Abstract
Triple-negative breast cancer is associated with a poor prognosis, and effective biomarkers for targeted diagnosis and treatment are lacking. The tumorigenicity of the provirus integration site for Moloney murine leukemia virus 1 (PIM-1) gene has been studied for many years. However, its significance in breast cancer remains unclear. In this review we briefly summarized the physiological characteristics and regulation of PIM-1 kinase, and subsequently focused on the role of PIM-1 in tumors, especially breast cancer. Oncogene PIM-1 was found to be upregulated in breast cancer, especially in triple-negative breast cancer. Moreover, it is involved in tumorigenesis and the development of drug resistance, and linked to poor prognosis. A highly selective probe targeting PIM-1 for imaging has emerged, suggesting that PIM-1 may be a potential biomarker for the accurate diagnosis and targeted therapy of triple-negative breast cancer.
Keywords: triple-negative breast cancer, biomarker, PIM-1
Introduction
Triple-negative breast cancer (TNBC), which is negative for the expression of ER, PR, and HER2, is associated with the poorer prognosis among all types of breast cancer. Since endocrine therapy is ineffective and targeted-therapy is currently unavailable, chemotherapy remains the mainstay of treatment after surgery for TNBC. Therefore, discovering specific biomarkers for the development of early precise diagnosis, effective targeted-therapy, and sensitive assessment of the treatment effect on TNBC is clinically urgent.
The PIM-1 (provirus integration site for Moloney murine leukemia virus 1) gene was identified as an oncogene in mice with leukemia induced by the Moloney murine leukemia virus in the1980s.1 PIM-1 kinase, encoded by the PIM-1 gene, is the most studied and important among all three members of the PIM kinase family. The other two members of the PIM kinase family discovered soon afterwards, PIM-2 and PIM-3, exhibit strong homology to PIM-1. Notably, in-vivo and in-vitro experiments suggested that they can be substitutes of PIM-1 to different extent.2–4 The human PIM-1 kinase shares >90% similarity at the primary structure level with the mouse PIM-1 protein.5,6 PIM-1 kinase was found to be overexpressed in human hematological malignancies,7–9 as well as in numerous human solid tumors such as breast cancer,10 prostate cancer,10 gastric cancer,11 and squamous cell carcinoma of the head and neck.12 The molecular mechanisms of PIM-1-induced tumorigenesis have been studied in great depth. Meanwhile, many different small-molecule inhibitors targeting PIM-1 kinase have been developed. In recent years, increasing attention has been paid to the value of PIM-1 in the treatment and diagnosis of tumors. This review aims to explore the potential of PIM-1 kinase as a biomarker of TNBC by briefly summarizing its physiological structure and function, and regulation of the kinase activities of PIM-1. In addition, the association of PIM-1 with tumors and its role in breast cancer are discussed.
Physiological structure and function of PIM-1 kinase
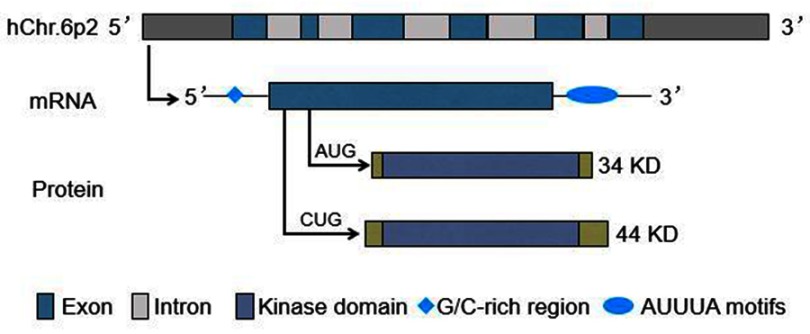
The human PIM-1 gene is located on chromosome 6p21, and consists of six exons and five introns. It produces a transcript which contains a G/C-rich sequence in the 5ʹ untranslated region (UTR) and five copies of AUUUA destabilizing motifs in the 3ʹ UTR.6,13 The use of alternative translation initiation sites (AUG or CUG) results in the synthesis of different protein isoforms: 34 KD and 44 KD PIM-1 kinase (Figure 1).14–16 The former isoform is comprised of 313 amino acids, while the latter includes 404 amino acids, both containing the kinase domain.17–20
Figure 1.
PIM-1 gene and its transcripts and proteins.
