Abstract
Since 1997, the highly pathogenic influenza H5N1 virus has spread from Hong Kong. According to the WHO bulletin report, the H5N1 virus is a zoonotic disease threat that has infected more than 850 humans, causing over 450 deaths. In addition, an outbreak of another new and highly pathogenic influenza virus (H7N9) occurred in 2013 in China. These highly pathogenic influenza viruses could potentially cause a worldwide pandemic. it is crucial to develop a rapid production platform to meet this surge demand against any possible influenza pandemic. A potential solution for this problem is the use of cell-based bioreactors for rapid vaccine production. These novel bioreactors, used for cell-based vaccine production, possess various advantages. For example, they enable a short production time, allow for the handling highly pathogenic influenza in closed environments, and can be easily scaled up. In this study, two novel disposable cell-based bioreactors, BelloCell and TideCell, were used to produce H5N1 clade II and H7N9 candidate vaccine viruses (CVVs). Madin-Darby canine kidney (MDCK) cells were used for the production of these influenza CVVs. A novel bench-scale bioreactor named BelloCell bioreactor was used in the study. All culturing conditions were tested and scaled to 10 L industrial-scale bioreactor known as TideCell002. The performances of between BelloCell and TideCell were similar in cell growth, the average MDCK cell doubling time was slightly decreased to 25 hours. The systems yielded approximately 39.2 and 18.0 μg/ml of HA protein with the 10-liter TideCell002 from the H5N1 clade II and H7N9 CVVs, respectively. The results of this study not only highlight the overall effectiveness of these bioreactors but also illustrate the potential of maintaining the same outcome when scaled up to industrial production, which has many implications for faster vaccine production. Although additional studies are required for process optimization, the results of this study are promising and show that oscillating bioreactors may be a suitable platform for pandemic influenza virus production.
Introduction
Since the avian influenza H5N1 outbreak of 2003, the H5N1 virus has caused over 450 deaths [1]. In addition, the avian influenza H7N9 virus has caused outbreak in China. The flu vaccine for unmatched strains of the virus is not expected to be cross-protective confirmed by data relating to the H5N1 pandemic strain. Many animal and clinical-trial studies have shown that the 2004 H5N1 influenza vaccine virus strain, which belongs to the first H5N1 genotype (clade I), does not provide cross-protection for the most recently isolated H5N1 virus from the Chinese mainland and Hong Kong, which belongs to the second H5N1 virus genotype (clade II) [2, 3]. To prevent influenza outbreaks from spreading, the most effective public health measure is vaccination [4]. Currently, influenza vaccine production heavily relies on traditional embryonated egg technology [5]. This process requires long and logistic planning that would severely delay the vaccine production to meet the surge demand in the event of a pandemic. Cell-based technology is considered as an alternative platform for influenza vaccine production, and it has piqued the interest of many in recent years [6, 7]. The common cell lines used for cell-based influenza vaccine production are MDCK (derived from Madin-Darby canine kidney) and Vero (derived from African green monkey kidney) cells, which are anchorage-dependent cells [8, 9]. For influenza vaccine production, it is crucial to choose a system, which is simple and robust, can produce high viral titers from a wide variety of influenza virus strains [10]. A number of cell culture systems were already used for their large-scale vaccine production potential, such as roller bottles and cell factories. These systems were originally designed for adherent cells; however, large-scale production with these systems is challenge to increase surface to volume ratio for cell proliferation. A solution to overcome this problem would be to use a microcarrier cell-lift bioreactor (New Brunswick Scientific, USA), by providing good mixing of the oxygen supply and a high concentration of microcarrier for more surface area. Other traditional bioreactors such as hollow-fiber bioreactors [11], the Celligen Plus bioreactor, [12] or bioreactors supplemented with microcarriers were already used for large-scale production [13]. However, all of these bioreactors involve complicated operations and are labor intensive. Since single-use (disposable) bioreactors were introduced, the traditional stainless-steel bioreactors slowly became obsolete in small-scale biotechnology and contract manufacturing companies [14]. Single-use bioreactors offer lower capital cost, easier operations, faster turn-around times, and fewer requirements for cleaning validation. Two novel bioreactors, BelloCell (bench-top scale) and TideCell002 (industrial scale), have recently been developed by Cesco Bioengineering, Taiwan. The BelloCell bioreactors have been successfully used to cultivate mammalian cells for the production of HDV-like particles [15], Japanese encephalitis virus [16], and insect cells for baculovirus [17]. In these studies, the bioreactors have consistently shown various beneficial characteristics: (1) Improved efficiency because they are pre-sterile and ready-to-use, (2) low shear stress because they move the liquid gently without the generation of gas bubbles, (3) large surface area to achieve a high-density growth of cells, and (4) the ability to collect whole cells or cell components [15, 18, 19]. At present, the use of these single-use bioreactors for influenza vaccine production has not been reported. In this study, we assessed the feasibility of commercially available disposable bioreactor systems for vaccine development. We used the H5N1 clade II and H7N9 candidate vaccine viruses cultured in both MDCK and Vero cells. The results of this study should illustrate the effectiveness of the disposable bioreactors and highlight the potential of their scalability for industrial use.
Materials and methods
Cells lines, virus strains and medium
Vero cells (CCL-81), a continuous African Green Monkey kidney cell line, and MDCK cells (ATCC CCL-34), a continuous Madin Darby Canine Kidney cell line, were purchased from the Food Industry Research and Development Institute, Hsinchu, Taiwan. For the Vero and MDCK cells growth and virus replication stages, VP-SFM and OptiPRO serum-free medium (Invitrogen, California, USA) supplemented with 4 mM L-glutamine was used, respectively. The MDCK cells were used at passage number between 63 and 69. The Vero cells were used for all experiments at passage number of 131 to 150. Two H5N1 candidate vaccine viruses, an RG6 strain derived from A/Anhui/01/2005(clade 2.3.4) and an RG30 strain derived from A/Hubei/1/2010 (clade 2.3.2.1) were obtained from the US CDC and were re-adapted for use in MDCK cells. The H7N9 strain (RG268) derived from A/Anhui/01/2013 was obtained from the NIBSC, UK and adapted for use in MDCK cells. The re-adapted strains were obtained by choosing better replication colonies in the plaque assay. The antigenicity of the chosen colonies was confirmed by the HI assay. Generally, the process needs to be repeated 3 to 10 times before obtaining the high growth CVVs in the MDCK cell culture. In this study, H5N1 adapted to MDCK cell for passage 10 times, and H7N9 adapted to MDCK cell for passage 5 times.
