Abstract
Background
We found an increase in the incidence rate (IR) of childhood thyrotoxicosis (CT) during the 1990s in central Sweden. The optimal treatment method for CT is a subject that is still debated upon.
Objectives
To investigate whether the increase in IR of CT in Sweden persists and to study the treatment outcome.
Method
Children <16 years of age diagnosed with CT during 2000–2009 and living in 1 of 5 counties in central Sweden were identified retrospectively using hospital registers. Data on clinical and biochemical characteristics and outcomes of treatment were collected from medical records. The corresponding data from 1990 to 1999 were pooled with the new data.
Results
In total, 113 children were diagnosed with CT during 1990–2009 in the study area. The overall IR was 2.2/100,000 person-years (95% CI 1.2–2.5/100,000 person-years). The IR was significantly higher during 2000–2009 than during 1990–1999 (2.8/100,000 [2.2–3.6] vs. 1.6/100,000 person-years [1.2–2.2], p = 0.006). The increase was significant for both sexes. Seventy percent of the patients who completed the planned initial treatment with antithyroid drugs (ATDs) and were not lost to follow-up relapsed within 3 years. Boys tended to relapse earlier than girls (6.0 months after drug withdrawal [95% CI 1.9–10.0] vs. 12.0 months [95% CI 6.8–17.3], p = 0.074).
Conclusions
The IR of CT is increasing in both girls and boys. Relapse rate after withdrawal of ATD treatment is 70%. Boys tend to relapse earlier than girls, and this needs to be further investigated.
Keywords: Children, Hyperthyroidism, Incidence rate, Relapse, Thyrotoxicosis
Introduction
Thyrotoxicosis is a clinical condition caused by excessive amounts of circulating thyroid hormones. It can be due to increased thyroid hormone synthesis and secretion, which is known as hyperthyroidism, or increased secretion of preformed thyroid hormones as in the initial phase of thyroiditis, or excessive ingestion of exogenous thyroid hormones [1].
The most common form of thyrotoxicosis in both children and adults is the Graves disease, which is caused by the stimulation of the thyroid stimulating hormone (TSH) receptor on the surface of thyroid follicular cells by autoantibodies called TSH receptor antibodies (TRAb) [2, 3]. Toxic adenomas and toxic multinodular goiter are other causes of thyrotoxicosis [1]. Transient neonatal hyperthyroidism can be due to transplacentally transported maternal antibodies affecting the fetal thyroid gland [2]. McCune-Albright syndrome, a rare condition characterized by café au lait macules of the skin, skeletal fibrous dysplasia, and autonomous endocrine hyperactivity, is associated with thyrotoxicosis in approximately one-third of the patients [4].
The majority of patients with childhood thyrotoxicosis (CT) are diagnosed during adolescence, and as in adults, CT is much more common in females than in males. There are 3 major treatment options for CT: antithyroid drugs (ATDs), thyroidectomy, and radioactive iodine treatment [2, 3]. Most patients with CT are treated initially with ATDs for approximately 2 years. However, there is a high risk for relapse during and especially after ATD treatment. Only <30% of pediatric patients remain in remission after discontinuation of on average 2 years ATD treatment [2]. In cases of relapse, a more definitive treatment, such as thyroidectomy or radioactive iodine treatment, can be given, or a new course of ATD [2, 5]. Repeated courses of ATD treatment have been shown to decrease the need for definitive treatment and to increase the remission rate to 40–50% of the cases [2, 6]. There is currently no consensus on optimal therapy for CT [2, 7].
It is also unclear what characterizes children with a poor prognosis. Previous studies have found associations of remission with different variables, such as thyroid hormone levels, age, levels of TRAb, or the presence of goiter at the onset of CT [8, 9, 10, 11]. However, a large study (1,138 children) was not able to find any predictors of prognosis, besides initial goiter size that showed a weak, but statistically significant, association with remission [12].
The incidence rate (IR) of CT has been investigated in only a few previous studies. They have shown an IR between 0.7 and 2.9/100,000 person-years in children <15 or 16 years of age in different European countries during 1982–2015 [13, 14, 15, 16, 17, 18]. However, a much higher IR for Graves disease in children <15 years (6.5/100,000 person-years) during 1994–1998 has been reported in Hong Kong [19]. We have earlier shown an increasing IR of CT in Sweden during the 1990s [13]. An increasing incidence has also been found in Hong Kong and Denmark [16, 19].
