Abstract
Objective.
To inform development of a core domain set for outcome measures for clinical trials in polymyalgia rheumatica (PMR), we conducted patient consultations, a systematic review, a Delphi study, and 2 qualitative studies.
Methods.
Domains identified by 70% or more of physicians and/or patients in the Delphi study were selected. The conceptual framework derived from the 2 qualitative research studies helped inform the meaning of each domain and its relationship to the others. The draft core domain set was refined by further discussion with patients and physicians who had participated in the Delphi study. At the Outcome Measures in Rheumatology (OMERACT) 2016, the domains were discussed and prioritized by 8 breakout groups. Formal voting took place at the end of the workshop and in the final plenary.
Results.
Ninety-three percent of voters in the final plenary agreed that the inner core of domains considered mandatory for clinical trials of PMR should consist the following: laboratory markers of systemic inflammation, pain, stiffness, and physical function. Patient’s global and fatigue were considered important but not mandatory (outer core). The research agenda included psychological impact, weakness, physical activity, participation, sleep, imaging, and health-related quality of life.
Conclusion.
This core domain set was considered sufficiently well-defined that the next step will be to apply the OMERACT Filter 2.0 Instrument Selection Algorithm to select candidate instruments for a subsequent “deeper dive” into the data. This will allow instruments to be mapped onto each of our core domains to derive a core outcome set for PMR.
Key Indexing Terms: POLYMYALGIA RHEUMATICA, OUTCOMES, OMERACT
Polymyalgia rheumatica (PMR) is an inflammatory disease of older people, causing pain and stiffness of the shoulders and hip girdles1. The prevalence of PMR is about 1% in people over 50 years in the United States2 and the United Kingdom3. Many patients with PMR are managed by general practitioners/family physicians rather than rheumatologists4,5. The mainstay of treatment is longterm therapy with glucocorticoids. This treatment approach has the potential for toxicity, depending on glucocorticoid dose and patient-specific factors such as age6,7. The most recent PMR treatment guidelines conditionally recommend early addition of methotrexate to glucocorticoids, especially if there are risk factors for relapse, for prolonged therapy, or for glucocorticoid-related adverse effects8. A stronger recommendation could not be made because the published randomized trials were small, with partly contradictory results. No high-quality evidence was identified evaluating any other potential glucocorticoid· sparing agent8. A systematic review of domains and instruments in 35 PMR trials and longitudinal observational studies, conducted by the Outcome Measures in Rheumatology (OMERACT) PMR Working Group, found inconsistency and poor clarity of outcome measures recorded for PMR9. The poor evidence base for management of PMR urgently requires improvement. Our objective is to produce guidance to researchers on a core outcome set for PMR: the minimal common set of outcome measurement instruments that should always be included in clinical trials of PMR, whether conducted in the community or specialist setting. Prior to recommending measurement instruments, it is necessary to define a core domain set of what it is that must be measured.
Here we report on the process that was used to generate a core domain set for clinical trials of PMR based on a combination of personal engagement, evidence synthesis, qualitative research, and a Delphi study. To our knowledge, this is the first core domain set developed for clinical trials of PMR; it has had strong patient involvement throughout. This core domain set will inform selection and validation of instruments to be used in clinical trials of PMR. It will also be relevant to design of observational studies and studies to develop a PMR-specific patient-reported outcome measure. Our report represents the culmination of a process reported in 2 prior OMERACT Special Interest Group reports10,11, work leading up to and during the 2016 OMERACT Workshop on PMR, and original primary research already published in full elsewhere9,12,13. The new matter in this report includes a description of the methods and results of the Delphi survey and the process that was used to bring together multiple different sources of information (patient consultations, systematic literature review, 1 Delphi survey, 2 qualitative studies, further patient and clinician consultation to refine the draft core domain set, and a workshop at OMERACT 2016) to arrive at a core domain set for PMR that was endorsed by 93% of voters in the final conference plenary, as well as highlighting areas that required further definition, such as psychological impact.
