Abstract
Material properties of implants such as volume porosity and nanoscale surface modification have been shown to enhance cell-material interactions in vitro and osseointegration in vivo. Porous tantalum (Ta) and titanium (Ti) coatings are widely used for non-cemented implants, which are fabricated using different processing routes. In recent years, some of those implants are being manufactured using additive manufacturing. However, limited knowledge is available on direct comparison of additively manufactured porous Ta and Ti structures towards early stage osseointegration. In this study, we have fabricated porous Ta and Ti6Al4V (Ti64) implants using laser engineered net shaping (LENS™) with similar volume fraction porosity to compare the influence of surface characteristics and material chemistry on in vivo response using a rat distal femur model for 5 and 12 weeks. We have also assessed whether surface modification on Ti64 can elicit similar in vivo response as porous Ta in a rat distal femur model for 5 and 12 weeks. The harvested implants were histologically analyzed for osteoid surface per bone surface. Field emission scanning electron microscopy (FESEM) was done to assess the bone-implant interface. The results presented here indicate comparable performance of porous Ta and surface modified porous Ti64 implants towards early stage osseointegration at 5 weeks post implantation through seamless bone-material interlocking. However, a continued and extended efficacy of porous Ta is found in terms of higher osteoid formation at 12 weeks post-surgery.
Keywords: Tantalum, Porosity, Titanium, Titania nanotubes, Osseointegration, Bone-healing
1. Introduction
Commercially available porous titanium [Tapered Screw-Vent® Implant, Zimmer Dental Inc., Carlsbad, CA, USA] and porous tantalum [Trabecular Metal material, Zimmer, Trabecular Metal Technology, Inc., Parsippany, NJ, USA] structures for orthopedic and dental implants are fabricated using different processing route and parameters [1,2]. The difference in optimized processing techniques stem from individual intrinsic material properties of Ti (and its alloys) and Ta such as density (ρ) and melting points (TM) of Ta (ρ = 16.4 gm/cc, TM = 3290 K) and Ti64 (ρ = 4.506 gm/cc, TM = 1941 K). As a result of customized fabrication, drawing a direct comparison based on biological response of porous Ti64 and Ta structures as a function of porosity and material chemistry becomes erroneous. This study is focused on direct comparison of material chemistry and porosity effects on Ti64 and Ta implants towards early-stage osseointegration. In order to do so, we have fabricated porous Ti64 and Ta structures under similar processing routine and same volume fraction porosity using Laser Engineered Net Shaping (LENS™). In addition, in an attempt to increase the biological response of Ti64, nanoscale surface modification in the form of titania nanotubes was implemented on porous Ti64 implants.
Since the mid-1900s, the biomedical industry has seen the emergence of numerous Class II and III medical devices fashioned from Ti alloys and Ta, including cranioplasty plates, pacemaker electrodes and radio markers [3]. While Ti64 is a well-established and relatively easy-to-fabricate osseous implant material [4–6], Ta is a newer addition in the industry [2,7,8]. Tantalum, is ductile and chemically resistant, which exhibits exceptional bio-compatibility when compared to surgical grade Ti64 alloy in both in vitro and in vivo applications [9–14]. Despite its brilliant candidacy, higher cost of Ta and difficulty in manufacturing have rendered it underappreciated in medical consumerism [9,15]. Porous Ti (and its alloys) and Ta coatings are manufactured under individually optimized parameters and commercially used for a large variety of load-bearing and dental implants [1,7,16]. Additive manufacturing processes like powder bed-based fabrication (Laser or Electron-beam) where the entire part is required to be made out of a single material such as Ta, is not a suitable choice for fabrication of coating on a finished part. Binder jetting is another option, but again this is a powder bed process that is good for single material design and cannot be used to add features on an existing part. In contrast, laser based directed energy deposition (DED) process can be used to manufacture porous Ta and Ti (both Ti and Ti64) coatings on implants to minimize processing complexity while allowing for flexibility in designs as well as on-demand manufacturing [17].
The first-generation evaluation on additively manufactured porous Ta on Ti towards biological response was done by Balla et. al. (2010) [9,16]. Since then, several studies have demonstrated in vitro and in vivo osteo-compatibility of porous Ti /Ti64 and Ta structures [18–22], and listed in Table 1. Table 1 also report discrete evaluation of biological performance of two different materials, porous Ti (and its alloys) and Ta, which are fabricated using different processing routes. However, after all this work, it is still unclear whether porous Ta shows better biological performance than porous Ti/Ti64 as perceived in the biomedical device industry. Therefore, a knowledge gap still exists concerning a direct and unbiased comparison of porous Ti64 and Ta coatings exclusively based on material chemistry towards in vivo efficacy when processing routine and volume fraction porosity are kept constant which essentially forms the objective of the current study. We hypothesize that when porosity is kept constant, material chemistry determines bone-tissue integration ability in vivo, particularly during early stage osseointegration which leads to faster healing for patients (Table 2).
Table 1.
Processing approach and porosities of additively fabricated Ti and Ta.
Table 2.
