Abstract
Severe hypertension can lead to irreversible kidney failure and end-stage renal disease (ESRD) and vice versa. Patients are often classified as hypertensive ESRD with no confirmative proof and the true cause of disease can therefore be missed, affecting outcomes. We present a case of chronic thrombotic microangiopathy (TMA) after kidney transplantation in a recipient who had been classified as hypertensive ESRD and found to have a genetic defect in CD46, a transmembrane protein that regulates complement activation, indicating atypical hemolytic uremic syndrome (HUS). The pathogenic variant in CD46 was also found in the mother who donated the kidney, indicating that the TMA occurred on the background of atypical HUS instead of severe hypertension. The patient died from disseminated cancer originated in the mother's kidney. Knowledge of the genetic background would have prevented recurrent disease and the cancer to occur. Patients classified as hypertensive ESRD suspect for TMA should therefore be screened for variants in complement genes to make informed decisions and save kidneys.
Keywords: Acute renal injury, Complement, End-stage renal disease, Genetics, Hemolytic uremic syndrome, Hypertension
Introduction
Kidney transplantation remains the preferred treatment for patients with end-stage renal disease (ESRD) [1]. Knowledge of the native kidney disease, however, is critical to the prognosis after transplantation.
Patients presenting with ESRD and severe hypertension often have been classified as hypertensive ESRD with, unfortunately, no confirmative proof, assuming that the kidneys are the victim rather than culprit of disease. Recently, we demonstrated that defects in complement regulation can be the key causative factor of ESRD among patients with severe hypertension and thrombotic microangiopathy (TMA) on kidney biopsy, indicating atypical hemolytic uremic syndrome (HUS) instead of hypertensive ESRD [2]. The complement defects can be caused by variants in genes encoding proteins that either regulate or activate complement and/or autoantibodies that inhibit regulatory proteins, lowering the threshold for TMA to manifest. Importantly, the risk of recurrent TMA and graft failure is high in patients with atypical HUS [3], underscoring the importance of a correct diagnosis in the native kidney.
Here, we present a patient with a clinical history of presumed hypertensive ESRD who developed recurrent TMA on the background of an ultrarare variant in CD46 after kidney transplantation, indicating atypical HUS. We discuss the implications of our observations to improve the prognosis of patients presenting with ESRD and severe hypertension.
Case Presentation
Clinical History and Initial Laboratory Data
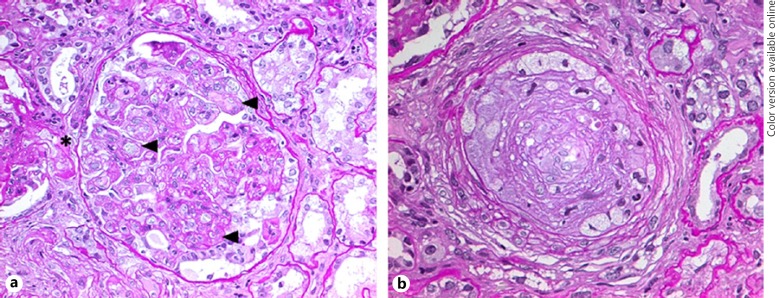
In 2006, a 40-year-old man with a history of controlled hypertension and urinary abnormalities for 10 years was admitted to our hospital because of severe hypertension, headache, fatigue, and ESRD. He used ramipril and no other drugs. Blood pressure (BP) and pulse were 205/114 mm Hg and 69 beats per minute, respectively; electron cardiograph showed characteristic changes of left ventricular hypertrophy. The laboratory data have been depicted in Table 1. Serum creatinine was 13.5 mg/dL, and Coombs negative microangiopathic hemolytic anemia was present. Proteinuria of 2,300 mg per day but no hematuria or pyuria was found on urinalysis. Nine months earlier, serum creatinine was 1.2 mg/dL. There was no known family history of kidney disease or hypertension. Malignant nephrosclerosis was clinically inferred and labetalol was administered, leading to rapid BP control. No renal response was achieved, and a kidney biopsy was performed. The key pattern of disease on kidney biopsy was membranoproliferative GN with secondary focal segmental glomerulosclerosis and typical features of severe as well as long-standing hypertension, that is, mucoid intimal edema and arteriolar hyalinosis (Fig. 1). Thrombi were not present. Testing for antinuclear and antiphospholipid antibodies, complement levels, and infections were unremarkable; the patient denied illicit drug use. Hypertensive ESRD was therefore diagnosed.
