2+ flux and translation of activation to the trigeminal brainstem nuclear complex (Patapoutian et al., 2009) and 2) temperature-induced tearing: detected by nociceptors that promote tear production in response to cold or hyperosmolarity of the ocular surface (Parra et al., 2010). These specialized thermoreceptors are present at corneal nerve endings and are activated by changes in tear content, temperature, and dryness (Parra et al., 2010). Upregulation in inflammatory mediators has also been associated with DED, including IL-1, −3, −6, and −13 (Solomon et al., 2001; Stern and Pflugfelder, 2004) and MMP-9 (Luo et al., 2004; Pflugfelder et al., 2005; Acera et al., 2008; Mori et al., 2012; Schargus et al., 2015). These factors are classical nociceptor sensitizers increasing baseline neuronal-responses to environmental factors (known as hyperalgesia) that may correspond to elevated pain detected (Belmonte et al., 2004; Belmonte et al., 2015; Belmonte et al., 2017).
TRP channels are involved in a wide range of biology, notably pain sensation, vasoregulation, mineralization, embryonic development, and thermogenesis (Hwang and Oh, 2007; Wu et al., 2010; Jin et al., 2012; Ye et al., 2012; Volkers et al., 2015; Dai, 2016). It is thus not surprising that dysfunction of TRP channels may lead to a broad array of pathology, including cardiovascular, musculoskeletal, genitourinary and nervous system ailments (Nilius and Szallasi, 2014). TRP channels play an indispensable role in relaying nociceptive stimuli for the perception of pain, from the periphery to the TG to the central nervous system.
Twenty-eight TRP ion channel genes are grouped in subfamilies based on sequence homology and are thought to work as homo- and hetero-tetramers (Moran and Szallasi, 2017). Structural similarities between family members are limited to the six transmembrane segments and a loop between segments five and six that forms the ion pore (Wu et al., 2010; Dai, 2016) (Fig. 5). Binding of a wide range of ligands, as well as thermal and mechanical stimuli, render TRP channels permeable to the major cations K+, Ca2+, and Na+ present in the extra- and intracellular fluids. The resulting net inward current may lead to generation of action potentials, whereas calcium signaling may also induce activity in postsynaptic neurons (Stucky et al., 2009; Parnas and Parnas, 2010). Collectively, these observations laid the foundation for pain management approaches that aim to reduce excitation of the peripheral nervous system by way of targeting TRP channels in a specific and selective manner (Sousa-Valente et al., 2014; Mickle et al., 2016; Moran and Szallasi, 2017). Clinical drug programs related to pain have been most extensively pursued for TRPA1 (inflammatory and neuropathic pain), TRPM8 (cold allodynia) and, with mixed results, TRPV1 with current approaches to management of neuropathic pain including corticosteroids and tricyclic antidepressants among others (Dieckmann et al., 2017a). These three TRP channels detect and transduce noxious, nociceptive, inflammatory and neuropathic stimuli and are highly expressed on nociceptors (Dai, 2016; Moran and Szallasi, 2017) (Fig. 5).
Fig. 5.
TRP-mediated signaling in sensory nerve fiber following exposure to noxious stimuli. TRPV1, TRPA1, and TRPM8 voltage-gated receptors are co-expressed on C and Aδ-nerve fibers and are responsive to chemical, mechanical, and thermal stimulation. Bradykinin release from resident inflammatory cells is known to increase sensitization of TRP receptors on sensory nerves acting primarily via phospholipase C (PLC) and protein kinase A (PKA) signaling pathways. Pictorials modified from Servier Medical Art under a Creative Commons Attribution 3.0 Unported License available at https://creativecommons.org/licenses/by/3.0/ .
The excitatory ion channel TRPA1 is expressed in dorsal root, trigeminal, nodose, geniculate and superior cervical ganglia and associated C and A??-fibers (Story et al., 2003; Smith et al., 2004; Kobayashi et al., 2005; Nagata et al., 2005; Katsura et al., 2006; Tatsumi et al., 2015). Crosstalk may occur between calcium signaling and other pathways, such as G-protein coupled receptors or TRPV1 (Moran and Szallasi, 2017), which is highly co-expressed with TRPA1 in C-fibers (Story et al., 2003).
The broad distribution of TRP receptors throughout the cornea leads to increased responsiveness of the tissue to pain detection. Selective agonists of the major TRP receptors present within the cornea have been studied (Table 2). The toxicity of these small molecules is dependent on receptor expression, concentration assayed, and duration of exposure. Moreover, effects at the sensory level are immediate with more chronic effects on nerve morphology and recurrent pain sensation dependent on exposure limits and may vary from patient-to-patient or model organism.
Table 2.
Known agonists of the voltage-gated channel receptors present within the human cornea. Chemical structure, major source of isolation, and target receptor are listed.
| Chemical | Structure | Source | Target |
|---|---|---|---|
| Capsaicin | Capsicum sp. (chili peppers) | TRPV1 | |
| Resiniferatoxin | 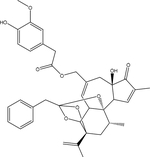 |
Euphorbia resinifera (cactus) | TRPV1 |
| Anandamide | 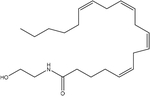 |
endogenous fatty acid metabolism | TRPV1 |
| Arachidonic acid | 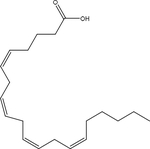 |
endogenous fatty acid metabolism | TRPV1 |
| Gingerol | 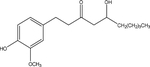 |
Z. officinale (ginger) | TRPV1 |
| Menthol | 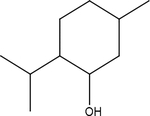 |
L. menthe, L. salvia | TRPM8 |
| Eucalyptol | 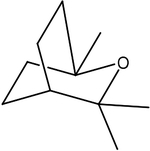 |
E. globulus | TRPM8 |
| Icilin | 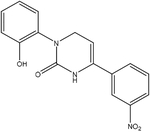 |
Synthetic | TRPM8, TRPA1 |
| Allyl Isothiocyanate | B. nigra, B. juncea, B. hirta | TRPA1 | |
| Cinnamaldehyde | 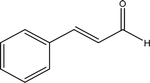 |
C. cassia | TRPA1 |
| Allicin | Alliaceae (garlic) | TRPA1, TRPV1 | |
| 2-chloro-acetophen | 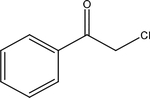 |
Synthetic (CN tear gas) | TRPA1 |
The TRP channels detect and respond to a broad range of exogenous chemicals (e.g. capsaicin, tear gases, aldehydes, cannabinoids) and endogenous signaling molecules that are released upon tissue damage, inflammation or oxidative or nitrative stress (e.g. prostaglandins, reactive oxygen and nitrogen species). Common TRPV1 agonists include the chili pepper constituent, capsaicin, resiniferatoxin, and endogenous fatty acid metabolites. Acute capsaicin application induces near immediate Ca2+-mediated depolarization (Chen et al., 1997) and release of neuropeptides SP and CGRP with chronic exposure leading to downregulation in TRPV1 receptor expression (Yang et al., 2010). Structural studies of the TRPV1 receptor have revealed characteristic binding motifs present that contribute to selective binding to potent ligands, capsaicin and resiniferatoxin (Elokely et al., 2016). Common chemical agonists of TRPA1 include thiosulfinate, isothiocyanate, and αβ unsaturated aldehyde-based irritants that are found in wasabi and mustard oils. In contrast, agents that lack a reactive group, such as capsaicin bind primarily via TRPV1 (Hinman et al., 2006; Macpherson et al., 2007). Activators of the TRPM8 channel include menthol and the synthetic chemical icilin, as well as pH changes during DED. Utilization of these stimulants in the study of pain mechanisms in tissue models may be useful to selectively activate TRP receptors autonomously, thereby establishing functional readouts for in vitro pain assessments. Additional activators of TRP receptors include hyperosmolar tears, light, cold-air, and select preservatives, such as benzalkonium chloride (Belmonte et al., 2017; Dieckmann et al., 2017a).
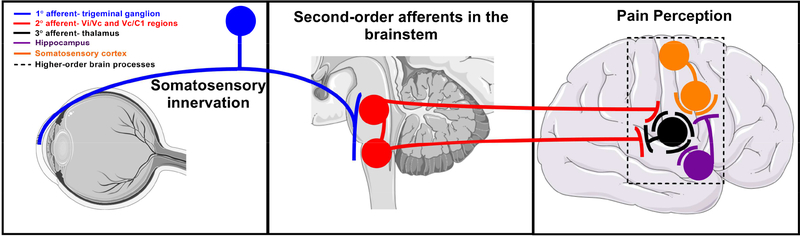
3.2. Propagation of sensory input to the trigeminal ganglion and brain
Lightly myelinated A??-fibers are very suitable to relay well-localized, fast pain because of their high conduction velocities, between 1.2 and 10 meters per second (Basbaum et al., 2009). The typical conduction velocity of <1.2 meters per second make unmyelinated, small-diameter C-fibers good candidates to transmit poorly localized, slow pain (Basbaum et al., 2009). Within the cornea, sensory perception propagates to the TG via both Aδ- and C-fibers (Belmonte et al., 2017). First-order neurons located in the TG receive nociceptive signals from the cornea from free ending termini and send projections to second-order neurons at two different locations of the brain stem nuclear complex: the Vi/Vc (trigeminal subnucleus interpolaris / caudalis transition region) and Vc/C1 (caudalis/upper cervical cord junction) areas of the trigeminal subnucleus caudalis region (Lazarov, 2002) (Fig. 6). First-order synapses play an important role in the process of pain signal amplification (Dubin and Patapoutian, 2010). Release of glutamate from these neurons activates voltage-gated sodium channels in second-order neurons (Peters et al., 2010). These neurons then send axonal projections to third-order neurons in the thalamus via the contralateral spinothalamic tract. Weak nociceptive signals cause a slight membrane depolarization via activation of AMPA type glutamate receptors (Courtney et al., 1990; Ben-Ari et al., 1997). In contrast, strong signals activate N-methyl D-aspartate (NMDA) receptors and presynaptic glutamate release from second-order neurons through complex signaling pathways that involve calcium influx, protein phosphorylation, upregulation of AMPA receptor expression and an increase in sodium ion conductance (Chittajallu et al., 1996; Cartmell and Schoepp, 2000). A second, longer lasting response is dependent on gene and protein expression, including that of TRP channels and release of neurotrophic factors (Hu et al., 2001). Spatial and temporal information related to the experienced noxious agent(s) ascend from the thalamus to the somatosensory cortex, the part of the brain responsible for pain perception (Fig. 6). These signals are further modified by input from subcortical structures associated with memory and emotions. Sustained, repeated, and intense nociceptive signals may elicit abnormal amplification of pain signals and a lowering of the threshold of activation by way of increasing synaptic function and membrane excitability and reduced inhibition. The net result is an enhancement of central excitatory activity leading to pain hypersensitivity during central sensitization (Almeida et al., 2004). Intricate negative feedback loops are in place to limit potentially uncontrolled activity of the ascending positive feed-forward loop. An additional safeguard is the activation of on and off interneurons in the TG and dorsal horn. These neurons release inhibitory glycine and GABA neurotransmitters, facilitating and inhibiting, respectively, second-order interneurons.
Fig. 6.
Transmission of sensory perception of stimuli detected at the ocular surface to the trigeminal ganglion, brainstem, and ultimately to the somatosensory cortex where pain is registered. Images of organs modified from Servier Medical Art under a Creative Commons Attribution 3.0 Unported License available at https://creativecommons.org/licenses/by/3.0/.
4. Corneal neuropathies associated with ocular pain
Neuropathic pain is defined by the International Association for the Study of Pain as pain caused by a lesion or disease of the somatosensory pathways in the peripheral and/or central nervous system (Jensen et al., 2011; Dworkin et al., 2013), in contrast with nociceptive pain produced by normal function of nociceptors (Belmonte et al., 2017). Neuropathic pain occurs in the eye as neuropathic ocular pain (NOP) (Galor et al., 2015a) and specifically in the cornea (NCP) (Rosenthal and Borsook, 2012; Aggarwal et al., 2015; Rosenthal and Borsook, 2016; Rosenthal et al., 2016; Dieckmann et al., 2017a; Hamrah et al., 2017) and can be perceived as pain or dysesthesias (unpleasant abnormal sensation), such as: discomfort (Theophanous et al., 2015b) (Galor et al., 2015a), photoallodynia (pain sensation to a non-painful stimulus; light) (Aggarwal et al., 2014; Aggarwal et al., 2015), burning (Galor et al., 2015b), irritation, dryness (Galor et al., 2015b) and grittiness. The diagnosis is clinical and requires a demonstrable damage or disease of the somatosensory nervous system (Jensen et al., 2011). Pain can be assessed with a variety of questionnaires, corneal nerves imaged with IVCM, and nerve functionality evaluated with esthesiometry or testing with topical anesthetics, such as proparacaine, to differentiate central from peripheral sensitization (Belmonte et al., 2017).
NCP can result from both peripheral nerve injury and systemic etiologies that can affect the somatosensory nerves and the pathway to the CNS. In addition, it has been categorized anatomically into peripheral and/or central, and etiologically into post-ocular surgery, ocular diseases and systemic diseases (Dieckmann et al., 2017a). Moreover, systemic comorbidities such as anxiety, depression and posttraumatic disorders have been shown to play an important role among NCP patients in modulating pain stimuli and transforming nociceptive pain into chronic pain (Galor et al., 2016) (Rosenthal and Borsook, 2012).
The corneal somatosensory system consists of nociceptors in the corneal epithelium (Marfurt et al., 2010), neural pathways to higher centers, thalamus and the somatosensory cortex, responsible for the conscious perception of pain. During homeostasis, nociceptors generate physiological responses to acute pain stimuli, however the persistence of inflammation may lead to nerve damage and release of pro-inflammatory cytokines and inflammatory mediators (Opree and Kress, 2000; Yamaguchi et al., 2014) that increase the peripheral signaling and cause peripheral sensitization (Belmonte et al., 2015). The abnormal peripheral signaling generated by nerve injury represents an altered sensory message transmitted to higher centers to relay in the CNS. Chronic pain and central sensitization often begins following an initial insult to the peripheral nerves that remains at minimal levels of peripheral activity for a long time (Hains et al., 2004; von Hehn et al., 2012; Baron et al., 2013). The hallmark of central sensitization is pain that is disconnected from ongoing peripheral signaling (Dieckmann et al., 2017a).
Diseases that lead to corneal nerve damage or injury may result in NCP and can be categorized into: infectious, chronic ocular surface disease, postsurgical, toxic, radiation keratopathy, trauma and systemic diseases. The most common clinical ocular comorbidity associated with NCP is DED, while the most two common associated ocular surgical condition for NCP are cataract and refractive surgeries (Dieckmann et al., 2017b). Systemic comorbidities associated with NCP include anxiety, depression and fibromyalgia, although diabetes and small fiber neuropathies also play a significant role (Dieckmann et al., 2017b).
Corneal nerves are part of the lacrimal functional unit (LFU), which is an integrated system composed of main and accessory lacrimal glands, ocular surface (cornea, conjunctiva, Meibomian glands) and eyelids. The integrity of LFU is essential for the maintenance of the tear film homeostasis and ocular surface integrity. Damage to corneal nerves due to inflammation leads to disruption of LFU integrity and ocular surface disease that express itself as DED (Cruzat et al., 2017). In DED, as part of a vicious cycle, the loss of tear film homeostasis as it occurs with increased tear film evaporation or decreased production of tear film, can lead to more inflammation and more peripheral nerve damage contributing to perpetuate the disease (Belmonte et al., 2017).