In this study, we aimed to follow-up the IR of CT in the same region in Sweden that we studied previously [13] to investigate whether the increase in IR persisted at the beginning of the 2000s. We also aimed to describe the clinical characteristics at presentation and the clinical course of CT, especially Graves disease, including treatment outcome, in a population-based setting.
Materials and Methods
Study Population
The study area consisted of 5 counties in central Sweden: Örebro, Värmland, Västmanland, Södermanland, and Dalarna. These counties provide all medical care for all children and adolescents living in the area. All treatment of CT is initiated and followed up by a senior paediatric endocrinologist at the Department of Paediatrics at the main hospital in every county.
In this study, the pediatric endocrinologists at the main hospitals retrospectively identified all children and adolescents <16 years of age diagnosed with CT in the study area from the January 1, 2000 until the December 31, 2009 using hospital registers. In these registers, all patients in each department are documented prospectively, including the unique personal number of each individual, the diagnosis codes, and the dates for each code. All other hospitals and outpatient paediatric departments in the study area were asked to report additional patients to ensure that no one was missed. Only patients with suppressed TSH concentrations and in need of ATD treatment as judged by the paediatric endocrinologist were included in the study. Most patients with Hashimoto thyroiditis do not need ATD treatment [20]. All patients with treatment-requiring CT in Sweden are treated initially with ATD. Newborns with transient neonatal thyrotoxicosis and patients with thyrotoxicosis due to McCune-Albright syndrome were excluded. Patients diagnosed with CT from the January 1, 1990 until the December 31, 1999 in the study area were already identified in the same way, as described previously [13]. In the previous study [13], 1 patient with McCune-Albright syndrome was included. In this follow-up study, that patient was excluded.
The total numbers of children below the age of 16 years living in the 5 counties in each year of the study time period were obtained from the Swedish governmental authority “Statistics Sweden.”
Data Collection
Information on the patients diagnosed with CT 1990–1999 in the study area was already collected, as described previously [13]. The data from that study were pooled with data from the patients diagnosed in 2000–2009. The old and new data were collected in the same way, as follows.
A careful review of each patient's medical record was performed to ensure that all patients fulfilled the inclusion, but none of the exclusion criteria. Subsequently, a standardized form was completed using data from the medical records of patients included in the study. The following data were recorded: age, sex, family history, clinical signs and symptoms, biochemical results, and comorbidity at the time of diagnosis. The duration and dosage of the initial treatment, its outcome, and reported side effects were also recorded. In cases of relapse, we registered the new treatment given. Each patient was followed for 3 years after the discontinuation of the initial ATD treatment or until definitive treatment was given, if earlier. We also collected data on all reported complications after surgical treatment.
Graves disease was defined as CT with elevated TRAb levels or CT with a relapse during the 3-year follow-up period [3], since CT due to Hashimoto thyroiditis do not relapse [21].
Laboratory Analyses
Serum concentrations of TSH and free thyroxine were measured at the Departments of Clinical Chemistry at each hospital. Initially, manual radio immune assays were used and from the mid-1990s automatized immunoassays were used. Serum levels of TRAb were initially measured at the Department of Clinical Chemistry and Pharmacology at Uppsala University Hospital, Uppsala, Sweden, and later at the Departments of Clinical Microbiology and Immunology at each hospital, where also serum levels of anti-thyroid peroxidase antibodies were measured. All values were compared with the reference ranges used for each method at the time of analysis.
Statistical Analysis and Ethics
Proportions are presented as absolute numbers and/or percentages. The continuous variables analysed in the study did not follow a normal distribution. Thus, they are presented as medians (min-max), and comparisons between groups were performed using the Mann-Whitney U test. The chi-square test and Fisher's exact test were used where appropriate. The statistical software program SPSS version 22 (IBM, Stockholm, Sweden) was used for these analyses.
The IRs and their 95% CIs were calculated for the entire 20-year period, as well as for the two 10-year periods: 1990–1999 and 2000–2009. The comparison of the IRs between the 2 periods was performed using the Poisson Regression method. Kaplan-Meier analysis was used to analyze the outcomes after ATD withdrawal. Possible predictive factors were analysed by the Cox regression method. The statistical software program Stata release 14 (Stata Corp. College Station, TX, USA) was used for these analyses. Statistical significance was set at p < 0.05 for two-sided tests.
The Regional Board of Ethics in Uppsala, Sweden, approved the study.
Results
Incidence Rates
In total, 113 children <16 years of age were diagnosed with CT after the neonatal period and as not having McCune-Albright syndrome during 1990–2009 in the 5 studied counties in central Sweden. The total population in the study area during this period was on average 256,909 children <16 years of age each year. The overall IR during 1990–2009 was 2.2/100,000 person-years (95% CI 1.2–2.5/100,000).
