Abstract
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an epidemic in the obese population. Bariatric surgery is known to reverse multiple metabolic complications of obesity such as diabetes, dyslipidemia, and NAFLD, but the timing of liver changes has not been well described.
Materials and Methods
This was an IRB-approved, two-institutional prospective study. Bariatric patients received MRIs at baseline and after a pre-operative liquid diet. Liver biopsies were performed during surgery and if NAFLD positive, the patients received MRIs at 1, 3, and 6 months. Liver volumes and proton-density fat fraction (PDFF) were calculated from offline MRI images. Primary outcomes were changes in weight, body mass index (BMI), percent excess weight loss (EWL%), liver volume, and PDFF. Resolution of steatosis, as defined as PDFF < 6.4% based on previously published cutoffs, was assessed. Secondarily, outcomes were compared between patients who underwent laparoscopic sleeve gastrectomy (LSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB).
Results
From October 2010 to June 2015, 124 patients were recruited. 49 patients (39.5%) completed all five scans. EWL% at 6 months was 55.6 ± 19.0%. BMI decreased from45.3 ± 5.9 to 34.4 ± 5.1 kg/m2 and mean liver volume decreased from 2464.6 ± 619.4 to 1874.3 ± 387.8 cm3 with a volume change of 21.4 ± 11.4%. PDFF decreased from16.6 ± 7.8 to 4.4 ± 3.4%. At 6 months, 83.7% patients had resolution of steatosis. Liver volume plateaued at 1 month, but PDFF and BMI continued to decrease. There were no statistically significant differences in liver volume or PDFF reduction from baseline to 6 months between the LSG versus LRYGB subgroups.
Conclusion
Patients with NAFLD undergoing bariatric surgery can expect significant decreases in liver volume and hepatic steatosis at 6 months, with 83.7% of patients achieving resolution of steatosis. Liver volume reduction plateaus 1-month post-bariatric surgery, but PDFF continues to decrease. LSG and LRYGB did not differ in efficacy for inducing regression of hepatosteatosis.
Keywords: Bariatric surgery, NAFLD, Liver volume, Sleeve gastrectomy, Gastric bypass
Nonalcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease in the United states, with about 20–30% of the adult population affected [1, 2]. NAFLD has been linked to other metabolic syndrome diseases such as type II diabetes, dyslipidemia, and cardiovascular disease. Additionally, patients with isolated steatosis can progress to a more histological aggressive form of NAFLD, known as nonalcoholic steatohepatitis (NASH) and eventually hepatic cirrhosis. Early detection and treatment can help slow or potentially reverse the progression of NAFLD. Since the most common risk factor for developing NAFLD is obesity, clinical management emphasizes weight loss, which can be achieved through diet modification, exercise, or surgical interventions. However, the current algorithms for NAFLD treatment suggest that while weight-loss surgery is beneficial, it is not recommended as a primary intervention in NAFLD [3].
Bariatric surgery with intensive medical management has been shown to be more effective when compared to intensive medical management alone for resolution of other metabolic diseases such as type II diabetes [4]. Studies have demonstrated an 85% pathological regression of steatosis after bariatric surgery with follow-up liver biopsy results [5]. However, there are few studies that analyze the time course of fatty liver resolution or attempt to correlate it with the degree of weight loss or reduction in liver size.
This study aims to characterize the timing of the reduction of liver fat and liver size following bariatric surgery, and to describe the resolution of hepatic steatosis. To perform this study, we performed magnetic resonance imaging (MRI) at multiple time points, measuring liver volume and MRI proton-density fat fraction (MRI-PDFF). The latter is a validated, noninvasive quantitative imaging biomarker of liver fat content; unlike conventional MRI, MRI-PDFF minimizes or corrects potential confounding factors to measure fat fraction accurately [6]. Previous PDFF studies have established values for different grades of hepatic steatosis, with a PDFF > 6.4% indicating grade 1 hepatosteatosis [7, 8]. Although liver biopsy is currently the gold standard for assessing liver fat content and diagnosing NAFLD, its invasiveness and associated risks precluded its use for the frequent measurements required by our study.