A multitude of etiologies can give rise to DED, including eyelid and blink abnormalities, as well as changes in tear film components and ocular surface. Based on the underlying etiology, DED can be categorized in subcategories that vary and frequently include complains of dysesthesias such as in NCP patients (Galor et al., 2018). Interestingly, DED patients without NCP and patients with NCP both demonstrate evidence of nerve injury as seen by IVCM. These patients may demonstrate similar symptoms, although in NCP patients, symptoms can be more severe. In chronic DED, persistent overt damage to peripheral nerves can lead to changes in the nociceptors firing abnormalities and symptoms like dysesthesias and pain. Indeed, the absence of clinical signs on slit-lamp examination, the lack of efficacy in the topical treatment with artificial tears, and the presence of microneuromas (disorganized mass of nerve cells that grow after nerve injury) in the IVCM has been shown to be present in NCP, but not among DED patients (Goyal and Hamrah, 2016; Dieckmann et al., 2017a; Moein et al., 2017).
There is a significant correlation between corneal nerve abnormalities (decreased corneal nerve density and nerve regeneration) and post-herpetic corneal sensation (Hamrah et al., 2010; Hamrah et al., 2013; Cruzat et al., 2016; Moein et al., 2018). Post-herpetic neuralgia (PHN) is a chronic and refractory neuropathic pain that persists for 3 months or more after an outbreak of acute herpes zoster infection. The varicella zoster virus is a neurotropic virus that remains dormant in the dorsal root ganglion and can reactivate resulting in acute herpes zoster. Patients complain about multiple types of pain including acute sharp pain, burning sensation, hyperalgesia and allodynia. Typically, the disease is unilateral following the correspondent dermatome and respecting the body medium line. Similar to PHN in non-ocular sites, the immune response triggered by the virus that propagates along the affected sensory nerves leads to peripheral nerve injury in the cornea. Once damaged, the peripheral nerve terminals (nociceptors) undergo changes that lower the threshold for nociceptive pain and deflagrate spontaneous ectopic pain signal. Subsequently, peripheral sensitization results in pain generated even in the absence of painful stimuli (Hadley et al., 2016). Continuous nociceptor stimulation also impairs descending pain inhibitory pathways, induces chronic excitability and depolarization of second-order neurons, which in turns leads to abnormal central processing involving activation of receptors like NMDA resulting in centralized post-herpetic neuropathic pain (Feller et al., 2005).
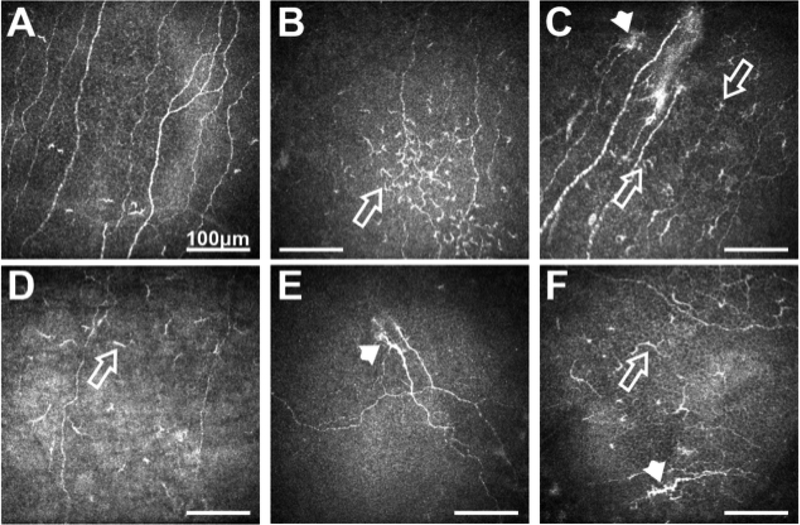
Although both α-herpes viruses, herpes simplex virus type 1 (HSV-1) and varicella zoster virus (HZV), can remain latent in the TG, reactivation from HZV in this site is rarely compared to HSV-1 (Theil et al., 2003). HSV-1 typically causes infection of the oral mucosa and persists latent in the sensory ganglion (TG). Reactivation of the HSV-1 in the TG generally affects cranial nerves causing herpes labialis, facial palsy, vestibular neuritis and corneal keratitis (Hamrah et al., 2010). As with HZV keratitis, after HSV-1 virus reactivation, damage to peripheral corneal nerves lead to inflammation and changes in nociceptors on the cornea. Recent IVCM studies have shown that peripheral corneal nerve density at the sub-basal corneal layer decreased in both viral infections compared to controls in both eyes (affected and contra-lateral eye) (Hamrah et al., 2010; Hamrah et al., 2013). These findings suggest bilateral changes in a clinically unilateral disease like HZV keratitis and reinforce the role of the CNS in bilateral neuronal ocular regulation (Fig. 7).
Fig. 7.
(A) Sub-basal nerve plexus in normal subject. (B) Patient with DED showing increased immune cells (arrow) and reduced nerve density. (C) Herpes simplex keratitis patient with increased immune cells (arrows), presence of micro-neuromas (arrow-head) and reduced nerve density. (D) Patient with herpes zoster ophthalmicus showing decreased nerve density and presence of immune cells. (E) Patient with NCP showing the presence of micro-neuromas (arrow-head). (F) Patient with diabetes showing increased nerve tortuosity (arrow-head), decreased nerve density, and presence of immune cells (arrow). All scale bars represent 100 μm (unpublished data).
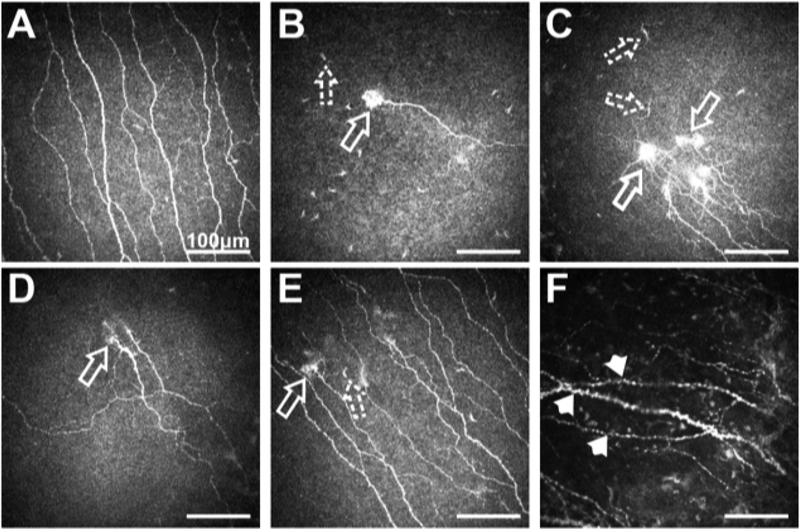
Systemic diseases that affect the peripheral nervous system and lead to small fiber neuropathy can also cause neuropathic pain. Small fiber neuropathies refer to a group of neuropathies defined by a selective or predominant lesion of peripheral poor myelinated Aδ-fibers and unmyelinated C-fibers (Fig. 8). The mechanism of injury is related to inflammation and axonal degeneration. The etiologies vary and include metabolic causes such as: diabetes, hypothyroidism, uremia, hypertriglyceridemia, vitamin B12 deficiency; neurotoxic exposure or vitamin intoxication such as antiretroviral agents, alcohol, chemotherapeutic agents; infections such as: hepatitis C virus, HIV, influenza, herpes virus; immunological causes such as Celiac disease and paraneoplastic syndrome, Sjögren’s disease, vasculitis; hereditary causes: Fabry disease, sensory and autonomic neuropathies; and idiopathic causes (Terkelsen et al., 2017). Interestingly, diabetes, Celiac disease, HIV and idiopathic small fiber neuropathies were the most common systemic diseases associated with NCP, after depression, anxiety, fibromyalgia and headache (Dieckmann et al., 2017b). Re-innervation and development of neuropathic corneal symptoms after a procedure may be affected by several factors, including type of surgical procedure, level of inflammation and systemic comorbidities (Richter et al., 1996; Dieckmann et al., 2017a). However, refractive surgery (in particular, laser-assisted in situ keratomileusis (LASIK)) and cataract surgery have been described as an associated risk factor for NCP (Theophanous et al., 2015b; Dieckmann et al., 2017b).
Fig. 8.
(A) Sub-basal nerve plexus in a normal subject; (B, C, D, E) NCP patient with different forms of micro-neuroma presentations (arrows) and presence of immune cells (dashed arrows); F. Sub-basal nerve plexus in NCP patient showing the presence of beading, axonal nerve swelling and nerve tortuosity (arrow-heads). All scale bars represent 100 μm (unpublished data).
The overlapping symptoms between neuropathic-like DED and the presence of non-ocular functional chronic pain have been recently showed by Galor and Crane (Galor et al., 2016; Crane et al., 2017). This close relationship emphasizes the idea of a systemic predisposition to central sensitivity through multiple mechanisms, including aberrations in signaling, glia/neuron interactions and genetic mechanisms of vulnerability. Neuropathic pain is often associated with comorbidities such as anxiety and depression resulting in a low health- related quality of life. The exact underlining neurobiological mechanism of overlap from those conditions is not clear, although neuroplasticity and gene expression changes were recently postulated as a possible mechanism (Post, 2016). Napadow et al., using functional magnetic resonance imaging (fMRI) data, have shown resting brain activity with multiple networks associated with spontaneous pain among patients with fibromyalgia in the brain stem (Napadow et al., 2010). These findings reinforce the interconnection between the associated comorbidities and pain perception areas. The temporal relationship between NCP and these comorbidities is not clear and may potentially be bidirectional. It is postulated that these two comorbidities play an important role modulating pain perception in the CNS (van Hecke et al., 2013; Dieckmann et al., 2017b).
5. Current models to study corneal pain
The indirect costs associated with chronic pain result from increased absenteeism and decreased productivity at work and have been estimated to total $100 billion each year in the United States. Neuropathic pain contributes substantially to these costs (McCarberg and Billington, 2006). Chronic pain can have detrimental effects on one’s quality of life. Current models to evaluate the propensity of chemicals to cause corneal irritation have focused heavily on toxicological assays using cell death as a marker of probable corneal damage. To protect against potential ocular harm, the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) was developed as an international treaty to define a consensus on chemical safety. The GHS serves to provide categorization of chemicals in terms of acute and chronic exposure on human and animal populations, as well environmental hazards, and protective measures that may need to be applied in the handling or transport of these agents (UN, 2009). The GHS categorization is based on animal testing and serves as a comparative means to evaluate the reliability of in vitro models. Due to the high-volume of chemicals continually in development, a shift from animal testing to more high-throughput, reliable cell culture methods have arisen over the years in an effort to “reduce, refine, and replace” the use of animals in assessing chemical safety and efficacy (Cotton, 1993).
5.1. In vivo neuropathic pain models
In vivo models to investigate neuropathic pain are commonly with rodents using surgical injury to a peripheral nerve. Sciatic nerve constriction is the most utilized technique in pain model development, as it produces a consistent and reproducible pain behavior (hyperalgesia) in response to stimulus. Models range in complexity and approach, and include chronic constriction injury (loose ligation) and ligation (tight) (Kim et al., 1997), axotomy, cryoneurolysis, resection, and laser-induced (Jaggi et al., 2011). The extent of hypersensitivity and pain is in turn determined by measuring response to mechanical (pressure and Von Frey Hair), heat and cold stimulation.
Current mouse models for corneal pain involve damage to the ocular surface and rely heavily on induction of corneal inflammation. Whilst none are neuropathic in nature, murine models to study corneal pain response include ocular alkali burn (Xiang et al., 2017) and photokeratitis (Tashiro et al., 2010), and induction of DED (Launay et al., 2016; Nicolle et al., 2016). The limitation of the ocular burn model, although a very clinically relevant complication present in patients, is that all terminal nerve endings are destroyed following an acute yet severe alcohol or alkali-based damage to the entire corneal epithelium. Such damage to the ocular surface results in the involvement and activation of resident immune cells of the cornea, as well as wound healing mechanisms, both likely to interfere with the investigation of underlying mechanisms behind pain. Similarly, UV irradiation of the ocular surface will likely result in damage to corneal nerves, as well as other corneal cell types including epithelial, keratocyte and endothelial cells (Kennedy et al., 1997; Wollensak et al., 2003; Thorsrud et al., 2012; Notara et al., 2015). Historically, induction of DED has included the use of scopolamine, and/or complete removal of the lacrimal gland, an invasive procedure severing the neural network involved in tear production and not necessarily clinically relevant. Further, DED, by definition, has inherent chronic inflammatory involvement, which will likely interfere with investigation of underlying mechanisms behind pain.
More recently, a murine NCP model whereby the ciliary branches innervating the cornea are ligated has been reported (Seyed-Razavi et al., 2017). This model utilizes a lateral conjunctival approach, previously employed to perform ciliary axotomy (Yamaguchi et al., 2013), to gain access and implement ligation of sensory nerve fibers supplied by the ophthalmic division of the trigeminal nerve (V), running parallel to the optic nerve, entering the eye retro-orbitally, and innervating the cornea by way of long and short ciliary nerves (Zander and Weddell, 1951; Schimmelpfennig, 1982). The partial compression injury to the ciliary nerves, in part due to axonal swelling, results in altered nerve function leading to peripheral nerve pain hypersensitivity, as also described in other models including sciatic nerve suture constriction (Maves et al., 1993; Ro and Jacobs, 1993; Jaggi et al., 2011; Austin et al., 2012). Slit-lamp analysis of the ocular surface revealed no signs of corneal fluorescein staining, opacity or neovascularization following ligation. Analysis of corneal sensation revealed the blink reflex is maintained following ciliary ligation. Interestingly, anatomical and density alterations were noted in corneal nerves with nerve beading and examples of micro-neuromas in the central cornea. Alteration in nociceptive response was noted with the number of nociceptive paw wipes to the affected eye following topical application of hyperosmolar saline (Hammond and Ruda, 1991; Farazifard et al., 2005) significantly increasing following ligation (Seyed-Razavi et al., 2017). Taken together, this novel NCP model demonstrates altered behavior, sensation and nerve density without epithelial damage, allowing investigation of underlying mechanism(s) behind NCP progression, as well as the study of therapeutic effect of drugs in the treatment of this debilitating disease.
5.3. In vitro tissue models
There are currently few 3D in vitro tissue models available to study corneal innervation and pain responses in concert with physical and structural changes in the cornea tissue. Corneal tissue models that more fully mimic the anatomy, mechanical properties, and cellular components of the human cornea would provide useful systems to study cellular interactions, corneal diseases, and provide options for improved drug screening.