Scoping the Problem
We intend our core outcome set to apply to interventional research studies conducted in any setting, with a study duration of at least 3 months and typically 1 year14. The selected domains would also be relevant to the design of observational studies, which could be much larger or of longer duration15. We began by consulting those involved on all outcomes they considered important for patients diagnosed with PMR; in later phases, we asked them to focus on clinical trials to give the context necessary for the prioritization of domains for a parsimonious core domain set.
Patient Involvement
Clinical management decisions relating to patients diagnosed with PMR are highly dependent on the patient’s symptoms; acute-phase laboratory markers are used as supportive evidence1. Defining what these symptoms are is therefore essential. Some of the patient research partners, including both co-authors of our current report, were involved over the life of this project and were deeply involved in patient support groups (telephone and/or Internet forums). Patient support groups were also helpful in identifying participants for our Delphi study.
Patient Consultations
To inform the scope of the problem, we started with a patient-driven consultation exercise11. A convenience sample of 104 English-speaking patients with PMR under the care of rheumatologists from the United Kingdom and elsewhere in Europe were included and a modified nominal group technique was used, involving group discussions about 3 prespecified topics (symptoms, diagnosis, and treatment), followed by sorting of cards to identify each patient’s “top ten” items for each topic. We reported these within the International Classification of Functioning, Disability and Health framework of impairments, disability, and participation11.
Comparing Outcome of Patient Consultations with Systematic Review Findings
Using the OMERACT Filter 2.0 framework16 we identified that outcomes reported in trials and observational studies of patients with PMR9 did not always map well onto the messages emerging from our patient consultations (Table 1). For example, patients preferred “stiffness” to “morning stiffness” and also considered fatigue to be important. Patients preferred to describe their experience of PMR in terms of its effect on activities such as getting out of bed, turning over in bed, getting up from the sofa or toilet, driving, picking items up from the floor, opening doors, walking, and dressing. They found the symptoms themselves hard to describe. The psychological impact of their condition was also mentioned. We noted that research studies had no standard definitions of key PMR symptoms; for example, in the literature it was frequently unclear exactly how patients had been asked about their pain severity, where that pain was, and what period of time was being asked about9. Similarly, the precise definition and meaning of morning stiffness in PMR appeared unclear in many published studies9. There was also no standard method used for reporting outcomes related to the burden of glucocorticoid therapy. Even the main daily dose and cumulative dose of glucocorticoids were not always well reported.
Table 1.
Comparison of domains reported as being important to patients with PMR. Outcomes measured in 35 clinical trials and longitudinal observational studies9, grouped according to the OMERACT Filter 2.0 Framework. Adapted from Duarte, et al. J Rheumatol 2015;42:2503–11; with permission9.
| Core Area | Domain | Instrument Used in Published Studies | No. Studies of PMR Reporting Data on This Domain |
|---|---|---|---|
| Pathophysiological manifestations | Laboratory markers of systemic inflammation | ESR, CRP, IL-6, fibrinogen | 30 |
| Elevation of upper limbs | Part of composite disease activity score | 5 | |
| Ability to carry out physical function tests | Grip strength, chair stand, 10-m walk | 1 | |
| Ultrasonography | Synovitis, bursitis in shoulder and hip regions | 4 | |
| Physician’s global | VAS,NRS | 14 | |
| Life impact | Pain | VAS,NRS | 17 |
| Morning stiffness | Duration (min), grade, severity (VAS) | 26 | |
| Fatigue | VAS | 1 | |
| Sleep disturbance | Not reported | 0 | |
| Patient’s global | VAS.NRS | 9 | |
| Physical functioning (in daily living) | HAQ | 9 | |
| Anxiety, depression | Part of SF-36 | 2 | |
| Health-related quality of life | SF-36, VAS | 3 | |
| Death | Mortality | Death registries, patient files | 1 |
| Resource use | Not reported | Not reported | 0 |
| Adverse events | Adverse effects of medication | Standard methods for clinical trials | 14 |
Bold face indicates highlighted in initial patient consultations11. PMR: polymyalgia rheumatic; OMERACT: Outcome Measures in Rheumatology; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; IL-6: interleukin 6; VAS, visual analog scale; NRS: numerical rating scale; HAQ: Health Assessment Questionnaire; SF-36: Medical Outcomes Study Short Form-36.