Both PIM-1 protein isoforms exhibit in vitro serine/threonine kinase activities, and mediate their physiological function by phosphorylating a wide range of cellular substrates,19,20 including: 1) cell cycle regulators, such as cyclin-dependent kinase inhibitor 1A/1B (CDKN1A/1B),21–24 cell division cycle 25A/C (CDC25A/C),25,26 checkpoint kinase 1 (CHK1),27 and forkhead box P3 (FOXP3);28 2) transcriptional regulators, such as MYC29 and MYB,30 runt-related transcription factor 1/3 (RUNX1/3),31 and high mobility group box transcription factor 1 (HBP1);32 3) signaling intermediates, such as Notch1,10 suppressor of cytokine signaling 1/3 (SOCS1/3),33 and mitogen-activated protein kinase kinase kinase 5 (MAP3K5);34 4) apoptosis signaling kinase 1 (ASK1)34 and apoptosis regulators, such as BCL-2-associated agonist of cell death (BAD);35 5) protein translation regulators, such as eukaryotic initiation factor 4B (EIF4B);36 and 6) others, such as breast cancer resistant protein (BCRP),24 ubiquitin-like with plant homeo domain and RING finger domains 1 (UHRF1),37 and androgen receptor (AR) (Table 1).38 Through the phosphorylation of the above substrates, PIM-1 kinase plays a role in cell cycle progression, survival, proliferation, differentiation, apoptosis, and senescence. Xie et al found that 44 KD PIM-1kinase (located primarily on the plasma membrane), instead of the 34 KD isoform (present in both the cytosol and nucleus), phosphorylated BCRP to protect prostate cancer cells from apoptosis induced by chemotherapeutic drugs.39,40 In addition, Katsube et al suggested that 44 KD PIM-1 kinase protected ATP-binding cassette transporter A1 (ABCA1) from lysosomal degradation in hepatocytes, and thereby regulated the circulating level of high-density lipoprotein.41 However, thus far, almost all research studies investigating human PIM-1 kinase do not consider the two PIM-1 isoforms independently. Further investigation is warranted to determine whether the two isoforms are involved in a single mechanism of regulation of protein expression, or play different biological roles.
Table 1.
PIM-1 kinase substrates
| Cell cycle regulators | Transcriptional regulators | Signaling intermediates | Apoptosis regulators | Protein translation regulators | Others |
|---|---|---|---|---|---|
| CDKN1A | MYC | SOCS1 | BAD | EIF4B | UHRF1 |
| CDKN1B | MYB | SOCS3 | ASK1 | BCRP | |
| CDC25A | RUNX1 | MAP3K5 | AR | ||
| CDC25C | RUNX3 | Notch1 | |||
| CHK1 | HBP1 | ||||
| FOXP3 |
Abbreviations: PIM-1, provirus integration site for Moloney murine leukemia virus 1; CDKN1A, cyclin-dependent kinase inhibitor 1A, also termed p21 and CIP1; CDKN1B, also termed p27KIP1; CDC25A, cell division cycle 25A, also termed MPIP1; CDC25C, also termed MPIP3; FOXP3, forkhead box P3; RUNX1, runt-related transcription factor 1; HBP1, high mobility group box transcription factor 1; SOCS1, suppressor of cytokine signaling 1; BAD, BCL-2-associated agonist of cell death; ASK1, apoptosis signaling kinase 1; EIF4B, eukaryotic initiation factor 4B; UHRF1, ubiquitin-like with plant homeo domain and RING finger domains 1; BCRP, breast cancer-resistant protein; AR, androgen receptor.
Regulation of PIM-1 kinase activities
Structural analysis revealed that the PIM-1 protein contains a constitutively active kinase conformation that does not require to be phosphorylated for activation.17 This means that the level of PIM-1 enzymatic activity in a cell is dependent on the absolute amount of protein present. Typically, both PIM-1 mRNA and protein have a relatively short half-life.14,39 The regulation of PIM-1 kinase activities largely depends on the induction of transcription and protein degradation, and varies among different cells. Numerous cytokines, growth factors, and mitogens can induce the expression of PIM-1 in hematological malignancies.42–44 In solid tumors, the expression of PIM-1 may also be induced by hypoxia through hypoxia-inducible factor 1α (HIF1α),45 DNA damage through Krüppel-like factor 5 (KLF5),46 and estrogen through the estrogen receptor.47 The majority of these factors transduce their signals through several common signaling pathways, such as the Notch pathway,10 Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway,43,48 and nuclear factor-κB (NF-κB) pathway.49
Both 5ʹ and 3ʹ UTRs of PIM-1 mRNA and the alternative translation initiation sites play a vital role in the regulation of PIM-1 expression.50 Eukaryotic translation initiation factor 4E (EIF4E) binds to the m7G cap structure in the 5ʹ UTR to regulate the expression of PIM-1; this process is termed cap-dependent translation.51 It was reported that miR328 specifically silenced the expression of PIM-1 through interaction with the PIM-1 mRNA 3ʹ UTR.52 Kim et al found that tristetraprolin bound to the adenylate-uridylate-rich element 2 in the 3ʹ UTR and enhanced the decay of PIM-1 mRNA in human prostate cancer.53 Recently, Blanco et al reported that mRNA stability factor HuR (Hu antigen R) interacted with the adenylate-uridylate-rich elements within the 3ʹ UTR in the context of hypoxia and stabilized the PIM-1 mRNA, resulting in overexpression of the PIM-1 protein in pancreatic cancer cells.54