LENS parameters used to fabricate 30Ti64, 30Ta and dense Ti64 samples.
| Sample | Laser Power (W) | Scan speed (mm/s) | Feed rate (g/min) | E (J/mm3) |
|---|---|---|---|---|
| Ti64 | 375 ± 3 | 10 | 14.8 | 98.42 |
| Ta | 430 ± 3 | 18 | 18 | 62.77 |
We further hypothesize that a combination of porosity and nanotube-based surface modification in Ti64 can elicit similar biological performance as porous Ta. Development of nano-architectural surface by oxidative anodization of Ti64 to form titania nanotubes have been explored in various studies towards enhancing in vitro and in vivo efficacy of Ti64 implants. Presence of titania (TiO2) nanotubes (TNT) provides a nanoscale surface roughness on Ti64 resulting in introduction of bioactivity through higher surface to volume ratio resulting in better bone tissue bonding ability [25–27]. TNT have also been shown to improve surface wettability of Ti, which in turn increases the affinity of cellular structures to attach and proliferate on such surfaces [28,29]. It is already established that presence of bulk porosity in a material can significantly reduce the mechanical mismatch in modulus of elasticity between the implant and surrounding bone while enhancing biological fixation thus avoiding likelihood of implant loosening. Keeping this in mind, several studies have been conducted on porous Ti (Ti64) implants in combination with TNT surface modification which demonstrated higher mechanical stability, in vitro cellular attachment and in vivo osseointegration as compared to dense Ti64 [21,30,31]. However, limited literature is available on whether this improvement in biological response of Ti64 through TNT surface modification can equate its in vivo performance to that of Ta. The key novelty of our research lies in understanding the effect of material chemistry of Ti64 and Ta towards early stage osseointegration, when volume fraction porosity and LENS™ fabrication are kept constant. We also present a novel evaluation of early stage in vivo response of TNT grown Ti64 implants in direct comparison to porous Ta.
In the current study we have used DED based LENS™ to manufacture dense Ti64, 30 vol % porous Ti64 (30Ti64), 30 vol % porous Ti64 with titania nanotube modified surface (30TNT), and 30 vol % porous Ta (30Ta) structures to directly compare in vivo efficacy as a function of material chemistry for early stage osseointegration using a rat distal femur model for 5 and 12 weeks.
2. Materials and methods
2.1. Fabrication of porous Ti64 and Ta implants using LENS™
Ti64 and Ta samples with 30% volume fraction porosity were fabricated using a commercially available LENS™ (Optomec Inc., Albuquerque, NM) system. The rods were additively fabricated using Ti64 (45–150 μm) and Ta (45–75 μm) powder based on previously optimized parameters [22–24]. Briefly, the Ta rods were engineered at a laser power of 375 ± 3 W, with a scan speed of 10 mm/s and a powder flow rate of 14.8 g/min. Similarly, the porous Ti64 samples were built using comparable build parameters, with a laser power of 430 ± 3 W, scan speed of 18 mm/s and a powder feed rate of 18 g/min. The slice thickness and hatch spacing were kept the same at 0.5 mm and 0.762 mm, respectively, in order to get similar volume fraction porosity. The designed volume fraction porosity was chosen to be 30% for all the samples based on previous literature [22]. Experimental pore volume calculations were done using Archimedes principle from weight and apparent volume of porous rods at room temperature.
The optimized LENS™ parameters based on the different laser energy inputs used in this work have been tabulated in Table 1. The total energy input per volume (E) was also calculated as a function of processing parameters [32]
where, P is laser power, ν is scan speed, h is the hatch distance and t is layer thickness.
2.2. Electrochemical anodic oxidation of porous Ti64 implants
Post build, all samples were ground using 1000 grit silicon carbide paper and then ultrasonicated using ethanol and DI water to get rid of the loosely attached particles. The LENS™ processed 30Ti64 rods were anodized using a standard electrochemical anodization process [33] to form nanotubes on the surface. The anodization setup consisted of platinum cathode and the sample as anode. An ethylene glycol electrolyte containing 1% hydrofluoric acid (HF) and 0.5% ammonium fluoride salt was used as source and buffer for fluoride ions (F−) in the electrolyte in order to initiate pit formation. The anodization was carried out for 1 h at 40 V to form nanotubes on the surface of the samples. The anodized samples were then further rinsed with DI water to get rid of any residual HF followed by cutting the rods into in vivo implants of dimension 3 mm in diameter and 5 mm length.