Analysis of composite outcomes used in studies of PMR17 showed that many included domains from both Patho-physiological Manifestations (acute-phase markers and/or ability to elevate upper limbs) and Life Impact (symptom or patient-reported component). Although none of these composite outcomes has yet been completely validated according to the OMERACT Filter, they are informative regarding what aspects of PMR are considered important by experts in PMR.
Delphi Study
To understand the differing perspectives of patients and physicians in prioritizing outcomes, we carried out a 3-round Delphi study10. We were advised by the National Research Ethics Service that ethical approval was not required. Although the disease (PMR) and its Life Impact may well be similar across countries, there are differences in the language used to describe this by patients. Whereas international English-speaking physicians are accustomed to using a common dialect (medical English) for accessing research studies and educational material, this is not necessarily the case for patients. To avoid potential misunderstanding arising from international differences in English vocabulary and usage, for our Delphi study we chose to recruit English-speaking patients from the United Kingdom.
The Delphi study started with 2 groups: patients (from UK patient organizations, self-identifying as diagnosed with PMR) and clinicians. Fifty-five patients with PMR took part. Of these, 46 completed round 2 and 34 completed round 3. Eighty-five clinicians with an interest in PMR were identified from PubMed searches and attendance at relevant sessions at international meetings (American College of Rheumatology, European League Against Rheumatism). Sixty clinicians replied to round 1, 55 to round 2, and 53 to round 3. Among the 60 clinicians in round 1, 21 were from the United Kingdom, 28 from elsewhere in Europe, 6 from North America, and 5 from Australasia. Self-reported expertise, other than clinical rheumatology and an interest in PMR, was clinical trials research (26), outcomes research (19), epidemiology (11), qualitative research (5), general practice (5), and the allied health professions (2). Potential domains were grouped using the framework of Filter 2.0 (including “Resource Use,” but omitting “Death” from the list, because the latter is always mandatory in Filter 2.0) and informed by the prior patient consultations and systematic review findings.
To avoid influence of the patients on the clinicians or vice versa, rounds 1 and 2 were conducted separately. However, to identify areas of consensus and disagreement, we started with the same list of domains for everyone, using plain language rather than rheumatology jargon wherever possible. In round 1, respondents selected their “top ten” domains and had the option of adding any further domains to generate an expanded list. In round 2, each group was presented with the domains selected by > 70% of respondents and were asked which other domains from the expanded list they considered essential for a core domain set for clinical trials of PMR. Those new domains selected by > 70% of respondents in round 2 were added to that group’s list. The 70% cutoff, while arbitrary, is conventional for Delphi studies as well as being the usual level of consensus for OMERACT voting. Because of the variety of potential domains that seemed more relevant to glucocorticoid exposure, a separate item for glucocorticoid-related adverse effect was added in round 2. Results of rounds 1 and 2 are given in Table 2. In round 3, the domains finally selected by both groups were presented and opinions sought on the combined domain set. Free-text feedback at each stage allowed participants to give their reasoning for including or not including particular domains. A total of 91% of respondents (85% clinicians, 97% patients) agreed with the draft core domain set, with the major divergence of opinion appearing to be in relation to different perceptions of the meaning of the words muscle weakness in medical English versus everyday English. It also became clear that morning stiffness [duration], a technical diagnostic term in rheumatology, is a different domain from “stiffness” as conceptualized by patients, who said that stiffness severity (rather than duration) was of key importance.
Table 2.
Results from rounds 1 and 2 of the Delphi study of core domains for PMR. Numbers given are percentages of respondents selecting each outcome. Domains with > 70% agreement are highlighted in bold face; for each group (clinicians or patients), only domains with 20%–69% agreement in round 1 went forward to round 2. Bold face denotes that with > 70% agreement from either group in round 1 or round 2, the domain went forward into the combined round 3.