Materials and Methods
Under institutional review board (IRB) approval, consecutive morbidly obese patients being considered for weight-loss surgery were recruited from October 2010 until June 2015 to participate in a prospective longitudinal observational study at two academic institutions. The inclusion criteria were baseline BMI ≥ 35 kg/m2, being considered for weight-loss surgery, and willingness to participate for follow-up. Exclusion criteria were contraindications to MRI and clinical or laboratory evidence of liver diseases other than NAFLD (such as viral hepatitis, hemochromatosis, and Wilson disease). At each center, enrolled subjects underwent evaluation by a multi-disciplinary bariatric team to determine eligibility for weight-loss surgery. Once patients were approved to undergo surgery, they received a baseline MRI prior to starting their pre-operative high protein, low carbohydrate liquid diet, then underwent a second MRI post-liquid diet (PLD). Within 2 days of PLD MRI, patients underwent weight-loss surgery, receiving either a laparoscopic sleeve gastrectomy (LSG), a laparoscopic Roux-en-Y gastric bypass (LRYGB), or a laparoscopic adjustable gastric band (LAGB) based on the surgeon’s recommendation. The patients then received follow-up MRI studies at 1, 3, and 6 months postoperatively.
For all patients, the following demographics, perioperative, and post-operative data were recorded: gender, ethnicity, age, weight, height, body mass index (BMI), ideal body weight (IBW) based on a BMI of 25, excess weight, excess weight loss (EWL), excess weight loss percentage decrease (EWL%), MRI-measured liver volume, and MRIPDFF. Relevant laboratory studies at all time points were also obtained including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (Tbili), triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL).
Imaging and liver volumetric analysis
At both centers, all MRIs were obtained at 3T scanner (GE Signa EXCITE HDxt or GE MR750, GE Healthcare, Waukesha, WI) utilizing a multi-channel torso phased-array coil placed over the liver with all subjects in the supine position. A six-echo spoiled gradient-recalled-echo magnitude-based fat quantification technique was performed without contrast using a low flip angle to minimize T1 effects. The collection of six echoes allowed for correction of T2 [7]. Liver volume measurements and MRI-PDFF calculations were performed with the OsiriX imaging software (Osirix Foundation, Geneva, Switzerland). Liver volume measurements were made by outlining liver boundaries on each slice and the final volume calculated by OsiriX. MRI-PDFF measurements were made by placing a circular region of interest (ROI) with a 1 cm radius in each Couinaud segment of the liver, with the PDFF values from each of these ROIs averaged to give the overall MRIPDFF. These averages were then used for data analysis.
Liver histology
Liver histology slides were prepared with hematoxylin and eosin (H&E), Masson’s trichrome, and iron stains. Each histology slide was labeled with a unique study identifier and sent to pathology for consensus interpretation. Histology features such as steatosis, iron, inflammation, ballooning, and fibrosis were scored using the NASH Clinical Research Network (CRN). The NASH CRN scores steatosis into broad ranges based on the fraction of cells containing intrahepatocellular fat droplets (<5% cells affected, 5–33%, 34–66%, >66%). Histological features important to the diagnosis of NAFLD included both the presence of 5% steatosis and the absence of findings consistent with and alternative diagnosis such as hemochromatosis or infectious hepatitis.
Outcome measures and statistical analysis
Statistical analysis was performed using SPSS (IBM, Armonk, NY). Paired-t test was used to calculate significance for normally distributed data and Wilcoxon signed-rank test for non-normally distributed dated comparing values during the different time points for liver volumes, PDFF values, BMI, excess weight loss, total body weight loss, and liver function tests.
As an exploratory analysis, patient outcomes between the surgical groups were compared. Since there were only three patients in the LAGB group they were excluded, and only LSG and LRYGB outcomes were compared. At baseline total body weight, excess weight, BMI, liver volume, and PDFF were compared. Then the percent changes in those five categories from baseline to the 6-months time point were compared between the LSG and LRYGB groups. Independent samples t test were performed for normally distributed data and Mann–Whitney U test was utilized for non-parametric data to compare differences between the two groups. A Chi square with Fisher’s exact test was performed comparing the proportion of patients in each group with hepatic resolution of steatosis, using a previously published PDFF cutoff of <6.4% for this determination [7, 8].