Spinner flask cultures
The Cytodex 1 microcarriers (GE Healthcare, USA) used for cell immobilization were made of cross-linked dextran matrix with a specific surface area of approximately 4400 cm2/g. The culturing method was described in the previous study [10]. The microcarriers were hydrated, autoclaved and preconditioned according to the manufacturer’s instructions before use. The cultures were performed in 1-L spinner flasks with a working volume of 400 mL, and each spinner flask contained 5 g/L of Cytodex 1 microcarriers. Each flask was initially inoculated with approximately 2 x 105 cells/mL. During cell growth, the pH, glucose, and glutamine concentrations were monitored daily, and the medium exchange rate was adjusted daily to maintain the glucose concentration at approximately 1 g/L. In addition, samples were taken daily to determine cell density. The concentration of cells for viral infection was approximately 2 x 106 cell/mL on day 4 after seeding. The cells were spun down, washed three times with 100 mL of PBS, and then approximately 75% of the culture medium (300 mL) was exchanged with fresh medium. In addition, the cultures were supplemented with 2 μg/mL of TPCK-treated trypsin (Invitrogen, USA) for viral growth. The cells were infected with candidate influenza H5N1 vaccine viruses with a multiplicity of infection (MOI) of 10−4. During the viral production, the culturing conditions were maintained at pH 7.2 and 34°C in a incubator with 5% CO2. All of the virus-containing broth, without microcarriers, was harvested when the viral titer reached the peaked on 3 days post-infection (dpi). Each batch run was repeated twice.
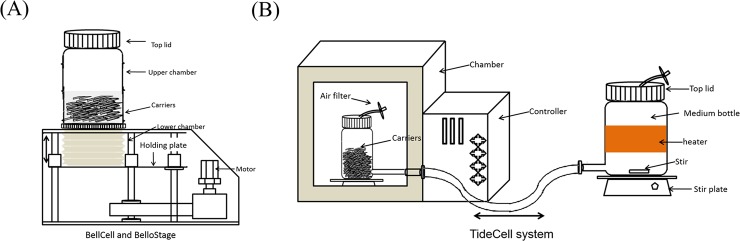
Bench-top scale BelloCell bioreactor run
The BelloCell bioreactor system has been used for the development of Vero cell-based Japanese encephalitis vaccines since the early 2000s. The BioNOC II (Cesco Bioengineering Co., Taiwan) carriers were used for cell attachment and were made out of 100% polyethyleneterephthalate (PET) non-woven fabric strips (width ~5 mm, length ~10 mm), with a specific surface area of approximately 2400 cm2/g. The fabric was surface-treated to make it hydrophilic and biocompatible. Each bottle of BelloCell-500 was pre-packed with ~865 BioNOC II carriers (approximately 5.5 g/pack, and ~100 cm3 bed volume). BioNOC II carriers were placed in a BelloCell-500 bottle and pre-sterilized by γ-irradiation. The BelloCell-500 bottle contains two parts, the upper chamber, used for cell immobilization, and the lower chamber, used to hold medium. There are two types of BelloCell-500 bioreactors, the BelloCell-500P and the BelloCell-500A. The BelloCell-500P bottle is equipped with an upper tubing (inlet) and a lower tubing (outlet) for perfusion culture use. Fresh medium was gradually introduced into the lower part of BelloCell-500P, and the medium was circulated by a peristaltic pump in the BelloFeeder. The BelloCell-500P system used a total of 2200 mL of medium. The BelloCell-500A bioreactor is designed for batch culture operation with working medium volume of 500 mL was used. Instead of the BelloFeeder, the movement and displacement of the lower chamber was controlled by a BelloStage console (Fig 1A). The concentration of inoculated cells was 2 x 105 cells/mL. During cell growth, the pH and glucose concentration were monitored once a day, and when glucose concentration dropped below 1 mM/L, the culture was replenished with fresh medium. The pH value was maintained at 7.0 by adjusting with 5% CO2 in the BelloCell-500 and/or adding NaHCO3 in the medium, with samples taken daily to determine cell density. When the cell concentration reached approximately 2 x 106 cells/mL, the medium was exchanged for fresh medium supplemented with 2 μg/mL of TPCK-treated trypsin. The cells were infected with influenza H5N1 vaccine viruses with an MOI of 10−4. During the virus harvest period, the medium was not changed, and the entire harvest was collected when the viral titer peaked. Each batch run was repeated twice.
Fig 1. Schematic diagram of the BelloCell and TideCell systems.
The BelloCell (A) and TideCell (B) systems consist of the control stage and the culturing bottle. The cultured medium level rises and descends periodically by the control stage motor. During the aeration, cells attached to the porous matrices are exposed to air for gas exchange. In the submerging phase, cells directly contact with liquid for fresh medium and waste exchange.
Industrial-scale TideCell002 bioreactor run
The TideCell002 bioreactor operates under the same principle as the BelloCell bottles. Each TideCell002 is pre-packed with approximately 55 g of BioNOC II carrier chips and pre-sterilized by γ-irradiation. The culture medium is periodically transferred from the external medium bottle into the carrier matrix vessel and is then withdrawn from the vessel using applied air pressure to export the matrices with the carriers. Thus, cells entrapped inside the matrices are able to receive sufficient nutrients and oxygen, while metabolic wastes, such as lactate and carbon dioxide, can be efficiently carried away in a gentle manner (Fig 1B). The culture medium was exchanged by the external mixing bottle, homogenized by different agitation methods, and circulated between the matrix vessel in the incubator and the mixing bag. This is accomplished by the switch between negative and positive air pressure, which submerges the matrices where the cells attach. In this study, the working liquid volume of the bioreactor was 10 L.