In vitro tissue models with functional innervation have the potential to replace in vivo animal testing and provide sophisticated tools to study ocular nociception. Therefore, attention has been focused on the development of innervated, 3D human corneal tissues grown and maintained under physiologically relevant culture conditions to study nociceptive-related responses. To address limitations of monoculture approaches, several multicellular models have been reported (Table 3). Recent studies have used trigeminal ganglia from chick embryos co-cultured with embryo corneas embedded in collagen matrix in culture medium supplemented with NGF (Lwigale and Bronner-Fraser, 2007; Kubilus and Linsenmayer, 2010b). Each culture is comprised of a cornea positioned with the epithelium side up in the collagen and two ganglia halves placed 1 to 2 mm on opposite side of the cornea. This co-culture system allows for the direct contact of neurites within the cornea, while incorporating regulatory factors that are either secreted or anchored to the cellular membrane. This in vitro system has been used to study the expression of TRP channels in response to stimuli, showing comparable responses to in vivo settings in terms of expression levels and timing (Canner et al., 2014). However, in this system all neurons within the ganglion can potentially interact with the explanted cornea, while in vivo only a portion of these neurons would innervate the corneal tissues. Therefore, the lack of selective interactions with the corneal tissue could alter the neuronal responses in the in vitro tissue. Another example includes a whole corneal in vitro model that was developed and populated with isolated primary bovine or rabbit stromal cells and endothelial cells (Minami et al., 1993). Furthermore, chicken DRG neurons have also been used in combination with corneal epithelium and stromal cells in a collagen hydrogel (Zieske et al., 1994; Suuronen et al., 2004).
Table 3.
Summary of current innervated 3D models to study corneal tissue biology in vitro. Based on references (Canner et al., 2014; Priyadarsini et al., 2017; Wang et al., 2017; Sharif, 2018).
| Collagen-Based Corneal Model | Silk-Based Corneal Model | Self-Assembled Stromal Model | |
|---|---|---|---|
| Cell types | Avian embryonic corneal buttons and ophthalmic division of TG explants | Primary human corneal epithelial cells, corneal stromal stem cells, and chick DRGs | Primary human corneal fibroblasts and human neuroblastoma-derived neurons |
| Scaffold | Type 1 collagen hydrogels | Functionalized silk scaffolds, rat-tail Collagen type 1 | none; de novo ECM production by corneal fibroblasts generates stromal layer Stimulated with a stable |
| Culture conditions | Maintained for 96 hours in DMEM:F-12 supplemented with 10% inactivated FBS | Maintained at an air-liquid interface for 4 weeks in defined media conditions | Vitamin C derivative to promote ECM secretion and assembly over 4 weeks; maintained on polycarbonate transwell membrane |
| Advantages | Inclusion of relevant cells types (epi, stroma, endo and neuronal); innervation into corneal tissue can be assessed | Inclusion of relevant cells types (epi, stroma, and neuronal); stable conditions permit chronic studies | Native ECM produced by resident stromal cells allows for studies involving ECM assembly in the context of development or repair |
Recently, a corneal tissue model was generated to include the stroma, epithelium, and innervation (Wang et al., 2017). A multi-layered construct based on thin silk protein film served as the scaffolding to support the corneal epithelium and stromal layers (Ghezzi et al., 2017; Gosselin et al., 2017), while a surrounding silk porous sponge has been applied to culture cortical neurons in a 3D environment (Tang-Schomer et al., 2014). The inclusion of three cell types in co-culture at an air-liquid interface provides an important advance for the field of in vitro corneal tissue engineering, in that it allows for the study of innervation and corneal tissue development, corneal disease, and tissue responses to environmental factors (Wang et al., 2017) (Fig. 9). Further advances in developing innervated corneal tissue systems in vitro include the application of a self-assembled stromal model that relies on de novo ECM production by corneal fibroblasts and addition of the bone-marrow derived neuroblastoma cell line, SH-SY5Y, differentiated to a neuronal lineage (Priyadarsini et al., 2017; Sharif, 2018). Collectively, application of these human-based in vitro models to study nerve responses to stimuli under homeostatic conditions, as well during disease states, will provide tremendous opportunity to discover novel biomarkers related to the onset and progression of chronic pain, as well as promote discovery of novel analgesics targeting the sensory nerve.
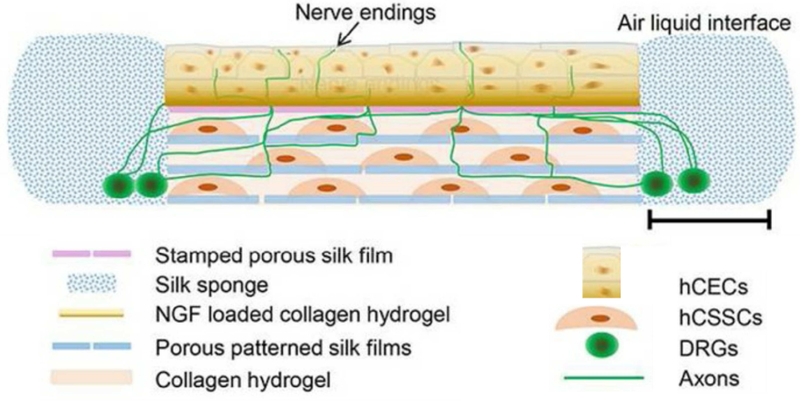
Fig. 9.
Tissue engineered innervated corneal model. Customized silk scaffolds containing appropriate cell types assembled to mimic the native cornea. The neural cell component (DRGs) are seeded in a silk sponge on the periphery with innervation into the stroma promoted using high NGF loading (50ng/mL) on the anterior film. The stromal layers are formed using Arg-Gly-Asp (RGD)-coated porous silk films assembled with interlaying collagen to develop biomimetic silk lamellae. (Abbreviations: hCECs (human corneal epithelial cells), hCSSCs (human corneal stromal stem cells), DRGs (dorsal root ganglion). Re-printed with permission (Wang et al., 2017).
5.6. Challenges with current approaches to predict ocular discomfort
Developing alternative models to in vivo approaches to predict ocular discomfort remains a common goal to increase screening potential, reduce animal use, and focus on human-based approaches. A need remains for consistent models to study the pain response, as well as development of comprehensive diagnostic tools with the prospect of discovering alternatives to opioids in the treatment of chronic pain. Retrospective analyses of the predictability of in vivo corneal toxicity assays in multiple databases suggest that significant inter- and intra- assay variability influences classification of chemical severity (Adriaens et al., 2014). Factors that contribute to accuracy of ocular discomfort predictability include persistence of the chemical on the corneal surface, animal behavior post-chemical application that may exacerbate ocular damage, and variances in clinical scoring. Inter-species differences in corneal structure may also affect the response of the tissue to chemical irritation suggesting that the choice of animal model, whether in vivo or ex vivo, may influence severity scores for chemicals. Physiological differences based on species may therefore impact the applicability of using animal responses to predict the human response. For example, the presence of the nictitating membrane, an inner, translucent membrane that can extend and retract over the corneal surface in the rabbit, may influence the corneal response to stimuli (Varsamidou et al., 2014) compared to the human, which lacks this structural feature. Inter-species variability is also exemplified by relative differences in corneal thickness with the bovine cornea having one of the thickest corneas (800–1000 μm) of animal models (Doughty, 2000). Utilization of isolated eyes from other animals, including the pig or chicken, may prove as useful substitutes for the cow cornea with more comparable thickness to the human cornea (500–700 μm).
While utilization of organotypic assays remain a common ex vivo approach to study ocular toxicity, nerve degeneration occurs near immediate post-cornea isolation at 1 hour that progresses to significant loss in sub-basal nerve fibers by 6 hours in the mouse (Stepp et al., 2014). Rapid degeneration of nerve endings in the cadaver cornea limits long-term studies of ocular irritation since nociceptive functionality is lacking. Furthermore, nerve loss of the excised cornea post-transplantation increases difficulty in attempting to study nerve structure in the human, thereby primarily relying on in vivo confocal microscopy in clinical settings.
The most common approaches to in vitro corneal models are based on the use of epithelial cells, as they act as a protective barrier for the eye, utilizing cultured human primary epithelial cells (hCECs) on tissue culture plastic for toxicology testing (Tripathi and Tripathi, 1988; Wilson et al., 2015). Alternatively, hCECs in fibrin gels form tissue sheets for ocular surface reconstruction (Han et al., 2002; Ramaesh and Dhillon, 2003). Immortalized human corneal epidermal keratinocytes cultured at the air-liquid interface on a polycarbonate membrane have been commercialized as an EpiOcular TM system (Van Goethem et al., 2006). Other commercially available corneal epithelium models include LabCyte CORNEA-MODEL (J-Tec), HCE (skinEthic) and Clonetics (Lonza) (Mohan et al., 2003; Shafaie et al., 2016). Notably, these epithelium-based models do not mirror the structure and multi-cellular population of the cornea, as well as their mutual interactions. Therefore, these models are useful to predict corneal toxicity, but due to lack of neural components are unable to be applied in the study of corneal pain. Further, these models do not recapitulate the alignment of the stromal cells, the multi-layer features of the epithelial cells, the sustained cultivation for chronic studies, the inclusion of tears, the exploitation of air-liquid interface culture environments, ocular pressure, or the native density of nerve endings (Roeder et al., 2002; Becker et al., 2006; Elsheikh et al., 2007). Provided the prominent role that these factors play in corneal tissue homeostasis, inclusion of these essential components normally present in the native microenvironment may prove important in developing dynamic models to study corneal sensory function.
6. Considerations for ideal model development
Understanding the multifaceted nature of pain caused by chemical, mechanical, or pathological irritation may lead to improved methods for predicting ocular toxicity of untested chemicals in vitro using human-based models, thereby limiting animal-use in chemical safety screening. However, in order for functional models to be applicable in the context of fulfilling requirements mandated by federal regulations, they must exceed well-defined metrics that show high-reproducibility, correlation to in vivo studies, and low false-negatives based on select chemical classes demonstrating the predictability of the system for unknown chemicals (ICCVAM, 2010). The difficulty in developing a functional in vitro system lies primarily with the cost, expertise, and timeframe required to assemble models that dictate the biochemical and structural cues found during corneal development in vivo. However, promising advances in tissue engineering approaches have been successfully applied in the development of dynamic systems of adipogenesis (Gerlach et al., 2012; Abbott et al., 2015), vascular flow (Lovett et al., 2007; Moya et al., 2013), and kidney function (Subramanian et al., 2010; Astashkina et al., 2014) with the inclusion of air- and liquid-perfusion, customized scaffolds, or co-culture techniques to ensure retention of the in vivo cellular phenotype in an assembled organotypic system. Likewise, similar approaches to developing functional corneal tissue models using silk scaffolds (Ghezzi et al., 2017; Wang et al., 2017), self-assembled stromal models (Zieske et al., 1994; Gouveia et al., 2017), and collagen hydrogels (Massie et al., 2014; Kureshi et al., 2015) have provided a basis for studying corneal tissue biology in vitro. Considerations regarding implementation of 2D versus 3D microenvironments, cell composition, and experimental readout are important in defining systems that may be useful in the study of pain mechanisms (Fig. 10).
Fig. 10.
Considerations for developing a functional corneal tissue model to study irritancy in vitro. A) NIH 3T3 fibroblasts grown in 3D collagen hydrogels or 2D polystyrene culture dishes. TRITC-conjugated phalloidin (red) and DAPI (blue). Adapted from (Bohm et al., 2017) with permission. B) Morphological differences between human corneal fibroblasts (hCFs) (elongated, expanded cytoplasm) and keratocytes (dendritic-like) depending on cell culture conditions maintained in vitro (Adapted from (Wilson et al., 2012) with permission). Immunofluorescence imaging of BIII-tubulin of DRGs grown on silicon micro-pillar substrates (Repic et al., 2016) and stem cell-derived nociceptors (Wainger et al., 2015) reproduced with permission. C) Dynamic maintenance of ex vivo tissue showing higher tight-junctions maintenance in the dynamic environment comparable to native lamellar corneas (NLC) in auto-tissue-engineered lamellar cornea (ATELC). Adapted from (Wu et al., 2014b) and reproduced with permission. D) Electrophysiological experiments in stem-cell derived nociceptors post-chemical (250 μm menthol, 100 μm mustard oil, 1 μm capsaicin, or 40 mM KCl) application. The number of nociceptors responding to stimuli is shown in a Venn diagram with capsaicin exhibiting the most robust response comparative to the extracellular microelectrode array recording. Adapted from (Wainger et al., 2015) and reproduced with permission. Potential modeling of pain responses will depend on exposure time and epithelial, stromal, and nerve contributions to the biochemical and electrophysiological responses to specific chemicals of interest (unpublished). Ideal validation of in vitro responses should correspond to reported in vivo responses, thereby establishing predictability for unknown chemicals.
6.1. 2D versus 3D
2D monocultures provide a relatively easy approach to study cellular interactions in vitro. However, 3D culture systems can provide a simplified variant of the native tissue architecture, where cells are embedded and surrounded within a hydrated matrix (Cummings et al., 2004; Yamane et al., 2005). Most of the cells, in particular those subjected to mechanical stimuli, demand a 3D environment in order to organize into a physiological tissue-like structure under in vitro conditions (Griffith and Swartz, 2006). Cell adhesion molecules distributed over the entire cell surface are able to interact with the surrounding matrix in 3D environments, significantly expanding the spatial organization of integrin receptors that mediate these cell-ECM linkages, in comparison to 2D substrates, which do not resemble the cellular arrangement found in native tissues. Moreover, the additional dimension provided by 3D substrates modulates integrin ligation, cell contraction, and associated intracellular signalling (Roskelley et al., 1994). Therefore, the dimensionality of the culture environment strongly affects the extent of external mechanical stimuli transferral to the resident cells.
Numerous studies have highlighted the importance of 3D constructs in the context of the corneal stroma with substrate stiffness influencing keratocyte and fibroblast morphology (Lakshman et al., 2010; Petroll et al., 2012; Miron-Mendoza et al., 2017). Maintenance of the native cellular phenotype has been shown to be influenced by culture conditions with higher expression of keratocyte markers, keratocan, lumican, and aldehyde dehydrogenase, in 3D systems compared to 2D monocultures (Ghezzi et al., 2017). The presence of stress fibers indicative of fibroblast differentiation may be driven by changes in ECM content, as well as secretion of biochemical factors, such as TGF-β and PDGF, by the epithelium or stroma (Zieske et al., 2001; Jester et al., 2002). These features not only influence the wound healing response, but also may modulate sensory nerve sensitivity in development of hyperalgesia (as discussed in Section 2). Furthermore, the ECM plays a fundamental role in regulating growth and functionality of peripheral nerves, as reviewed in (Martini, 1994; Chen et al., 2015). These studies emphasize the need to implement 3D model approaches in conjunction with in vivo models in the study of pain mechanisms.
6.2. Cell type
Given the multicellular nature of the cornea, choice of cell type to utilize in vitro may depend on media requirements for sustaining co-cultures, purpose of the assay, and expected readouts that may require accessibility or transparency. The corneal epithelium and stromal keratocytes contribute to hyperalgesia through sensitization of sensory nerves mediated by production of pro-inflammatory factors (Wilson et al., 2001). This contribution to the pain response necessitates careful thought as to the appropriate cell type for developing a functional model to predict corneal irritation. The most common cell choice in the study of the corneal stroma in vitro is the human corneal fibroblast (hCF) due to the relative ease of isolation from corneal buttons, proliferative ability, and significant ECM production. The utilization of hCFs in the development of 3D models includes a stable Vitamin C derivative as a required co-factor for collagen production to promote significant ECM deposition (~36 μm thick) in a relatively short period of time (4 weeks) (Guo et al., 2007). In stromal models, hCFs secrete and assemble a native ECM high in collagen type I and V with discrete proteoglycan secretion over time (Karamichos et al., 2010; Wu et al., 2014a). Further applications using isolated keratocytes from corneal buttons have been reported (Beales et al., 1999) but may prove difficult in obtaining large subcultures due to the maintenance of cells in low serum conditions, which limits cell migration from the corneal explant.