Results
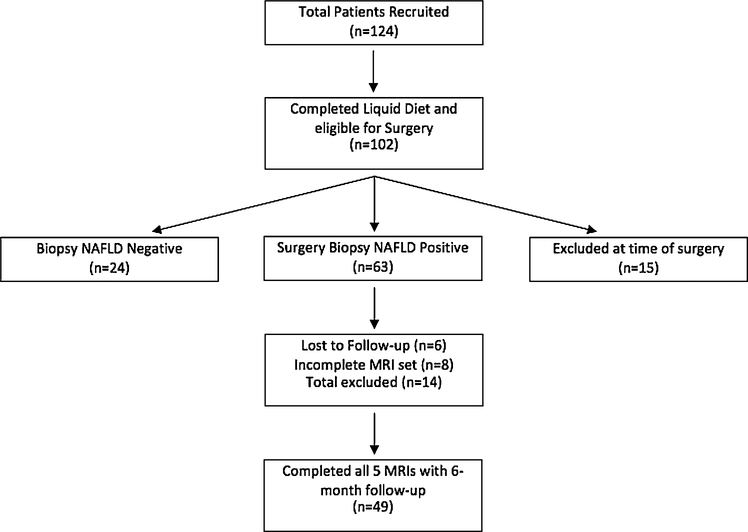
From October 2010 until June 2015, a total of 124 patients were enrolled. 102 patients completed the liquid diet and underwent bariatric surgery with planned concomitant liver biopsy. 22 patients were either unable to fit into the MRI scanner at baseline, and proceeded to surgery but were excluded from this analysis, or were disqualified by the multi-disciplinary team for either psychologically or medically related pathology. Of the 102 patients that received bariatric surgery—63 patients had positive intraoperative biopsies diagnostic for NAFLD, 24 patients had negative biopsies. 15 patients did not receive biopsies for the following reasons: 1 patient had significant hepatic bleeding just from the surgery alone, 1 patient the surgeon failed to perform the biopsy, 9 patients the surgeon deemed it unsafe to perform the biopsy, 3 patients were aborted at the time of surgery, and 1 patient canceled on the day of surgery. The 63 patients with NAFLD-positive liver biopsies were then allowed to continue in the study and receive follow-up MRIs. 14 patients subsequently were excluded from analysis with 6 lost to follow-up and 8 who missed at least one of the three follow-up MRIs. A total of 49 (39.5%) out of the initial 124 patients completed all five MRI time periods and the data were analyzed. A flowchart of patient enrollment and progress through the study is shown in Fig. 1.
Fig. 1.
Flowchart of patient selection
Patients were predominantly female (85.7%) and Caucasian (92.0%) with a mean age of 50.9 ± 10.8 years, mean weight of 123.2 ± 20.4 kg, and mean body mass index (BMI) of 45.3 ± 5.9 kg/m2 (Table 1). Twenty-six (53.1%) patients underwent LRYGB, 20 (41.8%) LSG, and 3 (6.1%) LAGB.
Table 1.
Patient demographics
| Demographics | NAFLD positive (n = 49) |
|---|---|
| Female gender, n (%) | 42 (85.7%) |
| Age (years) | 50.9 ± 10.8 |
| Weight (kg) | 123.2 ± 20.4 |
| Height (cm) | 164.7 ± 8.5 |
| Body mass index (kg/m2) | 45.3 ± 5.9 |
| Ethnicity, n (%) | |
| White | 45 (92%) |
| American Indian or Alaskan native | 1 (2.0%) |
| Unknown other | 3 (6.0%) |
Patients received a liquid diet for a mean duration of 15.5 ± 5.5 days. BMI decreased significantly from baseline to PLD (45.3 ± 5.9 vs. 43.5 ± 6.0 kg/m2 p < 0.001) and at 6 months had fallen to 34.4 ± 5.1 kg/m2. EBW decreased significantly from baseline to PLD (66.5 ± 16.7 vs. 61.5 ± 16.5 kg, p < 0.001) and at 6 months had decreased to 36.7 ± 13.5 kg. Excess weight-loss percentage (EWL%) from baseline to PLD was 9.5 ± 4.8%, and at post-operative 1, 3, and 6 months was 28.2 ± 9.0, 43.5 ± 12.5, and 55.6 ± 19.0%, respectively.
