Abstract
The economic issues related to medical treatments in youth with type 2 diabetes (T2D) are rarely reported and thus not fully understood. The TODAY clinical trial of youth recently diagnosed with T2D collected healthcare and related cost information from the largest cohort studied to date.
Costs related to medical treatments and expenses faced by caregivers were identified over a two-year period from 496 participants. Data were collected by surveys and diaries to document frequency of use of diabetes care (excluding study laboratory tests), non-diabetes care services and treatments, caregiver time, and expenses related to exercise and dietary activities recommended for patients. Economic costs were derived by applying national cost values to the reported utilization frequency data.
Annual medical costs in the first year varied by treatment group, averaging $1798 in those assigned to metformin alone (M), $2971 to combination drug therapy with metformin + rosiglitazone (M+R), and $2092 to metformin + an intensive lifestyle and behavior change program (M+L). Differences were primarily due to costs related to combination drug therapy. Adult caregiver support costs were higher for participants in the lifestyle program, which was delivered in weekly sessions in the first six months. Expenses for purchases to enhance diet and exercise change did not vary by treatment assignment. In year 2, medication costs increased in M and M+L due to the initiation of insulin in subjects who failed to maintain glycemic control on the assigned treatment.
Data are reported for use by researchers and those providing healthcare to this vulnerable patient population.
Keywords: Diabetes mellitus, Type 2, Cost analysis, Drug costs, Caregivers, Delivery of healthcare
INTRODUCTION
Profound increases in obesity in the United States over the last 30 years have led to a higher frequency of type 2 diabetes (T2D) in adolescents and youth (1–4). Most attention on T2D in youth has focused on identifying the best treatment strategies to attain glycemic control and address risk factors for complications (5–7). The economic issues, costs, and burdens related to treatment of T2D in youth have not yet been fully explored.
The overall costs of diabetes have been outlined extensively. In the United States, the direct and indirect medical costs totaled $327 billion in 2017, with a large proportion related to costs for hospital care, medicines, and disability (8). On average, an individual with diabetes spent about $9,600 on medical expenses in 2012 (8). Many face a large financial burden from covering these expenses out-of-pocket (9). In youth, the direct costs of treating type 1 diabetes were estimated at $4,750 per annum (10). Another report noted treatment costs in youth with either type 1 or type 2 diabetes of $9,333 per year (in 2007 dollars) for those using insulin and $5,683 per year for those on oral medications (11). These costs relate only to those with private health insurance and do not clearly identify the care patterns and non-medical factors related to managing T2D in youth.
A secondary aim of the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) clinical trial (12) was to investigate the economic resources involved in the treatment and management of T2D in youth and to outline the economic implications of these treatment options for families and caregivers (13). This report uses data gathered in the first two years of TODAY to describe the predominant economic costs and issues related to three treatment options for youth and adolescents with T2D: metformin alone (M), metformin plus rosiglitazone (M+R), and metformin plus a lifestyle and behavior modification program (M+L) (14). The primary outcome was loss of glycemic control, defined as a glycated hemoglobin level of at least 8% for 6 months or sustained metabolic decompensation requiring insulin. While TODAY showed that M+R was significantly better than M but that M+L was not different from M (5), by the time these results were reported rosiglitazone use was restricted by the FDA (since lifted) and it was no longer considered a viable treatment option; therefore, a more formal cost-benefit analysis was moot. Descriptive summary data are presented here by treatment group for purposes of reporting this unique collection of economic and cost data to other investigators and to the healthcare community addressing treatment and management of T2D in youth.
METHODS
At enrollment from 2004–2009, youth in the study were aged 10–17 years, diagnosed with type 2 diabetes for <2 years, with BMI ≥85th percentile (overweight/obese), and negative for diabetes autoantibodies (5,12,15). All participants were followed with regular clinic visits (every 2 months in year 1 and every 3 months thereafter) for an average of 3.86 years. The TODAY protocol was approved by all participating Institutional Review Boards; informed consent was provided by parents/guardians and youth assent was sought per local practices.
Information on healthcare utilization and caregiver burden was assessed at baseline and in the first two years of follow-up. Complete data were available from 496 of the participants, or 71% of the total cohort, including 163 assigned to M, 164 to M+R, and 169 to M+L. The entire cohort is not represented because economic data were collected periodically and not all participants had visits during those periods. The analysis sample was representative of the total cohort at baseline (15). Comparison of the 496 participants with the 203 not included in this report showed no differences in gender, randomized treatment group assignment, duration of T2D, or indicators of socioeconomic status (household education and annual income). There was a greater percentage of Hispanics in the analysis sample (42.1% versus 34.0%, p=0.0047), and the analysis sample was younger by 8.4 months (13.7 versus 14.6 years, p<0.0001). The complete comparison is reported in the on-line supplementary appendix Table A1.
Resource Utilization
Major economic resources related to the care of youth with T2D included those used for routine diabetes care practices, the treatment of acute events related to diabetes control, non-diabetes healthcare issues, and those related to caregiver burden. TODAY gathered information on the frequency of use for a number of direct medical resources, including: hospital, emergency department, urgent care, and outpatient physician healthcare services; contact with clinical personnel; insulin, prescription drugs, and diabetes equipment and supplies. Costs related to laboratory tests were not collected; these tests were performed according to standard best practice in all three treatment groups and clinics were charged rates for federally funded research. Non-medical factors related to the treatment options were assessed, including caregiver time in providing and supervising treatment, expenses related to diet and exercise recommendations, and travel for healthcare visits. Costs and resources related to conducting the research were not included.