| Domain | Round 1 Clinicians, n = 60 | Round 1 Patients, n = 55 | Round 2 Clinicians, n = 55 | Round 2 Patients, n = 46 |
|---|---|---|---|---|
| Pain/ache | 90 | 78 | — | — |
| Stiffness severity | 56 | 56 | 53 | 74 |
| Morning stiffness duration | 68 | 36 | 85 | 43 |
| Muscle weakness | 12 | 53 | — | 80 |
| Fatigue/tiredness | 49 | 73 | 65 | — |
| Sleep disturbance | 10 | 35 | — | 57 |
| Mood problems, low or “high” | 3 | 20 | — | 37 |
| Anxiety | 3 | 33 | — | 30 |
| Weight loss/gain | 25 | 33 | 27 | 48 |
| Appetite loss/gain | 3 | 11 | — | — |
| Balance problems | 2 | 7 | — | — |
| Fevers/shivers/sweats/flu-like symptoms | 12 | 15 | — | — |
| Ability to do everyday activities | 51 | 55 | 65 | 74 |
| Lack of mobility | 25 | 35 | 7 | 59 |
| Dependence on stick, wheelchair, etc. | 0 | 2 | — | — |
| Dependence on other people | 5 | 9 | — | — |
| Ability to carry out usual roles (work, caring for others, etc.) | 20 | 35 | 16 | 37 |
| Health-related quality of life | 50 | 24 | 76 | 39 |
| Overall quality of life | 27 | 51 | 13 | 72 |
| Change in appearance of face or body | 8 | 22 | — | 41 |
| Fluid retention/ankle swelling | 2 | 9 | — | — |
| Bruising, poor healing, or other skin change | 5 | 24 | — | 50 |
| Doctor’s assessment of activity/severity of PMR | 59 | 60 | 71 | 83 |
| Patient’s assessment of activity/severity of PMR | 76 | 42 | — | 76 |
| Blood tests | 86 | 40 | — | 70 |
| Abnormalities identified by physical examination by a doctor | 22 | 7 | 20 | — |
| Abnormalities identified by imaging tests | 36 | 20 | 29 | 33 |
| Bone fragility | 27 | 13 | 29 | — |
| New or worsening diabetes mellitus | 14 | 5 | — | — |
| High blood pressure | 12 | 11 | — | — |
| Cost of treatments used in the study | 31 | 18 | 13 | — |
| Overall costs to the healthcare provider | 36 | 24 | 22 | 11 |
| Overall costs to society | 56 | 40 | 55 | 20 |
| Any glucocorticoid-related adverse effect in judgment of doctor | — | — | 46 | 63 |
| Any glucocorticoid-related adverse effect in judgment of patient | — | — | 23 | 80 |
“ — ” means the question was not asked in that round. PMR: polymyalgia rheumatica.
Qualitative Research on Core PMR Symptoms of Pain and Stiffness
A qualitative study13 analyzed in more depth what stiffness means to patients and how it relates to pain. Fifty patients with a clear, rheumatologist-confirmed diagnosis of PMR took part in 8 focus groups; this convenience sample was recruited from 3 UK rheumatology clinics. Pain and stiffness usually represented related but different symptoms. Pain (“ache, hurt”) was an unpleasant experience, not necessarily related to movement. Stiffness (the experience of being prevented from movement) had profound consequences for daily functioning. Many patients suggested that measuring physical function would be the best way to measure stiffness itself. Fatigue was seen as separate from either pain or stiffness, but affecting the broader experience of PMR.
Qualitative Research on the Broader Patient Experience in PMR
A second qualitative study analyzed the broader experience of PMR for patients treated in the community12. The analysis of the study proceeded in parallel with the activities of the PMR Working Group and discussions before its publication informed the group’s thinking. At OMERACT 2016, the methodology and findings were presented. Based on the conceptual framework derived from the qualitative data, we added the domain “Psychological Impact,” which had emerged as a surprisingly strong theme from the interviews.