Calculation of cell numbers and distribution on BioNOC II carriers
To count cells on the BioNOC II carriers, a nucleus-staining crystal violet dye (CVD) method was used. To count cells on BelloCell-500A, six carrier chips were sampled from different locations (top, middle, and bottom) of the bottle and placed in three 1.5 mL Eppendorf tubes, with two carrier strips in each vial. After adding 1.0 mL of CVD reagent, each vial was shaken and incubated at 37°C for 20 min, which was repeated three times to ensure that the cell membranes ruptured and that the nuclei were released from the strips. To analyze MDCK cell distribution on BioNOC II carriers during the culturing period, the four bottles of BelloCell-500 bioreactors were seeded at the same cell concentration. Each BioNOC II carrier (approximately 865 carriers) of each bottle was counted daily and monitored by the CVD staining method in a 96-well plate from day 1 to 4. A hemocytometer was used to perform the nucleus count, allowing the cell numbers on each BioNOC II carrier to be indirectly counted.
Analysis of cell metabolites in the BelloCell bioreactor
The pH value and the concentration of glucose, lactate, glutamine, glutamate and ammonia in the culture supernatant was measured offline using a NOVA Bioprofile 400 biochemical analyzer (Nova Biomedical Corporation, USA). The glucose uptake rate (GUR) was monitored on the growth curve of MDCK cells and was calculated every day.
Virological assays
Hemagglutinin (HA) titration was conducted in 96-well microplates using turkey red blood cells (RBC) following standard protocols [20]. Virus infectious titers were measured using the 50% tissue culture infectious doses (TCID50) assay based on the cytopathic effect (CPE) in MDCK cells. A positive control with a pre-specified acceptable range was included for conducting HA and TCID50 assays [21].
Results
Cell growth in the BelloCell bioreactor
In the cell-based platform for growth of influenza viruses, the common cell lines used are MDCK and Vero. We first wanted to evaluate to the growth of these two cell lines in the BelloCell bioreactor. The MDCK cells were grown in a 500 ml BelloCell-500A bioreactor with the OptiPRO-SFM, with an initial cell density 1 x105 cells/ml. The density of the harvested cells reached 2.62 x106 cells/ml on day 5. Vero cells were cultured in the VP-SFM using the BelloCell-500A, with an initial cell density 2 x105 cells/ml. The density of the harvested cells reached 1.54 x106 cells/ml on day 5. For the Vero and MDCK cells, the number of cells increased by 7.7- and 26.2-fold, respectively. This result showed that the MDCK cells can reach a higher cell density than Vero cells on the BelloCell-500A systems (Table 1). To confirm the ability of the MDCK cells to attach to the matrix and assess their degree of distribution, the cellular distribution of the matrices was analyzed in the BelloCell-500A. The MDCK cells were inoculated into the four bottles of the BelloCell-500A bioreactors, with an initial inoculum of 1 x108 cells for each BioNOC II carrier (approximately 865 carriers) for each BelloCell-500A bottle, with cells counted and monitored daily. The cell number analysis showed that the total number of cells increase to 3.10x108, 5.58 x108, 9.34 x108 and 1.62 x109 cells on days 1, 2, 3 and 4, respectively. The cultured cell distribution had a coefficient of variation (% CV) of 2.54, 1.61, 1.36, and 1.03 on days 1, 2, 3, and 4, respectively. The time course data showed that the cellular distribution tended to become more uniform with increasing culture time (Fig 2). These results indicate that the MDCK cells showed good growth ability in the BelloCell bioreactor. Thus, subsequent experiments used the MDCK cells as a host for influenza virus production.
Table 1. Performance of MDCK and Vero cell growth in a BelloCell-500A bioreactor.
| Total surface area (cm2) | 15,600 | |
|---|---|---|
| Working volume (mL) | 500 | |
| Cell line | MDCK cell | Vero cell |
| Seeding cell density (cells/mL) | 2.0x105 | 2.0x105 |
| Total usage medium (mL) | 2,000 | 1,500 |
| Cell doubling time (hour)1 | 25.87 | 44.39 |
| Harvest cell density (cells/mL) | 2.62x106 | 1.54x106 |
| Fold increase of cell growth | 13.1 | 7.7 |
1. Calculate the cell doubling time with the following formula: Cell doubling time = during time (T) x ln2/ln (Xe/Xb); T is the incubation time in any units.; Xb is the cell number at the beginning of the incubation time.; Xe is the cell number at the end of the incubation time.
Fig 2. Time dependent analysis of MDCK cells distribution on BelloCell.
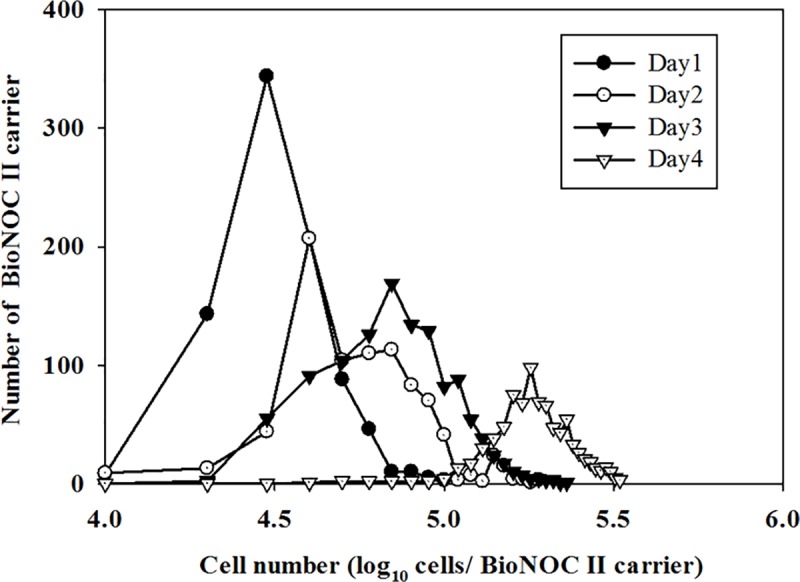
The MDCK cells were inoculated with a cell density of 2x105 cells/mL in Bellocell. The cells that counting the number of cells per day. The cell numbers were counted in each fabric on day 1(●), 2(○),3(▲) and 4(△). The total number of fabric are about 860 in the Bellocell.