Ubiquitylation and subsequent proteasomal degradation are the primary post-translational regulation mechanisms of PIM-1 kinase. Research studies showed that heat shock protein 90β (HSP90β) can protect PIM-1 kinase from proteasomal degradation and thus, stabilize the protein level.55 In contrast, protein phosphatase 2A (PP2A) promotes the ubiquitylation and proteasomal degradation of PIM-1.56 PIM-1 kinase has been shown to be constitutively active and does not require prior post-translational modifications for activation. However, Iyer et al57 revealed that, in vitro and in cultured cells, PIM-1 was modified by the small ubiquitin-like modifier (SUMO), and SUMOylation promoted the ubiquitin-mediated degradation of PIM-1 via recruitment of the SUMO-targeted ubiquitin ligase RNF4. Additionally, SUMOylated PIM-1 showed enhanced protein kinase activity in vitro. Hence, SUMOylation may govern PIM1 substrate specificity in certain contexts.57
PIM-1 kinase and tumors
Following its identification as an oncogene, the relationship between PIM-1 and tumors has been the emphasis of research. Firstly, upregulation of PIM-1 has been found in prostate cancer,10,58,59 squamous cell carcinoma of the head and neck,12 breast cancer,24,35 pancreatic cancer,60–62 gastric cancer,11,63 oral squamous cell cancer,64 colorectal cancer,65 hepatocellular carcinoma,66 bladder cancer,67 and non-small cell lung cancer,68,69 in addition to hematological malignancies. Secondly, dysregulation of PIM-1 has been associated with the invasiveness of cancer cells and patient prognosis. Although earlier studies suggested that upregulation of PIM-1 predicted favorable prognosis in patients with prostate cancer,58 pancreatic cancer,61 and non-small cell lung cancer,69 overexpression of PIM-1 has been linked to increasing invasiveness and/or poor prognosis in a large number of cancers (Table 2). Furthermore, the mechanisms of PIM-1-induced tumorigenesis have been studied in great depth. Eμ-Pim-1 transgenic mice overexpressing PIM-1 alone developed lymphoma with long latency and low incidence;70 thus PIM-1 is considered to be a weak oncogene. However, transgenic mice co-expressing PIM-1 and MYC succumbed to lymphomas in utero or around birth.71 Moreover, MYC has been shown to induce tumorigenesis depending on the expression of PIM-1 kinase in lymphoma, prostate cancer, and breast cancer.24,70,72,73 In prostate cancer, PIM-1 phosphorylated the serine-62 of c-MYC to induce tumorigenesis,73 while in breast cancer PIM-1 phosphorylated p27 and BAD, in addition to MYC.24 Furthermore, PIM-1 was also reported to phosphorylate AKT, facilitating the glycolysis of hepatocellular carcinoma and promoting tumor growth and metastasis.66 These findings suggest that PIM-1 may be involved in the development, progression, and maintenance of tumors via different mechanisms. This is consistent with the varied regulation of PIM-1 expression within different types of cells mentioned above. Of note, PIM-1 induced tumorigenesis in a variety of tumors, PIM-1 knockouts exerted only subtle effects without influence on growth and reproduction,74 and mice deficient for all members of the PIM family (PIM-1, 2, 3) are viable and fertile, displaying only reduced body size and impaired responses to hematopoietic growth factors.75 Based on this evidence, several research groups have generated structurally different small-molecule inhibitors targeting PIM kinases, with currently ongoing preclinical and clinical trials testing their potency for tumor inhibition.42
Table 2.
PIM-1 and prognosis of tumors
| Tumors | Dysregulation | Prognosis |
|---|---|---|
| Lymphoma7–9 | Up | Correlated to poor prognosis |
| Prostate cancer58 | Up | Correlated to favorable prognosis |
| Prostate cancer59 | Up | Related to the grade and neoplastic transformation |
| Pancreatic cancer60,62 | Up | Correlated to poor prognosis |
| Pancreatic cancer61 | Up | Has a positive prognostic impact |
| Non-small cell lung cancer68 | Up | Associated with an increase in pathological grade, lymph node metastasis, and advanced clinical stage |
| Non-small cell lung cancer69 | Down | Associated with the occurrence of lymph node metastases |
| Gastric cancer11,63 | Up | Inversely correlated to the presence of lymphovascular invasion |
| Squamous cell carcinoma of head and neck12 | Up | Correlated to poor response to radiation therapy |
| Oral squamous cell cancer64 | Up | NR |
| Breast cancer24,35 | Up | Associated with a significantly higher risk of recurrence |
| Colorectal cancer62 | Up | NR |
| Hepatocellular carcinoma65 | Up | Promoted tumor growth and metastasis |
| Bladder cancer67 | Up | Plays a role in the initiation and progression of bladder cancer |
Abbreviation: NR, not reported.