Domain Prioritization
OMERACT presents domains using an “onion” diagram of 3 nested circles, with the domains in the innermost circle (“Inner Core”) being mandatory for every clinical trial; the middle circle is labeled “Important” and the outer circle “Research Agenda”18. The Inner Core should contain at least 1 domain chosen from each of the core areas including Pathophysiological Manifestations and Life Impact. It was recognized that the list of candidate domains derived from the Delphi was likely too long to be suitable for an Inner Core. Therefore, in the run-up to OMERACT 2016, informal e-mail engagement was carried out with patients and physicians who had participated in the Delphi study. A longlist of domains that might be eligible for the Inner Core was proposed, based on all of the evidence presented above, and feedback was invited. This resulted in removal of the domain of Physician Global because several physicians said it is a composite construct, principally consisting of information from laboratory markers of inflammation and the patient’s global (both of which were already on the longlist of domains). There were also questions about whether the underlying construct of Physician Global would genuinely be a scalar quantity or if it was better conceptualized as a binary decision to escalate or reduce glucocorticoid dose, closer to the concept of relapse/remission. Because the only remaining “Pathophysiological Manifestations” domain was Systemic Inflammation (Laboratory Blood Tests), the breakout discussions at the OMERACT Workshop focused on the Life Impact aspect of PMR.
Breakout Group Discussions
To encourage the discussion at breakout groups to draw on authentic patient experience, quotes from the qualitative interview were printed onto cards; each individual participant in the breakout group received a randomly chosen card. Breakout group facilitators then asked their groups to arrange the domains by priority, based on the results of the research described and cited in the preconference reading, the work presented in the plenary, and the quotes they had on their individual cards.
Synthesis of Advice from Breakout Groups
Consistent with the conceptual model that emerged from both qualitative studies, breakout groups gave the highest priority to pain/ache, stiffness, and physical function in regard to Life Impact (Table 3).
Table 3.
Votes for inner core at OMERACT PMR workshop. Percentage votes are presented to the nearest whole number.
| Domain | No. Breakout Groups Selecting Domain among “Top 3” Life Impact Domains | Voted Yes | Voted No | Voted Insufficient Evidence or Information |
|---|---|---|---|---|
| Systemic inflammation, laboratory blood tests | Not asked | 123/142 (87%) | 4/142 (3%) | 15/142 (11%) |
| Physical function | 8/8 | 137/145 (94%) | 3/145 (2%) | 5/145 (3%) |
| Patient global | 1/8 | 66/144 (46%) | 41/144 (28%) | 37/144 (26%) |
| Pain/ache | 8/8 | 133/145 (92%) | 4/145 (3%) | 8/145 (6%) |
| Stiffness | 7/8 | 131/145 (90%) | 3/145 (2%) | 11/145 (8%) |
| Fatigue | 0/8 | 74/145 (51%) | 35/145 (24%) | 36/145 (25%) |
| Psychological impact | 0/8 | 50/146 (34%) | 57/146 (39%) | 39/146 (27%) |
OMERACT: Outcome Measures in Rheumatology; PMR: polymyalgia rheumatica.
Feedback from several breakout groups suggested that including Patient’s Global in addition to the “top three” Life Impact domains could introduce redundancy, because the qualitative data suggested such a strong overlap with physical function. Given the strong drive toward parsimony for this patient population, and given the lack of quantitative evidence to confirm or refute this suggestion, it was decided to provisionally rank this as important rather than core.
Psychological Impact was considered important, but to require further clarification of its meaning before inclusion in the Inner Core. The 2 candidate “psychological” domains that were drawn from the literature and entered into the Delphi (Mood problems — low or “high,” Anxiety) reached the 70% threshold in the patient arm of the Delphi study. However, the qualitative study data suggested that Psychological Impact goes beyond the clinical constructs of simple anxiety or mood disturbance and in fact describes complex, evolving, and pervasive effects on patients’ psychological state (for example, pre-diagnosis fears, relief at diagnosis followed by an ongoing sense of loss12, and “PMR always on one’s mind”13) that are not necessarily well described by the clinical constructs of anxiety or depression or indeed well understood by clinicians. This was identified as a clear priority for further patient-centered research, perhaps with a view to developing a PMR-specific patient-reported outcome measure encompassing the psychological impact relating to this disease.
Breakout groups also advised adding to the research agenda the following domains: Participation, Weakness, Glucocorticoid Exposure, Physical Activity, Sleep, Imaging, and Health-related Quality of Life. Some attendees also pointed out that some caution was required in the interpretation of the qualitative research because of the limited geographical area (United Kingdom) from which the participants were drawn.