Metabolic kinetics of the Vero and MDCK cells in the BelloCell-500A
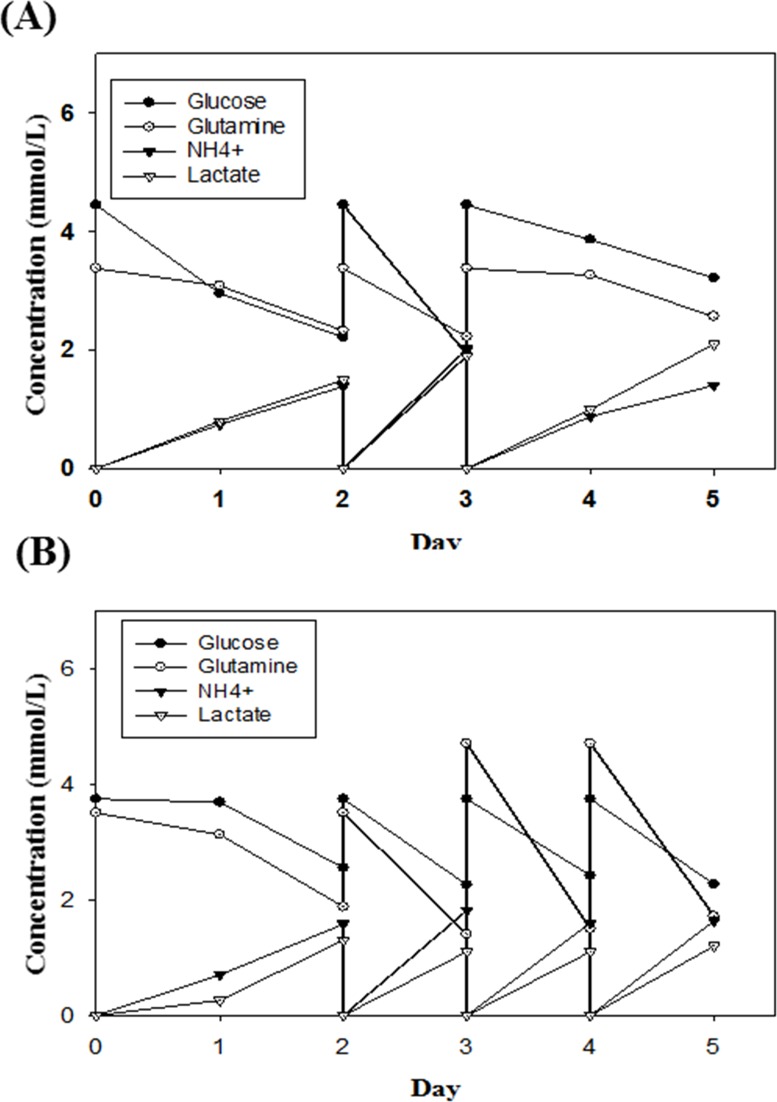
To investigate culture conditions for the two cell lines in the BelloCell-500A bioreactor, the metabolic kinetics of MDCK cells using OptiPRO™-SFM were first evaluated. The initial glucose and glutamine concentrations were approximately 3.5 and 3.8 mmol/L, respectively, with the glucose concentration maintained at 1.0 mmol/L throughout the experiment. The medium was exchanged on days 2, 3 and 4 to avoid a shortage of essential nutrients and the accumulation of toxic metabolites (Fig 3A). During culturing of the MDCK cells, a total of 2 L of fresh OptiPRO-SFM was used to maintain sufficient nutrients and waste reduction in the culture medium. The GUR peaked after the second day and rapidly declined thereafter. The metabolic kinetics of Vero cells in VP-SFM were also evaluated in the BelloCell-500A, with initial glucose and glutamine concentrations maintained at 3.5 and 4.5 mmol/L, respectively. The prevention of nutrient depletion and waste accumulation in the Vero cells was followed by a similar protocol as the MDCK cell cultures, with the medium exchanged on days 2 and 3 (Fig 3B). During growth of the Vero cells, old medium was replenished with 1.5 L of fresh medium. The GUR increased over time. These data showed that the metabolic kinetics of the MDCK cells was more exuberant than that of Vero cells under the same culture conditions, and this phenomenon was correlated with the observation of fold increase in cell growth.
Fig 3.
Metabolic profile of MDCK (A) and Vero (B) cells grown in the BelloCell-500A bioreactors. The MDCK (A) and Vero cells (B) cells were cultured in 500 mL BelloCell. The MDCK and Vero cells were inoculated with a cell density of 2x105 cells/mL. The Metabolic profile (○): Glucose, (●): Glutamine, (▲): NH4+, (△): Lactate. were monitored during the culture period.
Evaluation of the production of different influenza vaccine strains using the BelloCell-500A bioreactor
In cell culture experiments, the MDCK cells were suitable for virus replication using the Beollcell-500A. To determine the production of influenza vaccine strains in the Bellocell-500A, different pandemic influenza vaccine strains were used in this study and the result were compared with a spinner-flask production system. The MDCK cells were grown in a 500 ml BelloCell-500A system with OptiPRO-SFM. The initial cell density was 2x105 cells/mL, and the average cell density reached 2.62x106 cells/mL on day 4. MDCK cells were also cultured in a 400 mL spinner flask containing a 5 g/L Cytodex 1 microcarrier, and the average cell density reached 1.98x106 cells/mL on day 4. The MDCK cells in the BelloCell-500A were infected with influenza vaccine viruses using a very low MOI of 10−4. For virus production, the temperature was adjusted to 34°C, and the total cytopathic effect (CPE) was observed in the MDCK cells on day 3. The MDCK cells were infected with influenza H5N1 clade II vaccine strains (RG6 and RG30) and influenza H7N9 vaccine strains (RG268) from cell-adapted virus candidate strains. The results showed that the HA titer of RG6, RG30, RG268 was 1024, 64 and 512, respectively, and the TCID50 titer was 3.98x109, 6.00x106 and 1.00 x 107 virion/ml, respectively. The ability of the 400-ml spinner flask containing 5 g/L Cytodex 1 microcarriers system and the BelloCell-500A to produce viruses was compared. The data revealed that TCID50 titer in the spinner-flask system with the three tested strains was higher than that in the BelloCell-500A, but the data did not correlate well to the HA titers. The results showed that the HA titers were similar between the BelloCell-500A and the spinner flask culture (Table 2). The results of this analysis showed that the BelloCell-500A bioreactor had a good ability to produce pandemic influenza virus vaccine strains.