Information on the patterns and use of diabetes care as applied throughout the trial were identified from case report forms. All participants received metformin (up to 1000 mg bid), one-third also received rosiglitazone (4 mg bid), and one-third also received a lifestyle behavior change intervention (14). All participants were instructed to test blood glucose concentrations (using lancets and testing strips) twice daily.
When participants experienced the primary study outcome (defined as treatment failure due to loss of glycemic control), metformin use and lifestyle program visits continued, rosiglitazone use was terminated in the M+R group, and study-provided insulin was initiated. Once on insulin, self-monitoring of blood glucose involved lancet and test strip use four times daily and insulin syringe use twice daily.
The use of prescription medications for common comorbid conditions was also identified at each visit. Specific drugs identified during the study include angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB), diuretics, statins, sequestrants, fibrates, oral steroids, inhaled steroids, hormonal contraceptives, antidepressants or mood stabilizers, psychotropic stimulants, and thyroid medicine. As the overall use of each non-diabetes related prescription medication was negligible, total costs are reported for this as a combined category.
Unscheduled visits to the TODAY medical centers for treatment and management of diabetes and related conditions were also documented. Common reasons for unscheduled visits included poor glycemic control, education on insulin use, blood pressure checks following hypertension, follow-up visits due to abnormal test results, and pregnancy. The frequency of unscheduled visits was spread evenly across the 3 treatment arms. Overall, unscheduled visits occurred in 28% of the sample in year 1 and 38% in year 2; of those who had interim visits, 77.5% had 2 visits at most. Because of the minimal contribution to annual healthcare and because full details on the scope and use of services during these visits beyond that necessitated by the research protocol were not gathered, costs related to unscheduled visits were not included.
The time participants spent in contact with clinical personnel in diabetes related activities was identified from time diaries completed by health professionals during the study. The one week diaries assessed time involved in both direct and indirect contact with participants and were completed by physicians, educators, nurses, dietitians, psychologists, and the lifestyle program coaches.
While routine diabetes care was provided as part of the TODAY protocol, participants were free to access other healthcare venues. Self-report surveys were administered at baseline, 12 months, and 24 months to caregivers of the study participants to document the use of healthcare services outside of TODAY. Specific questions addressed hospitalization, emergency room visits, urgent care visits, regularly scheduled outpatient visits, and calls to healthcare providers for medical issues.
The survey interview also captured information on the time and expenses incurred by adult caregivers related to participant care and management, including time related to assisting in and monitoring of diabetes treatment and time related to assisting with recommended diet and exercise plans. Purchases of exercise-related items (shoes, equipment, classes, and gym/team memberships) and food costs were also assessed. Purchased food was categorized as either (a) regular expenses made in grocery stores, specialty stores (e.g., bakery, delicatessen), or for school lunch or (b) meals eaten at or carried out from restaurants, vending machines, or fast-food establishments. Further details on these methods and the specific items captured in the assessments are available in a prior publication (13).
Cost Identification and Estimation
Monetary costs were estimated for the documented services used by participants by applying a nationally representative cost figure for a given resource to the utilization data collected. As TODAY participants were recruited from 15 medical centers in 10 states across the country, cost figures were identified for specific medical and non-medical categories from national sources to reflect estimated costs, on average, from a nationwide perspective. Specific sources and the identified costs applied in this analysis are provided in the on-line supplementary appendix Table A2. As data were collected from participants from 2004 to 2011, costs for those associated years were adjusted to 2014 dollars using the Bureau of Labor Statistics Consumer Price Index for medical healthcare and treatment (16) and the overall Consumer Price Index for non-medical items (17).
For healthcare costs related to diabetes care visits, information from the time diaries was used to characterize the amount of time spent in care related activities. Costs related to the identified time by specific medical personnel were estimated based on the mean hourly wage without benefits published by the Occupational Employment Statistics program (18) for May 2013 and adjusted to 2014. Benefits, calculated as 31.3% of wage, were included per Bureau of Labor Statistics recommendation based on information from the National Compensation Survey (19).
The costs of medications were determined by using the specific drug or identification of the most common/probable drug (when no specific drug was mentioned) and dose prescribed and wholesale price (20), adjusted downward to reflect 64% of average wholesale price for brand name drugs and 27% of average wholesale price for generic drugs (21). Costs for diabetes care supplies were also estimated based upon average wholesale prices adjusted downward as described.
Healthcare services used outside of the TODAY clinics were valued by common practices. Costs related to physician services for outpatient visits and telephone calls were estimated using Medicare Relative Value Units (MRVU) based on current procedural terminology (CPT) codes (22–24). CPT-based MRVU and Agency for Healthcare Research and Quality (AHRQ) Medical Expenditure Panel Survey (MEPS) estimates were used to estimate costs for emergency room visits (25). Healthcare Cost and Utilization Project (HCUP) mean costs for diabetes hospital stays (26,27) were applied to estimate costs related to hospitalizations.
Caregiver time spent in study related activities was valued based upon the human capital method, which considers the wage that a person could earn in the associated time if that person was not involved in care activities. For caregivers who were working, a standardized wage was identified commensurate to the household education and income levels reported at baseline. Seven occupational profiles mirroring these education and income levels were identified from the Bureau of Labor Statistics (28) and an average wage across these profiles and associated industries was identified as $13.53 per hour in 2014 dollars. This figure falls within the same range as the most frequent income range in total household income for the study participants at baseline. For caregivers not in the workforce, costs related to time spend in household chores was estimated as $14.23 per hour in 2014 dollars based upon a study focused on valuing household activities (29).