Table 1.
Relevant laboratory data
| Laboratory test | Baseline, −9 months | Native kidney, first presentation | Donor kidney, recurrent TMA |
|---|---|---|---|
| Hematology | |||
| Platelets, ×103/μL | 242 | 158 | 208 |
| Hemoglobin, g/dL | 15.6 | 9.1 | 12.2 |
| Peripheral blood smear | Schistocytes, 3+ | ||
| Coombs test | Negative | ||
| Serology | |||
| Serum creatinine, mg/dL | 1.2 | 13.5 | 2.8 |
| Sodium, mmol/L | 139 | 140 | 142 |
| Potassium, mmol/L | 4.00 | 4.38 | 5.63 |
| Lactate dehydrogenase, U/L | 366 | 1,104 | 220 |
| Haptoglobin, mg/dL | <0.10 | ||
| Urinalysis | |||
| Proteinuria, g/day | 1.785 | 2.300 | 2.200 |
| Miscellaneous | |||
| Thyroid-stimulating hormone, μU/L | 2.3 | ||
| C4, g/L (ref., 0.11–0.35) | 0.28 | ||
| C3, g/L (ref., 0.75–1.35) | 0.89 | ||
| CPFA, % (ref., >75) | 97 | ||
Conversion factors for units: serum creatinine mg/dL toμmol/L, ×88.4.
CPFA, classical pathway functional activity; TMA, thrombotic microangiopathy.
Fig. 1.
Kidney biopsy findings. Periodic Acid Schiff stain of a glomerulus showing double contours of the glomerular basement membrane (arrowhead), subtle margination of neutrophils, and hyalinosis (asterisk) of an adjacent arteriole (a, 400×); mucoid intimal edema was seen in arteries, occluding the lumen (b, 400×). Endotheliosis, thrombi, and mesangiolysis were not present.
In 2007, he received an allograft from his mother with immediate function; tacrolimus, sirolimus, and prednisolone were started to suppress allograft rejection. Five years after transplantation (i.e., 2012) when on tacrolimus monotherapy (trough levels, > 4 ng/mL), nonhypertensive acute allograft failure (serum creatinine 2.8 mg/dL), and proteinuria of 2,200 mg per day developed (Table 1). Donor specific alloantibodies and infections (i.e., cytomegalovirus, hepatitis C virus, and parvovirus B19) were not found. Because of a persistent and unexplained increase in serum creatinine, a kidney allograft biopsy was performed. Again, membranoproliferative GN with moderate-to-severe interstitial fibrosis, tubular atrophy, and intimal fibrosis were present; thrombi, tubulitis, and capillaritis of the peritubular capillaries were not found. Neither C4d nor immune-complex deposits were found, suggesting transplant glomerulopathy.