Table 2. Cell-specific productivity comparison of MDCK cell grown on BelloCell 500A or spinner flask bioreactors.
| BelloCell 500A | Spinner-flask | |||||||
|---|---|---|---|---|---|---|---|---|
| Cell-specific productivities3 | Cell-specific productivities | |||||||
| Virus strain1 | HA2 units/100μL |
TCID50 virions/mL |
HA units/cell | TCID50 virions/cell |
HA2 units/100μL |
TCID50 virions/mL |
HA virions/cell | TCID50 virions/cell |
| H5N1 (RG6) | 1024 | 3.98 x 109 | 7817 | 1519 | 1024 | 1.00 x 1010 | 10343 | 5050 |
| H5N1 (RG30) | 64 | 6.00 x 106 | 489 | 2 | 64 | 3.98 x 107 | 646 | 20 |
| H7N9 (RG268) | 512 | 1.00 x 107 | 3908. | 4 | 256 | 5.62 x 107 | 2589 | 28 |
1. The virus strains (RG6, RG30 and RG268) were already adapted in MDCK cells.
2. Maximum HA titer expressed as HA units/100μL.
3. Cell-specific productivities were calculated from HA or TCID50 value and cell concentration at time of infection: HA was converted to virions/mL, assuming the binding of one virus particle per red blood cell (RBC) at a given RBC concentration of 2.0x107cells/ml; the other calculation method was calculated from TCID50 titer as live virus particle number and cell concentration at time of infection.
Evaluation of the influenza H5N1 clade II vaccine strain on perfusion run
The BelloCell bioreactor has the ability to provide a large surface area to achieve high-density cell growth. However, the high-density cell growth causes the initial medium to be insufficient. The BelloCell-500A uses medium replacement to solve the nutrient deficiencies during cell culturing. Another system, the BelloCell-500P, has a total medium volume of up to 2200 mL via the perfusion system. The perfusion system provides another way to maintain sufficient nutrients and waste reduction in the culture system. To evaluate the productivity of both systems, the influenza H5N1 clade II (RG6) vaccine strain was used as an example to compare the different BelloCell systems. The MDCK cells were grown in OptiPRO-SFM using the 500-ml BelloCell-500A system and the BelloCell-500P system. The initial cell density was 2 x105 cells/mL, and after four days, the cell density reached 2.6 x106 cells/mL and 7.65 x105 cells/mL for the BelloCell-500A and BelloCell-500P systems, respectively. The data showed that the cell density in the BelloCell-500P system was lower than that in the BelloCell-500A system. However, the total number of cells was higher in the BelloCell-500P system than in the BelloCell-500A (Table 3). The MDCK cells in the BelloCell-500A and BelloCell-500P systems were infected with influenza H5N1 clade II (RG6) virus, and the total CPE was observed on day 3. The virus titer was analyzed by HA and TCID50 assays. The virus titer data indicated that the virus productivity in the BelloCell-500P system was higher than that in the BelloCell-500A under the same conditions (Table 3). The productivity results showed that perfusion systems are more suitable for influenza vaccine production.
Table 3. Evaluation of influenza H5N1 clade II virus (RG6M13C4) production in different BelloCell-500 systems.
| Volume (mL) | HA titer1 (units/100μL) |
TCID50 (virions/mL) |
Cell destiny (cells/mL) |
Cell-specific productivities2 | ||
|---|---|---|---|---|---|---|
| HA virions/cell |
TCID50 virions/cell |
|||||
| T75 flask | 20 | 128 | 2.93 x107 | 5.00 x105 | 5120 | 59 |
| BelloCell-500A | 500 | 512 | 3.16 x107 | 2.60 x106 | 3938 | 12 |
| BelloCell-500P | 2200 | 512 | 2.50 x107 | 7.65 x105 | 13386 | 33 |
1. Maximum HA titer expressed as HA units/100μL.
2. Cell-specific productivities were calculated from HA or TCID50 value and cell concentration at time of infection: HA was converted to virions/mL, assuming the binding of one virus particle per red blood cell (RBC) at a given RBC concentration of 2.0x107cells/ml; the other calculation method was calculated from TCID50 titer as live virus particle number and cell concentration at time of infection.
Scaling-up study from the BelloCell-500P to the TideCell002 bioreactor
A good ability to produce the pandemic influenza vaccine strains was obtained in the BelloCell-500P bioreactor. To evaluate the linear capability of a larger production scale, the TideCell002 bioreactor was assessed for virus production. The TideCell002 was able to scale-up to over a 10-L production capacity. The MDCK cells were grown in a TideCell002 (with 55 g of BioNOC II carriers) bioreactor system with OptiPRO-SFM. The initial cell density was 2 x105 cells/mL, and the average doubling time of MDCK cells was 27.57 hours. The cell density reached 2.62 x106 cells/mL on day 4. The metabolic kinetics of MDCK cells in OptiPRO-SFM were also evaluated in the TideCell002. The initial glucose concentration was approximately 3.5 mmol/L and was maintained at 1.0 mmol/L. The lactate concentration was also monitored to ensure that the lactate concentration did not go over 1.5 mmol/L during the experiment. Ten liters of medium was replaced on day 3 to avoid a shortage of essential nutrients and avoid the accumulation of toxic metabolites. Twenty liters of OptiPRO-SFM was used in total for MDCK cells. The GUR of MDCK cells was assessed and ranged from 120 to 370 mg/h in the TideCell002 system. The maximal GUR was calculated to be 370 mg/h at 96 hours and remained steady thereafter. According to our pilot study, the conditions for the infection of MDCK cells in the TideCell002 bioreactor were similar to the BelloCell, except the total cytopathic effect (CPE) was observed on day 4. The MDCK cells were infected with the influenza H5N1 clade II (RG6) and influenza H7N9 (RG268) virus candidate strains. The results showed that the HA and TCID50 of RG6M13C4 were 512 and 1 x108 virion/mL, respectively, while the HA and TCID50 of RG268M5 were 512 and 3.16 x107 virion/mL, respectively (Table 4). These results demonstrated that high-yield MDCK cell-based influenza H5N1 and H7N9 vaccine production is possible using the TideCell002 bioreactor system and has a good linear amplification scalability.
