T cells normally recognize cognate antigens in the context of major histocompatibility complex class I (MHCI) and MHCII molecules. Effective T-cell immunity requires concordance among T-cell receptor (TCR) specificity, coreceptor phenotype and cellular function, such that T cells with MHCI-specific TCR express CD8 coreceptor proteins and possess cytotoxic function, whereas T cells with MHCII-specific TCR express CD4 coreceptor proteins and possess helper function.1 How TCR/coreceptor/MHC concordance is generated in the thymus has intrigued immunologists for many years. CD4 and CD8 T cells are generated from a bi-potential precursor population of double-positive (DP) thymocytes that express both CD4 and CD8 coreceptor proteins. DP thymocytes express TCRs with low affinities for antigen/MHC complexes, such that TCR signaling usually requires co-engagement by CD4 or CD8 proteins. Thus, immature DP thymocytes face the dilemma of which coreceptor expression to extinguish and which cellular function to acquire in accordance with the MHC specificity of their TCR.
We recently addressed the issue of CD4/CD8 lineage choice in the thymus by shifting focus away from the coreceptor proteins themselves and shifting focus to the Cd4 and Cd8 coreceptor gene loci which encode them.2 Using gene “knock-in” technology, murine CD4 cDNA was introduced into the Cd8a coreceptor gene locus, effectively replacing CD8α coding sequences with CD4 coding sequences. In such “4in8” knock-in mice, the Cd8a coreceptor gene locus encoded MHCII-specific CD4 coreceptor proteins instead of MHCI-specific CD8 coreceptor proteins. If lineage choices in the thymus were determined by coreceptor proteins themselves, then it would not matter which coreceptor gene locus encoded CD4 coreceptor proteins, and MHCII-specific CD4 T cells would always possess helper function. Remarkably, thymic selection and development in 4in8 mice yielded MHCII-specific CD4 T cells with cytotoxic function, not helper function. Indeed, unlike Cd4-encoded CD4 coreceptor proteins, which induced ThPOK (the helper lineage-specifying nuclear factor),3,4 Cd8-encoded CD4 coreceptor proteins induced Runx3 (the cytotoxic lineage-specifying factor)5,6 in developing MHCII-specific thymocytes. Thus, lineage fate in the thymus is determined by the coreceptor gene locus (Cd4 or Cd8) encoding the coreceptor protein that the TCR utilizes regardless of which coreceptor protein it is (Fig. 1). This concept is referred to as coreceptor gene “imprinting” and signifies that the Cd8 gene locus co-opts any coreceptor protein encoded within it to promote cytotoxic lineage fate, whereas the Cd4 gene locus co-opts any coreceptor protein encoded with it to promote helper lineage fate.2
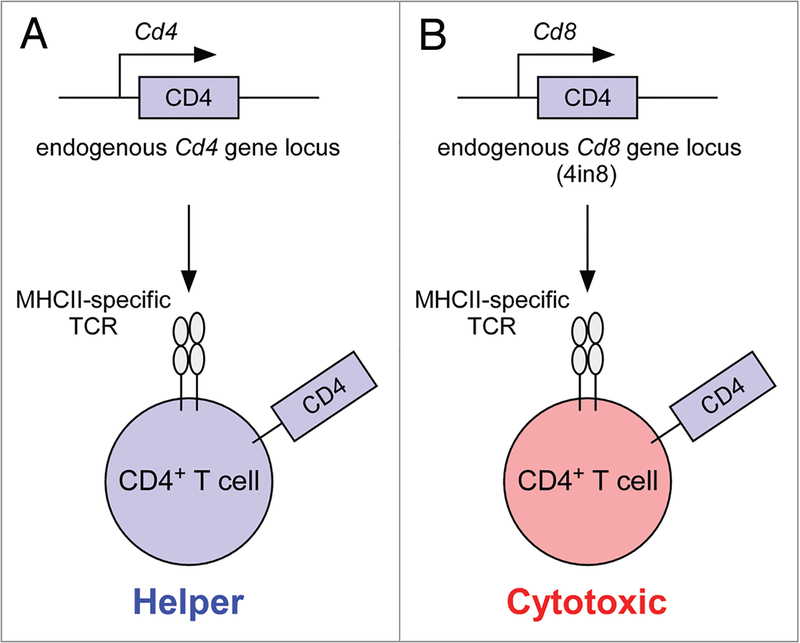
Figure 1.