2.3. In vivo study
2.3.1. Surgery and implantation procedure
Male Sprague-Dawley rats with average weights between 280 and 300 g were used for the in vivo study. The surgeries were performed on a distal femur model according to Institutional Animal Care and Use Committee (IACUC) of Washington State University (Pullman, WA) protocol for the experimental and surgical procedure. The rats were acclimatized in a controlled environment with alternate light and dark hours for a week before surgery. The animals were shaved around the implantation area prior to surgery and scrubbed with an alternate application of chlorohexidine and isopropyl alcohol to disinfect the area. At least 30 min. prior to anaesthesia, the animals were administered Buprenorphine (0.03 mg kg−1) subcutaneously as an analgesic and post-surgery, a pain reducing medication (meloxicam, 0.2 mg/kg: 24 h frequency). Following that, the animals were anesthetized using a previously determined dose of IsoFlo® (isoflurane, USP, Abbott Laboratories, North Chicago, IL, USA) coupled with oxygen (Oxygen USP, A–L Compressed Gases Inc., Spokane, WA, USA). Animals were intermittently monitored by pedal reflex and respiration rate to maintain continued anaesthesia. An incision at the fascia was made to expose the underlying condyle and epicondyle region of the distal femur followed by creating a defect comparable to the size of the implant using a gradual diametric increment in drill bit. The defect site was rinsed with saline and the implant is press-fit in the defect. The study was conducted through an incomplete randomized block design to distribute the placement of the implants in the rats in a statistically relevant manner. Post-procedure, the fascia at the incision site was sutured underneath the skin using an undyed braided coated MONOCRYL-polyglactin 910 (Ethicon Inc., Somerville, NJ, USA) and the outer skin was stapled using surgical staples. Betadine was applied over the sutured incision site to prevent superficial infection. 30Ti64, 30Ta, 30TNT and dense Ti64 treatments were implanted in vivo for the 5-weeks study. However, only 30TNT, 30Ta and dense Ti64 treatments were considered for 12 weeks in vivo study.
2.3.2. Evaluation of bone-material interlocking
Mechanical interlocking between bone and material was calculated using push out experiments with a load cell (136.07 kgs) (Instron 3433, Norwood, MA). A clamping arrangement to hold the specimens in place was used to avoid any translational displacement in X and Y directions while the load was applied along the Z-direction. Shear modulus calculations from stress-strain plots generated from load – displacement data provides an idea of bonding strength between the material and the host tissue.
2.3.3. Histology and field emission scanning electron microscopy (FESEM) characterization
Post 5 weeks and 12 weeks of implantation, samples were harvested for histological analysis. After fixation in 10% formalin for 72 h and serial dehydration of ethanol and acetone, bone-implant samples were embedded in Spurrs resin. They were further sliced into thin sections using diamond saw. After mounting on glass slides, modified Masson Goldner’s trichrome staining was used for the histological analysis. Stained implant-tissue sections were then observed under light microscope (Olympus BH-2, Olympus America Inc., USA) at a low voltage of 5 kV and FESEM (FEI Quanta 200, FEI Inc., OR, USA) under low vacuum conditions.
2.3.4. Histomorphometric analysis of optical microscope images from histology
The optical images of the stained histology slides were used for histomorphometric analysis. The histomorphometric evaluation was carried out using Gimp 2.8 software. A standardized region of interest of 0.25 mm thickness was estimated radially from the bone-implant interface which was determined based on earlier studies [4,5,34,35] (Supplementary Fig. 1). The reddish-orange stain for osteoid tissue was isolated from the other stain colors and osteoid surface per bone surface (OS/BS%) was calculated for both 5 and 12-week histology data. Example of detailed calculation for histomorphometry from histology micrographs for dense Ti64 treatment on 5 and 12 weeks has been provided in Supplementary Figs. 2 and 3.
2.4. Statistical analysis
The calculated data from histomorphometric analysis has been reported as the mean ± standard deviation (error bars). A statistical ANOVA was performed using Minitab 17 (Minitab Inc, PA) for treatments and time points. Pairwise comparisons between multiple treatments were done using a Dunnet’s simulation with α = 0.05. A p-value < α was marked as significant. Power analysis of the in vivo study-design was performed using Minitab 17.
3. Results
3.1. LENS™ fabrication of the porous Ta and Ti64 samples
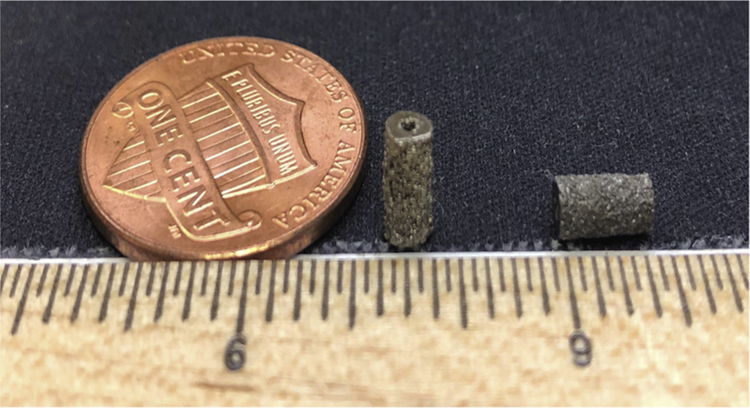
Ti64 and Ta samples were additively manufactured using LENS™ and shown in Fig. 1. The laser power was varied for the two metals based on their physical and chemical properties such as thermal conductivity, laser absorptivity and melting temperature. The thermal conductivities of Ti64 and Ta at 20 °C are 7.2 W/mK and 54 W/mK, respectively, while laser absorptivity for Ti64 and Ta powders is 0.30 and 0.32, respectively [36]. The fabricated Ti64 and Ta samples had 30% volume fraction porosity. The laser energy input per volume (E) based on equation mentioned in section 2.1 for Ti64 and Ta were calculated to be 98.42 J/mm3 and 62.77 J/mm3, respectively. The designed porosity was determined to be 30 vol % for the LENS™ operation based on our earlier study [22]. Experimental calculations from Archimedes principle showed actual volume fraction porosity of 25 ± 3% for all compositions which is in accordance to our earlier finding of 25–32% porosity in Ti64 exhibited modulus close to natural bone as well as resulted in highest Ca2+ ion deposition indicating better facilitation of fluid/ion exchange [22].