Laboratory values were compared from baseline to 6 months. AST and ALT both decreased significantly from 31.0 ± 29.1 to 15.9 ± 5.7 U/L (p = 0.002) and 42.5 ± 30.5 to 22.4 ± 9.4 U/L (p < 0.001), respectively. ALP increased from 78.6 ± 23.4 to 84.3 ± 28.9 U/L, although this was not statistically significant (p = 0.122). Tbili increased slightly from 0.47 ± 0.20 to 0.54 ±0.23 mg/dL (p = 0.02). TG decreased significantly from 184.6 ± 111.5 to 127.34 ± 47.2 mg/dL (p < 0.001). Both TC and LDL trended downward from 177.1 ± 41.6 to 166.5 ± 40.0 mg/dL and 97.4 ± 29.3 to 92.6 ± 34.9 mg/dL although neither were statistically significant (p = 0.142 and 0.411). Finally, HDL increased significantly from 44.1 ± 9.9 to 49.2 ± 10.2 mg/dL (p < 0.001).
Liver volume
From baseline to PLD, mean liver volume decreased 12.21%, from 2464.6 ± 619.4 to 2148.0 ± 527.2 cm3 (p < 0.001). Mean liver volume decreased a total of 21.37% from baseline to 1-month post-operatively. When comparing PLD with 1-month post-operatively, the volume decreased from 2148.0 ± 527.2 to 1909.9 ± 433.3 cm3 (p < 0.001). Liver volume plateaued with nonsignificant changes from 1 to 3 months (1909.9 ± 433.3 vs. 1917.0 ± 433.2 cm3, p = 0.775) and from 3 to 6 months (1917.0 ± 433.2 vs. 1874.3 ± 387.8 cm3, p = 0.067).
PDFF
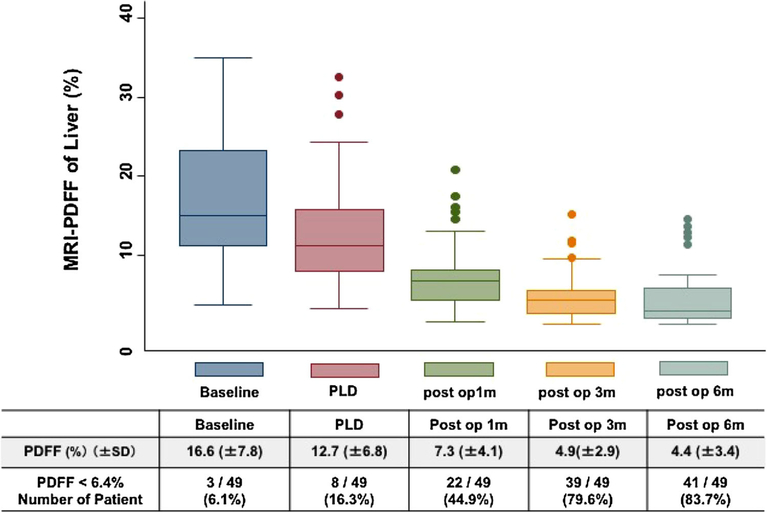
Mean PDFF decreased significantly between each pair of successive timepoints: baseline to PLD (16.6 ± 7.8 vs.12.7 ± 6.8%, p < 0.001), PLD to 1 month post-operatively (12.7 ± 6.8 vs. 7.3 ± 4.1%, p < 0.001), 1–3 months(7.3 ± 4.1 vs. 4.9 ± 2.9%, p < 0.001), and 3–6 months(4.9 ± 2.9 vs. 4.4 ± 3.4%, p = 0.007). All primary endpoints for all five time points are described in Table 2. Using a previously established MRI-PDFF cutoff for hepatic steatosis of <6.4%, the percentage of patients negative for steatosis based on MRI increased from baseline to 6 months (6.1 vs. 83.7%). Only 8 (16.3%) patients had hepatic steatosis based on MRI at 6 months post-operatively (Fig. 2).