Schematic of coreceptor gene imprinting. Helper vs. cytotoxic lineage fate is determined by Cd4 and Cd8 coreceptor gene loci and not by the coreceptor protein each encodes. CD4 coreceptor proteins promote thymic generation of mature CD4 T cells with MHCII-specific TCRs. Importantly, however, CD4 proteins encoded in the Cd4 gene locus induce CD4 T cells to acquire helper function (A), whereas CD4 proteins encoded in the Cd8 gene locus induce CD4 T cells to acquire cytotoxic function (B).
Coreceptor gene imprinting sheds light on how transcriptional regulation by coreceptor gene loci might couple TCR-specific positive selection signals to the specific expression of either ThPOK or Runx3. Cytokines such as interleukin-7 provide the intrathymic signals that induce Runx3 and instruct the cytotoxic lineage fate.7 However, cytokine signaling during positive selection requires the transient disruption of TCR signaling, because persistent TCR signaling prevents cytokine signaling.8,9 Coreceptor gene imprinting demonstrates that the kinetics of Cd8 transcription transiently disrupt coreceptor-dependent TCR signaling during positive selection to permit signaling by cytokines that induce Runx3 and impose the cytotoxic lineage fate. Specifically, TCR-signaled DP thymocytes initially terminate Cd8 transcription, which causes them to steadily lose Cd8-encoded coreceptor proteins from the cell surface. As a result, TCR signals that require Cd8-encoded coreceptor proteins are disrupted, permitting TCR-signaled thymocytes to then be signaled by intrathymic cytokines to express Runx3.7,9 Indeed, transgenic expression of SOCS1, a suppressor of cytokine signaling, impaired generation of 4in8 CD4 T cells with cytotoxic function but did not impair generation of WT CD4 T cells with helper function.2 In our perspective, the reason that cytotoxic T cells in normal mice express MHCI-specific TCRs is that their Cd8 gene locus encodes MHCI-specific CD8 proteins. If the Cd8 gene locus instead encoded MHCII-specific CD4 proteins, then cytotoxic T cells would express MHCII-specific TCRs. Thus, regardless of which coreceptor protein the Cd8 gene locus encodes, the transcriptional kinetics of the Cd8 gene locus during positive selection imposes cytotoxic lineage choice by inducing a transient disruption of coreceptor-dependent TCR signaling during positive selection (Fig. 1).
What is the genetic basis of coreceptor gene imprinting? Cd4 gene expression is chiefly regulated by a silencer element, and Cd8a gene expression is regulated by stage-specific enhancers E8I–E8V.10 E8II and E8III enhancers are active in pre-selection DP thymocytes10 but are terminated by TCR signaling, resulting in the transient termination of Cd8 gene transcription.2 In contrast, TCR signaling of DP thymocytes appears to increase Cd4 gene transcription, as evidenced by progressively increasing CD4 surface protein expression during conventional CD4 T-cell development.2 Increasing CD4 expression ensures that TCR signaling persists throughout MHCII-specific positive selection to induce ThPOK and prevent cytokine signaling of Runx3.9,11
In conclusion, helper-vs.-cytotoxic lineage choice involves the intricate interplay of a host of transcription factors whose expression is tightly coordinated with Cd4 or Cd8 coreceptor gene shutoff. Coreceptor gene imprinting provides an explanation of how coreceptors might influence this interplay. By their dynamic activity, regulatory elements in each coreceptor gene locus modulate the duration of TCR signaling, which leads to the induction of lineage-specific transcription factors. These findings set the stage for future investigations of the cis and trans factors involved in coreceptor gene regulation.
References
- 1.Starr TK, et al. Annu Rev Immunol 2003; 21:139–76; 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Adoro S, et al. EMBO J 2011; 31:366–77; 10.1038/emboj.2011.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He X, et al. Nature 2005; 433:826–33; 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 4.Sun G, et al. Nat Immunol 2005; 6:373–81; 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- 5.Egawa T, et al. J Exp Med 2007; 204:1945–57; 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato T, et al. Immunity 2005; 22:317–28; 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, et al. Nat Immunol 2010; 11:257–64; 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugnera E, et al. Immunity 2000; 13:59–71; 10.1016/S1074-7613(00)00008-X. [DOI] [PubMed] [Google Scholar]
- 9.Singer A, et al. Nat Rev Immunol 2008; 8:788–801; 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kioussis D, et al. Nat Rev Immunol 2002; 2:909–19; 10.1038/nri952. [DOI] [PubMed] [Google Scholar]
- 11.He X, et al. Immunity 2008; 28:346–58; 10.1016/j.immuni.2008.02.006. [DOI] [PubMed] [Google Scholar]