Fig. 1.
Macroscopic image of LENS™ processed porous Ti64 and Ta samples.
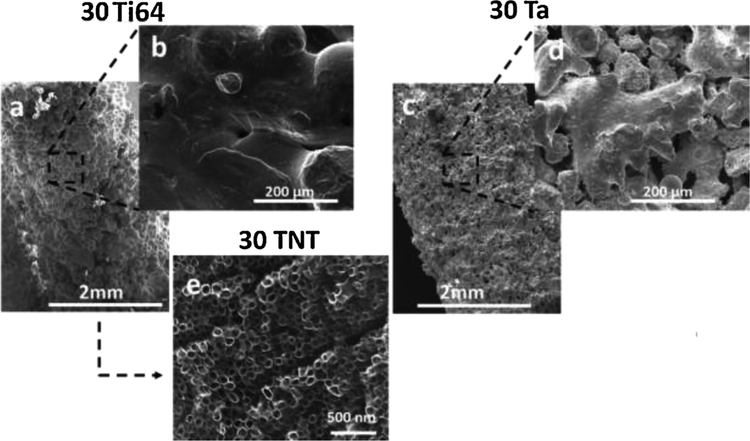
Fig. 2 shows the FESEM surface morphology of 30Ti64, 30TNT and 30Ta. Abundance of pores on the surface of 30Ta and 30Ti64 can be seen in Fig. 2a and c. The surface of 30TNT shows the presence of nanoscale pores and a smoother topography due to the presence of TiO2 nanotubes, shown in Fig. 2e. Bimodal distribution of macro and micro pores can be seen in Ta and Ti64 surfaces, ranging from 20 μm to 200 μm as shown in Fig. 2b and d. The nanotubes formed on the sample surface were measured to have average diameter of 93.83 ± 5 nm.
Fig. 2.
Shows the FESEM micrographs of the three treatments. All three surfaces show presence of pores. The surface morphology of 30Ta shows a grained appearance while 30TNT shows smoother surface due to the presence of TiO2 nanotubes (e).
3.2. Histological analysis of stained cross-section of explanted bone-implant conjugates
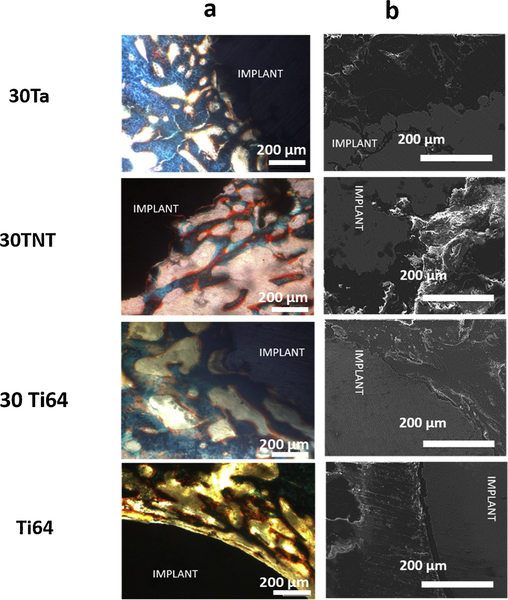
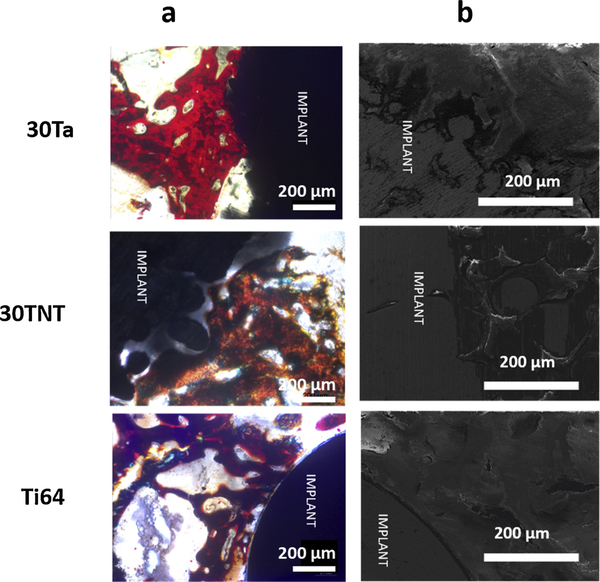
Figs. 3a and 4 a show the cross-sectional optical micrographs of the control (dense Ti64) and three treatment groups from the harvested bone-implant samples at 5- and 12-weeks post implantation, respectively. Fig. 3a shows comparable osteoid formation at 5 weeks between both 30Ta and 30TNT samples, which were higher than the control Ti64 as well as 30Ti64. Bone interlocking was also observed under microscope for 30Ta and 30TNT treatments in comparison to clear interfacial gaps visible in the Ti64 and 30Ti64 treatments. On the other hand, the optical micrographs at 12 weeks post implantation, shown in Fig. 4a, showed increase in the amount of osteoid surface per bone surface (OS/BS) for 30Ta treatment in comparison to all the rest of the compositions. Better bone-material interlocking was observed in all three treatments in contrast to clear interfacial gaps in Ti64.