Table 2.
Results for all 5 time points: baseline (BL), post-liquid diet (PLD), 1-, 3-, 6-months with p-values comparing with prior time point
| Variable | Baseline | PLD | 1-month | 3-month | 6-month |
|---|---|---|---|---|---|
| TBW (kg) mean ± SD | 123.2 ± 20.4 | 118.2 ± 19.8* | 108.5 ± 18.7* | 100.3 ± 17.1* | 92.9 ± 16.0*† |
| EBW (kg) mean ± SD | 55.24 ± 16.94 | 50.25 ± 16.70* | 40.49 ± 15.51* | 32.29 ± 14.05* | 25.79 ± 13.82*† |
| BMI (kg/m2) mean ± SD | 45.3 ± 5.9 | 43.5 ± 6.0* | 40.0 ± 5.5* | 36.9 ± 5.1* | 34.4 ± 5.1*† |
| Liver volume (cm3) mean ± SD | 2464.6 ± 619.4 | 2148.0 ± 527.2* | 1909.9 ± 433.3* | 1917.0 ± 433.2 (p = 0.775) | 1874.3 ± 387.8 (p = 0.067)† |
| PDFF (%) mean ± SD | 16.56 ± 7.78 | 12.71 ± 6.83* | 7.30 ± 4.14* | 4.86 ± 2.94* | 4.37 ± 3.38 (p = 0.007)† |
p-value for baseline comparison with 6-months timepoint provided. TBW total body weight, EBW excess body weight, BMI body mass index, PDFF proton-density fat fraction
p < 0.001 when compared with previous timepoint
p < 0.001 when compared with baseline
Fig. 2.
Proton-density fat fraction (PDFF) changes and percent of patients below the 6.4% cutoff for radiographic diagnosis of steatosis at all timepoints
LSG versus LRYGB
There were no statistically significant differences at baseline between the LSG and the LRYGB group for total body weight (121.6 ± 21.3 vs. 124.5 ± 20.8 kg, p = 0.352), excess body weight (53.7 ± 16.6 vs. 57.4 ± 18.0 kg, p = 0.288), BMI (44.8 ± 5.5 vs. 46.2 ± 6.3 kg/m2, p = 0.358), liver volume (2467.4 ± 623.7 vs. 2481.7 ± 612.8 cm3, p = 0.947), or PDFF (15.5 ± 7.9 vs.17.8 ± 7.8%, p = 0.245).
AST, Tbili, and Alk Phos were not statistically significantly different between the two groups. However, ALT (LSG 36.5 ± 37.2 vs. LRYGB 45.2 ± 24.4, p < 0.008) and INR (LSG 1.1 ± 0.1 vs. LYRGB 1.0 ± 0.1, p = 0.019) differed significantly. LFT comparison between the two groups is shown in Table 3.
Table 3.