Costs related to travel for study visits were estimated by identifying the most common method of travel to the TODAY clinical site by the caregiver in the self-report surveys. Public transportation and parking fees were identified and included. Mileage for private transportation was identified and valued at Internal Revenue Service federal standard mileage rates (30).
Statistical Methods
Distributions of reported healthcare utilization and cost items were largely skewed to the right; zero or low amounts of time and cost were valid responses and were included in this analysis. Extreme outliers (above the 99th percentile) of the distribution were not included at their reported values, but were set to the 99th percentile value. Descriptive statistics of mean and standard deviation (SD) are reported per recommendation for cost evaluations (31). Cost and utilization data are reported by year in study and the assigned treatment group at randomization. As the purpose of this analysis is to report the costs and treatment patterns for T2D in youth to an audience that will find them of practical use, no hypothesis testing was performed, although findings are interpreted in terms of clinical significance.
RESULTS
Annual Costs Related to Diabetes Care:
The care of an adolescent with T2D requires regular oversight by clinical staff, appropriate adjustments of medications and other interventions, and a commitment to daily management from the patient and family. During TODAY, the amount of time that clinical staff spent providing diabetes care was monitored. Table 1 shows the total time spent by clinical staff in care activities and associated costs in the first and second year of treatment. In general, average time and costs were not different between treatment groups M (from 87 minutes/$84 in year 1 to 78 minutes/$75 in year 2) and M+R (from 77 minutes/$73 to 76 minutes/$79) but were appreciably higher for the M+L group (from 277 minutes/$172 to 144 minutes/$99). In year 1 of the lifestyle program, the lifestyle coach introduced and reinforced behavior change through weekly in-person meetings for 6–8 months, followed by bi-weekly meetings for 6–8 months; the program focused on maintenance in year 2 with monthly meetings (14). Decrease in time and cost spent by the lifestyle coach reflects the change in focus from year 1 (218 minutes/$110) to year 2 (101 minutes/$51). Aside from the lifestyle coach, the time and costs related to treatment did not differ meaningfully between year 1 and year 2 overall or by each type of provider.
Table 1.
Time (minutes) and Cost (2014 US$)* of Clinical Staff to Provide Diabetes Care per Participant Visit by Treatment Group† and Year in Study
| Clinical Staff Position | Year 1 | Year 2 | ||||
| M | M+R | M+L | M | M+R | M+L | |
| All Staff‡ | ||||||
| time (minutes) | 87 (82) | 77 (77) | 277 (198) | 78 (87) | 76 (85) | 144 (125) |
| cost ($) | 84 (82) | 73 (73) | 172
(126) w/o lifestyle coach 90 (95) 83 (87) |
75 (88) | 79 (95) | 99 (89) w/o lifestyle coach 72 (65) 69 (66) |
| Physician | ||||||
| time (minutes) | 29 (19) | 24 (15) | 24 (17) | 21 (13) | 23 (19) | 30 (21) |
| cost ($) | 52 (33) | 42 (26) | 43 (31) | 38 (23) | 42 (34) | 53 (38) |
| NP/PA | ||||||
| time (minutes) | 27 (29) | 41 (29) | 35 (31) | 48 (57) | 50 (56) | 26 (31) |
| cost ($) | 28 (31) | 43 (30) | 37 (33) | 51 (59) | 52 (58) | 27 (32) |
| Educator | ||||||
| time (minutes) | 30 (32) | 36 (29) | 37 (28) | 48 (50) | 33 (36) | 34 (48) |
| cost ($) | 19 (20) | 23 (19) | 23 (17) | 30 (32) | 21 (23) | 22 (31) |
| Dietitian | ||||||
| time (minutes) | 40 (41) | 44 (53) | 37 (51) | 32 (41) | 17 (18) | 47 (48) |
| cost ($) | 24 (25) | 27 (32) | 22 (31) | 19 (25) | 10 (11) | 28 (29) |
| Lifestyle coach | > | > | > | > | ||
| time (minutes) | --- | --- | 218 (152) | --- | --- | 101 (88) |
| cost ($) | --- | --- | 110 (76) | --- | --- | 51 (44) |
All values reported as mean (SD) based on 1515 observations in 399 participants; costs expressed in 2014 US dollars for salary plus benefits. If position was not involved in the visit, time and cost = 0.
M = Metformin alone, M+R = Metformin + Rosiglitazone, M+L = Metformin + Lifestyle Program.
Includes: physician, study coordinator, nurse, nurse practitioner/physician assistant (NP/PA), diabetes educator, dietitian, lifestyle coach, and psychologist. Data refer to direct and indirect management and visit preparation time; research-related activities are not included. For M+L, data are also given for all staff excluding lifestyle coach.
Table 2 gives the annual costs related to the use of diabetes medications and supplies by the participants. By protocol, metformin was distributed at the prescribed dose for each participant in all treatment groups; adherence estimated by pill count remained high (5). Annual metformin treatment costs were estimated at $331 in each year. The combination therapy of metformin and rosiglitazone involved higher costs ($972 in year 1 to $957 in year 2). Rosiglitazone costs varied over time as a result of the occurrence of treatment failure (primary outcome), at which time insulin was added and rosiglitazone was discontinued. Insulin use and costs increased over time in all three treatment regimens because of progressive increase in treatment failures, with higher insulin costs in the first year in the M group due to higher rates of failure occurring earlier. In the second year, treatment failure rates were higher in M and M+L, with resultant higher annual costs for insulin compared to participants in M+R.