Table 4. Scaling-up evaluation of pandemic influenza virus production in TideCell002 bioreactor.
| Virus strain | Volume (mL) | HA titer1 (units/100μL) |
TCID50 (virions/mL) | Cell destiny (cells/mL) |
Cell-specific productivities2 | |
|---|---|---|---|---|---|---|
| HA virions/cells |
TCID50 virions/cells |
|||||
| H7N9 | 10,000 | 512 | 1.00 x108 | 1.98x106 | 5120 | 51 |
| H5N13 | 10,000 | 512 | 3.16 x107 | 1.98 x106 | 5120 | 16 |
1. Maximum HA titer expressed as HA units/100μL.
2. Cell-specific productivities were calculated from HA or TCID50 value and cell concentration at time of infection: HA was converted to virions/mL, assuming the binding of one virus particle per red blood cell (RBC) at a given RBC concentration of 2.0x107cells/ml; the other calculation method was calculated from TCID50 titer as live virus particle number and cell concentration at time of infection.
Discussion
Cell culture processes have played an increasingly important role in the development of influenza vaccines. MDCK cells have been used in the formulation of the vaccine Flucelvax Quadrivalent (Seqirus, Inc), which is approved for use in Europe and the USA [22]. However, the development of an efficient cell culture process is still needed. It is important that cell-based influenza vaccine manufacturing processes use a highly efficient bioreactor that can produce high cell densities, high virus yields and high HA titers from a wide variety of influenza virus strains. In a study by Hu et al., the BelloCell was shown to have a good gas exchange ability and high cell density culturing [23]. In this study, we confirmed the ability of the BelloCell system to culture mammalian cells at high cell densities. The BioNOC II carriers in the BelloCell allow the cells to attach and proliferate (Fig 3). In recent years, commercially available cell-based influenza vaccines are commonly produced from either Vero or MDCK cells. In this study, we showed that MDCK cells achieved a higher cell density than Vero cells in the BelloCell system, suggesting that the MDCK cell line is more suitable for use with the BelloCell system (Table 1). In addition, recent studies have shown that MDCK cells are the most suitable for propagating influenza viruses, as the cells not only enable the growth of different influenza virus strains but also produce good virus yields with relatively high HA titers [24–26].
The cell culturing and virus replication conditions for influenza vaccine production were established using the method described in the microcarrier process by Hu et al., 2011. In this study, the results show that the HA titers were similar between the BelloCell and microcarrier processes (Table 2). We confirmed that the BelloCell system has the ability to produce high yields of influenza viruses and showed that the production system has a high potential for cell production capacity. The observed level of cell production is similar to that observed in previous studies and has been successfully applied to the production of different viruses [15, 16, 27]. In addition, about spinner-flask has a consistently higher TCID50 titer than that in the BelloCell-500A.This is an interesting phenomenon. The TCID50 titer results indicated that the total number of live virus, not total the effective antigen. The HA titer could be explaining to the total number of effective antigen in this study. In the virus production curve data showed the TCID50 titer was decreasing from 48 hours. However, the HA titer was still remaining high till 72 hrs (S1 Fig). These data maybe can explain that the spinner-flask have a more live virus, but the total number of the virus was similar to BelloCell-500A.
In previous reports [10, 28], MDCK and Vero cells could produce influenza viruses using SFM in spinner flasks. In this study, the same cells were used for the production of H5N1 influenza clade II and H7N9 vaccine strains using SFM in the BelloCell systems, as well as in spinner flasks. The cell density reached in the BelloCell system was higher than that of the spinner flasks; however, the virus titers were similar in both systems. This suggests that cell density does not correlate with virus titer well. This may be due to the “cell density effect”, which was first reported by Wood et al., 1982. The cell culture was observed to be "nutrient-limited", and this nutrient limitation caused total cell yield and health to be compromised. The nutrient limitation and unknown inhibitory factors may have contributed to the decrease in virus productivity in the culture medium [29]. Another study encountered a similar problem when dealing with adenovirus [30] and influenza virus [31, 32]. In this study, the ability of the BelloCell-500P (2200-mL) and the BelloCell-500A (500-mL) to produce influenza virus was compared. It was observed that increasing the volume of the culture medium resulted in a three-fold increase of influenza virus production per unit cell in the BelloCell-500P (Table 3). Thus, we confirmed that the cell density effect played a key role in BelloCell experiments, and nutrient limitation may be one of the important factors affecting the yield. In addition, the volume of the culture medium also reflection on the price. It could be compared by the usage of medium and HA titer produced. The data showed that Beollcell-500p bioreactors maybe have cheaper than flask system and Beollcell-500A in well-control condition. This information was shown in Table 5.
Table 5. Productivity comparison of the virus production in different systems.
| BelloCell 500P | BelloCell 500A | Spinner flask | |
|---|---|---|---|
| Medium name | OptiPro-SFM3 | ||
| Working volume (L) | 2.2 | 0.5 | 0.4 |
| HA titer (HA units/100μl)1 | 512 | 512 | 512 |
| Medium usage volume (L) | 4.4 | 2.0 | 2.0 |
| Specific productivity (HA unit/L)2 | 256.0 | 128.0 | 102.4 |
1. HA titer as HA units/100μL (RBC concentration:2.0x107cells/ml)
2. The specific productivity is based on the calculation of viral titer * working volume /medium usage.
3. The cost based on OptiPro-SFM only, about 100 US/L.
As described by Merten et al., 2015 [33], the difference in bioreactor system design has caused bench-scale process parameters did not correlated well to industrial-scale bioreactors. The shear force generated from the agitating blade and the uniformity of the cell adhesion are often an issue, resulting in a decrease in the production process. In this study, different influenza virus vaccine strains were cultured in the BelloCell and in spinner flasks with microcarrier. The TideCell002 was also used, and its entire process is amplified in volume by almost 20-fold compared to the BelloCell-500A system. Based on similar culture conditions, the HA titer did not differ significantly. In many previous reported, these virus production systems have been widely used to produce a vaccine[34]. However, since an influenza pandemic usually spreads quickly and is highly contagious, a single-use system with rapid vaccine-production capabilities seems to be preferable. The results of this study have highlighted the potential for rapid and cost-effective vaccine manufacturing using the TideCell002 system. TideCell002 can produce 10 to 20 L of vaccine per run. In addition, the TideCell002 system has shown a good linear scale-up, and is easy to switch to larger scale bioreactors (TideCell100, equivalent to 500 to 1000 L, industrial scale). This novel single-use oscillating bioreactor is able to produce large quantities of vaccine antigen at an industrial scale (up to 1000 L).