Additional Investigations
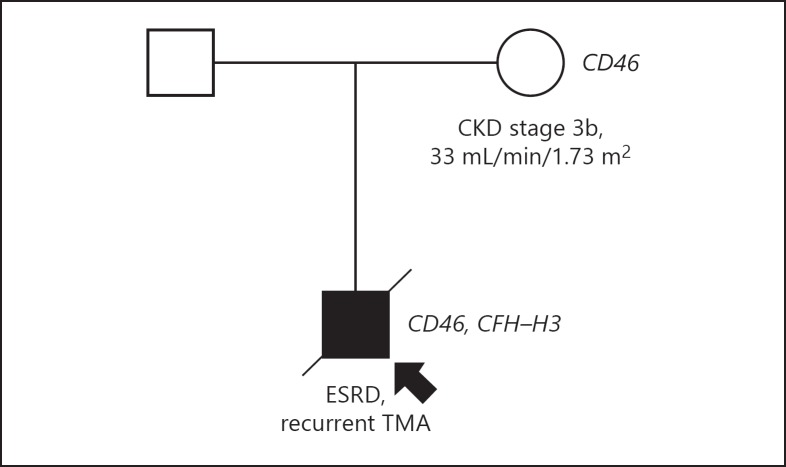
The morphologic pattern in the native and donor kidney, however, can be found in the setting of chronic TMA. Most cases of recurrent TMA have been linked to genetic complement defects, and thus atypical HUS [3]. DNA sequencing of complement genes, indeed, revealed a heterozygous frameshift variant in CD46 (c.811_816delGACAGT, leading to p.Asp271_Ser272del) and a missense variant in CFH (c.2,850G>T, leading to p.Gln950His). The ultrarare (i.e., minor allele frequency of < 0.01%) variant in CD46 has been classified as pathogenic [4], while the variant in CFH is rather common (i.e., minor allele frequency of ≥0.1%) and not linked to a defect in complement regulation [5]. Also, the CFH-H3 haplotype and ΔCFHR1-CFHR3 were found in heterozygosity. No variants were found in CFI, CFB, C3, THBD (i.e., thrombomodulin), and DGKE (i.e., diacylglycerol kinase ε) as well as rearrangements in the CFH-CFHR1-5 genomic region.
TMA recurrence after transplantation is uncommon in patients with variants in CD46, since CD46 is expressed by the donor kidney's endothelium. The CD46 variant but not CFH-H3 haplotype was traced in the donor who is nowadays 81 years of age and never developed TMA (Fig. 2); chronic kidney disease stage 3b, however, developed after kidney donation and remained stable ever since.
Fig. 2.
DNA sequencing of complement genes in the patient and his mother who donated the kidney. ESRD, end-stage renal disease; TMA, thrombotic microangiopathy.
Diagnosis
Recurrent atypical HUS after kidney transplantation on the background of a variant in CD46.
Clinical Follow-Up
The patient had been retransplanted with a living-unrelated allograft and because the variant in CFH is not linked to atypical HUS, prophylactic therapeutic measures were not needed. Macroscopic hematuria, however, developed, while the allograft function remained stable. The urologic work-up demonstrated a urothelial carcinoma originating from the mother's kidney as confirmed by fluorescence in situ hybridization. Prior to retransplantation, however, a routine computed tomography scan of the abdomen showed no tumor mass in the donor kidney. The patient died from disseminated disease 2 years later.
Discussion
We present a case of chronic TMA that reoccurred after kidney transplantation on the background of a defect in complement regulation, that is, atypical HUS, in a patient assigned to have hypertensive ESRD. The complicated disease course, however, would have been prevented by a correct diagnosis and proper donor selection. Unfortunately, in our experience, several patients with atypical HUS have been misdiagnosed as hypertensive ESRD [2], affecting kidney survival.
TMA within the renal vasculature can induce severe hypertension, and vice versa, making the identification of atypical HUS in patients with severe hypertension challenging; this is particularly the case in patients not presenting with systemic hemolysis [6]. In these cases, a confirmatory kidney biopsy is the first step to detect the TMA. TMA warrants screening for thrombotic thrombocytopenic purpura and Shiga toxin-induced HUS. In addition, secondary causes, including autoimmune disease, infection, cancer, and drug use, should be screened for. If none of these causes can be identified, atypical HUS should be considered and differentiated from TMA due to severe hypertension as the sole cause of disease. The differentiation between both, however, has not been delineated in clinical algorithms [7].