Comparison of liver function tests (LFT) at baseline between laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass
| Variable | LSG (n = 20) | LRYGB (n = 26) | p-value |
|---|---|---|---|
| AST (U/L) | 27.9 ± 29.8 | 32.4 ± 28.6 | 0.236* |
| ALT (U/L) | 36.5 ± 37.2 | 45.2 ± 24.4 | 0.008* |
| Tbili (mg/dL) | 0.40 ± 0.20 | 0.50 ± 0.20 | 0.060† |
| ALP (U/L) | 78.4 ± 26.6 | 78.4 ± 25.8 | 0.922* |
| [NR | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.019† |
AST Aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, Tbili total bilirubin, and INR international normalized ratio
Mann–Whitney U test performed
Unpaired t test performed
When comparing the LSG and LRYGB groups for change from baseline to 6 months, mean liver volume percentage decrease (LSG 20.2 vs. LRYGB 25.4%, p = 0.106), mean EBW percentage decrease (LSG 52.9 vs. LRYGB 60.2%, p = 0.268), and median PDFF percentage decrease (LSG 68.0 vs. LRYGB 74.5%, p = 0.084) did not differ significantly. Total body weight percentage change (LSG 22.3 vs. LRYGB 26.3%, p = 0.044) and BMI percentage change (LSG 22.0 vs. LRYGB 26.3%, p = 0.024) differed significantly between the groups with LRYGB achieving higher percentage changes in both categories. The comparison between the percentage changes between the LSG and LRYGB is outlined in Table 4. At 6 months, 16/20 (80.0%) of LSG patients had no steatosis based on MRI compared to 24/26 (92.3%) of LYRG patients, a nonsignificant difference (p = 0.215).
Table 4.
Comparison of percentage difference from baseline to 6-months between laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB) groups
| Variable | LSG | RYGB | p-value |
|---|---|---|---|
| TBW% change | 22.31 ± 6.47 | 26.32 ± 5.93 | 0.044* |
| EBW% change | 52.86 ± 18.70 | 60.19 ± 17.80 | 0.268* |
| BMI% change | 21.98 ± 6.36 | 26.33 ± 5.94 | 0.024* |
| Liver volume% change | 20.22 ± 9.21 | 25.37 ± 13.1 | 0.106* |
| PDFF% change | 67.95 ± 18.68 | 74.47 ± 21.46 | 0.084* |
TBW total body weight, EBW excess body weight, BMI body mass index, PDFF proton-density fat fraction
Mann–Whitney U test performed
Discussion
NASH, which is a form of advanced liver disease on the NAFLD spectrum can progress to cirrhosis in as high as 20% of patients, and is more prevalent in weight-loss surgery patients than the general population. NASH currently occurs in 2–5% of the general population with about 20% of patients developing cirrhosis and in severe cases progressing to liver failure [1]. Weight loss has been proven to decrease liver volume and fat composition, and previous studies have demonstrated similar decreases in PDFF values with liquid diets [5, 9–16]. However, our study longitudinally follows patients after bariatric surgery and provides 6-month data. Our key findings were that liver volume decreases 12.1% after a 2-week liquid diet, and 21.4% at 1-month post-operatively. At 6-months post-operatively, 83.7% of patients had regression of their hepatosteatosis, determined by having PDFF values <6.4%.
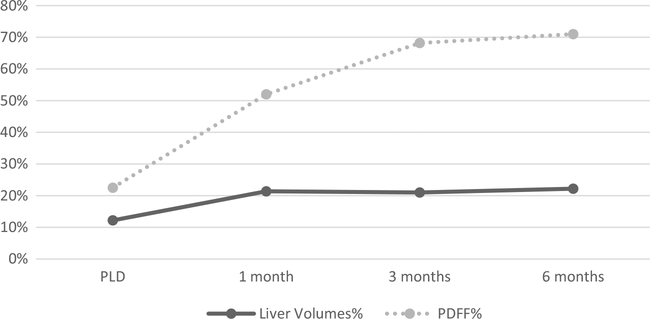
Despite the liver volume percentage changes plateauing statistically from post-operative 1 month onward, there continued to be statistically significant decreases in the PDFF (Fig. 3). This would make sense intuitively as the liver volume and the fat volume decreases dramatically at first, but the liver volume itself can only decrease to a certain percentage. After this either parenchymal architectural changes or cellular rearrangement causes the fat fraction to decrease further but the liver volume to remain stable.
Fig. 3.