The Workshop concluded with a formal vote on whether each of our longlist domains should be included in the inner core for clinical trials (Table 3). Based on these votes, which was also in line with the results of our qualitative studies, we entered the 3 Life Impact domains plus Systemic Inflammation (Laboratory Blood Tests) into the proposed Inner Core.
Summary
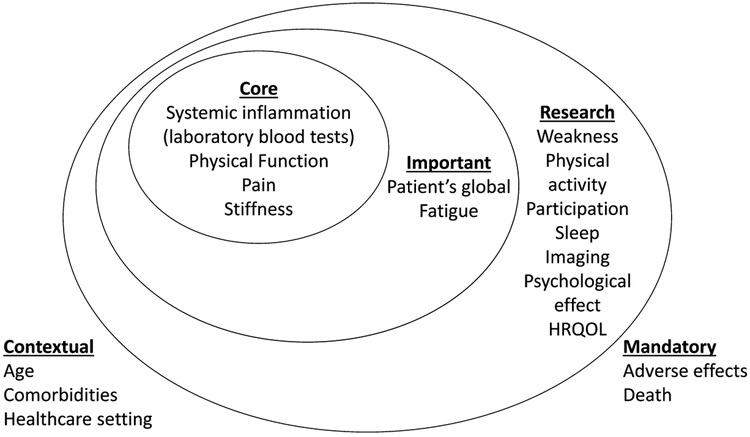
Based on all the quantitative and qualitative feedback received during the whole process, a diagram (Figure 1) was presented at the final plenary session of the conference. Ninety-three percent of voters agreed with the final proposed Inner Core Domain Set (laboratory markers of systemic inflammation, pain, stiffness, physical function).
Figure 1.
Proposed core domain set for PMR clinical trials. This “onion” diagram uses nested circles with the innermost circle denoting the Inner Core (mandatory to measure in all clinical trials of PMR), the middle circle denoting Important Outcomes (strongly recommended to measure in PMR), and the outer circle denoting the Research Agenda (domains that require further investigation in PMR). Mandatory domains (bottom right) are those that should be reported by default in all clinical trials of any condition. The proposed contextual factors (bottom left) are suggestions we received regarding possible contextual factors and represent hypothesized factors only. PMR: polymyalgia rheumatica; HRQOL: health-related quality of life.
Future Work
Although there was substantial agreement on the inner core domains, the limitations of the voting procedure should be acknowledged; the system of 1 vote per attendee meant that clinicians’ votes outnumbered patients’ votes. The process also identified a substantial list of potential outcomes requiring further research in PMR. It will also be important to conduct further work with patients outside the United Kingdom, including non-English speakers, to assess generalizability of the concepts presented here. The OMERACT Handbook describes the next step, which will be to apply the OMERACT Filter 2.0 Instrument Selection Algorithm (the “eyeball test”), a systematic screening process to select candidate instruments for a subsequent “deeper dive” into the data to finally determine whether each selected instrument should be included in the core outcome set.
ACKNOWLEDGMENT
We thank all the patients who so generously contributed in so many ways to all stages of this project. We also thank all the members of the rheumatology research community who have provided important feedback during the latter stages of the development of this core domain set. Those not already listed as co-authors include (but are not limited to): Drs. Toby Helliwell, Elisabeth Brouwer, Joanna Robson, Christina Duftner, Mar Pujades-Rodriguez, Pereira da Silva, Maria Cid, Lyn March, and Maarten Boers. Dr. John Kirwan provided invaluable leadership, expertise, and guidance, including in the design of the Delphi.
Supported by the NIHR Clinician Scientist Fellowship to Sarah Mackie; unrestricted grant from Horizon Pharmaceuticals. Rachel Black is the recipient of an Australian Rheumatology Association Outcome Measures in Rheumatology (OMERACT) Fellowship 2016. Financial support for patient travel was received from the OMERACT charity DINORA, which received an unrestricted grant from Horizon Pharmaceuticals.