Table 2.
Use (%) and Cost (2014 US$)* per Participant for Diabetes Related Medications† and Supplies‡ by Treatment Group§ and Year in Study
| Year 1 | Year 2 | |||||
| M | M+R | M+L | M | M+R | M+L | |
| Metformin | ||||||
| % used | 100% | 100% | 100% | 100% | 100% | 100% |
| cost ($) | 331 (0) | 331 (0) | 331 (0) | 331 (0) | 331 (0) | 331 (0) |
| Rosiglitazone | ||||||
| % used | 0% | 100% | 0% | 0% | 91% | 0% |
| cost ($) | --- | 972 (119) | --- | --- | 957 (149) | --- |
| Insulin | ||||||
| % used | 11% | 8% | 11% | 25% | 18% | 24% |
| cost in users only ($) | 725 (448) | 655 (355) | 612 (370) | 1,339 (442) | 1,234 (528) | 1,415 (413) |
| cost overall ($)⁋ | 80 (270) | 52 (202) | 69 (229) | 337 (623) | 226 (528) | 335 (635) |
| Supplies | ||||||
| % used | 100% | 100% | 100% | 100% | 100% | 100% |
| cost ($) | 157 (49) | 151 (42) | 157 (49) | 182 (75) | 170 (66) | 180 (74) |
All cost figures presented as mean and (SD) expressed in 2014 dollars.
Use of diabetes medications not supplied by the study (e.g., TZD, sulfonylurea, glitinide) was only reported once by participants in the first 24 months and is not included.
Supplies used per protocol: lancets, test strips, and insulin syringes twice a day if not on insulin ($139/participant/year); lancets and test strips four times per day and insulin syringes twice a day if on insulin ($320/participant/year).
M = Metformin alone, M+R = Metformin + Rosiglitazone, M+L = Metformin + Lifestyle Program.
Metformin was used by every participant throughout the study, both before and after occurrence of treatment failure (primary outcome); rosiglitazone use was terminated once the primary outcome occurred and the participant started insulin.
Includes 100% of sample, participants with no use assigned cost = $0.
Non-study Related Healthcare Costs:
The use of healthcare services from sources outside of the TODAY clinic providers was examined, with a focus on identifying the frequency of use of these services and non-diabetes medications. Table 3 shows the frequency of use and related costs for these items. In year 1, calls to a healthcare provider (48–57%), outpatient visits (61–62%), urgent care visits (27–39%), and emergency room visits (20–24%) were relatively common among the study participants and showed no appreciable difference across the treatment groups. Related costs were also similar. In general, the use and costs related to these resources declined over time from year 1 to year 2. Hospitalization was an infrequent event in this young cohort of participants in both years.
Table 3.
Use of Healthcare Services Outside of Study Setting and Cost* per Participant by Treatment Group† and Year in Study
| Type of Healthcare Use | Year 1 | Year 2 | ||||
| M | M+R | M+L | M | M+R | M+L | |
| Call to healthcare provider | ||||||
| % used | 57% | 49% | 48% | 47% | 47% | 46% |
| % ≥ 4 calls | 10% | 7% | 9% | 10% | 8% | 9% |
| cost in users only ($) | 64 (54) | 53 (40) | 70 (61) | 64 (51) | 58 (42) | 64 (46) |
| cost overall ($)‡ | 38 (52) | 26 (39) | 34 (55) | 30 (48) | 27 (41) | 29 (44) |
| Outpatient visit | ||||||
| % used | 61% | 62% | 62% | 60% | 56% | 59% |
| % ≥ 4 visits | 12% | 9% | 15% | 11% | 9% | 17% |
| cost in users only ($) | 226 (187) | 181 (142) | 261 (220) | 223 (201) | 209 (196) | 265 (247) |
| cost overall ($)‡ | 137 (183) | 112 (142) | 160 (214) | 134 (190) | 117 (180) | 157 (230) |
| Urgent care visit | ||||||
| % used | 39% | 27% | 35% | 36% | 22% | 31% |
| % ≥ 2 visits | 13% | 9% | 15% | 18% | 12% | 11% |
| cost in users only ($) | 422 (332) | 442 (261) | 464 (245) | 505 (274) | 607 (379) | 461 (311) |
| cost overall ($)‡ | 166 (252) | 121 (240) | 165 (266) | 183 (294) | 133 (307) | 145 (276) |
| Emergency room visit | ||||||
| % used | 24% | 20% | 23% | 25% | 25% | 28% |
| % ≥ 2 visits | 4% | 4% | 7% | 4% | 4% | 8% |
| cost in users only ($) | 1,286 (560) | 1,267 (570) | 1,581 (955) | 1,223 (462) | 1,274 (596) | 1,401 (588) |
| cost overall ($)‡ | 308 (614) | 255 (569) | 365 (809) | 308 (580) | 319 (627) | 390 (701) |
| Hospital stay | ||||||
| % used | 4% | 5% | 3% | 4% | 2% | 8% |
| cost in users only ($) | 6,330 (0) | 7,121 (2,238) | 7,596 (2,831) | 6,330 (0) | 9,595 (6,330) | 6,817 (1,756) |
| cost overall ($)‡ | 233 (1,196) | 347 (1,607) | 225 (1,363) | 272 (1,287) | 232 (1,702) | 524 (1,881) |
| Nondiabetes medication§ | ||||||
| % used | 40% | 30% | 36% | 45% | 45% | 49% |
| cost in users ($) | 136 (163) | 256 (415) | 183 (223) | 273 (269) | 239 (317) | 266 (329) |
| cost overall ($)‡ | 55 (123) | 77 (254) | 66 (160) | 122 (225) | 106 (242) | 129 (264) |
All cost figures presented as mean and (SD) costs in 2014 US dollars.