Conclusions
The goal of this study was to evaluate cell-based influenza virus production platforms using novel, single-use bioreactors, particularly for newly emerging influenza viruses, such as H5N1 and H7N9. BelloCell, a new type of bioreactor, has high cell density and viral production capabilities. The BelloCell is easily scaled up to the size of the TideCell system using similar operational conditions. This novel bioreactor shown good performance for adhesion cells for influenza virus production. This study highlights the ability of disposable bioreactors to manufacture vaccines quickly and efficiently. TideCell bioreactor could be quick and easy producing large amounts of the virus is a major point of the study. In future studies, The TideCell002 also shown a good scalability performance from the BelloCell bioreactor. If the TideCell bioreactor can also scaled to 1000 L production scale (harvest volume). Rapid production of influenza virus would be possible to meet the surge demand against any possible pandemic outbreak. In addition, this novel escalatory bioreactor also applies to other vaccine processes such as rabies polio and EV71.
Supporting information
The MDCK cells were cultured in beollcell-500A and spinner flask. These cultured cells were infected by H5N1. During the infection period, the HA titer (■) and TCID50 (●) were monitored.
(TIF)
Acknowledgments
The authors would like to thank Mr. Anthony Chang for English editing, and the US CDC for supplying the egg-derived vaccine strains. The authors would also like to thank the funding support from the Ministry of Science and Technology (R.O.C. 102-2622-B-400-001-CC2; 103-2622-B-400-001-CC2). Chia-Chun Lai carried out his PhD study under the auspices of the Graduate Program of Biotechnology in Medicine, National Tsing Hua University and National Health Research Institutes.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors would like to thank Mr. Anthony Chang for English editing, and the US CDC for supplying the egg-derived vaccine strains. The authors would also like to thank the funding support from the Ministry of Science and Technology (R.O.C. 102-2622-B-400-001-CC2; 103-2622-B-400-001-CC2). Mr. Chia-Chun Lai is currently pursuing his Ph.D. studies under the Graduate Program of Biotechnology in Medicine, National Tsing Hua University and the National Health Research Institutes. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Organization WH. Cumulative Number of Confirmed Human Cases of Avian Influenza A(H5N1) Reported to WHO. 2017.
- 2.To KK, Ng KH, Que TL, Chan JM, Tsang KY, Tsang AK, et al. Avian influenza A H5N1 virus: a continuous threat to humans. Emerg Microbes Infect. 2012;1(9):e25 10.1038/emi.2012.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu WL, Chen Y, Wang P, Song W, Lau SY, Rayner JM, et al. Antigenic profile of avian H5N1 viruses in Asia from 2002 to 2007. J Virol. 2008;82(4):1798–807. 10.1128/JVI.02256-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH. Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. 2017. [Google Scholar]
- 5.Eisfeld AJ, Neumann G, Kawaoka Y. Influenza A virus isolation, culture and identification. Nat Protoc. 2014;9(11):2663–81. Epub 2014/10/17. 10.1038/nprot.2014.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milian E, Kamen AA. Current and emerging cell culture manufacturing technologies for influenza vaccines. BioMed research international. 2015;2015:504831 10.1155/2015/504831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MS, Hu AY. A cell-based backup to speed up pandemic influenza vaccine production. Trends Microbiol. 2012;20(3):103–5. Epub 2012/01/20. 10.1016/j.tim.2011.12.002 . [DOI] [PubMed] [Google Scholar]
- 8.Perdue M.L, AF, Li S, Donabedian A, Cioce V, Warf T, Huebner R. The future of cell culture-based influenza vaccine production. Expert Rev Vaccines. 2011;10(8):1183–94. 10.1586/erv.11.82 [DOI] [PubMed] [Google Scholar]
- 9.Genzel Y, Dietzsch C, Rapp E, Schwarzer J, Reichl U. MDCK and Vero cells for influenza virus vaccine production: a one-to-one comparison up to lab-scale bioreactor cultivation. Appl Microbiol Biotechnol. 2010;88(2):461–75. Epub 2010/07/10. 10.1007/s00253-010-2742-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu AY, Tseng YF, Weng TC, Liao CC, Wu J, Chou AH, et al. Production of inactivated influenza H5N1 vaccines from MDCK cells in serum-free medium. PloS one. 2011;6(1):e14578 Epub 2011/02/02. 10.1371/journal.pone.0014578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapia F, Vogel T, Genzel Y, Behrendt I, Hirschel M, Gangemi JD, et al. Production of high-titer human influenza A virus with adherent and suspension MDCK cells cultured in a single-use hollow fiber bioreactor. Vaccine. 2014;32(8):1003–11. Epub 2013/11/26. 10.1016/j.vaccine.2013.11.044 . [DOI] [PubMed] [Google Scholar]
- 12.Souza MC, Freire MS, Schulze EA, Gaspar LP, Castilho LR. Production of yellow fever virus in microcarrier-based Vero cell cultures. Vaccine. 2009;27(46):6420–3. Epub 2009/06/30. 10.1016/j.vaccine.2009.06.023 . [DOI] [PubMed] [Google Scholar]
- 13.Rourou S, van der Ark A, Majoul S, Trabelsi K, van der Velden T, Kallel H. A novel animal-component-free medium for rabies virus production in Vero cells grown on Cytodex 1 microcarriers in a stirred bioreactor. Appl Microbiol Biotechnol. 2009;85(1):53–63. Epub 2009/06/13. 10.1007/s00253-009-2064-y . [DOI] [PubMed] [Google Scholar]