Significant work in the application of limbal stromal stem cells isolated from the corneal-scleral rim of corneal buttons has highlighted their potential to secrete and assemble a native stroma in vitro (Karamichos et al., 2014; Kureshi et al., 2014; Kureshi et al., 2015; Ghezzi et al., 2017). Advantages of the use of limbal stromal stem cells over commonly used corneal fibroblasts is linked to retention of a quiescent phenotype with high expression of the dominant crystallins present in the corneal stroma that promote tissue transparency, such as aldehyde dehydrogenase 3a, keratocan, and lumican, which is heavily reduced in the activated fibroblast (Wu et al., 2014a). Likewise, other stem cell-derived sources, including periodontal ligament stem cells, can be differentiated to keratocytes using defined culture conditions to promote stromal assembly high in collagen type I and V expression (Chen et al., 2017). In terms of predicting the stromal response to noxious stimuli, numerous studies have correlated increased α-SMA and collagen type III with corneal scarring both in vitro and in vivo mediated via TGF-β1 signalling (Zieske et al., 2001; Guo et al., 2016; Gupta et al., 2017). Therefore, upregulation in myofibroblast markers, α-SMA and collagen type III, may prove useful as potential predictors of the ability of a chemical to cause corneal scarring in vivo.
In developing models to study pain, the neuronal contribution is essential to detecting irritancy and mitigating pain signal propagation. The most heavily studied neuronal cell type for pain studies utilizes DRGs, due to the relative ease of isolation and high innervation into 3D models (Wang et al., 2015). Use of DRGs has been applied in developing an innervated corneal tissue model (Wang et al., 2017) highlighting the potential to apply whole-tissue systems of the cornea to study ocular pain. Other cell types that may prove useful in developing innervated tissues include the use of human induced neural stem cells (hiNSCs), which can be differentiated to sensory neurons (Chambers et al., 2012; Cairns et al., 2016) and implemented in similar co-culture conditions to develop a functional sensory component. Utilization of stem cell sources to generate select cell types may also be applied to develop genetic models for select diseases that may exhibit the pathological phenotype in vitro. Examples of this application include development of models for neurodegenerative diseases or metabolic conditions associated with nerve loss.
The inclusion of inflammatory cell types or appropriate pro-inflammatory factors may also be needed in pain model development given the contributory role of inflammation in hyperalgesia and acute and chronic pain responses. This area remains relatively unexplored in corneal tissue models but may be important in studying ocular infection and inflammatory pain in vitro. Addition of resident and invading immune cells (neutrophils, macrophages, and dendritic cells) may be useful to study nerve-immune cell crosstalk in a system readily adaptable to temporal studies that may be difficult to delineate in vivo due to the rapid and complex responses that occur following exposure to noxious stimuli.
6.3. Perfusion and environmental control
As an external tissue, the cornea requires frequent tear washing to prevent microbial growth and remove debris, as well as to supply oxygen and growth factors secreted by the tear glands. To mimic this dynamic nature in vitro, bioreactors have been developed to enable controlled regimes for the delivery of multiple growth factors and mechanical stimuli to direct cell growth and differentiation (Freed et al., 2006). Specifically, a bioreactor is a tissue culture tool to provide a mechanically active environment where physical, chemical and mechanical stimuli can be independently monitored and controlled. This multi-variable culture system has been proposed as a translational step from the traditional static culture environment to the in vivo animal model, to study construct maturation and integration with the capability to control physiological equivalent stimuli. However, the choice of the bioreactor regime is strongly related to the ability of the 3D substrate to respond to and transfer the applied stresses to the resident cells. Emphasis has been placed on a dedicated bioreactor for corneal tissues to recreate the intraocular pressure of the resident tissue and potentially extend the transplant shelf life by recreating physiological conditions (Guindolet et al., 2017). The importance of the tear film and its respective constituents in regulating cellular function in vivo has set benchmarks for functional requirements in a physiologically-relevant in vitro system. Most importantly, the presence of a tear film in the in vitro model would allow assessments of reversible damage upon irritant exposure, while reducing the occurrence of false positive due to the static culture environment as in mono-culture systems. In addition, the opportunity to extend the temporal window to study acute and chronic responses would have a tremendous impact on the clinical relevance of such tissue models.
6.4. Electrophysiological readouts and high-throughput approaches
Development of high-throughput approaches to evaluate irritancy potential has focused primarily on TRP receptor activation in 2D monocultures. Indirect measures of electrophysiological responses may be applied to assess cellular depolarization via inclusion of intracellular fluorescent probes that detect changes in ion flux. Prolonged over-expression of TRP receptors gives rise to an amplified signal for detection, but has been shown to affect cell viability and cell membrane integrity (Caudle et al., 2003), thereby limiting usefulness of this approach in high-throughput screening. However, large-scale transiently transfected cell lines exhibit over-expression and viability for the duration of the assay (24–72 hours) or up to 35 weeks frozen (Chen et al., 2007). Studies using large-scale transiently transfected HEK293 cells have been utilized to measure effects of topical drugs on TRPA1 activation in a 96-well plate format with the inclusion of a fluorescent calcium sensor (Bianchi et al., 2007). This approach may prove useful in identifying agonists of select receptors from large chemical libraries. Other interests have focused on opioid receptors as alternative targets to ameliorate persistent pain or anxiety, including κ-opioid receptor. Application of this approach has been shown with screening of >80,000 compounds performed on the hamster-derived epithelial cell line, CHO-OP2 with stable expression of κ-opioid receptor, using the LANCE™ cAMP assay as an experimental readout for receptor activation (Wang et al., 2014).
Significant developments in high-throughput patch clamp assays have enabled screening of thousands of compounds per day in a 384-well plate format using stable, over-expressing cell lines highlighting the potential for identifying novel therapeutics targeting select pain receptors (Chambers et al., 2016). However, further validation of analgesic effects must be performed in animal models given the limitations associated with drug degradation, tissue-specificity, and blood-brain barrier permeability that may influence therapeutic effectiveness in vivo. Dose-dependent effects of drug application and electrophysiological responses give promise to utilizing this approach in detecting irritancy potential of unknown chemicals. Further developments in applying these techniques to 3D constructs will serve as a useful means to study real-time responses to pain stimuli in addition to drug screening for novel analgesics (Frega et al., 2014).
7. Future perspectives
Growing advances in molecular imaging, electrophysiological approaches, and tissue engineering have led to the development of novel models to study physiological and disease processes related to nociception. Emphasis on potential applications is placed on novel approaches in tissue engineering that may enable in vivo extrapolation to predict the human response to an irritant depending on variations in biochemical and electrophysiological cues. Also compelling is the replacement of animal testing with human-equivalent options for development and evaluation of ocular pharmaceuticals in settings of inflammation and infection, as well as toxicology. Requirements for an in vitro tissue engineered cornea system range from cellular composition and tissue architecture, appropriate mechanics, long-term sustained culture conditions, and ultimately functional innervation mimicking the native tissue.
While the collective pain response occurs in the CNS, a novel approach to accelerate discovery of pain therapeutics is to establish functional in vitro systems that re-capitulate the milieu required for sensory perception. The overarching goal is to identify select afferent neuronal pathways that are involved in neuropathic pain at the level of the peripheral nervous system independent of physiological nociceptive processes that are required for tissue maintenance. This application may fill a gap in knowledge regarding sensory nerve biology, distinguishing acute and chronic pain mechanisms, with the prospect of developing alternatives to current opioid treatments by targeting select receptors present on sensory nerves that may contribute to morphological and biochemical changes that promote neuropathic pain development. Furthermore, research and development in this area may set the benchmark for modeling peripheral nerve signaling, distinguishing molecular differences between ocular itch and pain, and perhaps defining the neural networks required in higher-order brain processes using tissue culture systems that lend to easier visual evaluation and malleability in conjunction with in vivo models.
Highlights:
Corneal nerves detect mechanical, chemical, and thermal stimuli
Resident immune cells influence neuronal sensitization to noxious stimuli
Transient receptor potential channels expressed on nerves detect sensory input
Current corneal pain models focus on toxicity, structural, and functional changes
Advances in tissue engineering potentiate the ability to study corneal pain in vitro
Acknowledgments
We thank the various lab members who have contributed to our own research on cornea tissues over the years. We also thank the NIH (P41EB002520 (DK), R01AR055993 (DK), R01EY020856 (DK), R01EY022695 (PH), R01EY029602 (PH), and R21EY025393 (PH)) for support of our research related to the topic in this review. We would also like to acknowledge Rachael Parker for critical editing of the manuscript.
Abbreviations:
- α-SMA
alpha-smooth muscle actin
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- APCs
antigen-presenting cells
- BCOP
bovine corneal opacity and permeability assay
- BDNF
brain-derived neurotrophic factor
- BM
bone marrow
- cDCs
conventional dendritic cells
- CGRP
calcitonin gene related peptide
- CNS
central nervous system
- CNT
ciliary neurotrophic factor
- DCs
dendritic cells
- DED
dry eye disease
- DRG
dorsal root ganglion
- ECM
extracellular matrix
- EGF
epithelial growth factor
- EPA
Environmental Protections Agency
- FDA
Food and Drug Administration
- FOX
Forkhead transcription factors
- GABA
gamma-aminobutyric acid
- GDNF
glial cell-derived neurotrophic factor
- GHS
Globally Harmonized System of Classification and Labelling of Chemicals
- hCECs
human corneal epithelial cells
- hCFs
human corneal fibroblasts
- hCSSCs
human corneal stromal stem cells
- hiNPCs
human induced neuronal precursor cells
- IFN-γ
interferon-γ
- IGF-1
insulin-related growth factor-1
- IL
interleukins
- IVCM
in vivo confocal microscopy
- LASIK
laser-assisted in situ keratomileusis
- LFU
lacrimal function unit
- LVET
low-volume eye test
- MAPK
mitogen-activated protein kinases
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- MDSCs
myeloid derived suppressor cells
- NCP
neuropathic corneal pain
- NGF
nerve growth factor
- NK
natural killer
- NK1
neurokinin 1
- NMDA
N-methyl-D-aspartate
- NPY
neuropeptide Y
- PDCs
plasmacytoid dendritic cells
- PHN
post-herpetic neuralgia
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- SP
Substance P
- TG
trigeminal ganglion
- TGF-β
transforming growth factor-
- TNF-α
tumor necrosis factor-α
- TRKB
tropomyosin receptor kinase B
- TRP
transient receptor potential channel
- TRPA1
transient receptor potential cation channel subfamily A member 1
- TRPM8
transient receptor potential cation channel subfamily M member 8
- TRPV1-4
transient receptor potential cation channel subfamily V member 1–4
- VEGF
vascular endothelial growth factor
- 5-HT
5-hydroxytrypatmine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- 2007. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5, 179–193. [DOI] [PubMed] [Google Scholar]
- Abbott RD, Raja WK, Wang RY, Stinson JA, Glettig DL, Burke KA, Kaplan DL, 2015. Long term perfusion system supporting adipogenesis Methods (San Diego, Calif: ) 84, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acera A, Rocha G, Vecino E, Lema I, Durán JA, 2008. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic research 40, 315–321. [DOI] [PubMed] [Google Scholar]
- Acosta MC, Luna C, Quirce S, Belmonte C, Gallar J, 2013. Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain 154, 2353–2362. [DOI] [PubMed] [Google Scholar]
- Adriaens E, Barroso J, Eskes C, Hoffmann S, McNamee P, Alepee N, Bessou-Touya S, De Smedt A, De Wever B, Pfannenbecker U, Tailhardat M, Zuang V, 2014. Retrospective analysis of the Draize test for serious eye damage/eye irritation: importance of understanding the in vivo endpoints under UN GHS/EU CLP for the development and evaluation of in vitro test methods. Archives of toxicology 88, 701–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Colon C, Kheirkhah A, Hamrah P, 2014. Efficacy of autologous serum tears for treatment of severe corneal pain in patients with corneal neuropathy: An in vivo confocal microscopy study. Investigative ophthalmology & visual science 55, 1468. [Google Scholar]
- Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, Borsook D, Pruss H, Hamrah P, 2015. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf 13, 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AR, Arroz-Madeira S, Fonseca-Pereira D, Ribeiro H, Lasrado R, Pachnis V, Veiga-Fernandes H, 2012. RET/GFRalpha signals are dispensable for thymic T cell development in vivo. PLoS One 7, e52949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida TF, Roizenblatt S, Tufik S, 2004. Afferent pain pathways: a neuroanatomical review. Brain research 1000, 40–56. [DOI] [PubMed] [Google Scholar]
- Alven A, L.C.R.R., 2015. The Role of Damage Associated Molecular Patterns in Dry Eye Inflammation. Optom Vis Sci 85. [Google Scholar]
- Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G, 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nature immunology 2, 1144–1150. [DOI] [PubMed] [Google Scholar]
- Astashkina AI, Jones CF, Thiagarajan G, Kurtzeborn K, Ghandehari H, Brooks BD, Grainger DW, 2014. Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. Biomaterials 35, 6323–6331. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Moalem-Taylor G, 2010. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol 229, 26–50. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Wu A, Moalem-Taylor G, 2012. Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban Y, Dota A, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Mochida C, Kinoshita S, 2003. Tight junction-related protein expression and distribution in human corneal epithelium. Exp Eye Res 76, 663–669. [DOI] [PubMed] [Google Scholar]
- Barabino S, Chen Y, Chauhan S, Dana R, 2012. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res 31, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Hans G, Dickenson AH, 2013. Peripheral input and its importance for central sensitization. Ann Neurol 74, 630–636. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D, 2009. Cellular and molecular mechanisms of pain. Cell 139, 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G, Labetoulle M, Rolando M, 2013. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf 11, 246–258. [DOI] [PubMed] [Google Scholar]
- Beales MP, Funderburgh JL, Jester JV, Hassell JR, 1999. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Investigative ophthalmology & visual science 40, 1658–1663. [PubMed] [Google Scholar]
- Becker RA, Borgert CJ, Webb S, Ansell J, Amundson S, Portier CJ, Goldberg A, Bruner LH, Rowan A, Curren RD, 2006. Report of an ISRTP Workshop: Progress and barriers to incorporating alternative toxicological methods in the US. Regulatory Toxicology and Pharmacology 46, 18–22. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Gallar J, 2004. Neural basis of sensation in intact and injured corneas. Experimental eye research 78, 513–525. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J, 2015. What Causes Eye Pain? Curr Ophthalmol Rep 3, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, Dartt DA, Galor A, Hamrah P, Ivanusic JJ, Jacobs DS, McNamara NA, Rosenblatt MI, Stapleton F, Wolffsohn JS, 2017. TFOS DEWS II pain and sensation report. Ocul Surf 15, 404–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa J-L, 1997. GABAA, NMDA and AMPA receptors: a developmentally regulatedmenage a trois’. Trends in neurosciences 20, 523–529. [DOI] [PubMed] [Google Scholar]
- Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J, 2004. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Investigative ophthalmology & visual science 45, 3030–3035. [DOI] [PubMed] [Google Scholar]
- Bianchi BR, Moreland RB, Faltynek CR, Chen J, 2007. Application of large-scale transiently transfected cells to functional assays of ion channels: different targets and assay formats. Assay and drug development technologies 5, 417–424. [DOI] [PubMed] [Google Scholar]
- Bianchi ME, 2007. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology 81, 1–5. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA, 2008. Nociceptors are interleukin-1beta sensors. J Neurosci 28, 14062–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorck P, 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98, 3520–3526. [DOI] [PubMed] [Google Scholar]
- Bohm S, Strauss C, Stoiber S, Kasper C, Charwat V, 2017. Impact of Source and Manufacturing of Collagen Matrices on Fibroblast Cell Growth and Platelet Aggregation Materials (Basel, Switzerland: ) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL, 2002. Identification of a novel macrophage population in the normal mouse corneal stroma. Investigative ophthalmology & visual science 43, 2264–2271. [PMC free article] [PubMed] [Google Scholar]
- Bucher F, Schneider C, Blau T, Cursiefen C, Fink GR, Lehmann HC, Heindl LM, 2015. Small-Fiber Neuropathy Is Associated With Corneal Nerve and Dendritic Cell Alterations: An In Vivo Confocal Microscopy Study. Cornea 34, 1114–1119. [DOI] [PubMed] [Google Scholar]
- Cairns DM, Chwalek K, Moore YE, Kelley MR, Abbott RD, Moss S, Kaplan DL, 2016. Expandable and Rapidly Differentiating Human Induced Neural Stem Cell Lines for Multiple Tissue Engineering Applications. Stem cell reports 7, 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canner JP, Linsenmayer TF, Kubilus JK, 2014. Developmental regulation of trigeminal TRPA1 by the cornea. Investigative ophthalmology & visual science 56, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragnano M, Tortorella P, Bergami A, Ruggieri M, Livrea P, Specchio LM, Martino G, Trojano M, Furlan R, Avolio C, 2012. Monocytes P2X7 purinergic receptor is modulated by glatiramer acetate in multiple sclerosis. J Neuroimmunol 245, 93–97. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD, 2000. Regulation of neurotransmitter release by metabotropic glutamate receptors. Journal of neurochemistry 75, 889–907. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Karai L, Mena N, Cooper BY, Mannes AJ, Perez FM, Iadarola MJ, Olah Z, 2003. Resiniferatoxin-induced loss of plasma membrane in vanilloid receptor expressing cells. Neurotoxicology 24, 895–908. [DOI] [PubMed] [Google Scholar]
- Cavalcanti BM, Cruzat A, Sahin A, Pavan-Langston D, Samayoa E, Hamrah P, 2018. In vivo confocal microscopy detects bilateral changes of corneal immune cells and nerves in unilateral herpes zoster ophthalmicus. Ocul Surf 16, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik MO, Labuz D, Henning K, Busch-Dienstfertig M, Gaveriaux-Ruff C, Kieffer BL, Zimmer A, Machelska H, 2016. Leukocyte opioid receptors mediate analgesia via Ca(2+)-regulated release of opioid peptides. Brain, behavior, and immunity 57, 227–242. [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L, Zust R, Weber F, Spiegel M, Lang KS, Akira S, Thiel V, Ludewig B, 2007. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood 109, 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C, Witton I, Adams C, Marrington L, Kammonen J, 2016. High-Throughput Screening of Na(V)1.7 Modulators Using a Giga-Seal Automated Patch Clamp Instrument. Assay and drug development technologies 14, 93–108. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH, Studer L, 2012. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nature biotechnology 30, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DK, Frizzi KE, Guernsey LS, Ladt K, Mizisin AP, Calcutt NA, 2013. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. Journal of the peripheral nervous system : JPNS 18, 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lake MR, Sabet RS, Niforatos W, Pratt SD, Cassar SC, Xu J, Gopalakrishnan S, Pereda-Lopez A, Gopalakrishnan M, Holzman TF, Moreland RB, Walter KA, Faltynek CR, Warrior U, Scott VE, 2007. Utility of large-scale transiently transfected cells for cell-based high-throughput screens to identify transient receptor potential channel A1 (TRPA1) antagonists. Journal of biomolecular screening 12, 61–69. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang W, Kelk P, Backman LJ, Danielson P, 2017. Substance P and patterned silk biomaterial stimulate periodontal ligament stem cells to form corneal stroma in a bioengineered three-dimensional model. Stem Cell Research & Therapy 8, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Cescon M, Bonaldo P, 2015. The Role of Collagens in Peripheral Nerve Myelination and Function. Molecular neurobiology 52, 216–225. [DOI] [PubMed] [Google Scholar]
- Chen X, Belmonte C, Rang HP, 1997. Capsaicin and carbon dioxide act by distinct mechanisms on sensory nerve terminals in the cat cornea. Pain 70, 23–29. [DOI] [PubMed] [Google Scholar]
- Chinnery HR, Leong CM, Chen W, Forrester JV, McMenamin PG, 2015. TLR9 and TLR7/8 activation induces formation of keratic precipitates and giant macrophages in the mouse cornea. Journal of leukocyte biology 97, 103–110. [DOI] [PubMed] [Google Scholar]
- Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, McMenamin PG, 2007. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Investigative ophthalmology & visual science 48, 1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM, 1996. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 379, 78. [DOI] [PubMed] [Google Scholar]
- Chiu IM, von Hehn CA, Woolf CJ, 2012. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nature neuroscience 15, 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrai SS, Patton TF, Mehta A, Robinson JR, 1973. Lacrimal and instilled fluid dynamics in rabbit eyes. Journal of pharmaceutical sciences 62, 1112–1121. [DOI] [PubMed] [Google Scholar]
- Clark AK, Malcangio M, 2014. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci 8, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Wodarski R, Guida F, Sasso O, Malcangio M, 2010. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia 58, 1710–1726. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP, 2000. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407, 1011–1015. [DOI] [PubMed] [Google Scholar]
- Colonna M, 2006. Toll-like receptors and IFN-alpha: partners in autoimmunity. J Clin Invest 116, 2319–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton P, 1993. Animals and science benefit from ‘replace, reduce, refine’ effort. Jama 270, 2905–2907. [PubMed] [Google Scholar]
- Courtney M, Lambert J, Nicholls D, 1990. The interactions between plasma membrane depolarization and glutamate receptor activation in the regulation of cytoplasmic free calcium in cultured cerebellar granule cells. Journal of Neuroscience 10, 3873–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A, 2017. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol 101, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, Hamrah P, Cavalcanti BM, Zheng L, Colby K, Pavan-Langston D, 2016. Corneal Reinnervation and Sensation Recovery in Patients With Herpes Zoster Ophthalmicus: An In Vivo and Ex Vivo Study of Corneal Nerves. Cornea 35, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, Pavan-Langston D, Hamrah P, 2010. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Seminars in ophthalmology 25, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, Qazi Y, Hamrah P, 2017. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf 15, 15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, Schrems WA, Schrems-Hoesl LM, Cavalcanti BM, Baniasadi N, Witkin D, Pavan-Langston D, Dana R, Hamrah P, 2015. Contralateral Clinically Unaffected Eyes of Patients With Unilateral Infectious Keratitis Demonstrate a Sympathetic Immune Response. Investigative ophthalmology & visual science 56, 6612–6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, Chodosh J, Pavan-Langston D, Dana R, Hamrah P, 2011. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Investigative ophthalmology & visual science 52, 5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M, Dearman RJ, Kimber I, 1997. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology 92, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CL, Gawlitta D, Nerem RM, Stegemann JP, 2004. Properties of engineered vascular constructs made from collagen, fibrin, and collagen‚ fibrin mixtures. Biomaterials 25, 3699–3706. [DOI] [PubMed] [Google Scholar]
- Dai Y, 2016. TRPs and pain. Semin Immunopathol 38, 277–291. [DOI] [PubMed] [Google Scholar]
- De Armentia ML, Cabanes C, Belmonte C, 2000. Electrophysiological properties of identified trigeminal ganglion neurons innervating the cornea of the mouse. Neuroscience 101, 1109–1115. [DOI] [PubMed] [Google Scholar]
- De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD 3rd, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC, 2009. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann G, Goyal S, Hamrah P, 2017a. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 124, S34–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann G, Neslihan DK, Jamali A, Lopez JM, Moein H, Hamrah P, 2017b. Demographics and Risk Factors of Patients with Neuropathic Corneal Pain, American Academy of Ophtalmology, New Orleans [Google Scholar]
- Doughty MJ, 2000. Swelling of the collagen-keratocyte matrix of the bovine corneal stroma ex vivo in various solutions and its relationship to tissue thickness. Tissue & cell 32, 478–493. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Patapoutian A, 2010. Nociceptors: the sensors of the pain pathway. J Clin Invest 120, 3760–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, O’Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, Levy RM, Backonja M, Baron R, Harke H, Loeser JD, Treede RD, Turk DC, Wells CD, International Association for the Study of Pain Neuropathic Pain Special Interest, G., 2013. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 154, 2249–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron N, 2011. The Glenn A. Fry award lecture 2010: Ophthalmic markers of diabetic neuropathy. Optom Vis Sci 88, 661–683. [DOI] [PubMed] [Google Scholar]
- Elokely K, Velisetty P, Delemotte L, Palovcak E, Klein ML, Rohacs T, Carnevale V, 2016. Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin. Proceedings of the National Academy of Sciences of the United States of America 113, E137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikh A, Wang D, Brown M, Rama P, Campanelli M, Pye D, 2007. Assessment of corneal biomechanical properties and their variation with age. Current eye research 32, 11–19. [DOI] [PubMed] [Google Scholar]
- Farazifard R, Safarpour F, Sheibani V, Javan M, 2005. Eye-wiping test: a sensitive animal model for acute trigeminal pain studies. Brain Res Brain Res Protoc 16, 44–49. [DOI] [PubMed] [Google Scholar]
- Feller L, Jadwat Y, Bouckaert M, 2005. Herpes zoster post-herpetic neuralgia. SADJ 60, 432, 436–437. [PubMed] [Google Scholar]
- Fernandes ES, Liang L, Smillie SJ, Kaiser F, Purcell R, Rivett DW, Alam S, Howat S, Collins H, Thompson SJ, Keeble JE, Riffo-Vasquez Y, Bruce KD, Brain SD, 2012. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J Immunol 188, 5741–5751. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Bignami F, Giacomini C, Capitolo E, Comi G, Chaabane L, Rama P, 2014. Ocular surface injury induces inflammation in the brain: in vivo and ex vivo evidence of a corneal-trigeminal axis. Invest. Ophthalmol. Vis. Sci 55, 6289–6300. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S, 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 183, 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frascoli M, Marcandalli J, Schenk U, Grassi F, 2012. Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral gammadelta cells. J Immunol 189, 174–180. [DOI] [PubMed] [Google Scholar]
- Freed LE, Guilak F, Guo XE, Gray ML, Tranquillo R, Holmes JW, Radisic M, Sefton MV, Kaplan D, Vunjak-Novakovic G, 2006. Advanced tools for tissue engineering: Scaffolds, bioreactors, and signaling. Tissue Engineering 12, 3285–3305. [DOI] [PubMed] [Google Scholar]
- Freedman BD, Fleischmann BK, Punt JA, Gaulton G, Hashimoto Y, Kotlikoff MI, 1995. Identification of Kv1.1 expression by murine CD4-CD8- thymocytes. A role for voltage-dependent K+ channels in murine thymocyte development. The Journal of biological chemistry 270, 22406–22411. [DOI] [PubMed] [Google Scholar]
- Frega M, Tedesco M, Massobrio P, Pesce M, Martinoia S, 2014. Network dynamics of 3D engineered neuronal cultures: a new experimental model for in-vitro electrophysiology. Scientific reports 4, 5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galor A, Covington D, Levitt AE, McManus KT, Seiden B, Felix ER, Kalangara J, Feuer W, Patin DJ, Martin ER, Sarantopoulos KD, Levitt RC, 2016. Neuropathic Ocular Pain due to Dry Eye is Associated with Multiple Comorbid Chronic Pain Syndromes. The journal of pain : official journal of the American Pain Society 17, 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD, 2015a. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 29, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galor A, Levitt RC, Felix ER, Sarantopoulos CD, 2015b. Understanding the true burden of dry eye disease. Expert Rev Ophthalmol 10, 403–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galor A, Moein HR, Lee C, Rodriguez A, Felix ER, Sarantopoulos KD, Levitt RC, 2018. Neuropathic pain and dry eye. Ocul Surf 16, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NB, Su Z, Zhang X, Volpe EA, Pelegrino FS, Rahman SA, Li DQ, Pflugfelder SC, de Paiva CS, 2013. Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress. Journal of leukocyte biology 94, 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR, 2010. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacology & therapeutics 126, 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Suarez O, Blanco-Gelaz MA, Lopez ML, Germana A, Cabo R, Diaz-Esnal B, Silos-Santiago I, Ciriaco E, Vega JA, 2002. Massive lymphocyte apoptosis in the thymus of functionally deficient TrkB mice. J Neuroimmunol 129, 25–34. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, 2012. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature immunology 13, 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautreau L, Chabannes D, Heslan M, Josien R, 2011. Modulation of regulatory T cell-Th17 balance by plasmacytoid dendritic cells. Journal of leukocyte biology 90, 521–527. [DOI] [PubMed] [Google Scholar]
- Gerlach JC, Lin YC, Brayfield CA, Minteer DM, Li H, Rubin JP, Marra KG, 2012. Adipogenesis of human adipose-derived stem cells within three-dimensional hollow fiber-based bioreactors. Tissue engineering. Part C, Methods 18, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi CE, Marelli B, Omenetto FG, Funderburgh JL, Kaplan DL, 2017. 3D Functional Corneal Stromal Tissue Equivalent Based on Corneal Stromal Stem Cells and Multi-Layered Silk Film Architecture. PloS one 12, e0169504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette TE, Chandler JW, Greiner JV, 1982. Langerhans cells of the ocular surface. Ophthalmology 89, 700–711. [DOI] [PubMed] [Google Scholar]
- Gold MS, Gebhart GF, 2010. Nociceptor sensitization in pain pathogenesis. Nat Med 16, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez O, Bech F, Gallar J, Merayo-Lloves J, Belmonte C, 2017. Functional Properties of Sensory Nerve Terminals of the Mouse Cornea. Investigative ophthalmology & visual science 58, 404–415. [DOI] [PubMed] [Google Scholar]
- Gosselin EA, Torregrosa T, Ghezzi CE, Mendelsohn AC, Gomes R, Funderburgh JL, Kaplan DL, 2017. Multi-layered silk film coculture system for human corneal epithelial and stromal stem cells. Journal of tissue engineering and regenerative medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Oh SB, Takeda M, Shinoda M, Sato T, Gunjikake KK, Iwata K, 2016. Recent advances in basic research on the trigeminal ganglion. J Physiol Sci 66, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia RM, Gonzalez-Andrades E, Cardona JC, Gonzalez-Gallardo C, Ionescu AM, Garzon I, Alaminos M, Gonzalez-Andrades M, Connon CJ, 2017. Controlling the 3D architecture of Self-Lifting Auto-generated Tissue Equivalents (SLATEs) for optimized corneal graft composition and stability. Biomaterials 121, 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S, Hamrah P, 2016. Understanding Neuropathic Corneal Pain--Gaps and Current Therapeutic Approaches. Seminars in ophthalmology 31, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LG, Swartz MA, 2006. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7, 211–224. [DOI] [PubMed] [Google Scholar]
- Guindolet D, Crouzet E, He Z, Herbepin P, Jumelle C, Perrache C, Dumollard JM, Forest F, Peoc’h M, Gain P, Gabison E, Thuret G, 2017. Storage of Porcine Cornea in an Innovative Bioreactor. Investigative ophthalmology & visual science 58, 5907–5917. [DOI] [PubMed] [Google Scholar]
- Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW, 2007. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Investigative ophthalmology & visual science 48, 4050–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Hutcheon AE, Zieske JD, 2016. Molecular insights on the effect of TGF-beta1/-beta3 in human corneal fibroblasts. Exp Eye Res 146, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Rodier JT, Sharma A, Giuliano EA, Sinha PR, Hesemann NP, Ghosh A, Mohan RR, 2017. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS One 12, e0172928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley GR, Gayle JA, Ripoll J, Jones MR, Argoff CE, Kaye RJ, Kaye AD, 2016. Post-herpetic Neuralgia: a Review. Curr Pain Headache Rep 20, 17. [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG, 2004. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J Neurosci 24, 4832–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond DL, Ruda MA, 1991. Developmental alterations in nociceptive threshold, immunoreactive calcitonin gene-related peptide and substance P, and fluoride-resistant acid phosphatase in neonatally capsaicin-treated rats. The Journal of comparative neurology 312, 436–450. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Cruzat A, Dastjerdi MH, Pruss H, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D, 2013. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology 120, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D, 2010. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology 117, 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Dana MR, 2007. Corneal antigen-presenting cells. Chemical immunology and allergy 92, 58–70. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Dana R, 2010. Antigen-Presenting Cells in the Eye and Ocular Surface A2 - Dartt, Darlene A, Encyclopedia of the Eye. Academic Press, Oxford, pp. 120–127. [Google Scholar]
- Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR, 2003a. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. Journal of leukocyte biology 74, 172–178. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Liu Y, Zhang Q, Dana MR, 2003b. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol 121, 1132–1140. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Liu Y, Zhang Q, Dana MR, 2003c. The corneal stroma is endowed with a significant number of resident dendritic cells. Investigative ophthalmology & visual science 44, 581–589. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Qazi Y, Shahatit B, Dastjerdi MH, Pavan-Langston D, Jacobs DS, Rosenthal P, 2017. Corneal nerve and epithelial cell alterations in corneal allodynia: An in vivo confocal microscopy case series. Ocul Surf 15, 139–151. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Seyed-Razavi Y, Yamaguchi T, 2016. Translational Immunoimaging and Neuroimaging Demonstrate Corneal Neuroimmune Crosstalk. Cornea 35 Suppl 1, S20–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Zhang Q, Liu Y, Dana MR, 2002. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Investigative ophthalmology & visual science 43, 639–646. [PubMed] [Google Scholar]
- Han B, Schwab IR, Madsen TK, Isseroff RR, 2002. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea 21, 505–510. [DOI] [PubMed] [Google Scholar]
- Han KY, Tran JA, Chang JH, Azar DT, Zieske JD, 2017. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Scientific reports 7, 40548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L, 1998. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proceedings of the National Academy of Sciences of the United States of America 95, 10896–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Chauhan SK, Lee H, Ueno H, Dana R, Kaplan DH, Saban DR, 2011. Characterization of Langerin-expressing dendritic cell subsets in the normal cornea. Investigative ophthalmology & visual science 52, 4598–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiner I, Eisfeld J, Halaszovich CR, Wehage E, Jungling E, Zitt C, Luckhoff A, 2003. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. The Biochemical journal 371, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D, 2006. TRP channel activation by reversible covalent modification. Proceedings of the National Academy of Sciences of the United States of America 103, 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Mizerska K, Marfurt CF, Rosenblatt MI, 2015. Hyperosmolar Tears Induce Functional and Structural Alterations of Corneal Nerves: Electrophysiological and Anatomical Evidence Toward Neurotoxicity. Investigative ophthalmology & visual science 56, 8125–8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Rosenblatt MI, 2014. Hyperosmolar tears enhance cooling sensitivity of the corneal nerves in rats: possible neural basis for cold-induced dry eye pain. Investigative ophthalmology & visual science 55, 5821–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockley JR, Tranter MM, McGuire C, Boundouki G, Cibert-Goton V, Thaha MA, Blackshaw LA, Michael GJ, Baker MD, Knowles CH, Winchester WJ, Bulmer DC, 2016. P2Y Receptors Sensitize Mouse and Human Colonic Nociceptors. J Neurosci 36, 2364–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao L-R, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, 2001. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. Journal of Neuroscience 21, 9585–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho T, Levine JD, 2007. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55, 365–376. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Oh U, 2007. Current concepts of nociception: nociceptive molecular sensors in sensory neurons. Curr Opin Anaesthesiol 20, 427–434. [DOI] [PubMed] [Google Scholar]
- Ian Zagon TR, Alphonse Leure-duPree, Joseph Sassani, Patricia McLaughlin, 2003. Immunoelectron microscopic localization of the opioid growth factor receptor (OGFr) and OGF in the cornea. Brain Research 967, 37–47. [DOI] [PubMed] [Google Scholar]
- ICCVAM, 2010. ICCVAM Test Method Evaluation Report: Current Validation Status of In Vitro Test Methods Proposed for Identifying Eye Injury Hazard Potential of Chemicals and Products, in: 10–7553, N.P.N. (Ed.). National Institute of Environmental Health Sciences, Research Triangle Park, NC. [Google Scholar]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O, 1997. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530. [DOI] [PubMed] [Google Scholar]
- Immke DC, Gavva NR, 2006. The TRPV1 receptor and nociception. Semin Cell Dev Biol 17, 582–591. [DOI] [PubMed] [Google Scholar]
- Ito G, Suekawa Y, Watanabe M, Takahashi K, Inubushi T, Murasaki K, Hirose N, Hiyama S, Uchida T, Tanne K, 2013. P2X7 receptor in the trigeminal sensory nuclear complex contributes to tactile allodynia/hyperalgesia following trigeminal nerve injury. Eur J Pain 17, 185–199. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Jain V, Singh N, 2011. Animal models of neuropathic pain. Fundam Clin Pharmacol 25, 1–28. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, Treede RD, 2011. A new definition of neuropathic pain. Pain 152, 2204–2205. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Matthew Petroll W, Dwight Cavanagh H, 2002. TGFβ Induced Myofibroblast Differentiation of Rabbit Keratocytes Requires Synergistic TGFβ, PDGF and Integrin Signaling. Experimental Eye Research 75, 645–657. [DOI] [PubMed] [Google Scholar]
- Jha A, Singh AK, Weissgerber P, Freichel M, Flockerzi V, Flavell RA, Jha MK, 2015. Essential roles for Cavbeta2 and Cav1 channels in thymocyte development and T cell homeostasis. Science signaling 8, ra103. [DOI] [PubMed] [Google Scholar]
- Jin J, Wu LJ, Jun J, Cheng X, Xu H, Andrews NC, Clapham DE, 2012. The channel kinase, TRPM7, is required for early embryonic development. Proceedings of the National Academy of Sciences of the United States of America 109, E225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ME, Murphy PJ, 2004. Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res 23, 449–474. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR, 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Molecular and cellular biology 20, 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Funderburgh ML, Hutcheon AE, Zieske JD, Du Y, Wu J, Funderburgh JL, 2014. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS One 9, e86260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AE, Zieske JD, 2010. Human corneal fibrosis: an in vitro model. Investigative ophthalmology & visual science 51, 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Tsuzuki K, Noguchi K, Sakagami M, 2006. Differential expression of capsaicin-, menthol-, and mustard oil-sensitive receptors in naive rat geniculate ganglion neurons. Chem Senses 31, 681–688. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Kim KH, Harten B, Brown J, Planck S, Meshul C, Edelhauser H, Rosenbaum JT, Armstrong CA, Ansel JC, 1997. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Investigative ophthalmology & visual science 38, 2483–2491. [PubMed] [Google Scholar]
- Kidd BL, Urban LA, 2001. Mechanisms of inflammatory pain. Br J Anaesth 87, 3–11. [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S, 2010. Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain 149, 305–315. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM, 1997. Comparison of three rodent neuropathic pain models. Exp Brain Res 113, 200–206. [DOI] [PubMed] [Google Scholar]
- Knickelbein JE, Buela KA, Hendricks RL, 2014. Antigen-presenting cells are stratified within normal human corneas and are rapidly mobilized during ex vivo viral infection. Investigative ophthalmology & visual science 55, 1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, Shapland E, Kucera G, Mogan J, Humann J, Lenz LL, Morrison AD, Perraud AL, 2011. Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proceedings of the National Academy of Sciences of the United States of America 108, 11578–11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K, 2005. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. The Journal of comparative neurology 493, 596–606. [DOI] [PubMed] [Google Scholar]
- Kowtharapu BS, Stahnke T, Wree A, Guthoff RF, Stachs O, 2014. Corneal epithelial and neuronal interactions: role in wound healing. Exp Eye Res 125, 53–61. [DOI] [PubMed] [Google Scholar]
- Kubilus JK, Linsenmayer TF, 2010a. Developmental corneal innervation: interactions between nerves and specialized apical corneal epithelial cells. Investigative ophthalmology & visual science 51, 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubilus JK, Linsenmayer TF, 2010b. Developmental guidance of embryonic corneal innervation: roles of Semaphorin3A and Slit2. Dev Biol 344, 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureshi AK, Dziasko M, Funderburgh JL, Daniels JT, 2015. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Scientific reports 5, 16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureshi AK, Funderburgh JL, Daniels JT, 2014. Human corneal stromal stem cells exhibit survival capacity following isolation from stored organ-culture corneas. Investigative ophthalmology & visual science 55, 7583–7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman N, Kim A, Petroll WM, 2010. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp Eye Res 90, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Micera A, Sacchetti M, Mantelli F, Bonini S, 2011. Toll-like receptors in ocular surface diseases: overview and new findings. Clin Sci (Lond) 120, 441–450. [DOI] [PubMed] [Google Scholar]
- Launay PS, Reboussin E, Liang H, Kessal K, Godefroy D, Rostene W, Sahel JA, Baudouin C, Melik Parsadaniantz S, Reaux Le Goazigo A, 2016. Ocular inflammation induces trigeminal pain, peripheral and central neuroinflammatory mechanisms. Neurobiol Dis 88, 16–28. [DOI] [PubMed] [Google Scholar]
- Lazarov NE, 2002. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Progress in neurobiology 66, 19–59. [DOI] [PubMed] [Google Scholar]
- Lee S, Mattingly A, Lin A, Sacramento J, Mannent L, Castel MN, Canolle B, Delbary-Gossart S, Ferzaz B, Morganti JM, Rosi S, Ferguson AR, Manley GT, Bresnahan JC, Beattie MS, 2016. A novel antagonist of p75NTR reduces peripheral expansion and CNS trafficking of pro-inflammatory monocytes and spares function after traumatic brain injury. Journal of neuroinflammation 13, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, Kim EK, Seo KY, 2013. Analysis of tear cytokines and clinical correlations in Sjogren syndrome dry eye patients and non-Sjogren syndrome dry eye patients. Am J Ophthalmol 156, 247–253 e241. [DOI] [PubMed] [Google Scholar]
- Leiske DL, Raju SR, Ketelson HA, Millar TJ, Fuller GG, 2010. The interfacial viscoelastic properties and structures of human and animal Meibomian lipids. Exp Eye Res 90, 598–604. [DOI] [PubMed] [Google Scholar]
- Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, Baltrusch S, Eule JC, Vollmar B, 2014. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Investigative ophthalmology & visual science 55, 3603–3615. [DOI] [PubMed] [Google Scholar]
- Li Z, Burns AR, Han L, Rumbaut RE, Smith CW, 2011. IL-17 and VEGF Are Necessary for Efficient Corneal Nerve Regeneration. The American Journal of Pathology 178, 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Rumbaut RE, Smith CW, 2007. gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. The American journal of pathology 171, 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Smith CW, 2006. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Investigative ophthalmology & visual science 47, 1947–1955. [DOI] [PubMed] [Google Scholar]
- Linna TU, Vesaluoma MH, Perez-Santonja JJ, Petroll WM, Alio JL, Tervo TM, 2000. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Investigative ophthalmology & visual science 41, 393–397. [PubMed] [Google Scholar]
- Liu J, Xue Y, Dong D, Xiao C, Lin C, Wang H, Song F, Fu T, Wang Z, Chen J, Pan H, Li Y, Cai D, Li Z, 2017. CCR2(−) and CCR2(+) corneal macrophages exhibit distinct characteristics and balance inflammatory responses after epithelial abrasion. Mucosal Immunol 10, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Smith CW, Zhang W, Burns AR, Li Z, 2012. NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing. The American journal of pathology 181, 452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lin H, Huang C, Chen W, Xiang W, Geng Y, Chen W, 2015. Development and Effects of FTY720 Ophthalmic Solution on Corneal Allograft Survival. Scientific reports 5, 16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Cabanes C, Belmonte C, 2000. Electrophysiological properties of identified trigeminal ganglion neurons innervating the cornea of the mouse. Neuroscience 101, 1109–1115. [DOI] [PubMed] [Google Scholar]
- Lovett M, Cannizzaro C, Daheron L, Messmer B, Vunjak-Novakovic G, Kaplan DL, 2007. Silk fibroin microtubes for blood vessel engineering. Biomaterials 28, 5271–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, 2006. Stress-induced corneal epithlial apoptosis mediated by K+ channel activation. Prog. Retin. Eye Res. 25, 515–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Li L, Liu G, van Rooijen N, Mukaida N, Zhang X, 2009. Opposite roles of CCR2 and CX3CR1 macrophages in alkali-induced corneal neovascularization. Cornea 28, 562–569. [DOI] [PubMed] [Google Scholar]
- Lund JM, Linehan MM, Iijima N, Iwasaki A, 2006. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol 177, 7510–7514. [DOI] [PubMed] [Google Scholar]
- Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC, 2004. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investigative ophthalmology & visual science 45, 4293–4301. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Bronner-Fraser M, 2007. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol 306, 750–759. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A, 2007. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545. [DOI] [PubMed] [Google Scholar]
- Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F, 2009. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci 29, 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Micera A, Sacchetti M, Bonini S, 2010. Neurogenic inflammation of the ocular surface. Curr Opin Allergy Clin Immunol 10, 498–504. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, 1988. Sympathetic innervation of the rat cornea as demonstrated by the retrograde and anterograde transport of horseradish peroxidase-wheat germ agglutinin. The Journal of comparative neurology 268, 147–160. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, Dvorscak L, 2010. Anatomy of the human corneal innervation. Experimental eye research 90, 478–492. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Jones MA, Thrasher K, 1998. Parasympathetic innervation of the rat cornea. Exp Eye Res 66, 437–448. [DOI] [PubMed] [Google Scholar]
- Martin Minns GT, Celeste Rich, Vickery Trinkaus-Randall, 2016. Purinoreceptor P2X7 Regulation of Ca2+ Mobilization and Cytoskeletal Rearrangement is Required for Corneal Reepithelialization after Injury. The American Journal of Pathology 186, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, 1994. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. Journal of neurocytology 23, 1–28. [DOI] [PubMed] [Google Scholar]