REFERENCES
- 1.Mackie SL, Mallen CD. Polymyalgia rheumatica. BMJ 2013;347:f6937. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. ; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayward RA, Rathod T, Muller S, Hider SL, Roddy E, Mallen CD. Association of polymyalgia rheumatica with socioeconomic status in primary care: a cross-sectional observational study. Arthritis Care Res 2014;66:956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barraclough K, Liddell WG, du Toit J, Foy C, Dasgupta B, Thomas M, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract 2008;25:328–33. [DOI] [PubMed] [Google Scholar]
- 5.Kremers HM, Reinalda MS, Crowson CS, Zinsmeister AR, Hunder GG, Gabriel SE. Use of physician services in a population-based cohort of patients with polymyalgia rheumatica over the course of their disease. Arthritis Rheum 2005;53:395–403. [DOI] [PubMed] [Google Scholar]
- 6.Harris E, Tiganescu A, Tubeuf S, Mackie SL. The prediction and monitoring of toxicity associated with long-term systemic glucocorticoid therapy. Curr Rheumatol Rep 2015;17:513. [DOI] [PubMed] [Google Scholar]
- 7.Strehl C, Bijlsma JW, de Wit M, Boers M, Caeyers N, Cutolo M, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis 2016;75:952–7. [DOI] [PubMed] [Google Scholar]
- 8.Dejaco C, Singh YP, Perel P, Hutchings A, Camellino D, Mackie S, et al. ; European League Against Rheumatism; American College of Rheumatology. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015;74:1799–807. [DOI] [PubMed] [Google Scholar]
- 9.Duarte C, Ferreira RJ, Mackie SL, Kirwan JR, Pereira da Silva JA; OMERACT Polymyalgia Rheumatica Special Interest Group. Outcome measures in polymyalgia rheumatica. A systematic review. J Rheumatol 2015;42:2503–11. [DOI] [PubMed] [Google Scholar]
- 10.Helliwell T, Brouwer E, Pease CT, Hughes R, Hill CL, Neill LM, et al. Development of a provisional core domain set for polymyalgia rheumatica: report from the OMERACT 12 Polymyalgia Rheumatica Working Group. J Rheumatol 2016;43:182–6. [DOI] [PubMed] [Google Scholar]
- 11.Mackie SL, Arat S, da Silva J, Duarte C, Halliday S, Hughes R, et al. Polymyalgia rheumatica (PMR) special interest group at OMERACT 11: outcomes of importance for patients with PMR. J Rheumatol 2014;41:819–23. [DOI] [PubMed] [Google Scholar]
- 12.Twohig H, Mitchell C, Mallen C, Adebajo A, Mathers N. “I suddenly felt I’d aged”: a qualitative study of patient experiences of polymyalgia rheumatica (PMR). Patient Educ Couns 2015; 98:645–50. [DOI] [PubMed] [Google Scholar]
- 13.Mackie SL, Hughes R, Walsh M, Day J, Newton M, Pease C, et al. “An impediment to living life”: why and how should we measure stiffness in polymyalgia rheumatica? PloS One 2015;10:e0126758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchings A, Hollywood J, Lamping DL, Pease CT, Chakravarty K, Silverman B, et al. Clinical outcomes, quality of life, and diagnostic uncertainty in the first year of polymyalgia rheumatica. Arthritis Rheum 2007;57:803–9. [DOI] [PubMed] [Google Scholar]
- 15.Mackie SL, Hensor EM, Haugeberg G, Bhakta B, Pease CT. Can the prognosis of polymyalgia rheumatica be predicted at disease onset? Results from a 5-year prospective study. Rheumatology 2010;49:716–22. [DOI] [PubMed] [Google Scholar]
- 16.Boers M, Kirwan JR, Gossec L, Conaghan PG, D’Agostino MA, Bingham CO 3rd, et al. How to choose core outcome measurement sets for clinical trials: OMERACT 11 approves Filter 2.0. J Rheumatol 2014;41:1025–30. [DOI] [PubMed] [Google Scholar]
- 17.Leeb BF, Bird HA. A disease activity score for polymyalgia rheumatica. Ann Rheum Dis 2004;63:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boers M, Kirwan JR, Tugwell P, Beaton D, Bingham CO III, Conaghan PG, et al. The OMERACT Handbook. [Internet. Accessed May 17, 2017.] Available from: www.omeract.org/pdf/OMERACT_Handbook.pdf