The IR was significantly higher during 2000–2009 than during 1990–1999 (p = 0.006, Table 1). The increases in IRs were significant for both girls and boys when those groups were analysed separately (Table 1).
Table 1.
The IRs of thyrotoxicosis in children <16 years of age in central Sweden in the 1990s compared with the 2000s
| IRs per 100,000 person−years | 1990–1999 | 2000–2009 | p value |
|---|---|---|---|
| Total population | 1.6 (1.2–2.2) | 2.8 (2.2–3.6) | 0.006 |
| Girls | 2.9 (2.1–4.0) | 4.5 (3.4–5.9) | 0.041 |
| Boys | 0.4 (0.2–1.0) | 1.2 (0.7–2.0) | 0.038 |
The values in parentheses are the 95% CIs.
IR, incidence rate.
Clinical and Biochemical Characteristics at the Onset of CT
The majority (81%) of the 113 children were female. The median age was 13.5 years (2.5–16), and pubertal development had started in 56% of the children at onset of the disease. Six patients had Down syndrome. Most of the patients (n = 110) had Graves disease indicated by the presence of raised levels of TRAb at onset or a relapse during the follow-up period. The type of CT could not be defined in 3 patients as they did not relapse during the follow-up period and information on their antibody status were missing in 2 of them and the third patient did not have elevated levels of TRAb. These 3 patients were female, 15–16 years old, had goiter and undetected TSH levels at presentation. They were treated by ATD for 2 years. One of them was lost to follow-up. None of the 113 patients was considered to have Hashitoxicosis.
Twenty-seven of the patients had another autoimmune disease at the onset of CT, such as type 1 diabetes mellitus (n = 7), coeliac disease (n = 6), inflammatory bowel disease (n = 3), vitiligo (n = 3), or rheumatoid disease (n = 2). Boys were known with another autoimmune disease more often than girls (48 vs. 20%, p = 0.009). Girls had goiter at presentation more often than boys (79 vs. 48%, p = 0.003). Girls tended to have entered puberty before the diagnosis of CT more often than boys (62 vs. 39%, p = 0.070), but the age at onset did not differ significantly between girls and boys. There were no other differences in clinical presentation between the sexes.
There were no differences in clinical characteristics between the two 10-year periods. The clinical and biochemical characteristics at the time of diagnosis of patients defined as having Graves disease are presented in Table 2.
Table 2.
Clinical and biochemical characteristics at the onset of Graves disease in 110 children
| Clinical and biochemical parameters | Proportion of patients with a positive finding, % |
|---|---|
| Tachycardia (n = 97)* | 37 |
| Goiter (n = 110) | 73 |
| Tremor(n = 99) | 43 |
| Exophthalmus (n = 108) | 32 |
| Co-morbidity with other autoimmune diseases(n = 108) | 26 |
| Positive family history of thyrotoxicosis (n = 102) | 19 |
| Raised levels of TRAb (n = 105) | 93 |
| Raised levels of TPO ab (n = 90) | 77 |
| Levels of FT4: | |
| ≥25 pmol/L (n = 109) | 92 |
| ≥50 pmol/L (n = 109) | 61 |
Tachycardia defined as heart rate >140/min in children aged 2–5 years, >120/min in children aged 5–12 years and >100/min in children aged >12 years.
TRAb, TSH receptor stimulating antibody; TPO ab, anti-thyroid peroxidase antibody; FT4, free thyroxine.
Initial Treatment
All children were treated initially with ATDs as expected by the inclusion criteria in the study. The block-replace regimen was used in all patients. Thiamazole was used in 98% of the cases. Only 2 children were treated with carbimazole, which is a pro drug of thiamazole, and they were both diagnosed and treated during the first study period, 1990–1999. No child was treated with propylthiouracil. The initial daily dose of thiamazole was 0.52 (0.13–1.20) mg/kg body weight, and the duration of treatment was 28.9 months (4.8–90). Neither initial dose nor treatment duration differed significantly between the 2 decades. Mild side effects such as rashes (reported in 7% of the patients), arthralgia (3%), and elevated liver enzymes (1%) were reported. No severe side effects, such as neutropenia, sepsis, or severe liver injury, were observed.