Routine complement tests and markers of complement activation, such as C5a and soluble C5b9, lack sensitivity and specificity and are therefore not suitable to diagnose atypical HUS [6, 8]. It remains to be established whether features on kidney biopsy can define etiology [9]. Larsen et al. [10] proposed that patients with mucoid intimal edema, which has been thought to be characteristic for severe hypertension, are at risk for atypical HUS only if active lesions, including glomerular thrombi, are present. This, however, can lead to failure to identify patients with atypical HUS. As in the current case, pathogenic variants in complement genes can confirm the diagnosis in ∼60% of patients [11, 12].
Complement activation via the alternative pathway is a physiological process that is tightly controlled by complement regulatory proteins to prevent damage to the self. Factor H, factor I, and/or CD46 among other proteins are required to control activation of the alternative pathway and prevent the formation of new convertases and C5 activation. The activation of C5 is key for atypical HUS to manifest [13]. The patient's mutation in CD46 has been demonstrated to reduce the expression of CD46 on the endothelium [4], lowering the threshold for C5 activation. Moreover, the CFH-H3 haplotype affects the penetrance of atypical HUS in carriers of complement gene mutations [14], which might explain the benign phenotype of the patient's mother who lack the at-risk haplotype.
BP control, obviously the cornerstone of treatment, is effective for patients with TMA caused by severe hypertension [15] but not for those with atypical HUS [6]. ESRD can be expected in about half the patients with atypical HUS, if left untreated, during the first year [8, 11]. The phenotype of patients with a single defect in CD46, however, is rather benign and often is localized to the kidneys, although relapses are common [11, 12]. In our case, we therefore assume that ESRD developed on the background of smoldering disease activity as substantiated by his previous medical history and the presence of chronic rather than active TMA on kidney biopsy; hypertension, however, can aggravate complement activation [16] and TMA, leading to a vicious circle via ischemia and activation of the renin-angiotensin system. Furthermore, renin can enhance complement activation via cleavage of C3 [17].
TMA recurrence after kidney transplantation has been linked to atypical HUS and, in particular, to patients with rare variants in CFH, CFB, C3, and to a lesser extent CFI [3]. Patients with an isolated defect in the gene encoding the transmembrane protein CD46 have a low risk of recurrence because the donor kidney typically expresses wild-type CD46, correcting the defect. The expression of CD46, however, was reduced in the kidney donated by the recipient's mother, explaining disease recurrence. In potential recipients with “hypertensive” ESRD and proof or a high suspicion of TMA, genetic testing is instrumental to make informed decisions and to adopt suitable prophylactic measures. Living kidney donation should be preferred to limit ischemia-reperfusion injury and complement activation, reducing the risk of early recurrence. Living-related kidney donation, however, carries a risk for recurrence in the recipient and de novo disease in the donor and is therefore feasible only if the potential donor does not carry an at-risk genetic variant. In addition, the importance of lower target level tacrolimus and adequate antihypertensive treatment should be acknowledged [18].
The higher cancer risk in kidney transplantation has been attributed to the long-term use of immunosuppressive agents and infections [1]. The normal appearance of the donor kidney on computed tomography scan prior to retransplantation indicated that the malignant transformation occurred on the background of immunosuppression. In the current case, a correct diagnosis in the native kidney and consequently, proper donor selection, would have prevented the cancer to occur.
In conclusion, atypical HUS can be missed in patients assigned to have hypertensive ESRD. Pathologic data often are needed to detect the TMA but cannot differentiate atypical HUS from TMA caused by severe hypertension. Patients classified as hypertensive ESRD suspect for atypical HUS and those selected for kidney transplantation should be screened for rare variants in complement genes to make informed decisions. Furthermore, genetic screening of potential living-related donors is indicated prior to kidney donation.
Ethics Statement
The family of the patient provided informed consent prior to manuscript submission. The current study did not require review/approval by the appropriate Ethics Committee.
Disclosure Statement
The authors declare that they have no relevant financial interests.