Roles of PIM-1 kinase in breast cancer
Although research on the expression and function of PIM-1 kinase in breast cancer has not been as extensive as that for other solid tumors (eg, prostate cancer), great progress in determining its roles in this type of cancer has been achieved in recent years. In 2006, Gapter et al demonstrated that the levels of both PIM-1 mRNA and protein were upregulated in breast cancer cells compared with those reported in normal breast epithelial cells.21 A subsequent study also reported that the expression of PIM-1 mRNA in human breast cancer was higher than that observed in benign breast tumors. Moreover, elevated PIM-1 expression was associated with malignancy and a higher tumor grade.47 Recently, a study performed by Jimenez–Garcia et al and based on several public databases revealed that the expression of PIM-1 was significantly increased in breast cancer compared with normal breast epithelium. Patients with higher PIM-1 expression were associated with worse prognosis in relapsed and treatment-resistant tumors.76 This evidence indicates that upregulation of PIM-1 may be a biomarker of breast cancer.
In 2016, Braso–Maristany et al35 noticed that oncogene PIM-1 is located on a genomic recurrent amplification region of 6p21-p25 in TNBC. They investigated whether the copy-number status and expression levels of PIM-’ are increased in TNBC by analyzing three independent published datasets. The results showed that the levels of PIM-1 mRNA were significantly higher in TNBC compared with those measured in non-TNBC. Furthermore, PIM-1 gene expression was significantly correlated with its copy number in TNBC.35 Meanwhile, Horiuchi et al24 identified nine kinases that were selectively required for the survival of MYC-activated non-immortalized human mammary epithelial cells, among which PIM-1 exhibited the greatest efficacy in maintaining survival. Analysis of four distinct clinical cohorts revealed that PIM-1 mRNA expression was significantly elevated in TNBC compared with that reported in non-TNBC. In addition, increased PIM-1 expression was associated with poor prognosis in patients with hormone receptor-negative tumors.24 These findings, related to the functions of PIM-1 in TNBC, indicated that PIM-1 mediated survival, tumor growth, and response to chemotherapy in cooperation with MYC in TNBC. Moreover, small-molecule inhibitors of PIM kinase halted the growth of human TNBC in a mouse model and sensitized TNBC to standard-of-care chemotherapy.24,35
Recently, Guo et al generated a highly selective red-emitting fluorescent probe targeting PIM-1 kinase for imaging cancer cells.77 This probe successfully distinguished breast cancer cells overexpressing PIM-1 kinase from normal cells in vitro and in vivo. In summary, the aforementioned findings demonstrate that upregulation of PIM-1 may be an important molecular event during the development and progression of TNBC. Thus, PIM-1 may be a reliable biomarker for the diagnosis, treatment, and prognosis of TNBC.
Conclusion and perspectives
After nearly 40 years of research, we have developed a deep understanding of the physiological structure and function of PIM-1. The role of PIM-1 in tumorigenesis has been determined to a certain extent. Exciting results have been obtained from studies involving treatment targeted to PIM-1 in lymphoma78 and prostate cancer.79 PIM-1 is overexpressed in TNBC and associated with cell survival, tumor growth, and resistance to chemotherapy in this setting.24,35 Therefore, PIM-1 may be a reliable biomarker of TNBC. Further studies are warranted to investigate the relationship between PIM-1 and MYC in TNBC, and develop highly selective compounds against PIM-1. Such investigations will provide a new opportunity for the diagnosis and treatment of TNBC.
Acknowledgment
This study was funded by the National Natural Science Foundation of China (No 81871325, 81801656).