Treatment Outcome and Definitive Treatment
The initial treatment with ATD was successful in most patients. However, in 25% of them, thyroidectomy was performed prior to the completion of the initial ATD treatment course. The reasons for this were relapse with thyrotoxicosis during the treatment, lack of response to the treatment, problems with compliance, or the family's own wish. One patient, a boy with Down syndrome, switched treatment from ATD to radioactive iodine due to elevated liver enzymes.
The rest of the patients (n = 84) completed the initial planned ATD treatment course. The treatment was withdrawn at the discretion of each paediatric endocrinologist. By tradition and according to national guidelines, the ATD treatment is stopped after approximately 24 months in Sweden and especially if TRAb levels have normalized.
Two patients (one girl and one boy) were lost to follow-up. Of the remaining 82 patients (80% girls), 57 relapsed during the 3-year follow-up (Fig. 1). This gives a relapse rate of 70%. Forty-four (77%) of the relapsing patients were girls and 13 (23%) were boys.
Fig. 1.
Flowchart of the clinical course of the patients. Each patient was followed for 3 years after withdrawal of the initial ATD treatment course or until definitive treatment was given, if earlier. ATD, antithyroid drug.
After exclusion of the 3 patients, who could not be labeled as having Graves disease as explained above, the relapse rate increased to 72%.
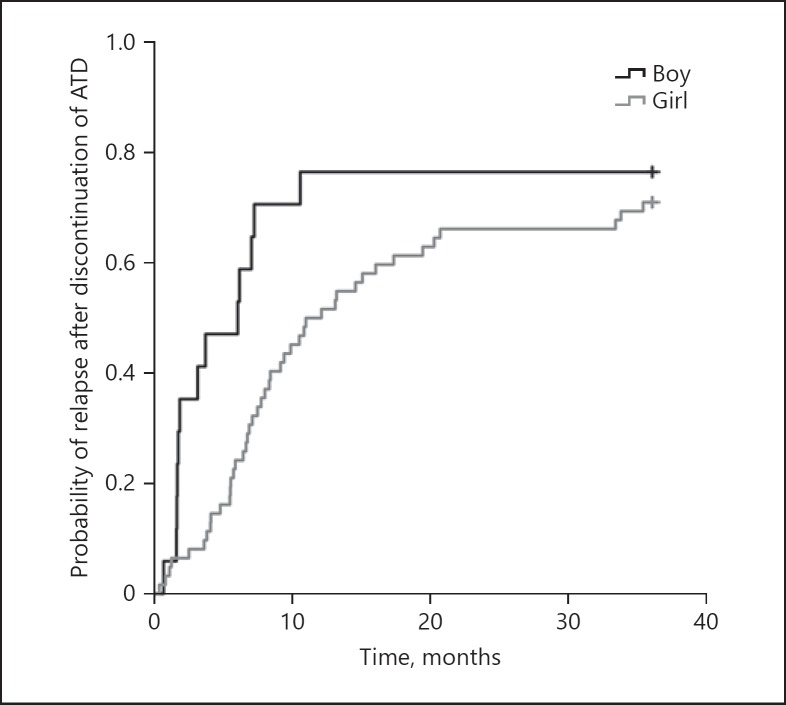
Most relapses occurred during the first year after drug withdrawal (Fig. 2). Boys tended to relapse earlier than girls (median 6.0 months after drug withdrawal [95% CI 1.9–10.0] vs. 12.0 months [95% CI 6.8–17.3], p = 0.074).
Fig. 2.
Time to relapse after ATD withdrawal in girls and boys. ATD, antithyroid drug.
The treatment chosen by the clinician after the relapse varied: a new treatment course with ATD (n = 14), thyroidectomy (n = 39), or radioactive iodine treatment (n = 3; Fig. 1).
In total, thyroidectomy was the definitive treatment for 67 (59%) of the patients. The most common complication of thyroidectomy was persistent hypocalcaemia (n = 5). Transient paralysis of the recurrent laryngeal nerve and bleeding were also reported. In 2 patients (1 girl aged 10 years at presentation of CT and 1 boy aged 15 years), the histopathological examination of the removed thyroid tissue unexpectedly revealed a papillary carcinoma. These patients were treated by total thyroidectomy and subsequent radioactive iodine. Thus, approximately 3% of the patients treated by surgery had an incidental thyroid carcinoma.
One of the patients with Down syndrome was treated early by radioactive iodine (see above), another was treated early by surgery due to insufficient response to ATD treatment, and the other 4 patients with Down syndrome relapsed after the termination of the initial ATD treatment course.