Fig. 3.
a shows optical and b shows FESEM micrographs of bone-implant interface 5 weeks post implantation. Osteoid formation is shown in reddish orange color and mineralized bone in bluish-green color. Presence of gaps between the host bone and implant interface has been observed in the Dense Ti64 samples. 30Ta, 30Ti64 and 30TNT samples show very good bone-implant interlocking with comparable osteoid formation in 30TNT and 30Ta treatments (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 4.
a shows optical micrograph and b shows FESEM micrograph of bone-implant interface 12 weeks post implantation. Presence of gaps between the host bone and implant interface has been observed in the Dense Ti64 samples. 30Ta treatment show comparatively higher osteoid formation than 5 weeks as well as 30TNT treatment.
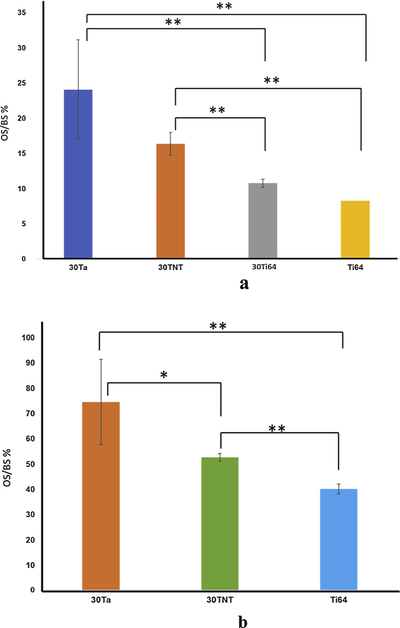
Fig. 5a and b show the histomorphometric calculation for explants at 5 and 12 weeks. All four treatments show extremely significant interaction within the treatments. Pairwise comparison evaluated through t-test for means reveals detailed insight into osteoid formation capability of individual treatments in contrast to others through OS/BS % measurement. 30TNT show comparable osteoid formation to 30Ta around the implant which is significantly higher than the Ti64 and 30Ti64 controls at 5 weeks. However, all three compositions show increase in osteoid formation from 5 weeks to 12 weeks. 30Ta was observed to outperform 30TNT and Ti64 at 12 weeks.
Fig. 5.
a and b show the histomorphometric plots of OS/BS% for 5 weeks and 12 weeks histology data, respectively. *- stand of significant difference (P value < 0.05) while **- stand for extremely significant difference in means (P value < 0.01).
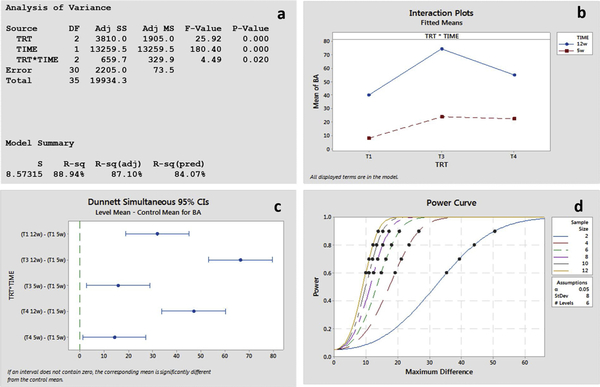
Fig. 6 shows statistical analysis on obtained histomorphometric data. ANOVA (Fig. 6d) and interaction plot (Fig. 6a), show significant interaction between treatment and time points. Dunnett’s simulation at 95% confidence interval indicates difference in means between treatments and control at 5 and 12 weeks (Fig. 6b). The post-hoc power curve, for a sample size of 6 with a standard deviation estimate of 8.08 for 80% effect size, detected a difference of 18.5 (Fig. 6c).
Fig. 6.
a ANOVA results showing significant interaction between treatment and time points, b. Interaction plot for 5 and 12 weeks for dense Ti64 (T1), 30Ta (T3) and 30TNT (T4), c. Dunnett simulation for multiple comparisons showing difference in means between control and treatments, d. post-hoc power analysis curve for effective sample size 80%.
3.3. Analysis of bone-implant interface to evaluate osseointegration
Push out tests were performed to evaluate mechanical interlocking at the bone-material interface. However, bone broke for all samples, which indicates that the mechanical interlocking was similar if not stronger than the strength of the bone. FESEM analyses were performed as a measure of evaluating morphological characteristics of the bone- implant interface. Figs. 3b and 4 b show the FESEM micrographs of the bone tissue- material cross sectional area for the control (Ti64) and the treatment groups (30Ti64, 30Ta, 30TNT) at 5- and 12-week post-surgery. Clear interfacial gaps are seen in Ti64 at both 5 and 12 weeks. 30Ta and 30TNT treated groups show very well defined and seamless interface between implant and bone tissue in contrast to 30Ti64 which shows presence of reduced gaps. The 30Ta treated group shows very well ingrown bone into the pores of the implant demonstrating bone-material interlocking.