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Cuypers HT, Selten G, Quint W, et al. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984;37(1):141. doi: 10.1016/0092-8674(84)90309-X [DOI] [PubMed] [Google Scholar]
- 2.Mikkers H, Allen J, Knipscheer P, et al. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat Genet. 2002;32(1):153–159. doi: 10.1038/ng950 [DOI] [PubMed] [Google Scholar]
- 3.Feldman JD, Vician L, Crispino M, et al. KID-1, a protein kinase induced by depolarization in brain. J Biol Chem. 1998;273(26):16535–16543. doi: 10.1074/jbc.273.26.16535 [DOI] [PubMed] [Google Scholar]
- 4.Baytel D, Shalom S, Madgar I, Weissenberg R, Don J. The human Pim-2 proto-oncogene and its testicular expression. Biochim Biophys Acta. 1998;1442(2–3):274–285. [DOI] [PubMed] [Google Scholar]
- 5.Domen J, Von Lindern M, Hermans A, Breuer M, Grosveld G, Berns A. Comparison of the human and mouse PIM-1 cDNAs: nucleotide sequence and immunological identification of the in vitro synthesized PIM-1 protein. Oncogene Res. 1987;1(1):103–112. [PubMed] [Google Scholar]
- 6.Zakut-Houri R, Hazum S, Givol D, Telerman A. The cDNA sequence and gene analysis of the human pim oncogene. Gene. 1987;54(1):105–111. doi: 10.1016/0378-1119(87)90352-0 [DOI] [PubMed] [Google Scholar]
- 7.Bellon M, Lu L, Nicot C. Constitutive activation of Pim1 kinase is a therapeutic target for adult T-cell leukemia. Blood. 2016;127(20):2439–2450. doi: 10.1182/blood-2015-11-685032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lilly M, Le T, Holland P, Hendrickson SL. Sustained expression of the pim-1 kinase is specifically induced in myeloid cells by cytokines whose receptors are structurally related. Oncogene. 1992;7(4):727. [PubMed] [Google Scholar]
- 9.Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354(23):2431–2442. doi: 10.1056/NEJMoa055759 [DOI] [PubMed] [Google Scholar]
- 10.Santio NM, Landor SK, Vahtera L, et al. Phosphorylation of Notch1 by Pim kinases promotes oncogenic signaling in breast and prostate cancer cells. Oncotarget. 2016;7(28):43220–43238. doi: 10.18632/oncotarget.9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnecke-Eberz U, Bollschweiler E, Drebber U, et al. Prognostic impact of protein overexpression of the proto-oncogene PIM-1 in gastric cancer. Anticancer Res. 2009;29(11):4451–4455. [PubMed] [Google Scholar]
- 12.Peltola K, Hollmen M, Maula SM, et al. Pim-1 kinase expression predicts radiation response in squamocellular carcinoma of head and neck and is under the control of epidermal growth factor receptor. Neoplasia. 2009;11(7):629–636. doi: 10.1593/neo.81038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meeker TC, Nagarajan L, ar-Rushdi A, Rovera G, Huebner K, Croce CM. Characterization of the human PIM-1 gene: a putative proto-oncogene coding for a tissue specific member of the protein kinase family. Oncogene Res. 1987;1(1):87–101. [PubMed] [Google Scholar]
- 14.Saris CJ, Domen J, Berns A. The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO J. 1991;10(3):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padma R, Nagarajan L. The human PIM-1 gene product is a protein serine kinase. Cancer Res. 1991;51(9):2486–2489. [PubMed] [Google Scholar]
- 16.Telerman A, Amson R, Zakut-Houri R, Givol D. Identification of the human pim-1 gene product as a 33-kilodalton cytoplasmic protein with tyrosine kinase activity. Mol Cell Biol. 1988;8(4):1498–1503. doi: 10.1128/mcb.8.4.1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian KC, Wang L, Hickey ER, et al. Structural basis of constitutive activity and a unique nucleotide binding mode of human Pim-1 kinase. J Biol Chem. 2005;280(7):6130–6137. doi: 10.1074/jbc.M409123200 [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Mandiyan V, Suzuki Y, et al. Crystal structures of proto-oncogene kinase Pim1: a target of aberrant somatic hypermutations in diffuse large cell lymphoma. J Mol Biol. 2005;348(1):183–193. doi: 10.1016/j.jmb.2005.02.039 [DOI] [PubMed] [Google Scholar]
- 19.Bullock AN, Debreczeni J, Amos AL, Knapp S, Turk BE. Structure and substrate specificity of the Pim-1 kinase. J Biol Chem. 2005;280(50):41675–41682. doi: 10.1074/jbc.M510711200 [DOI] [PubMed] [Google Scholar]
- 20.Bachmann M, Moroy T. The serine/threonine kinase Pim-1. Int J Biochem Cell Biol. 2005;37(4):726–730. doi: 10.1016/j.biocel.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Gapter LA, Magnuson NS, Ng KY, Hosick HL. Pim-1 kinase expression during murine mammary development. Biochem Biophys Res Commun. 2006;345(3):989–997. doi: 10.1016/j.bbrc.2006.04.110 [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS. Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim Biophys Acta. 2002;1593(1):45–55. doi: 10.1016/s0167-4889(02)00347-6 [DOI] [PubMed] [Google Scholar]