Predictors of Relapse
We investigated the impact of several variables on relapse risk in patients who completed the initial planned ATD treatment course, were considered to have Graves disease and not lost to follow-up (n = 80). The variables were sex, presence of another autoimmune disease, family history of thyrotoxicosis, presence of puberty, goiter, exophthalmus, age, and free thyroxine concentrations at presentation and initial ATD treatment duration. We found no association for any of these variables with the occurrence of a relapse during the follow-up.
Discussion
The time period of this study – 20 years – is the longest period used for the evaluation of the incidence of CT together with a description of the clinical picture, as far as we know. We found that the incidence of CT in central Sweden is increasing, which is in accordance with findings in Denmark and Hong Kong [16, 19]. However, to the best of our knowledge, we show for the first time an increasing IR of CT in both girls and boys.
The proportion of boys in this study does not differ significantly from the proportions found in previous studies [8, 10, 12, 18] and it did not change between the first and the second 10-year study periods.
A rising trend in incidence and prevalence has been seen over recent decades in other autoimmune diseases, such as coeliac disease, inflammatory bowel diseases, type 1 diabetes, and myasthaenia gravis [22, 23, 24, 25, 26, 27]. This trend may be due to environmental changes, such as infections and nutrition [22, 23, 27]. It is possible that some of the etiological factors responsible for the increase in the incidence and prevalence of other autoimmune diseases are the same for CT.
The IR found in this study, 2.2/100,000 person-years, is higher than that found in Denmark (1.6/100,000 person-years) during 1998–2012 [16], which to some extent may be explained by the slightly different age groups included in these studies. We included all children aged <16 years, and the Danish study included all children aged <15 years.
The IR found in children <15 years of age in the United Kingdom and Ireland 2004–2005 (0.9/100,000 person-years) [15] was lower than those found both in this study (2.8/100,000 person-years during 2000–2009) and in the Danish study (1.6/100,000 person-years during 1998–2012) [16]. Differences in the identification of patients between these studies may partly explain the different IRs found. It is possible that the identification procedure used in the British study [15] misses more cases than the procedures used in our study and the Danish study [16], which were based on hospital registers. Other possible explanations for the differences in IRs between different European countries include potential genetic and environmental differences in the populations. The IRs of childhood type 1 diabetes also differ between countries [28], indicating that there may be genetic or environmental differences between countries contributing to different susceptibility to autoimmune, endocrine diseases in children.
The IR reported from Hong Kong in children aged <15 years during 1989–1998 was 6.5/100,000 person-years [19], which is much higher than the IRs described in Europe. The reasons for this difference are not known, but differences in iodine intake may be involved [1, 19].
Compared to the IR of thyrotoxicosis observed in patients of all ages in Sweden, 27.6/100,000 person-years [29], the incidence in the pediatric population in Sweden remains low.
The clinical signs at onset found in this study correspond rather well to previous reports [15, 16, 19, 30]. However, we found a lower frequency of goiter than reported earlier in Graves disease in adolescence [31], which may partly be due to the fact that thyroid volume was mainly assessed clinically through manual palpation in our study. On the other hand, Léger and Carel [2] report that thyroid volume is highly variable in Graves disease in childhood.
The block-replace regimen was used in all patients in this study, which was in accordance with national guidelines in Sweden during the study period. Only a few patients reported side effects, and they were mostly mild, which may be explained by the use of thiamazole or carbimazole in this study. These ATDs have fewer severe side effects than propylthiouracil [32], which was not used at all in this study. However, the block-replace regimen has been found to be associated with a higher frequency of adverse events than the titration regimen [33]. From 2016, when revised national guidelines were published in Sweden, the titration regimen is recommended as the first-line treatment for CT in Sweden.
Six patients in this study had Down syndrome and they all had Graves disease. The clinical course of Graves disease in children with Down syndrome has been reported to be less severe than that in other children, seldom requiring definitive treatment and having a lower relapse rate [34]. However, all patients with Down syndrome in this study were either treated early by a definitive method or relapsed after ATD withdrawal. Our findings need to be interpreted with caution due to the low number of patients with Down syndrome.
Seventy-two percent of the patients with Graves disease in our study relapsed within 3 years after the discontinuation of the ATD treatment course, which is in accordance with the literature [2].
Fifty-nine percent of all patients in our study were treated by surgery either before or after the completion of the planned initial ATD treatment course. This proportion is slightly higher than the proportion (43%) found in a recent Scottish study [35]. In that study, radioactive iodine treatment was given much more frequently than in our study. These differences reflect that there is still no international consensus regarding the choice of definitive treatment in CT [2, 5, 7].