4. Discussion
The present work involves a direct comparison of biological response from porous Ti64 and Ta fabricated using singular additive manufacturing approach with uniform 30% volume fraction porosities. The input laser energies are calculated to be 98.42 J/mm3 and 62.77 J/mm3, respectively using Simchi’s equation [37]. These values directly correlate the intrinsic laser absorption coefficient of Ti64 and Ta, which is a material property, to an external parameter such as the laser power input. Higher laser absorption coefficient of Ta (0.32) justifies the lower E (62.77 J/mm3) needed to melt the powder required to fabricate the porous structure. The purpose of this study is to use similar approaches to built porous structures of Ti64 and Ta to directly compare their biological efficacy as a function of surface architecture and material chemistry.
Fig. 2e and d, show difference in porosity and surface morphology between 30TNT and 30Ta. The surface of 30TNT exhibited a smoother texture in contrast to 30Ta due to the presence of TiO2 nanotubes which impart a nanoscale roughness. This variation in texture can be correlated to the effect of surface topography on cell attachment for the treatments. Studies have shown that chemical properties, texture and energy of the surface of a material have significant effect on cell-material interactions [38]. It has been reported that substrates with more grained microstructure have higher surface energy than smoother surfaces [39]. Therefore, it is plausible that the specific microtexture of 30Ta surface can promote osteoconduction and hence osseointegration through surface roughness [24] and upregulation of specific integrins which results in higher recruitment of osteoprogenitor cells [8].
Histological evaluation through OS/BS percent as a measure of osteoid formation at 5 and 12-weeks post implantation validates our findings from the FESEM analyses. Fig. 3a, shows comparable osteoid formation for 30TNT and 30Ta (1.56fold increase from 30TNT) at 5 weeks which were higher than dense Ti64 by an average of 12-fold OS. These values substantiate shorter healing time of as early as 5 weeks as well as faster bone-material interlocking for both 30Ta and 30TNT treatment groups as a function of new bone formation. The influence of Ta chemistry is also evident at this early time point in terms of OS as shown in Figs. 4a and 5 a. The observation of continued and higher osteoid formation in Figs. 4b and 5 b at 12 weeks for 30Ta, 30TNT as well as Ti64 was made. Significant interaction in Fig. 6b shows interdependency of treatments and time-points, which signify that new bone formation through OS/BS is dependent not only on composition but also on the time points. These results signify the effect of Ta chemistry towards excellent in vivo biocompatibility by altering the bone-remodelling cycle at early time point. The inherent biological activity shown by Ta [3,9,12,13] supported by higher surface area and rough topography of the pores, based on built parameters and powder characteristics, provide better cellular attachment and bone interlocking through porous channels in the bulk of the implant.
Figs. 3b and 4 b show the FESEM micrographs for the Ti64 (control) in contrast to the other treatments at 5- and 12-weeks post implantation, respectively. The clear gaps seen in dense Ti64 results from very poor to almost no bone-implant interlocking due to inherent inertness of Ti64 and poor biocompatibility of Ti64 [40]. 30Ti64 shows reduced gap width at the bone-implant interface due to the introduction of porosity resulting in biological fixation compared to dense Ti64. Al-though, presence of gaps still reflects the bio-inert nature of pure Ti64 which results in inadequate bioactive fixation. On the other hand, 30Ta and 30TNT show seamless bone ingrowth and interlocking at the bone-material interface as early as 5 weeks of implantation.
It is clear from the histological and FESEM micrographs at 5 and 12 weeks that volume fraction porosity plays a key role in early stage osseointegration of porous metallic implants. These observations provide a distinct insight into the direct comparison between Ti64 and Ta as osseous implant materials as well as influence of surface characteristics and chemistry on osseointegration when fabrication approach and volume fraction porosity are kept constant. It is evident from our results that 30Ta and 30TNT show similar efficacy in early stage osseointegration (at 5 weeks) while 30Ta shows the influence of material chemistry in extended and continued healing.
Our experimental results on fabrication and direct comparison of in vivo biological response of porous Ta and Ti64 through qualitative histological and quantitative histomorphometry correlation displays the influence of surface architecture and material chemistry on early stage osseointegration. Thus, proving the efficiency of 30TNT, as a comparable candidate to 30Ta for orthopedic applications in terms of in vivo osseointegration at early time point such as 5 weeks post-surgery.
5. Conclusions
Porous metallic bone implants of Ta and Ti64 were fabricated using Laser Engineered Net Shaping (LENS™) with 30% volume porosity while keeping a similar processing conditions. A comparative evaluation on in vivo osseointegration of three treatments (30Ti64, 30Ta, 30TNT) was conducted in contrast with a control group (dense Ti64) through FESEM and histological characterizations. Porous TNT showed comparable candidacy to porous Ta as an orthopedic implant material owing to its adequate biological performance at both 5 and 12 weeks among the treatment groups. Comparable interfacial integration of both Ta and TNT with host bone at 5 weeks is an indication of faster bone-interlocking, and hence shorter healing time as a function of bulk porosity and surface characteristics. The higher osteoid formation at 12 weeks for all three compositions show better biocompatibility and longevity of these materials in terms of continued healing, although, with time the influence of bulk porosity and surface modifications reduces towards bone-healing. At this extended period, the intrinsic material chemistry drives the biological response from Ta. Overall, porous TNT showed a comparable efficacy to Ta as well as exhibited efficient performance as a biomaterial for arthroplasty through the influence of porosity, chemistry and surface modification on early stage osseointegration. Our results not only demonstrated a way to directly compare biological efficacy of porous Ti64 and Ta through laser based additive manufacturing but also, exhibited reliable and beneficial findings such as the comparable efficiency of porous TNT to porous Ta, which also makes arthroplasty more cost effective.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR067306-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.addma.2019.04.025.