- 23.Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N. Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res. 2008;68(13):5076–5085. doi: 10.1158/0008-5472.CAN-08-0634 [DOI] [PubMed] [Google Scholar]
- 24.Horiuchi D, Camarda R, Zhou AY, et al. PIM1 kinase inhibition as a targeted therapy against triple-negative breast tumors with elevated MYC expression. Nat Med. 2016;22(11):1321–1329. doi: 10.1038/nm.4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachmann M, Kosan C, Xing PX, Montenarh M, Hoffmann I, Moroy T. The oncogenic serine/threonine kinase Pim-1 directly phosphorylates and activates the G2/M specific phosphatase Cdc25C. Int J Biochem Cell Biol. 2006;38(3):430–443. doi: 10.1016/j.biocel.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y. Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway. J Biol Chem. 1999;274(26):18659–18666. doi: 10.1074/jbc.274.26.18659 [DOI] [PubMed] [Google Scholar]
- 27.Yuan LL, Green AS, Bertoli S, et al. Pim kinases phosphorylate Chk1 and regulate its functions in acute myeloid leukemia. Leukemia. 2014;28(2):293–301. doi: 10.1038/leu.2013.168 [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Lin F, Zhuo C, et al. PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity under inflammation. J Biol Chem. 2014;289(39):26872–26881. doi: 10.1074/jbc.M114.586651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang Z, Li X, Magnuson NS. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27(35):4809–4819. doi: 10.1038/onc.2008.123 [DOI] [PubMed] [Google Scholar]
- 30.Winn LM, Lei W, Ness SA. Pim-1 phosphorylates the DNA binding domain of c-Myb. Cell Cycle. 2003;2(3):258–262. [PubMed] [Google Scholar]
- 31.Aho TL, Sandholm J, Peltola KJ, Ito Y, Koskinen PJ. Pim-1 kinase phosphorylates RUNX family transcription factors and enhances their activity. BMC Cell Biol. 2006;7:21. doi: 10.1186/1471-2121-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Cao Z, Xue J, et al. A positive feedback loop between Pim-1 kinase and HBP1 transcription factor contributes to hydrogen peroxide-induced premature senescence and apoptosis. J Biol Chem. 2017;292(20):8207–8222. doi: 10.1074/jbc.M116.768101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peltola KJ, Paukku K, Aho TL, Ruuska M, Silvennoinen O, Koskinen PJ. Pim-1 kinase inhibits STAT5-dependent transcription via its interactions with SOCS1 and SOCS3. Blood. 2004;103(10):3744–3750. doi: 10.1182/blood-2003-09-3126 [DOI] [PubMed] [Google Scholar]
- 34.Gu JJ, Wang Z, Reeves R, Magnuson NS. PIM1 phosphorylates and negatively regulates ASK1-mediated apoptosis. Oncogene. 2009;28(48):4261–4271. doi: 10.1038/onc.2009.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braso-Maristany F, Filosto S, Catchpole S, et al. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat Med. 2016;22(11):1303–1313. doi: 10.1038/nm.4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Wang J, Chen K, et al. eIF4B phosphorylation by pim kinases plays a critical role in cellular transformation by Abl oncogenes. Cancer Res. 2013;73(15):4898–4908. doi: 10.1158/0008-5472.CAN-12-4277 [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Liu K, Yang J, et al. PIM1 induces cellular senescence through phosphorylation of UHRF1 at Ser311. Oncogene. 2017;36(34):4828–4842. doi: 10.1038/onc.2017.96 [DOI] [PubMed] [Google Scholar]
- 38.Ha S, Iqbal NJ, Mita P, et al. Phosphorylation of the androgen receptor by PIM1 in hormone refractory prostate cancer. Oncogene. 2013;32(34):3992–4000. doi: 10.1038/onc.2012.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y, Xu K, Dai B, et al. The 44 kDa Pim-1 kinase directly interacts with tyrosine kinase Etk/BMX and protects human prostate cancer cells from apoptosis induced by chemotherapeutic drugs. Oncogene. 2006;25(1):70–78. doi: 10.1038/sj.onc.1209058 [DOI] [PubMed] [Google Scholar]
- 40.Xie Y, Xu K, Linn DE, et al. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J Biol Chem. 2008;283(6):3349–3356. doi: 10.1074/jbc.M707773200 [DOI] [PubMed] [Google Scholar]
- 41.Katsube A, Hayashi H, Kusuhara H. Pim-1L protects cell surface-resident ABCA1 from lysosomal degradation in hepatocytes and thereby regulates plasma high-density lipoprotein level. Arterioscler Thromb Vasc Biol. 2016;36(12):2304–2314. doi: 10.1161/ATVBAHA.116.308472 [DOI] [PubMed] [Google Scholar]