Liver volume and PDFF percent decrease compared to baseline
As we move toward an era where bariatric surgery becomes increasingly utilized as a metabolic surgery rather than a purely weight-loss procedure, it is important to note these improvements in NAFLD patients before the disease progresses to NASH. Our study appears to indicate that there is no difference clinically significant difference at 6 months between LSG and LRYGB outcomes for decreases in PDFF values and resolution of NAFLD, however, there was a trend toward improved outcomes in LRYGB patients.
When LSG and LRYGB was compared, it did not surprise our group to discover that the LRYGB group achieved higher weight loss with a higher BMI and total body weight percentage change as compared to the LSG group as weight loss is known to be superior in LRYGB patients [17, 18]. What was interesting was that the liver volume percentage decrease and PDFF percentage decrease did not differ significantly between the two groups. This is possibly due to the fact that the amount of excess weight loss achieved in the LSG group was sufficient to create the cellular changes necessary to achieve regression of hepatosteatosis. This finding is consistent with recent data comparing NAFLD activity scores (NAS) between LSG and LRYGB, demonstrating no statistical differences in post-operative decreases in NAS between the two surgical groups [19]. However, there was a trend toward improved outcomes in LRYGB patients in both liver volume and PDFF.
Limitations
There were multiple limitations to this study. First, there was only a surgical treatment arm and there was no randomization to a best medical management arm, nor was there randomization of patients into the different surgical options. Although the overall cohort size was adequate for statistical analysis, once the cohorts were divided into the three procedures the individual group sizes were small. The patient population was also fairly homogenous with the patient’s predominately being Caucasian, and a majority of them female making this less generalizable to the United States population as a whole.
Another limitation of this study was despite recruiting 124 patients, only 49 (39.5%) patients ultimately completed all 5 MRIs. The 14 patients who had both BL and PLD MRIs with a positive biopsy who were either lost to follow-up or missed an MRI were contacted extensively, but unfortunately they either relocated or changed insurance coverage making this time sensitive coordination of imaging difficult.
6.1% of baseline patients were negative for hepatosteatosis based on an MRI-PDFF value of less than 6.4% but subsequently had positive liver biopsies for hepatosteatosis. This discordance was consistent with previously established liver-PDFF parameters [7, 8] and should be taken into consideration if utilizing the MRIPDFF biomarker in the future in lieu of liver biopsy. The last limitation was that there were no liver biopsies at the completion of the study to fully characterize the histological changes throughout the study and to correlate pathology with the final liver volume and PDFF data.
Conclusion
Bariatric surgery appears to be an effective treatment for NAFLD. Liver volume decreased 21.4% at 1-month postoperatively and plateaus, while PDFF continued to decrease with 83.7% of patients achieving radiologic resolution of hepatosteatosis at 6 months. Further research is needed to understand how liver fat can continue to regress while liver volume remains stable. When LSG and LRYGB was compared, there were no statistically significant differences in liver volume or PDFF changes, however, there was a trend toward improved liver imaging outcomes in patients who underwent LYRGB.
Funding
National Institute of Health (NIH) Grants: R01DK088925, R01DK083380, R01DK100651, K24DK102595.
Footnotes
Presented at the SAGES 2017Annual Meeting, March 22–25, 2017, Houston, Texas.
Disclosures Dr. Reeder reports other from Cellectar Biosciences, other from Elucent Medical, other from Calimetrix, LCC, personal fees from Parexel International, outside the submitted work. Dr. Sandler reports personal fees from W.L Gore, personal fees from Bard/Davol, personal fees from ValenTx, Inc. outside the submitted work. Dr. Horgan reports personal fees from Johnson and Johnson/ Ethicon, personal fees from W.L. Gore, personal fees from Torax/Ethicon, personal fees from ValenTx, Inc outside the submitted work. Dr. Sirlin reports grants from National Institute of Health, during the conduct of the study; grants from Bayer, grants from Guerbet, grants from Siemens, grants from General Electric, grants from Supersonic, grants from Arterys, personal fees from Alexion, personal fees from AstraZeneca, personal fees from Bioclinica, personal fees from BMS, personal fees from Bracco, personal fees from Celgene, personal fees from Fibrogen, personal fees from Galmed, personal fees from Genentech, personal fees from Genzyme, personal fees from Gilead, personal fees from Icon, personal fees from Intercept, personal fees from Isis, personal fees from Janssen, personal fees from NuSirt, personal fees from Perspectum, personal fees from Pfizer, personal fees from Profil, personal fees from Sanofi, personal fees from Shire, personal fees from Synageva, personal fees from Tobira, personal fees from Takeda, personal fees from Virtual Scopics, outside the submitted work. Dr. Jacobsen reports personal fees from W.L.Gore, personal fees from Davol/Bard, personal fees from Viasite, personal fees from Ethicon, outside the submitted work. Drs. Luo, Suzuki, Liu, Schwimmer, Funk, Greenberg and Campos, Mr. Hooker, Ms. Schlein, and Ms. Covarrubias have no conflicts of interest or financial ties to disclose.