Several factors have been associated with a higher relapse frequency, such as younger age, higher goiter volume, higher levels of TRAb, and absence of other autoimmune conditions at the time of diagnosis [6, 8, 9, 10, 11]. However, in the present study, no predictor of relapse was found, and this may be due to the relatively low number of patients in the study.
The probability of relapse in CT in this study steadily increased during the first 2 years after drug withdrawal (Fig. 2), emphasizing the need for frequent follow-up during this time period. This early follow-up may be even more important in boys than in girls, as we found that boys tended to relapse earlier than girls. This possible difference needs to be further explored in a larger sample set.
As boys in this study more often had autoimmune comorbidity, less often had goiter and tended to have entered puberty less often than girls at the presentation of CT, we speculate that CT in boys may in part have a different pathogenesis or pathophysiology than in girls. This potential difference may also influence the time to relapse after ATD withdrawal. These speculations need to be investigated in future studies.
Our finding of incidental papillary carcinoma in 2 patients treated by surgery is in accordance with previous reports in adults. Approximately 7% of patients with hyperthyroidism and treated by surgery are found to have an incidental thyroid malignancy [36, 37] and the most common form in Graves disease is papillary carcinoma [36]. However, there are only a few previous reports on this in children. For example, out of 64 children treated by thyroidectomy and reviewed for surgery-related complications, only 14 children had Graves disease and none of them had an incidental finding of thyroid malignancy [38]. Before our findings may influence treatment recommendations for patients with CT more studies are needed on the prevalence and clinical impact of incidental thyroid malignancy in CT.
We conclude that the IR of CT has increased in Sweden during a 20-year period, both in girls and boys. The relapse frequency after withdrawal of initial ATD treatment is high. Boys tend to relapse earlier after drug withdrawal than girls and approximately 3% of children treated by surgery are found to have an incidental thyroid papillary carcinoma. These findings need to be further explored in future investigations.
Statement of Ethics
Patients' consent was not needed in this study, since data were collected only from the medical records and only health professionals at each patient's department reviewed the records. After the review, all data were handled in a coded manner. The study was conducted in agreement with the Declaration of Helsinki and Swedish laws. The Regional Board of Ethics in Uppsala, Sweden, approved of the study.
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
Funding Sources
This study was performed using financial support from ALF funding at Region Örebro County, Sweden.
Author Contribution
M.R. contributed to the study design, the statistical analyses and the interpretation of the findings. She collected data and had the main responsibility of writing the manuscript. M.L. contributed to the statistical analyses and the interpretation of the findings and finalized the manuscript. K.F. collected data, contributed to the interpretation of the findings and to the writing of the manuscript. C.-G.A. and M.F. collected data and contributed to the interpretation of the findings and the drafting of the manuscript. J.Å. had the main responsibility for the study design. He contributed to the interpretation of the findings and to the writing of the manuscript. All authors agreed on the finalized manuscript.
Acknowledgments
We acknowledge the contribution of Mathias Röjås and Annika Ljungwald for including patients in the study and the help rendered by Lars-Göran Jansson for developing Figure 2.
References
- 1.Franklyn JA, Boelaert K. Thyrotoxicosis. Lancet. 2012 Mar;379((9821)):1155–66. doi: 10.1016/S0140-6736(11)60782-4. [DOI] [PubMed] [Google Scholar]