- 42.Blanco-Aparicio C, Carnero A. Pim kinases in cancer: diagnostic, prognostic and treatment opportunities. Biochem Pharmacol. 2013;85(5):629–643. doi: 10.1016/j.bcp.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 43.Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koskinen PJ, Julkunen I. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc, and pim-1 genes in human T cells. Blood. 1999;93(6):1980–1991. [PubMed] [Google Scholar]
- 44.Wang Z, Bhattacharya N, Weaver M, et al. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J Vet Sci. 2001;2(3):167–179. [PubMed] [Google Scholar]
- 45.Casillas AL, Toth RK, Sainz AG, et al. Hypoxia-inducible PIM kinase expression promotes resistance to antiangiogenic agents. Clin Cancer Res. 2018;24(1):169–180. doi: 10.1158/1078-0432.CCR-17-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Hamza MS, Leong HS, et al. Kruppel-like factor 5 modulates p53-independent apoptosis through Pim1 survival kinase in cancer cells. Oncogene. 2008;27(1):1–8. doi: 10.1038/sj.onc.1210625 [DOI] [PubMed] [Google Scholar]
- 47.Malinen M, Jaaskelainen T, Pelkonen M, et al. Proto-oncogene PIM-1 is a novel estrogen receptor target associating with high grade breast tumors. Mol Cell Endocrinol. 2013;365(2):270–276. doi: 10.1016/j.mce.2012.10.028 [DOI] [PubMed] [Google Scholar]
- 48.Shirogane T, Fukada T, Muller JM, Shima DT, Hibi M, Hirano T. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 1999;11(6):709–719. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Parsanejad M, Huang E, et al. Pim-1 kinase as activator of the cell cycle pathway in neuronal death induced by DNA damage. J Neurochem. 2010;112(2):497–510. doi: 10.1111/j.1471-4159.2009.06476.x [DOI] [PubMed] [Google Scholar]
- 50.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer. 2011;11(1):23–34. doi: 10.1038/nrc2986 [DOI] [PubMed] [Google Scholar]
- 51.Hoover DS, Wingett DG, Zhang J, Reeves R, Magnuson NS. Pim-1 protein expression is regulated by its 5ʹ-untranslated region and translation initiation factor elF-4E. Cell Growth Differ. 1997;8(12):1371–1380. [PubMed] [Google Scholar]
- 52.Eiring AM, Harb JG, Neviani P, et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140(5):652–665. doi: 10.1016/j.cell.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HK, Kim CW, Vo MT, et al. Expression of proviral integration site for Moloney murine leukemia virus 1 (Pim-1) is post-transcriptionally regulated by tristetraprolin in cancer cells. J Biol Chem. 2012;287(34):28770–28778. doi: 10.1074/jbc.M112.376483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanco FF, Jimbo M, Wulfkuhle J, et al. The mRNA-binding protein HuR promotes hypoxia-induced chemoresistance through posttranscriptional regulation of the proto-oncogene PIM1 in pancreatic cancer cells. Oncogene. 2016;35(19):2529–2541. doi: 10.1038/onc.2015.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizuno K, Shirogane T, Shinohara A, Iwamatsu A, Hibi M, Hirano T. Regulation of Pim-1 by Hsp90. Biochem Biophys Res Commun. 2001;281(3):663–669. doi: 10.1006/bbrc.2001.4405 [DOI] [PubMed] [Google Scholar]
- 56.Ma J, Arnold HK, Lilly MB, Sears RC, Kraft AS. Negative regulation of Pim-1 protein kinase levels by the B56beta subunit of PP2A. Oncogene. 2007;26(35):5145. doi: 10.1038/sj.onc.1210323 [DOI] [PubMed] [Google Scholar]
- 57.Iyer RS, Chatham L, Sleigh R, Meek DW. A functional SUMO-motif in the active site of PIM1 promotes its degradation via RNF4, and stimulates protein kinase activity. Sci Rep. 2017;7(1):3598. doi: 10.1038/s41598-017-03775-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dhanasekaran SM, Barrette TR, Ghosh D, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412(6849):822–826. doi: 10.1038/35090585 [DOI] [PubMed] [Google Scholar]
- 59.Xu Y, Zhang T, Tang H, et al. Overexpression of PIM-1 is a potential biomarker in prostate carcinoma. J Surg Oncol. 2005;92(4):326–330. doi: 10.1002/jso.20325 [DOI] [PubMed] [Google Scholar]
- 60.Xu J, Zhang T, Wang T, You L, Zhao Y. PIM kinases: an overview in tumors and recent advances in pancreatic cancer. Future Oncol. 2014;10(5):865–876. doi: 10.2217/fon.13.229 [DOI] [PubMed] [Google Scholar]