References
- [1].Bencharit S, Byrd WC, Altarawneh S, Hosseini B, Leong A, Reside G, Morelli T, Offenbacher S, Development and applications of porous tantalum trabecular metal-enhanced titanium dental implants, Clin. Implant Dent. Relat. Res 16 (2014) 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bencharit S, Byrd WC, Hosseini B, Immediate placement of a porous-tantalum, trabecular metal-enhanced titanium dental implant with demineralized bone matrix into a socket with deficient buccal bone: a clinical report, J. Prosthet. Dent 113 (2015) 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Black J, Biologic performance of tantalum, Clin. Mater 16 (1994) 167–173. [DOI] [PubMed] [Google Scholar]
- [4].Li K, Wang C, Yan J, Zhang Q, Dang B, Wang Z, Yao Y, Lin K, Guo Z, Bi L, Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: an in vivo study, Sci. Rep 8 (2018) 1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].He T, Cao C, Xu Z, Li G, Cao H, Liu X, Zhang C, Dong Y, A comparison of micro-CT and histomorphometry for evaluation of osseointegration of PEO-coated titanium implants in a rat model, Sci. Rep 7 (2017) 16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gnedenkov SV, Sinebryukhov SL, Egorkin VS, Kostiv RE , In vivo study of osteogenerating properties of calcium-phosphate coating on titanium alloy Ti–6Al–4V, Biomed. Mater. Eng 27 (2016) 551–560. [DOI] [PubMed] [Google Scholar]
- [7].Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ, Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial, J. Bone Joint Surg. Br 81 (1999) 907–914. [DOI] [PubMed] [Google Scholar]
- [8].Sagomonyants KB, Hakim-Zargar M, Jhaveri A, Aronow MS, Gronowicz G, Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients, J. Orthop. Res 29 (2011) 609–616. [DOI] [PubMed] [Google Scholar]
- [9].Balla VK, Bose S, Davies NM, Bandyopadhyay A, Tantalum—a bioactive metal for implants, JOM 62 (2010) 61–64. [Google Scholar]
- [10].Kato H, Nakamura T, Nishiguchi S, Matsusue Y, Kobayashi M, Miyazaki T, Kim H-M, Kokubo T, Bonding of alkali-and heat-treated tantalum implants to bone, J. Biomed. Mater. Res. A 53 (2000) 28–35. [DOI] [PubMed] [Google Scholar]
- [11].Matsuno H, Yokoyama A, Watari F, Uo M, Kawasaki T, Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium, Biomaterials 22 (2001) 1253–1262. [DOI] [PubMed] [Google Scholar]
- [12].Findlay DM, Welldon K, Atkins GJ, Howie DW, Zannettino AC, Bobyn D, The proliferation and phenotypic expression of human osteoblasts on tantalum metal, Biomaterials 25 (2004) 2215–2227. [DOI] [PubMed] [Google Scholar]
- [13].Stiehler M, Lind M, Mygind T, Baatrup A, Dolatshahi-Pirouz A, Li H, Foss M, Besenbacher F, Kassem M, Bünger C, Morphology, proliferation, and osteogenic differentiation of mesenchymal stem cells cultured on titanium, tantalum, and chromium surfaces, J. Biomed. Mater. Res. A 86 (2008) 448–458. [DOI] [PubMed] [Google Scholar]
- [14].Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ, Experimental and clinical performance of porous tantalum in orthopedic surgery, Biomaterials 27 (2006) 4671–4681. [DOI] [PubMed] [Google Scholar]
- [15].Helsen JA, Missirlis Y, Biomaterials: A Tantalus Experience, Springer Science & Business Media, 2010. [Google Scholar]
- [16].Balla VK, Banerjee S, Bose S, Bandyopadhyay A, Direct laser processing of a tantalum coating on titanium for bone replacement structures, Acta Biomater. 6 (2010) 2329–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay A, Additive manufacturing of biomaterials, Prog. Mater. Sci (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taniguchi N, Fujibayashi S, Takemoto M, Sasaki K, Otsuki B, Nakamura T, Matsushita T, Kokubo T, Matsuda S, Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: an in vivo experiment, Mater. Sci. Eng. C 59 (2016) 690–701, 10.1016/j.msec.2015.10.069. [DOI] [PubMed] [Google Scholar]
- [19].Ponader S, von Wilmowsky C, Widenmayer M, Lutz R, Heinl P, Körner C, Singer RF, Nkenke E, Neukam FW, Schlegel KA, In vivo performance of selective electron beam-melted Ti-6Al-4V structures, J. Biomed. Mater. Res. A 92A (2010) 56–62, 10.1002/jbm.a.32337. [DOI] [PubMed] [Google Scholar]