- 14.Rao G, Moreira A, Brorson K. Disposable bioprocessing: the future has arrived. Biotechnol Bioeng. 2009;102(2):348–56. Epub 2008/12/19. 10.1002/bit.22192 . [DOI] [PubMed] [Google Scholar]
- 15.Chen YH, Wu JC, Wang KC, Chiang YW, Lai CW, Chung YC, et al. Baculovirus-mediated production of HDV-like particles in BHK cells using a novel oscillating bioreactor. J Biotechnol. 2005;118(2):135–47. Epub 2005/06/14. 10.1016/j.jbiotec.2005.02.018 . [DOI] [PubMed] [Google Scholar]
- 16.Toriniwa H, Komiya T. Japanese encephalitis virus production in Vero cells with serum-free medium using a novel oscillating bioreactor. Biologicals. 2007;35(4):221–6. Epub 2007/04/03. 10.1016/j.biologicals.2007.02.002 . [DOI] [PubMed] [Google Scholar]
- 17.Yu-Chen Hu J-TLaY-CC. High-density cultivation of insect cells and production of recombinant baculovirus using a novel oscillating bioreactor. Cytotechnology. 2003;(42):4 145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu JT, Chung YC, Chan ZR, Hu YC. A novel oscillating bioreactor BelloCell: implications for insect cell culture and recombinant protein production. Biotechnol Lett. 2005;27(15):1059–65. Epub 2005/09/01. 10.1007/s10529-005-8450-3 . [DOI] [PubMed] [Google Scholar]
- 19.Yu-Chi Wang, K-M C, F-L L, Mei-Lin Wu, Lewis Ho. Novel Disposable Bioreactor for Mammalian Cell Cultures and Virus Production. CESCO Bioengineering Inc., 2003. [Google Scholar]
- 20.WHO. WHO manual on animal influenza diagnosis and surveillance. Geneva: 2002. [Google Scholar]
- 21.Genzel Y, Behrendt I, Rodig J, Rapp E, Kueppers C, Kochanek S, et al. CAP, a new human suspension cell line for influenza virus production. Appl Microbiol Biotechnol. 2013;97(1):111–22. Epub 2012/07/24. 10.1007/s00253-012-4238-2 . [DOI] [PubMed] [Google Scholar]
- 22.Manini I, Domnich A, Amicizia D, Rossi S, Pozzi T, Gasparini R, et al. Flucelvax (Optaflu) for seasonal influenza. Expert Rev Vaccines. 2015;14(6):789–804. 10.1586/14760584.2015.1039520 . [DOI] [PubMed] [Google Scholar]
- 23.Yu-Chen Hu J-TLaY-CC. High-density cultivation of insect cells and production of recombinant baculovirus using a novel oscillating bioreactor. Cytotechnology. 2003;(42):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin D, Park KJ, Lee H, Cho EY, Kim MS, Hwang MH, et al. Comparison of immunogenicity of cell-and egg-passaged viruses for manufacturing MDCK cell culture-based influenza vaccines. Virus Res. 2015;204:40–6. Epub 2015/04/22. 10.1016/j.virusres.2015.04.005 . [DOI] [PubMed] [Google Scholar]
- 25.Huang D, Peng WJ, Ye Q, Liu XP, Zhao L, Fan L, et al. Serum-Free Suspension Culture of MDCK Cells for Production of Influenza H1N1 Vaccines. PloS one. 2015;10(11):e0141686 Epub 2015/11/06. 10.1371/journal.pone.0141686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamamoto I, Takaku H, Tashiro M, Yamamoto N. High yield production of influenza virus in Madin Darby canine kidney (MDCK) cells with stable knockdown of IRF7. PloS one. 2013;8(3):e59892 10.1371/journal.pone.0059892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang KS, Lo WH, Chung YC, Lai YK, Chen CY, Chou ST, et al. Combination of baculovirus-mediated gene delivery and packed-bed reactor for scalable production of adeno-associated virus. Hum Gene Ther. 2007;18(11):1161–70. Epub 2007/10/20. 10.1089/hum.2007.107 . [DOI] [PubMed] [Google Scholar]
- 28.Tseng YF, Hu AY, Huang ML, Yeh WZ, Weng TC, Chen YS, et al. Adaptation of high-growth influenza H5N1 vaccine virus in Vero cells: implications for pandemic preparedness. PloS one. 2011;6(10):e24057 10.1371/journal.pone.0024057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bock A, Schulze-Horsel J, Schwarzer J, Rapp E, Genzel Y, Reichl U. High-density microcarrier cell cultures for influenza virus production. Biotechnol Prog. 2011;27(1):241–50. Epub 2011/02/12. 10.1002/btpr.539 . [DOI] [PubMed] [Google Scholar]
- 30.Kamen A, Henry O. Development and optimization of an adenovirus production process. J Gene Med. 2004;6 Suppl 1:S184–92. Epub 2004/02/24. 10.1002/jgm.503 . [DOI] [PubMed] [Google Scholar]
- 31.Chen A, Poh SL, Dietzsch C, Roethl E, Yan ML, Ng SK. Serum-free microcarrier based production of replication deficient influenza vaccine candidate virus lacking NS1 using Vero cells. BMC Biotechnol. 2011;11:81 Epub 2011/08/13. 10.1186/1472-6750-11-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genzel Y, Vogel T, Buck J, Behrendt I, Ramirez DV, Schiedner G, et al. High cell density cultivations by alternating tangential flow (ATF) perfusion for influenza A virus production using suspension cells. Vaccine. 2014;32(24):2770–81. Epub 2014/03/04. 10.1016/j.vaccine.2014.02.016 . [DOI] [PubMed] [Google Scholar]
- 33.Merten OW. Advances in cell culture: anchorage dependence. Philos Trans R Soc Lond B Biol Sci. 2015;370(1661):20140040 Epub 2014/12/24. 10.1098/rstb.2014.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shukla AA, Gottschalk U. Single-use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 2013;31(3):147–54. Epub 2012/11/28. 10.1016/j.tibtech.2012.10.004 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The MDCK cells were cultured in beollcell-500A and spinner flask. These cultured cells were infected by H5N1. During the infection period, the HA titer (■) and TCID50 (●) were monitored.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.