References
- 1.Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34(3):274–285 [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ (2013) The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10(11):686–690 [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55(6):2005–2023 [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA et al. (2017) Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 376(7):641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J et al. (2015) Bariatric surgery reduces features of non-alcoholic steatohepatitis in morbidly obese patients. Gastroenterology 149(2):379–388 [DOI] [PubMed] [Google Scholar]
- 6.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA et al. (2013) Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 58(6):1930–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang A, Tan J, Sun M et al. (2013) Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267(2):422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J et al. (2015) Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 274(2):416–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang GH, Cruite I, Shiehmorteza M, Wolfson T, Gamst AC, Hamilton G et al. (2011) Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging 34(4):928–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edholm D, Kullberg J, Karlsson FA, Haenni A, Ahlstrom H, Sundbom M (2015) Changes in liver volume and body composition during 4 weeks of low calorie diet before laparoscopic gastric bypass. Surg Obes Relat Dis. 11(3):602–606 [DOI] [PubMed] [Google Scholar]
- 11.González-Pérez J, Sánchez-Leenheer S, Delgado AR, González-Vargas L, Díaz-Zamudio M, Montejo G et al. (2013) Clinical impact of a 6-week preoperative very low calorie diet on body weight and liver size in morbidly obese patients. Obes Surg 23(10):1624–1631 [DOI] [PubMed] [Google Scholar]
- 12.Tang A, Chen J, Le TA, Changchien C, Hamilton G, Middleton MS et al. (2015) Cross-sectional and longitudinal evaluation of liver volume and total liver fat burden in adults with nonalcoholic steatohepatitis. Abdom Imaging 40(1):26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel NS, Doycheva I, Peterson MR, Hooker J, Kisselva T, Schnabl B et al. (2015) Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 13(3):561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson L, Roos M, Kullberg J, Weis J, Ahlström H, Sundbom M et al. (2008) Lipid mobilization following Roux-en-Y gastric bypass examined by magnetic resonance imaging and spectroscopy. Obes Surg 18(10):1297–1304 [DOI] [PubMed] [Google Scholar]
- 15.Edholm D, Kullberg J, Haenni A, Karlsson FA, Ahlström A, Hedberg J et al. (2011) Preoperative 4-week low-calorie diet reduces liver volume and intrahepatic fat, and facilitates laparoscopic gastric bypass in morbidly obese. Obes Surg 21(3):345–350 [DOI] [PubMed] [Google Scholar]
- 16.Iannelli A, Martini F, Schneck AS, Ghavami B, Baudin G, Anty R et al. (2013) Preoperative 4-week supplementation with omega-3 polyunsaturated fatty acids reduces liver volume and facilitates bariatric surgery in morbidly obese patients. Obes Surg 23(11):1761–1765 [DOI] [PubMed] [Google Scholar]
- 17.Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, Krause KR et al. (2013) The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg 257(5):791–797 [DOI] [PubMed] [Google Scholar]
- 18.Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults. Cochrane Database Syst Rev 8:CD003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froylich D, Corcelles R, Daigle C, Boules M, Brethauer S, Schauer P (2016) Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: a comparative study. Surg Obes Relat Dis 12(1):127–131 [DOI] [PubMed] [Google Scholar]