M = Metformin alone, M+R = Metformin + Rosiglitazone, M+L = Metformin + Lifestyle Program.
Includes 100% of sample, for persons with no use cost = $0.
Medications include: angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), diuretic, statin, sequestrant, fibrate, oral steroid, inhaled steroid, hormonal contraceptive, antidepressant or mood stabilizer, psychotropic, stimulant, thyroid medicine.
In year 1, 30–40% of participants reported using non-diabetes medications, including lipid lowering and antihypertensive medications. Use was higher in M (40%) and M+L (36%) compared to M+R (30%). There was a notable increase in reported use and costs of medications in year 2 across all three treatment groups, with average costs roughly doubling. Use of non-diabetes medications did not differ by treatment group in year two.
Non-medical Costs Borne by Families of Participants:
Treatment of diabetes in youth in TODAY involved meaningful parent/caregiver oversight. Adult caregivers were involved in multiple activities that could affect the success of an intervention. These activities included oversight of diabetes treatment and assisting with recommended diet and exercise plans. The amount of time and associated expenses related to these non-medical issues among caregivers are shown in Table 4. Overall, caregivers of participants in M+L spent more time in year 1 helping the participant with care management (327 hours) than in the drug therapy arms (M 233 hours and M+R 262 hours). M+L arm caregivers also spent more time in exercise related activities with their child. Caregiver time related to assisting participants in M+L declined in year 2, but remained at higher levels than the caregiver time associated with the drug interventions. Overall, the related economic cost of caregiver time was $1400 to $1900 higher in year 1 and $900 to $1600 higher in year 2 for caregivers of M+L participants relative to M or M+R.
Table 4.
Adult Caregiver Time and Resources* Spent Per Participant for Non-Medical Issues Affecting Diabetes Care by Treatment Group† and Year in Study
| Resource | Year 1 | Year 2 | ||||
| M | M+R | M+L | M | M+R | M+L | |
| Assistance with T2D treatment | ||||||
| time (hours) | 233 (372) | 262 (425) | 327 (421) | 201 (263) | 215 (352) | 278 (345) |
| wage loss ($) | 2967 (5072) | 3361 (5939) | 4389 (5861) | 2443 (3438) | 2893 (4979) | 3671 (4952) |
| Assistance with exercise‡ | ||||||
| time (hours) | 77 (125) | 100 (150) | 126 (171) | 77 (120) | 93 (137) | 102 (146) |
| wage loss ($) | 805 (1590) | 937 (1636) | 1317 (1923) | 645 (1238) | 919 (1500) | 1029 (1647) |
| Travel to clinic visits | ||||||
| travel cost ($) | 38 (43) | 35 (42) | 41 (42) | 40 (47) | 42 (46) | 45 (49) |
| wage loss ($) | 18 (16) | 16 (15) | 18 (15) | 18 (17) | 18 (18) | 19 (19) |
| Exercise related purchases ($)§ | 610 (1122) | 634 (1060) | 647 (809) | 531 (855) | 593 (978) | 580 (877) |
| Total purchased food ($)‖ | 9096 (5141) | 9843 (5062) | 9802 (5079) | 10,132 (5805) | 9908 (5372) | 9969 (5354) |
| Groceries | 6698 (3730) | 7423 (3648) | 7090 (3529) | 7489 (4290) | 7304 (3939) | 7492 (3804) |
| Eaten out | 2322 (2336) | 2408 (2203) | 2668 (2675) | 2452 (2456) | 2604 (2221) | 2417 (2497) |
All values reported as mean and (SD). Costs in 2014 US dollars. Adult caregiver cost expressed as related wages lost. Travel to clinic visits include private car (~75%) or public transportation (~25%).
M = Metformin alone, M+R = Metformin + Rosiglitazone, M+L = Metformin + Lifestyle Program.
Includes travel time to take to exercise venue plus time engaged in exercise with participant.
Includes shoes, equipment, classes and memberships.
Purchased food was categorized as either (a) bought in grocery stores, specialty stores (e.g., bakery, delicatessen), or school lunch or (b) meals eaten out in fast food, carryout, restaurant, vending machine, etc.
Purchases to support exercise activities and food expenses were also examined. Overall, purchases and expenses related to exercise items (equipment, clothing, and participation fees) did not differ across treatment arms or by year of the study. In year 1, annual food expenses were slightly lower in the M treatment group ($9096) compared to M+R ($9843) and M+L ($9802). This was related to lower grocery purchase expenses. In year 2, there was no difference in reported food expenses across the groups. Purchases of food for eating in restaurants did not differ by treatment group or time in the study.
Estimated Average Annual Healthcare System Costs:
Costs were totaled for 399 participants with data for Tables 1, 2, and 3; the sample is reduced because clinical staff data for Table 1 were collected periodically as a one-week diary and only participants with clinic visits scheduled during the week were recorded. Clinical staff costs per visit were multiplied by 4 to represent quarterly visits per year which is standard care. Nonuse of a service or therapy was included as $0.