- 2.Léger J, Carel JC. Diagnosis and management of hyperthyroidism from prenatal life to adolescence. Best Pract Res Clin Endocrinol Metab. 2018 Aug;32((4)):373–86. doi: 10.1016/j.beem.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Léger J, Oliver I, Rodrigue D, Lambert AS, Coutant R. Graves' disease in children. Ann Endocrinol (Paris) 2018 Dec;79((6)):647–55. doi: 10.1016/j.ando.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Brillante B, Guthrie L, Van Ryzin C. McCune-Albright Syndrome: An Overview of Clinical Features. J Pediatr Nurs. 2015 Sep-Oct;30((5)):815–7. doi: 10.1016/j.pedn.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Bauer AJ. Approach to the pediatric patient with Graves' disease: when is definitive therapy warranted? J Clin Endocrinol Metab. 2011 Mar;96((3)):580–8. doi: 10.1210/jc.2010-0898. [DOI] [PubMed] [Google Scholar]
- 6.Léger J, Gelwane G, Kaguelidou F, Benmerad M, Alberti C, French Childhood Graves' Disease Study Group Positive impact of long-term antithyroid drug treatment on the outcome of children with Graves' disease: national long-term cohort study. J Clin Endocrinol Metab. 2012 Jan;97((1)):110–9. doi: 10.1210/jc.2011-1944. [DOI] [PubMed] [Google Scholar]
- 7.De Luca F, Valenzise M. Controversies in the pharmacological treatment of Graves' disease in children. Expert Rev Clin Pharmacol. 2018 Nov;11((11)):1113–21. doi: 10.1080/17512433.2018.1546576. [DOI] [PubMed] [Google Scholar]
- 8.Gastaldi R, Poggi E, Mussa A, Weber G, Vigone MC, Salerno M, et al. Graves disease in children: thyroid-stimulating hormone receptor antibodies as remission markers. J Pediatr. 2014 May;164((5)):1189–1194.e1. doi: 10.1016/j.jpeds.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 9.Glaser NS, Styne DM, Organization of Pediatric Endocrinologists of Northern California Collaborative Graves' Disease Study Group Predicting the likelihood of remission in children with Graves' disease: a prospective, multicenter study. Pediatrics. 2008 Mar;121((3)):e481–8. doi: 10.1542/peds.2007-1535. [DOI] [PubMed] [Google Scholar]
- 10.Jevalikar G, Solis J, Zacharin M. Long-term outcomes of pediatric Graves' disease. J Pediatr Endocrinol Metab. 2014 Nov;27((11–12)):1131–1136. doi: 10.1515/jpem-2013-0342. [DOI] [PubMed] [Google Scholar]
- 11.Kaguelidou F, Alberti C, Castanet M, Guitteny MA, Czernichow P, Léger J, French Childhood Graves' Disease Study Group Predictors of autoimmune hyperthyroidism relapse in children after discontinuation of antithyroid drug treatment. J Clin Endocrinol Metab. 2008 Oct;93((10)):3817–26. doi: 10.1210/jc.2008-0842. [DOI] [PubMed] [Google Scholar]
- 12.Ohye H, Minagawa A, Noh JY, Mukasa K, Kunii Y, Watanabe N, et al. Antithyroid drug treatment for graves' disease in children: a long-term retrospective study at a single institution. Thyroid. 2014 Feb;24((2)):200–7. doi: 10.1089/thy.2012.0612. [DOI] [PubMed] [Google Scholar]
- 13.Forssberg M, Arvidsson CG, Engvall J, Lindblad C, Snellman K, Aman J. Increasing incidence of childhood thyrotoxicosis in a population-based area of central Sweden. Acta Paediatr. 2004 Jan;93((1)):25–9. doi: 10.1080/08035250310008069. [DOI] [PubMed] [Google Scholar]
- 14.Lavard L, Ranløv I, Perrild H, Andersen O, Jacobsen BB. Incidence of juvenile thyrotoxicosis in Denmark, 1982-1988. A nationwide study. Eur J Endocrinol. 1994 Jun;130((6)):565–8. doi: 10.1530/eje.0.1300565. [DOI] [PubMed] [Google Scholar]
- 15.Williamson S, Greene SA. Incidence of thyrotoxicosis in childhood: a national population based study in the UK and Ireland. Clin Endocrinol (Oxf) 2010 Mar;72((3)):358–63. doi: 10.1111/j.1365-2265.2009.03717.x. [DOI] [PubMed] [Google Scholar]
- 16.Havgaard Kjær R, Smedegård Andersen M, Hansen D. Increasing Incidence of Juvenile Thyrotoxicosis in Denmark: A Nationwide Study, 1998-2012. Horm Res Paediatr. 2015;84((2)):102–7. doi: 10.1159/000430985. [DOI] [PubMed] [Google Scholar]
- 17.Kumorowicz-Kopiec M, Dziatkowiak H, Starzyk J, Nizankowska-Błaz T, Rybakowa M. [Incidence of Graves disease in children in some regions of south-eastern Poland] Przegl Lek. 2004;61((8)):872–5. [PubMed] [Google Scholar]
- 18.Simon M, Rigou A, Le Moal J, Zeghnoun A, Le Tertre A, De Crouy-Chanel P, et al. Epidemiology of Childhood Hyperthyroidism in France: A Nationwide Population-Based Study. J Clin Endocrinol Metab. 2018 Aug;103((8)):2980–7. doi: 10.1210/jc.2018-00273. [DOI] [PubMed] [Google Scholar]