- Massie I, Levis HJ, Daniels JT, 2014. Response of human limbal epithelial cells to wounding on 3D RAFT tissue equivalents: effect of airlifting and human limbal fibroblasts. Exp Eye Res 127, 196–205. [DOI] [PubMed] [Google Scholar]
- Matundan HH, Mott KR, Allen SJ, Wang S, Bresee CJ, Ghiasi YN, Town T, Wechsler SL, 2016. Interrelationship of Primary Virus Replication, Level of Latency, and Time to Reactivation in the Trigeminal Ganglia of Latently Infected Mice. 90, 9533–9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P, 1998. An innate sense of danger. Semin Immunol 10, 399–415. [DOI] [PubMed] [Google Scholar]
- Maves TJ, Pechman PS, Gebhart GF, Meller ST, 1993. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. Pain 54, 57–69. [DOI] [PubMed] [Google Scholar]
- Mayer WJ, Irschick UM, Moser P, Wurm M, Huemer HP, Romani N, Irschick EU, 2007. Characterization of antigen-presenting cells in fresh and cultured human corneas using novel dendritic cell markers. Investigative ophthalmology & visual science 48, 4459–4467. [DOI] [PubMed] [Google Scholar]
- Mayer WJ, Mackert MJ, Kranebitter N, Messmer EM, Gruterich M, Kampik A, Kook D, 2012. Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr Eye Res 37, 1012–1018. [DOI] [PubMed] [Google Scholar]
- Mayo C, Ren R, Rich C, Stepp MA, Trinkaus-Randall V, 2008. Regulation by P2X7: epithelial migration and stromal organization in the cornea. Investigative ophthalmology & visual science 49, 4384–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarberg BH, Billington R, 2006. Consequences of neuropathic pain: quality-of-life issues and associated costs. The American journal of managed care 12, S263–268. [PubMed] [Google Scholar]
- McMahon SB, Malcangio M, 2009. Current challenges in glia-pain biology. Neuron 64, 46–54. [DOI] [PubMed] [Google Scholar]
- Medawar PB, 1948. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. British journal of experimental pathology 29, 58–69. [PMC free article] [PubMed] [Google Scholar]
- Medawar PB, 1961. Immunological tolerance. Nature 189, 14–17. [DOI] [PubMed] [Google Scholar]
- Meng ID, Kurose M, 2013. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Experimental eye research 117, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler S, Dannowski H, Bednarz J, Engelmann K, Hartmann C, Pleyer U, 2003. Calcium influx induced by activation of receptor tyrosine kinases in SV40-transfected human corneal endothelial cells. Experimental Eye Research 77, 485–495. [DOI] [PubMed] [Google Scholar]
- Mickle AD, Shepherd AJ, Mohapatra DP, 2016. Nociceptive TRP Channels: Sensory Detectors and Transducers in Multiple Pain Pathologies. Pharmaceuticals (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM, 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164, 6166–6173.10843666 [Google Scholar]
- Minami Y, Sugihara H, Oono S, 1993. Reconstruction of cornea in three-dimensional collagen gel matrix culture. Investigative ophthalmology & visual science 34, 2316–2324. [PubMed] [Google Scholar]
- Miron-Mendoza M, Graham E, Manohar S, Petroll WM, 2017. Fibroblast-fibronectin patterning and network formation in 3D fibrin matrices. Matrix biology : journal of the International Society for Matrix Biology 64, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein H-R, Dieckmann G, Abbouda A, Pondelis N, Jamali A, Salem Z, Hamrah P, 2017. In Vivo Confocal Microscopy Demonstrates the Presence of Microneuromas and may Allow Differentiation of Patients with Corneal Neuropathic Pain from Dry Eye Disease. Investigative ophthalmology & visual science 58, 2656–2656. [Google Scholar]
- Moein HR, Kheirkhah A, Muller RT, Cruzat AC, Pavan-Langston D, Hamrah P, 2018. Corneal nerve regeneration after herpes simplex keratitis:An in vivo confocal microscopy study. Ocul Surf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Possin DE, Mohan RR, Sinha S, Wilson SE, 2003. Development of genetically engineered tet HPV16-E6/E7 transduced human corneal epithelial clones having tight regulation of proliferation and normal differentiation. Experimental eye research 77, 395–407. [DOI] [PubMed] [Google Scholar]
- Moran MM, Szallasi A, 2017. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, De Lorenzo E, Torre E, Fragai M, Nativi C, Luchinat C, Arcangeli A, 2012. A highly soluble matrix metalloproteinase-9 inhibitor for potential treatment of dry eye syndrome. Basic & clinical pharmacology & toxicology 111, 289–295. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Becerra L, Rosenthal P, Borsook D, 2012. An Approach to Localizing Corneal Pain Representation in Human Primary Somatosensory Cortex. PLoS ONE 7, e44643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya ML, Hsu YH, Lee AP, Hughes CC, George SC, 2013. In vitro perfused human capillary networks. Tissue engineering. Part C, Methods 19, 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller LJ, Marfurt CF, Kruse F, Tervo TM, 2003. Corneal nerves: structure, contents and function. Experimental eye research 76, 521–542. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Pels L, Vrensen GF, 1996. Ultrastructural organization of human corneal nerves. Investigative ophthalmology & visual science 37, 476–488. [PubMed] [Google Scholar]
- Murasaki K, Watanabe M, Takahashi K, Ito G, Suekawa Y, Inubushi T, Hirose N, Uchida T, Tanne K, 2013. P2X7 receptor and cytokines contribute to extra-territorial facial pain. J Dent Res 92, 260–265. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J, 2005. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25, 4052–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Chiambaretta F, Sugar J, Sapin V, Yue BY, 2004. Developmentally regulated expression of KLF6 in the mouse cornea and lens. Investigative ophthalmology & visual science 45, 4327–4332. [DOI] [PubMed] [Google Scholar]
- Nakano H, Yanagita M, Gunn MD, 2001. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med 194, 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE, 2010. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 62, 2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Ambrosio R Jr., Hutcheon AE, Zieske JD, Wilson SE, 2005. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea 24, 509–522. [DOI] [PubMed] [Google Scholar]
- Nicolle P, Labbe A, Reboussin E, Kessal K, Melik-Parsadaniantz S, Baudouin C, Réaux-Le-Goazigo A, 2016. Extraorbital lacrimal and Harderian glands excision in mice: a new pre-clinical animal model of dry eye. Investigative ophthalmology & visual science 57, 5708–5708. [Google Scholar]
- Niederkorn JY, 2003. The immune privilege of corneal grafts. Journal of leukocyte biology 74, 167–171. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY, Stern ME, Pflugfelder SC, De Paiva CS, Corrales RM, Gao J, Siemasko K, 2006. Desiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitis. J Immunol 176, 3950–3957. [DOI] [PubMed] [Google Scholar]
- Nilius B, Szallasi A, 2014. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 66, 676–814. [DOI] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M, 1998. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS letters 429, 167–172. [DOI] [PubMed] [Google Scholar]
- Notara M, Refaian N, Braun G, Steven P, Bock F, Cursiefen C, 2015. Short-term uvb-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell Res 15, 643–654. [DOI] [PubMed] [Google Scholar]
- Obreja O, Schmelz M, Poole S, Kress M, 2002. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain 96, 57–62. [DOI] [PubMed] [Google Scholar]
- Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS, 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nature immunology 7, 652–662. [DOI] [PubMed] [Google Scholar]
- Okada Y, Shirai K, Reinach PS, Kitano-Izutani A, Miyajima M, Flanders KC, Jester JV, Tominaga M, Saika S, 2014. TRPA1 is required for TGF-beta signaling and its loss blocks inflammatory fibrosis in mouse corneal stroma. Laboratory investigation; a journal of technical methods and pathology 94, 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Soto L, Efron N, 2001. Morphology of corneal nerves using confocal microscopy. Cornea 20, 374–384. [DOI] [PubMed] [Google Scholar]
- Opree A, Kress M, 2000. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci 20, 6289–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Kyne BM, Saban DR, Stepp MA, 2015. Partial denervation of sub-basal axons persists following debridement wounds to the mouse cornea. Laboratory investigation; a journal of technical methods and pathology 95, 1305–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Tadvalkar G, Stepp MA, 2017. Alterations in Corneal Sensory Nerves During Homeostasis, Aging, and After Injury in Mice Lacking the Heparan Sulfate Proteoglycan Syndecan-1. Investigative ophthalmology & visual science 58, 4959–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos EJ, Sassetti C, Saeki H, Yamada N, Kawamura T, Fitzhugh DJ, Saraf MA, Schall T, Blauvelt A, Rosen SD, Hwang ST, 1999. Fractalkine, a CX3C chemokine, is expressed by dendritic cells and is up-regulated upon dendritic cell maturation. European journal of immunology 29, 2551–2559. [DOI] [PubMed] [Google Scholar]
- Parnas I, Parnas H, 2010. Control of neurotransmitter release: From Ca2+ to voltage dependent G-protein coupled receptors. Pflugers Arch 460, 975–990. [DOI] [PubMed] [Google Scholar]
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C, 2010. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med 16, 1396–1399. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ, 2009. Transient receptor potential channels: targeting pain at the source. Nature reviews. Drug discovery 8, 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan-Langston D, 2008. Herpes zoster antivirals and pain management. Ophthalmology 115, S13–20. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC, 2010. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, Lakshman N, Ma L, 2012. Experimental models for investigating intra-stromal migration of corneal keratocytes, fibroblasts and myofibroblasts. Journal of functional biomaterials 3, 183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Corrales RM, de Paiva CS, 2013. T helper cytokines in dry eye disease. Exp Eye Res 117, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC, Li DQ, Fini ME, 2005. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol 166, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, 2016. Epigenetic basis of sensitization to stress, affective episodes, and stimulants: implications for illness progression and prevention. Bipolar Disord 18, 315–324. [DOI] [PubMed] [Google Scholar]
- Postnikoff CK, Pintwala R, Williams S, Wright AM, Hileeto D, Gorbet MB, 2014. Development of a curved, stratified, in vitro model to assess ocular biocompatibility. PLoS One 9, e96448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prencipe G, Minnone G, Strippoli R, De Pasquale L, Petrini S, Caiello I, Manni L, De Benedetti F, Bracci-Laudiero L, 2014. Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J Immunol 192, 3345–3354. [DOI] [PubMed] [Google Scholar]
- Priyadarsini S, Rowsey TG, Ma JX, Karamichos D, 2017. Unravelling the stromal-nerve interactions in the human diabetic cornea. Exp Eye Res 164, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi Y, Aggarwal S, Hamrah P, 2014. Image-guided evaluation and monitoring of treatment response in patients with dry eye disease. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 252, 857–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H CE, Yoon KC, de Paiva CS, Shine HD, Jones DB, Pflugfelder SC, Li DQ, 2007. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Molecular Vision 13, 1934–1941. [PMC free article] [PubMed] [Google Scholar]
- Qi H, Li DQ, Shine HD, Chen Z, Yoon KC, Jones DB, Pflugfelder SC, 2008. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res 86, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quallo T, Vastani N, Horridge E, Gentry C, Parra A, Moss S, Viana F, Belmonte C, Andersson DA, Bevan S, 2015. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nature communications 6, 7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaesh K, Dhillon B, 2003. Ex vivo expansion of corneal limbal epithelial/stem cells for corneal surface reconstruction. European journal of ophthalmology 13, 515–524. [DOI] [PubMed] [Google Scholar]
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V, 2011. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 29, 163–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R, 2010. Interactions between the immune and nervous systems in pain. Nat Med 16, 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repic T, Madirazza K, Bektur E, Sapunar D, 2016. Characterization of dorsal root ganglion neurons cultured on silicon micro-pillar substrates. Scientific reports 6, 39560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Slowik C, Somodi S, Vick HP, Guthoff R, 1996. Corneal reinnervation following penetrating keratoplasty--correlation of esthesiometry and confocal microscopy. German journal of ophthalmology 5, 513–517. [PubMed] [Google Scholar]
- Ro LS, Jacobs JM, 1993. The role of the saphenous nerve in experimental sciatic nerve mononeuropathy produced by loose ligatures: a behavioural study. Pain 52, 359–369. [DOI] [PubMed] [Google Scholar]
- Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL, 2002. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. Journal of biomechanical engineering 124, 214–222. [DOI] [PubMed] [Google Scholar]
- Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH, 2000. Corneal structure and sensitivity in type 1 diabetes mellitus. Investigative ophthalmology & visual science 41, 2915–2921. [PubMed] [Google Scholar]
- Rosenthal P, Borsook D, 2012. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf 10, 2–14. [DOI] [PubMed] [Google Scholar]
- Rosenthal P, Borsook D, 2016. Ocular neuropathic pain. Br J Ophthalmol 100, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P, Borsook D, Moulton EA, 2016. Oculofacial pain: Corneal nerve damage leading to pain beyond the eye. Investigative ophthalmology & visual science 57, 5285–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ, 1994. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proceedings of the National Academy of Sciences 91, 12378–12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Chaudhary S, Jassim SH, Ozturk O, Chamon W, Ganesh B, Tibrewal S, Gandhi S, Byun YS, Hallak J, Mahmud DL, Mahmud N, Rondelli D, Jain S, 2013. CD11b+GR1+ myeloid cells secrete NGF and promote trigeminal ganglion neurite growth: implications for corneal nerve regeneration. Investigative ophthalmology & visual science 54, 5920–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Chaudhary S, Namavari A, Ozturk O, Chang JH, Yco L, Sonawane S, Khanolkar V, Hallak J, Jain S, 2012. Corneal neurotoxicity due to topical benzalkonium chloride. Investigative ophthalmology & visual science 53, 1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R-S, Hackel D, Morschel L, Sahlbach H, Wang Y, Mousa SA, Roewer N, Brack A, Rittner HL, 2014. Toll like receptor (TLR)-4 as a regulator of peripheral endogenous opioid-mediated analgesia in inflammation. Molecular Pain 10, 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schargus M, Ivanova S, Kakkassery V, Dick HB, Joachim S, 2015. Correlation of Tear Film Osmolarity and 2 Different MMP-9 Tests With Common Dry Eye Tests in a Cohort of Non–Dry Eye Patients. Cornea 34, 739–744. [DOI] [PubMed] [Google Scholar]
- Schimmelpfennig B, 1982. Nerve structures in human central corneal epithelium. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 218, 14–20. [DOI] [PubMed] [Google Scholar]
- Schreiter A, Gore C, Labuz D, Fournie-Zaluski MC, Roques BP, Stein C, Machelska H, 2012. Pain inhibition by blocking leukocytic and neuronal opioid peptidases in peripheral inflamed tissue. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 26, 5161–5171. [DOI] [PubMed] [Google Scholar]
- Serafini N, Dahdah A, Barbet G, Demion M, Attout T, Gautier G, Arcos-Fajardo M, Souchet H, Jouvin MH, Vrtovsnik F, Kinet JP, Benhamou M, Monteiro RC, Launay P, 2012. The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J Immunol 189, 3689–3699. [DOI] [PubMed] [Google Scholar]
- Seyed-Razavi Y, Chinnery HR, McMenamin PG, 2014. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Investigative ophthalmology & visual science 55, 1313–1320. [DOI] [PubMed] [Google Scholar]
- Seyed-Razavi Y, Lopez MJ, Yamaguchi T, Hamrah P, 2017. A Novel Experimental Model for Corneal Neuropathic Pain: The Ciliary Nerve Ligation Approach. Investigative ophthalmology & visual science 58, 3951–3951. [Google Scholar]