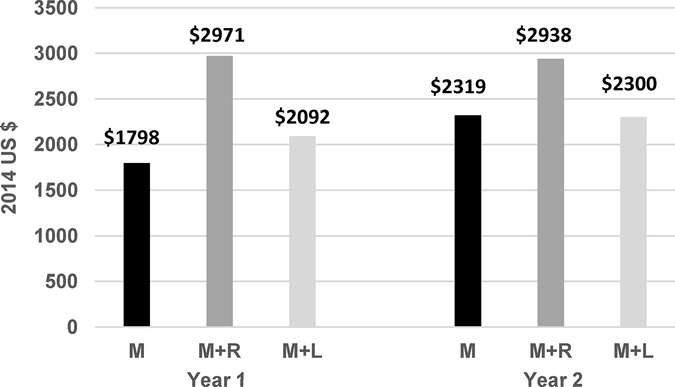
Figure 1 reflects the higher costs of combination medical therapy and increased costs from year 1 to 2 in M and M+L as more participants went on insulin therapy. Treatment failure rates in M started earlier in year 1 than in the other two groups, but the higher costs of administering the lifestyle program high intensity phase outweighed the higher costs of insulin over metformin. In year 2, costs between M and M+L evened out as the lifestyle program entered its less intense maintenance phase and failure rates in M+L increased.
Figure 1. Average Annual Cost of Healthcare Provided by Treatment and Year in Study.
Costs were totaled for 399 participants with data for Tables 1, 2, and 3; the sample is reduced because clinical staff data for Table 1 were collected periodically as a one-week diary and only participants with clinic visits scheduled during the week were recorded. Clinical staff costs per visit were multiplied by 4 to represent standard of care quarterly visits per year. Overall costs were used, i.e., no participant use of a service or medicine was included as $0. M = Metformin alone, M+R = Metformin + Rosiglitazone, M+L = Metformin + Lifestyle Program.
DISCUSSION
The results presented indicate notable patterns in the economic costs related to treatment of youth with T2D. The cost data reported in Tables 1, 2, and 3 can be combined to estimate healthcare costs in the first two years of clinical treatment and management of youth diagnosed with T2D. For purposes of planning and budgeting clinical resources and supplies, the overall costs averaged over users and nonusers may be used; for estimating anticipated case specific expenses, average costs based only on users may be more informative. While rosiglitazone is unlikely to be administered, the costs for the M+R group can be interpreted as representing ‘combination’ drug therapy. Per visit clinical staff costs in Table 1 can be multiplied by 4 to estimate annual costs based on the recommended quarterly visits.
Of note, use (and related costs) of insulin went up in all three treatment groups in year 2 related to the failure to maintain glycemic control on the assigned randomized treatment regimens. Insulin use and costs, however, were lower with combination therapy (M+R) due to lower treatment failure rates in this group.
The time and resources related to adult caregiver support for a child with T2D was also meaningful and varied by treatment regimen. Caregivers supporting a youth in the M+L arm spent 25–40% more time assisting with treatment recommendations than caregivers of a youth receiving mono (M) or combination (M+R) drug therapy regimens in year 1 and 29–38% more time in year 2. Caregiver time and costs were also higher in the combination therapy arm than in the mono therapy arm.
Studies addressing the economic impact of T2D in youth are not common. This report represents one of the first extensive collections of the economic costs related to the treatment and management of youth with T2D. The annual medical costs estimated from TODAY are substantially lower than reported by Shrestha et al. (11), who predicted mean annual total medical expenditure of $9,061 for youth with diabetes ($9,333 if treated with insulin, $5,683 if not) and $1,468 for youth without diabetes. Their data include both type 1 and type 2 diabetes in unknown proportions and their participants were privately insured. The TODAY cohort represents a particularly diverse population, with many participants having disadvantaged financial circumstances and public insurance plans. Annual food expenditures are categorized as low-cost for a family of four according to the USDA (32). The TODAY cohort also represents youth with a recent diagnosis of T2D (average duration 7.8 months at baseline). Likely the costs cited by Shrestha et al. for those treated with insulin are largely for T1D, and many patients would be using insulin pumps, a more expensive insulin delivery device requiring more frequent blood glucose testing. Other possible reasons for the disparity in estimated costs are that the TODAY analysis: (1) used cost for family and general practice physician rather than for pediatric endocrinologist; (2) did not collect costs for laboratory testing; (3) based medication costs largely on use of generics (see on-line appendix Table A2); (4) included only regularly scheduled clinical visits due to lack of appropriate data collection for unscheduled or interim visits.
Strengths related to this report include the prospective data collection of diverse cost factors, including caregiver related costs, the size of the cohort, and the inclusion of participants from across the US (5). In contrast, most economic reports detail one year cost estimates from administrative datasets. Complete data on economic parameters were available from 496 participants in the TODAY cohort over a two year time period. These data allow for the evaluation of trends in expenditures over time and identification of the factors that underlie them.
The report also was able to include details elucidating the large role and contribution that adult caregivers provide; this is a key component of care and management that is often lacking in reports of diseases in youth. The impact of diabetes care on caregivers was most pronounced in those supporting a child engaged in the intensive lifestyle and behavior change program. While all TODAY participants received standard diabetes education during a pre-randomization run-in period, the program administered to youth randomized to the M+L group likely placed larger demands on adult caregivers providing assistance with treatment, as evidenced by higher time and opportunity costs. The intensive lifestyle intervention administered in TODAY was based on a program proven to yield weight loss in adolescents (33,34). The future application of a lifestyle intervention for T2D in youth is likely to involve less demanding behavior change approaches.