References
- 1.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006 Dec;296((23)):2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 2.Timmermans SA, Abdul-Hamid MA, Vanderlocht J, Damoiseaux JG, Reutelingsperger CP, van Paassen P, et al. Limburg Renal Registry. Patients with hypertension-associated thrombotic microangiopathy may present with complement abnormalities. Kidney Int. 2017 Jun;91((6)):1420–1425. doi: 10.1016/j.kint.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Le Quintrec M, Zuber J, Moulin B, Kamar N, Jablonski M, Lionet A, et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant. 2013 Mar;13((3)):663–75. doi: 10.1111/ajt.12077. [DOI] [PubMed] [Google Scholar]
- 4.Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, et al. Mutations in human complement regulator membrane cofactor protein (CD46) predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2003 Oct;100((22)):12966–71. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohlin FC, Nilsson SC, Levart TK, Golubovic E, Rusai K, Müller-Sacherer T, et al. Functional characterization of two novel non-synonymous alterations in CD46 and a Q950H change in factor H found in atypical hemolytic uremic syndrome patients. Mol Immunol. 2015 Jun;65((2)):367–76. doi: 10.1016/j.molimm.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Timmermans SA, Abdul-Hamid MA, Potjewijd J, Theunissen RO, Damoiseaux JG, Reutelingsperger CP, et al. Limburg Renal Registry. C5b9 Formation on Endothelial Cells Reflects Complement Defects among Patients with Renal Thrombotic Microangiopathy and Severe Hypertension. J Am Soc Nephrol. 2018 Aug;29((8)):2234–43. doi: 10.1681/ASN.2018020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, et al. HUS International. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016 Jan;31((1)):15–39. doi: 10.1007/s00467-015-3076-8. [DOI] [PubMed] [Google Scholar]
- 8.Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014 Sep;124((11)):1715–26. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, et al. Conference Participants. Atypical hemolytic uremic syndrome and C3 glomerulopathy conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017 Mar;91((3)):539–551. doi: 10.1016/j.kint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Larsen CP, Wilson JD, Best-Rocha A, Beggs ML, Hennigar RA. Genetic testing of complement and coagulation pathways in patients with severe hypertension and renal microangiopathy. Mod Pathol. 2018 Mar;31((3)):488–94. doi: 10.1038/modpathol.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, et al. Genetics and outcome of atypical hemolytic uremic syndrome a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013 Apr;8((4)):554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010 Oct;5((10)):1844–59. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jorge EG, Macor P, Paixão-Cavalcante D, Rose KL, Tedesco F, Cook HT, et al. The development of atypical hemolytic uremic syndrome depends on complement C5. J Am Soc Nephrol. 2011 Jan;22((1)):137–45. doi: 10.1681/ASN.2010050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roumenina LT, Frimat M, Miller EC, Provot F, Dragon-Durey MA, Bordereau P, et al. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood. 2012 May;119((18)):4182–91. doi: 10.1182/blood-2011-10-383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González R, Morales E, Segura J, Ruilope LM, Praga M. Long-term renal survival in malignant hypertension. Nephrol Dial Transplant. 2010 Oct;25((10)):3266–72. doi: 10.1093/ndt/gfq143. [DOI] [PubMed] [Google Scholar]
- 16.Yin W, Ghebrehiwet B, Weksler B, Peerschke EI. Regulated complement deposition on the surface of human endothelial cells effect of tobacco smoke and shear stress. Thromb Res. 2008;122((2)):221–228. doi: 10.1016/j.thromres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Békássy ZD, Kristoffersson AC, Rebetz J, Tati R, Olin AI, Karpman D. Aliskiren inhibits renin-mediated complement activation. Kidney Int. 2018 Oct;94((4)):689–700. doi: 10.1016/j.kint.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Duineveld C, Verhave JC, Berger SP, van de Kar NC, Wetzels JF. Living Donor Kidney Transplantation in Atypical Hemolytic Uremic Syndrome. A Case Series. Am J Kidney Dis. 2017 Dec;70((6)):770–777. doi: 10.1053/j.ajkd.2017.06.024. [DOI] [PubMed] [Google Scholar]