- 19.Wong GW, Cheng PS. Increasing incidence of childhood Graves' disease in Hong Kong: a follow-up study. Clin Endocrinol (Oxf) 2001 Apr;54((4)):547–50. doi: 10.1046/j.1365-2265.2001.01252.x. [DOI] [PubMed] [Google Scholar]
- 20.Lorini R, Gastaldi R, Traggiai C, Perucchin PP. Hashimoto's Thyroiditis. Pediatr Endocrinol Rev. 2003 Dec;1(Suppl 2):205–11. [PubMed] [Google Scholar]
- 21.Wasniewska M, Corrias A, Salerno M, Lombardo F, Aversa T, Mussa A, et al. Outcomes of children with hashitoxicosis. Horm Res Paediatr. 2012;77((1)):36–40. doi: 10.1159/000334640. [DOI] [PubMed] [Google Scholar]
- 22.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002 Sep;347((12)):911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 23.Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007 Nov;26((9)):1217–25. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 24.Kang JY, Kang AH, Green A, Gwee KA, Ho KY. Systematic review: worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther. 2013 Aug;38((3)):226–45. doi: 10.1111/apt.12373. [DOI] [PubMed] [Google Scholar]
- 25.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142((1)):46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Binks S, Vincent A, Palace J. Myasthenia gravis: a clinical-immunological update. J Neurol. 2016 Apr;263((4)):826–34. doi: 10.1007/s00415-015-7963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Y, Xie Z, Huang G, Zhou Z. Incidence and trend of type 1 diabetes and the underlying environmental determinants. Diabetes Metab Res Rev. 2019 Jan;35((1)):e3075. doi: 10.1002/dmrr.3075. [DOI] [PubMed] [Google Scholar]
- 28.DIAMOND Project Group Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006 Aug;23((8)):857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 29.Abraham-Nordling M, Byström K, Törring O, Lantz M, Berg G, Calissendorff J, et al. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. 2011 Dec;165((6)):899–905. doi: 10.1530/EJE-11-0548. [DOI] [PubMed] [Google Scholar]
- 30.Bergman P, Auldist AW, Cameron F. Review of the outcome of management of Graves' disease in children and adolescents. J Paediatr Child Health. 2001 Apr;37((2)):176–82. doi: 10.1046/j.1440-1754.2001.00641.x. [DOI] [PubMed] [Google Scholar]
- 31.Muirhead S. Diagnostic approach to goitre in children. Paediatr Child Health. 2001 Apr;6((4)):195–9. doi: 10.1093/pch/6.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karras S, Tzotzas T, Krassas GE. Toxicological considerations for antithyroid drugs in children. Expert Opin Drug Metab Toxicol. 2011 Apr;7((4)):399–410. doi: 10.1517/17425255.2011.557068. [DOI] [PubMed] [Google Scholar]
- 33.Abraham P, Avenell A, McGeoch SC, Clark LF, Bevan JS. Antithyroid drug regimen for treating Graves' hyperthyroidism. Cochrane Database Syst Rev. 2010 Jan;((1)):CD003420. doi: 10.1002/14651858.CD003420.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Luca F, Corrias A, Salerno M, Wasniewska M, Gastaldi R, Cassio A, et al. Peculiarities of Graves' disease in children and adolescents with Down's syndrome. Eur J Endocrinol. 2010 Mar;162((3)):591–5. doi: 10.1530/EJE-09-0751. [DOI] [PubMed] [Google Scholar]
- 35.Kourime M, McGowan S, Al Towati M, Ahmed SF, Stewart G, Williamson S, et al. Long-term outcome of thyrotoxicosis in childhood and adolescence in the west of Scotland: the case for long-term antithyroid treatment and the importance of initial counselling. Arch Dis Child. 2018 Jul;103((7)):637–42. doi: 10.1136/archdischild-2017-313454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staniforth JU, Erdirimanne S, Eslick GD. Thyroid carcinoma in Graves' disease: A meta-analysis. Int J Surg. 2016 Mar;27:118–25. doi: 10.1016/j.ijsu.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 37.Jia Q, Li X, Liu Y, Li L, Kwong JS, Ren K, et al. Incidental thyroid carcinoma in surgery-treated hyperthyroid patients with Graves' disease: a systematic review and meta-analysis of cohort studies. Cancer Manag Res. 2018 May;10:1201–7. doi: 10.2147/CMAR.S164210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akkari M, Makeieff M, Jeandel C, Raingeard I, Cartier C, Garrel R, et al. Thyroid surgery in children and adolescents: a series of 65 cases. Eur Ann Otorhinolaryngol Head Neck Dis. 2014 Nov;131((5)):293–7. doi: 10.1016/j.anorl.2013.11.009. [DOI] [PubMed] [Google Scholar]