Higher caregiver time and opportunity costs were also observed for participants in combination drug therapy compared to mono drug therapy. At face value, increased attention to care seems likely when more than one drug is involved. However, the medications in this study were provided in capsules that looked the same whether there was one (metformin) or two (metformin and rosiglitazone) active agents administered. Thus, the greater caregiver time was not related to ensuring the participants took extra oral medications.
There are limitations to this report. First, the study is based upon a cohort recruited for a clinical trial. The characteristics of those who volunteer for research studies may differ from those of youth with T2D in general. Study eligibility criteria also required the successful completion of a run-in period prior to randomized treatment to demonstrate that the participants could maintain good glycemic control (HbA1c <8% [64 mmol/mol]) and be adherent to metformin monotherapy. The run-in period may have affected reported cost estimates, particularly with regard to the annual costs of care and diabetes and other medications.
Second, in the last year of TODAY, rosiglitazone was reviewed by the Federal Drug Administration for reported links to serious cardiac problems in adults with T2D and its clinical use was curtailed. While TODAY was permitted to continue using rosiglitazone to study completion, the action effectively negated future widespread use of this particular agent in either adults or adolescents. While cost estimates for metformin plus rosiglitazone may not be comparable to other drug combinations, the current findings can provide an important description of issues related to combination therapies. Newer oral agents widely used in adults with T2D may be considered for youth with T2D. This report suggests that their cost profiles in youth should consider impacts on caregivers and tertiary care resources.
Third, the estimates provided are based upon an assumption that the use of diabetes supplies was similar to the recommendations provided. We also assumed that the average wages attributable to adult caregivers and healthcare providers in this assessment mirrored those of the United States population. Estimated costs do not include routine laboratory tests, which followed standard care and were constant across the three treatment arms. Reported costs also represent a short-term experience (2 years) of youth with T2D. Complications related to diabetes in youth were not present in a meaningful manner. Longer term annual medical costs in T2D in youth are not yet clear. Finally, economic data collection was designed and implemented for the purpose of performing a standard cost assessment analysis across the three treatment groups. The findings are intended to help researchers and practitioners start to understand costs and burdens but do not represent complete coverage of the care, management, and treatment of youth-onset T2D.
In summary, the rising prevalence of obesity and T2D in youth brings focus to several healthcare and economic issues. At present, the healthcare and economic patterns of youth with T2D are poorly understood, and because of the demographics of these patients and limited FDA-approved medications, these patients are frequently inadequately treated. This report outlines the annual cost burden associated with recently diagnosed T2D in adolescents. It shows that the largest cost burden at this early stage is related to diabetes medication, lifestyle intervention, and caregiver burden. While TODAY and other clinical trials of T2D in adolescents have yet to identify a treatment regimen that will prove the most cost effective, the need to aggressively pursue better health outcomes through control of HbA1c, blood pressure, lipids, etc. persists. New treatments are emerging including lifestyle education and intervention as part of standard care recommended by the American Diabetes Association and International Society for Pediatric and Adolescent Diabetes. Future research is needed to inform the cost burdens associated with the ongoing healthcare needs of this vulnerable patient population.
Supplementary Material
ACKNOWLEDGMENTS
The TODAY Study Group would like to acknowledge the contribution of our dear colleague Leona Cuttler (Case Western Reserve University, Rainbow Babies and Children’s Hospital, Cleveland OH). As a member of the writing group, Leona had a significant impact on this manuscript before her untimely death.
This work was supported by funding provided by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254.
ABBREVIATIONS
- ACE
angiotensin-converting enzyme
- AHRQ
Agency for Healthcare Research and Quality
- ARB
angiotensin receptor blocker
- CPT
current procedural terminology
- HCUP
Healthcare Cost and Utilization Project
- MEPS
Medical Expenditure Panel Survey
- MRVU
Medicare Relative Value Units
- NP/PA
nurse practitioner/physician assistant
- T2D
type 2 diabetes
- TODAY
Treatments Options for type 2 Diabetes in Adolescents and Youth
Footnotes
Members of the TODAY Study Group are listed in the on-line supplementary appendix.
Conflict of Interest
LML has served as a consultant for Johnson & Johnson, Eli Lilly, Sanofi, NovoNordisk, Mannkind, Merck, Bristol-Myers Squibb, Astra-Zeneca, Roche, Dexcom, Unomedical-ConvaTec, Insulet, and Boehringer Ingelheim. The other authors have no conflicts of interest relevant to this article to disclose.
Contributor Information
Thomas J. Songer, University of Pittsburgh, Department of Epidemiology, Pittsburgh PA USA 15261
Morey W. Haymond, Baylor College of Medicine, Children’s Nutrition Research Center, Department of Pediatrics, Houston TX USA 77030
Judith E. Glazner, Colorado School of Public Health, Aurora CO USA 80045
Georgeanna J. Klingensmith, University of Colorado, Department of Pediatrics, Barbara Davis Center for Childhood Diabetes, Aurora CO USA 80045
Lori M. Laffel, Harvard Medical School, Joslin Diabetes Center, Adolescent and Young Adult Section, Section on Clinical, Behavioral and Outcomes Research, Boston MA USA 02215
Ping Zhang, Centers for Disease Control & Prevention, Division of Diabetes Translation, Atlanta GA USA 30333.