- [20].Wauthle R, Van Der Stok J, Yavari SA, Van Humbeeck J, Kruth J-P, Zadpoor AA, Weinans H, Mulier M, Schrooten J, Additively manufactured porous tantalum implants, Acta Biomater. 14 (2015) 217–225. [DOI] [PubMed] [Google Scholar]
- [21].Amin Yavari S, van der Stok J, Chai YC, Wauthle R, Tahmasebi Birgani Z, Habibovic P, Mulier M, Schrooten J, Weinans H, Zadpoor AA, Bone regeneration performance of surface-treated porous titanium, Biomaterials 35 (2014) 6172–6181, 10.1016/j.biomaterials.2014.04.054. [DOI] [PubMed] [Google Scholar]
- [22].Bandyopadhyay A, Espana F, Balla VK, Bose S, Ohgami Y, Davies NM, Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants, Acta Biomater. 6 (2010) 1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xue W, Krishna BV, Bandyopadhyay A, Bose S, Processing and biocompatibility evaluation of laser processed porous titanium, Acta Biomater. 3 (2007) 1007–1018. [DOI] [PubMed] [Google Scholar]
- [24].Balla VK, Bodhak S, Bose S, Bandyopadhyay A, Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties, Acta Biomater. 6 (2010) 3349–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tan AW, Pingguan-Murphy B, Ahmad R, Akbar SA, Review of titania nanotubes: fabrication and cellular response, Ceram. Int 38 (2012) 4421–4435. [Google Scholar]
- [26].Bjursten LM, Rasmusson L, Oh S, Smith GC, Brammer KS, Jin S, Titanium dioxide nanotubes enhance bone bonding in vivo, J. Biomed. Mater. Res. A 92 (2010) 1218–1224. [DOI] [PubMed] [Google Scholar]
- [27].Brammer KS, Oh S, Cobb CJ, Bjursten LM, van der Heyde H, Jin S, Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface, Acta Biomater. 5 (2009) 3215–3223. [DOI] [PubMed] [Google Scholar]
- [28].Das K, Bose S, Bandyopadhyay A, TiO2 nanotubes on Ti: influence of nanoscale morphology on bone cell–materials interaction, J. Biomed. Mater. Res. A 90 (2009) 225–237. [DOI] [PubMed] [Google Scholar]
- [29].Bose S, Roy M, Das K, Bandyopadhyay A, Surface modification of titanium for load-bearing applications, J. Mater. Sci. Mater. Med 20 (2009) 19. [DOI] [PubMed] [Google Scholar]
- [30].Bandyopadhyay A, Shivaram A, Tarafder S, Sahasrabudhe H, Banerjee D, Bose S, In vivo response of laser processed porous titanium implants for loadbearing implants, Ann. Biomed. Eng 45 (2017) 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Das K, Balla VK, Bandyopadhyay A, Bose S, Surface modification of laser-processed porous titanium for load-bearing implants, Scr. Mater 59 (2008) 822–825. [Google Scholar]
- [32].Simchi A, Pohl H, Direct laser sintering of iron–graphite powder mixture, Mater. Sci. Eng. A 383 (2004) 191–200. [Google Scholar]
- [33].Bose S, Bandyopadhyay A, Modified Metal Materials, Surface Modifications to Improve Cell Interactions and Antimicrobial Properties, and Methods for Modifying Metal Surface Properties, (2017).
- [34].Vandeweghe S, Coelho PG, Vanhove C, Wennerberg A, Jimbo R, Utilizing micro-computed tomography to evaluate bone structure surrounding dental implants: a comparison with histomorphometry, J. Biomed. Mater. Res. Part B Appl. Biomater 101 (2013) 1259–1266. [DOI] [PubMed] [Google Scholar]
- [35].Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Bone histomorphometry: standardization of nomenclature, symbols, and units: report of the ASBMR Histomorphometry Nomenclature Committee, J. Bone Miner. Res 2 (1987) 595–610. [DOI] [PubMed] [Google Scholar]
- [36].Rai R, Elmer JW, Palmer TA, DebRoy T, Heat transfer and fluid flow during keyhole mode laser welding of tantalum, Ti–6Al–4V, 304L stainless steel and vanadium, J. Phys. D Appl. Phys 40 (2007) 5753. [Google Scholar]
- [37].España FA, Balla VK, Bandyopadhyay A, Laser processing of bulk Al–12Si alloy: influence of microstructure on thermal properties, Philos. Mag 91 (2011) 574–588. [Google Scholar]
- [38].Popat KC, Leoni L, Grimes CA, Desai TA, Influence of engineered titania nanotubular surfaces on bone cells, Biomaterials 28 (2007) 3188–3197. [DOI] [PubMed] [Google Scholar]
- [39].Kieswetter K, Schwartz Z, Dean DD, Boyan BD, The role of implant surface characteristics in the healing of bone, Crit. Rev. Oral Biol. Med 7 (1996) 329–345. [DOI] [PubMed] [Google Scholar]
- [40].Niinomi M, Mechanical biocompatibilities of titanium alloys for biomedical applications, J. Mech. Behav. Biomed. Mater 1 (2008) 30–42, 10.1016/j.jmbbm.2007.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.