- 61.Reiser-Erkan C, Erkan M, Pan Z, et al. Hypoxia-inducible proto-oncogene Pim-1 is a prognostic marker in pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2008;7(9):1352–1359. doi: 10.4161/cbt.7.9.6418 [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Xiong G, Cao Z, et al. PIM-1 contributes to the malignancy of pancreatic cancer and displays diagnostic and prognostic value. J Exp Clin Cancer Res. 2016;35(1):133. doi: 10.1186/s13046-016-0406-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan B, Yau EX, Samanta S, et al. Clinical and therapeutic relevance of PIM1 kinase in gastric cancer. Gastric Cancer. 2012;15(2):188–197. doi: 10.1007/s10120-011-0097-2 [DOI] [PubMed] [Google Scholar]
- 64.Chiang WF, Yen CY, Lin CN, et al. Up-regulation of a serine-threonine kinase proto-oncogene Pim-1 in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2006;35(8):740–745. doi: 10.1016/j.ijom.2006.01.027 [DOI] [PubMed] [Google Scholar]
- 65.Peng YH, Li JJ, Xie FW, et al. Expression of pim-1 in tumors, tumor stroma and tumor-adjacent mucosa co-determines the prognosis of colon cancer patients. PLoS One. 2013;8(10):e76693. doi: 10.1371/journal.pone.0076693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leung CO, Wong CC, Fan DN, et al. PIM1 regulates glycolysis and promotes tumor progression in hepatocellular carcinoma. Oncotarget. 2015;6(13):10880–10892. doi: 10.18632/oncotarget.3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo S, Mao X, Chen J, et al. Overexpression of Pim-1 in bladder cancer. J Exp Clin Cancer Res. 2010;29:161. doi: 10.1186/1756-9966-29-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin Y, Tong DY, Chen JN, et al. Overexpression of osteopontin, alphavbeta3 and Pim-1 associated with prognostically important clinicopathologic variables in non-small cell lung cancer. PLoS One. 2012;7(10):e48575. doi: 10.1371/journal.pone.0048575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warnecke-Eberz U, Bollschweiler E, Drebber U, et al. Frequent down-regulation of pim-1 mRNA expression in non-small cell lung cancer is associated with lymph node metastases. Oncol Rep. 2008;20(3):619–624. [PubMed] [Google Scholar]
- 70.van Lohuizen M, Verbeek S, Krimpenfort P, et al. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989;56(4):673–682. doi: 10.1016/0092-8674(89)90589-8 [DOI] [PubMed] [Google Scholar]
- 71.Verbeek S, van Lohuizen M, van der Valk M, Domen J, Kraal G, Berns A. Mice bearing the E mu-myc and E mu-pim-1 transgenes develop pre-B-cell leukemia prenatally. Mol Cell Biol. 1991;11(2):1176–1179. doi: 10.1128/mcb.11.2.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Anderson PD, Luo W, Gius D, Roh M, Abdulkadir SA. Pim1 kinase is required to maintain tumorigenicity in MYC-expressing prostate cancer cells. Oncogene. 2012;31(14):1794–1803. doi: 10.1038/onc.2011.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Kim J, Roh M, et al. Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma. Oncogene. 2010;29(17):2477–2487. doi: 10.1038/onc.2010.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laird PW, van der Lugt NM, Clarke A, et al. In vivo analysis of Pim-1 deficiency. Nucleic Acids Res. 1993;21(20):4750–4755. doi: 10.1093/nar/21.20.4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mikkers H, Nawijn M, Allen J, et al. Mice deficient for all PIM kinases display reduced body size and impaired responses to hematopoietic growth factors. Mol Cell Biol. 2004;24(13):6104–6115. doi: 10.1128/MCB.24.13.6104-6115.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jimenez-Garcia MP, Lucena-Cacace A, Robles-Frias MJ, et al. Inflammation and stem markers association to PIM1/PIM2 kinase-induced tumors in breast and uterus. Oncotarget. 2017;8(35):58872–58886. doi: 10.18632/oncotarget.19438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo S, Fan J, Wang B, et al. Highly selective red-emitting fluorescent probe for imaging cancer cells in situ by targeting Pim-1 kinase. ACS Appl Mater Interfaces. 2018;10(2):1499–1507. doi: 10.1021/acsami.7b14553 [DOI] [PubMed] [Google Scholar]
- 78.Cortes J, Tamura K, DeAngelo DJ, et al. Phase I studies of AZD1208, a proviral integration Moloney virus kinase inhibitor in solid and haematological cancers. Br J Cancer. 2018;118(11):1425–1433. doi: 10.1038/s41416-018-0082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mologni L, Magistroni V, Casuscelli F, Montemartini M, Gambacorti-Passerini C. The novel PIM1 inhibitor NMS-P645 reverses PIM1-dependent effects on TMPRSS2/ERG positive prostate cancer cells and shows anti-proliferative activity in combination with PI3K inhibition. J Cancer. 2017;8(1):140–145. doi: 10.7150/jca.15838 [DOI] [PMC free article] [PubMed] [Google Scholar]