Kathryn Hirst, George Washington University Biostatistics Center, Rockville MD USA 20852.
REFERENCES
- 1.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin dependent diabetes mellitus among adolescents. Journal of Pediatrics 1996; 128:608–615. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Pettitt DJ, Jones KL, Arslanian SA. Type 2 diabetes mellitus in minority children and adolescents: an emerging problem. Endocrinol Metab Clin North Am 1999; 28:709–729. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care 1999; 22:345–354. [DOI] [PubMed] [Google Scholar]
- 4.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000; 136:664–672. [DOI] [PubMed] [Google Scholar]
- 5.TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. NEJM 2012; 366:2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeitler P, Arslanian S, Fu J, et al. Type 2 Diabetes mellitus in youth. Chapter 3 in ISPAD Clinical Practice Consensus Guidelines 2018, Maahs DM and Sperline M (eds). Pediatric Diabetes 2018: 19(S27):28–46. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Children and adolescents. Section 12 in Standards of Medical Care in Diabetes 2018. Diabetes Care 2018; 41(Suppl 1):S126–S136. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018; 41(5):917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Baker Le, Shrestha S, et al. Changes over time in high out-of-pocket healthcare burden in U.S. adults with diabetes, 2001–2011. Diabetes Care 2014; 37(6):1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying AK, Lairson DR, Giardino AP, et al. Predictors of direct costs of diabetes care in pediatric patients with type 1 diabetes. Pediatr Diabetes 2011; 12(3 Pt 1):177–182. [DOI] [PubMed] [Google Scholar]
- 11.Shrestha S, Zhang P, Albright A, Imperatore G. Medical expenditures associated with diabetes among privately insured U.S. youth in 2007. Diabetes Care 2011; 34(5):1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.TODAY Study Group. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatric Diabetes 2007; 8:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Songer T, Glazner J, Coombs L, et al. Examining the economic costs related to lifestyle and pharmacological interventions in youth with type 2 diabetes. Expert Rev Pharmacoeconomics Outcomes Res 2006; 6(3):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. International Journal of Obesity 2010; 34:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. Journal of Clinical Endocrinology & Metabolism 2011; 96(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bureau of Labor Statistics. Consumer Price Index – Medical care in U.S. city average, all urban consumers, not seasonally adjusted. United States Department of Labor, http://data.bls.gov/cgi-bin/surveymost, accessed March 2, 2015.
- 17.Bureau of Labor Statistics. Consumer Price Index – All items in U.S. city average, all urban consumers, not seasonally adjusted. United States Department of Labor, http://data.bls.gov/cgi-bin/surveymost, accessed March 2, 2015.
- 18.Bureau of Labor Statistics. Occupational Employment Statistics, May 2013. United States Department of Labor, https://www.bls.gov/oes/tables.htm, accessed March 2, 2015.
- 19.Bureau of Labor Statistics. National Compensation Survey Employer Costs for Employee Compensation. United States Department of Labor, https://www.bls.gov/news.release/archives/ecec_12102014.pdf, accessed May 12, 2017.
- 20.PDR. Red Book 2010 – Pharmacy’s Fundamental Reference. Montvale, NJ: Thomson Reuters, 2010. [Google Scholar]
- 21.VA Health Economics Resource Center. Determining the cost of pharmaceuticals for a cost-effectiveness analysis, http://www.herc.research.va.gov/include/page.asp?id=pharmaceutical-costs, accessed March 2, 2015.
- 22.Medicare RBRVS. The Physicians’ Guide, AMA, Chicago IL, 2015. [Google Scholar]
- 23.CMS. Physician Fee Schedule, www.cms.gov/apps/physician-fee-schedule, accessed March 2, 2015.
- 24.Mehrotra A, Liu H, Adams JL, et al. Comparing costs and quality of care at retail clinics with that of other medical settings for 3 common illnesses. Annals of Internal Medicine 2009; 151:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AHRQ. MEPSNET Query http://meps.ahrq.gov/mepsweb, accessed March 2, 2015.
- 26.AHRQ. HCUP Kids’ Inpatient Database, https://www.hcup-us.ahrq.gov/db/nation/kid/kiddbdocumentation.jsp, accessed March 2, 2015.
- 27.AHRQ. HCUP National Inpatient Sample, https://healthdata.gov/dataset/hcup-national-nationwide-inpatient-sample-nis-restricted-access-file, accessed March 2, 2015.
- 28.Bureau of Labor Statistics. Occupation Profiles, May 2013. United States Department of Labor, https://www.bls.gov/oes/tables.htm, accessed March 2, 2015.
- 29.Grosse SD, Krueger KV, Mvundura M. Economic productivity by age and sex 2007 estimates for the United States. Med Care 2009; 47:S94–S103. [DOI] [PubMed] [Google Scholar]
- 30.Internal Revenue Service. Standard mileage rates, http://www.irs.gov/Tax-Professionals/Standard-Mileage-Rates, accessed March 2, 2015.
- 31.Thompson SG, Barber JA. How should cost data in pragmatic randomized trials be analysed? BMJ 2000; 320:1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.USDA. Official USDA Food Plans: Cost of Food at Home at Four Levels, U.S. Average, November 2014, https://www.cnpp.usda.gov/sites/default/files/CostofFoodNov2014.pdf.
- 33.Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics 1998; 101(3):554–570. [PubMed] [Google Scholar]
- 34.Epstein L, Paluch R, Roemmich J, Beecher M. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol 2007; 26:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.