- Shafaie S, Hutter V, Cook MT, Brown MB, Chau DY, 2016. In Vitro Cell Models for Ophthalmic Drug Development Applications. BioResearch open access 5, 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R, Priyadarsini S, Rowsey TG, Ma JX, Karamichos D, 2018. Corneal Tissue Engineering: An In Vitro Model of the Stromal-nerve Interactions of the Human Cornea. J. Vis. Exp. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Barabino S, Taylor AW, Dana MR, 2007. Effect of the ocular microenvironment in regulating corneal dendritic cell maturation. Arch Ophthalmol 125, 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JJ, Rudd BD, Lukacs NW, 2006. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med 203, 1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MP, Beacham D, Ensor E, Koltzenburg M, 2004. Cold-sensitive, menthol-insensitive neurons in the murine sympathetic nervous system. Neuroreport 15, 1399–1403. [DOI] [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC, 2001. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Investigative ophthalmology & visual science 42, 2283–2292. [PubMed] [Google Scholar]
- Sommer C, Kress M, 2004. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361, 184–187. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schmidt C, George A, 1998. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol 151, 138–142. [DOI] [PubMed] [Google Scholar]
- Sousa-Valente J, Andreou AP, Urban L, Nagy I, 2014. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br J Pharmacol 171, 2508–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN, 2000. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 407, 1015–1017. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Marfurt C, Golebiowski B, Rosenblatt M, Bereiter D, Begley C, Dartt D, Gallar J, Belmonte C, Hamrah P, Willcox M, 2013. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on neurobiology. Investigative ophthalmology & visual science 54, TFOS71-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Machelska H, 2011. Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol Rev 63, 860–881. [DOI] [PubMed] [Google Scholar]
- Stephan Mergler MV, Taetz Katrin, Sahlmuller Monika, Fels Gabriele, Englemann Katrin, Pleye Uwe, 2011. Characterization of transient receptor potential vanilloid channel 4 (TRPV4) in human corneal endothelial cells. Experimental Eye Research 93, 710–719. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Tadvalkar G, Hakh R, Pal-Ghosh S, 2017. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia 65, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Zieske JD, Trinkaus-Randall V, Kyne BM, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A, 2014. Wounding the cornea to learn how it heals. Exp Eye Res 121, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ME, Pflugfelder SC, 2004. Inflammation in dry eye. The ocular surface 2, 124–130. [DOI] [PubMed] [Google Scholar]
- Stiemke M, McCartney M, Cantu-Crouch D, Edelhauser H, 1991. Maturation of the corneal endothelial tight junction. Investigative ophthalmology & visual science 32, 2757–2765. [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M, 2007. Differentiation and function of Th17 T cells. Curr Opin Immunol 19, 281–286. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A, 2003. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829. [DOI] [PubMed] [Google Scholar]
- Streilein JW, 1999. Regional immunity and ocular immune privilege. Chemical immunology 73, 11–38. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Ohta K, Mo JS, Taylor AW, 2002. Ocular immune privilege and the impact of intraocular inflammation. DNA and cell biology 21, 453–459. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Toews GB, Bergstresser PR, 1979. Corneal allografts fail to express Ia antigens. Nature 282, 326–327. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM, 2009. Roles of transient receptor potential channels in pain. Brain Res Rev 60, 2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian B, Rudym D, Cannizzaro C, Perrone R, Zhou J, Kaplan DL, 2010. Tissue-engineered three-dimensional in vitro models for normal and diseased kidney. Tissue engineering. Part A 16, 2821–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, Yu LN, Zhang FJ, Chen G, Yan M, 2013. CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. Journal of neuroscience research 91, 545–553. [DOI] [PubMed] [Google Scholar]
- Suuronen EJ, Nakamura M, Watsky MA, Stys PK, Müller LJ, Munger R, Shinozaki N, Griffith M, 2004. Innervated human corneal equivalents as in vitro models for nerve-target cell interactions. The FASEB journal 18, 170–172. [DOI] [PubMed] [Google Scholar]
- Takahiro Yamada TU, Ugawa Shinya, Ishida Yusuke, Imayasu Masaki, Koyama Satoshi, Shimada Shoichi 2010. Functional expression of transient receptor potential vanilloid 3 (TRPV3) in corneal epithelial cells: Involvement in thermosensation and wound healing. Experimental Eye Research 90, 121–129. [DOI] [PubMed] [Google Scholar]
- Takayama T, Kondo T, Kobayashi M, Ohta K, Ishibashi Y, Kanemaru T, Shimazu H, Ishikawa F, Nakamura T, Kinoshita S, Nakamura K, 2009. Characteristic morphology and distribution of bone marrow derived cells in the cornea. Anat Rec (Hoboken) 292, 756–763. [DOI] [PubMed] [Google Scholar]
- Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydon PG, Kaplan DL, 2014. Bioengineered functional brain-like cortical tissue. Proceedings of the National Academy of Sciences 111, 13811–13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Chang Z, Bereiter DA, 2010. Behavioral and neurophysiological correlates of nociception in an animal model of photokeratitis. Neuroscience 169, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi E, Katsura H, Kobayashi K, Yamanaka H, Tsuzuki K, Noguchi K, Sakagami M, 2015. Changes in transient receptor potential channels in the rat geniculate ganglion after chorda tympani nerve injury. Neuroreport 26, 856–861. [DOI] [PubMed] [Google Scholar]
- Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS, 2017. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 16, 934–944. [DOI] [PubMed] [Google Scholar]
- Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T, 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol 163, 2179–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theophanous C, Jacobs DS, Hamrah P, 2015a. Corneal Neuralgia after LASIK. Optom Vis Sci. [DOI] [PubMed] [Google Scholar]
- Theophanous C, Jacobs DS, Hamrah P, 2015b. Corneal Neuralgia after LASIK. Optometry and vision science : official publication of the American Academy of Optometry 92, e233–240. [DOI] [PubMed] [Google Scholar]
- Thorsrud A, Nicolaissen B, Drolsum L, 2012. Corneal collagen crosslinking in vitro: inhibited regeneration of human limbal epithelial cells after riboflavin-ultraviolet-A exposure. Journal of cataract and refractive surgery 38, 1072–1076. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW, 2009. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 29, 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi B, Tripathi R, 1988. Cytotoxic effects of benzalkonium chloride and chlorobutanol on human corneal epithelial cells in vitro. Lens and eye toxicity research 6, 395–403. [PubMed] [Google Scholar]
- Tsubota K, Fukagawa K, Fujihara T, Shimmura S, Saito I, Saito K, Takeuchi T, 1999. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Investigative ophthalmology & visual science 40, 28–34. [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K, 2003. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424, 778–783. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F, 2008. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28, 11263–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN, 2009. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). United Nations Publications. [Google Scholar]
- Utsunomiya T, Nagaoka T, Hanada K, Omae T, Yokota H, Abiko A, Haneda M, Yoshida A, 2015. Imaging of the Corneal Subbasal Whorl-like Nerve Plexus: More Accurate Depiction of the Extent of Corneal Nerve Damage in Patients With Diabetes. Investigative ophthalmology & visual science 56, 5417–5423. [DOI] [PubMed] [Google Scholar]
- Van Furth R, Diesselhoff-den Dulk MC, Mattie H, 1973. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med 138, 1314–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goethem F, Adriaens E, Alepee N, Straube F, De Wever B, Cappadoro M, Catoire S, Hansen E, Wolf A, Vanparys P, 2006. Prevalidation of a new in vitro reconstituted human cornea model to assess the eye irritating potential of chemicals. Toxicology in vitro 20, 1–17. [DOI] [PubMed] [Google Scholar]
- van Hecke O, Torrance N, Smith BH, 2013. Chronic pain epidemiology and its clinical relevance. Br J Anaesth 111, 13–18. [DOI] [PubMed] [Google Scholar]
- Varsamidou E, Markopoulou S, Karayannopoulou G, Kalpatsanidis A, Kokkas V, Karampatakis V, Goulas A, 2014. Acute exposure of rabbit eyes to artificial light in vivo: effect on corneal and third eyelid conjunctival histology and the gene expression of PAFR. Curr Eye Res 39, 512–517. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC, 2004. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. The European journal of neuroscience 20, 1150–1160. [DOI] [PubMed] [Google Scholar]
- Volkers L, Mechioukhi Y, Coste B, 2015. Piezo channels: from structure to function. Pflugers Arch 467, 95–99. [DOI] [PubMed] [Google Scholar]
- von Hehn CA, Baron R, Woolf CJ, 2012. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73, 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Myers RR, O’Brien JS, 1998. Prosaptide prevents hyperalgesia and reduces peripheral TNFR1 expression following TNF-alpha nerve injection. Neuroreport 9, 2827–2831. [DOI] [PubMed] [Google Scholar]
- Wainger BJ, Buttermore ED, Oliveira JT, Mellin C, Lee S, Saber WA, Wang AJ, Ichida JK, Chiu IM, Barrett L, Huebner EA, Bilgin C, Tsujimoto N, Brenneis C, Kapur K, Rubin LL, Eggan K, Woolf CJ, 2015. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nature neuroscience 18, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Peters N, Schwarze J, 2006. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol 177, 6263–6270. [DOI] [PubMed] [Google Scholar]
- Wang S, Ghezzi CE, Gomes R, Pollard RE, Funderburgh JL, Kaplan DL, 2017. In vitro 3D corneal tissue model with epithelium, stroma, and innervation. Biomaterials 112, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ghezzi CE, White JD, Kaplan DL, 2015. Coculture of dorsal root ganglion neurons and differentiated human corneal stromal stem cells on silk-based scaffolds. Journal of biomedical materials research. Part A 103, 3339–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yan M, Zheng GY, He L, Yang H, 2014. A cell-based, high-throughput homogeneous time-resolved fluorescence assay for the screening of potential kappa-opioid receptor agonists. Acta pharmacologica Sinica 35, 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE, 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25, 821–852. [DOI] [PubMed] [Google Scholar]
- Weil AM, Waite SE, Randall NJ, Gunthorpe A, J. M, 2005. Conservation of functional and pharmacological properties in the distantly related temperature sensors TRPV1 and TRPM8. Molecular Pharmacology 68, 518–527. [DOI] [PubMed] [Google Scholar]
- Wieser B, Tichy A, Nell B, 2013. Correlation between corneal sensitivity and quantity of reflex tearing in cows, horses, goats, sheep, dogs, cats, rabbits, and guinea pigs. Veterinary ophthalmology 16, 251–262. [DOI] [PubMed] [Google Scholar]
- Wilhelmus KR, 2001. The Draize eye test. Survey of ophthalmology 45, 493–515. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Hong J, Lee J, 2001. The Corneal Wound Healing Response:: Cytokine-mediated Interaction of the Epithelium, Stroma, and Inflammatory Cells. Progress in retinal and eye research 20, 625–637. [DOI] [PubMed] [Google Scholar]
- Wilson SL, Ahearne M, Hopkinson A, 2015. An overview of current techniques for ocular toxicity testing. Toxicology 327, 32–46. [DOI] [PubMed] [Google Scholar]
- Wilson SL, El Haj AJ, Yang Y, 2012. Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering. Journal of functional biomaterials 3, 642–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollensak G, Sporl E, Reber F, Pillunat L, Funk R, 2003. Corneal endothelial cytotoxicity of riboflavin/UVA treatment in vitro. Ophthalmic Res 35, 324–328. [DOI] [PubMed] [Google Scholar]
- Wu J, Du Y, Mann MM, Funderburgh JL, Wagner WR, 2014a. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp Eye Res 120, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE, 2010. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62, 381–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zhou Q, Duan H, Wang X, Xiao J, Duan H, Li N, Li C, Wan P, Liu Y, Song Y, Zhou C, Huang Z, Wang Z, 2014b. Reconstruction of auto-tissue-engineered lamellar cornea by dynamic culture for transplantation: a rabbit model. PLoS One 9, e93012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Zhou W, Wang P, Yang H, Gao F, Xiang H, Manyande A, Tian Y, Tian X, 2017. Alkali Burn Induced Corneal Spontaneous Pain and Activated Neuropathic Pain Matrix in the Central Nervous System in Mice. Cornea 36, 1408–1414. [DOI] [PubMed] [Google Scholar]
- Yagci A, Gurdal C, 2014. The role and treatment of inflammation in dry eye disease. Int Ophthalmol 34, 1291–1301. [DOI] [PubMed] [Google Scholar]
- Yam GH, Williams GP, Setiawan M, Yusoff NZ, Lee XW, Htoon HM, Zhou L, Fuest M, Mehta JS, 2017. Nerve regeneration by human corneal stromal keratocytes and stromal fibroblasts. Scientific reports 7, 45396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami S, Ebihara N, Usui T, Yokoo S, Amano S, 2006. Bone marrow-derived cells in normal human corneal stroma. Arch Ophthalmol 124, 62–69. [DOI] [PubMed] [Google Scholar]
- Yamagami S, Usui T, Amano S, Ebihara N, 2005. Bone marrow-derived cells in mouse and human cornea. Cornea 24, S71–S74. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Calvacanti BM, Cruzat A, Qazi Y, Ishikawa S, Osuka A, Lederer J, Hamrah P, 2014. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Investigative ophthalmology & visual science 55, 7457–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Turhan A, Harris DL, Hu K, Pruss H, von Andrian U, Hamrah P, 2013. Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy. PLoS One 8, e70908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y, 2008. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane S, Iwasaki N, Majima T, Funakoshi T, Masuko T, Harada K, Minami A, Monde K, Nishimura S, 2005. Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterials 26, 611–619. [DOI] [PubMed] [Google Scholar]
- Yang X, Gong H, Liu Z, Liu H, Wang H, Li Z, 2010. Similar and different effects of capsaicin and resiniferatoxin on substance P release and transient receptor potential vanilloid type 1 expression of cultured rat dorsal root ganglion neurons. Methods and findings in experimental and clinical pharmacology 32, 3–11. [DOI] [PubMed] [Google Scholar]
- Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Bostrom P, Mepani RJ, Laznik D, Kamenecka TM, Song X, Liedtke W, Mootha VK, Puigserver P, Griffin PR, Clapham DE, Spiegelman BM, 2012. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 151, 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf A, Klinger F, Schicker K, Boehm S, 2011. Nucleotides control the excitability of sensory neurons via two P2Y receptors and a bifurcated signaling cascade. Pain 152, 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon IS, Donahue RN, Bonneau RH, McLaughlin PJ, 2011. T lymphocyte proliferation is suppressed by the opioid growth factor ([Met(5)]-enkephalin)-opioid growth factor receptor axis: implication for the treatment of autoimmune diseases. Immunobiology 216, 579–590. [DOI] [PubMed] [Google Scholar]
- Zander E, Weddell G, 1951. Observations on the innervation of the cornea. Journal of anatomy 85, 68–99. [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Yang H, Wang Z, Mergler S, Liu H, Kawakita T, Tachado SD, Pan Z, Capó-Aponte JE, Pleyer U, Koziel H, Kao WWY and Reinach PS, 2007. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. Cell Physiology 213, 730–739. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu Z, Hayashi Y, Okada R, Nakanishi H, 2014. Peripheral role of cathepsin S in Th1 cell-dependent transition of nerve injury-induced acute pain to a chronic pain state. J Neurosci 34, 3013–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Kainz V, Burstein R, Levy D, 2011. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain 152, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Andrian U.H.v., Hamrah P, 2010. Identification of Novel Subsets of Plasmacytoid and Conventional Dendritic Cells in the Cornea. Investigative ophthalmology & visual science 51, 1544–1544. [Google Scholar]
- Zhivov A, Stave J, Vollmar B, Guthoff R, 2005. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 243, 1056–1061. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR, 2007. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain, behavior, and immunity 21, 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Hutcheon AE, Guo X, Chung EH, Joyce NC, 2001. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Investigative ophthalmology & visual science 42, 1465–1471. [PubMed] [Google Scholar]
- Zieske JD, Mason VS, Wasson ME, Meunier SF, Nolte CJ, Fukai N, Olsen BR, Parenteau NL, 1994. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Experimental cell research 214, 621–633. [DOI] [PubMed] [Google Scholar]
- Zollner C MS, Klinger A, 2008. Topical fentanyl in a randomized, double-blind study in patients with corneal damage. Clinical Journal of Pain 24, 690–696. [DOI] [PubMed] [Google Scholar]