Abstract
A juvenile White-headed woodpecker (Dryobates albolarvatus) fitted with a radio tag was located dead at approximately 22-days post-fledging in Yakima county in central Washington in July 2015. Postmortem examination revealed an enlarged liver and spleen plus evidence of iron sequestration. Microscopic examination observed young gametocytes within the cytoplasm of erythrocytes, and exo-erythrocytic meronts within the cytoplasm of capillary endothelial cells, hepatocytes, and myocytes, and free in the tissues. These attributes implicated a haemosporidian infection that likely resulted in mortality. Subsequent sampling results of local woodpecker species in the same area during the breeding season in June–July 2016 and May–July 2017 showed other individuals infected with Haemoproteus parasites. Nested Polymerase Chain Reaction (PCR), sequencing, and microscopic analyses for avian haemosporidians revealed infections with Haemoproteus velans (Haemosporida, Haemoproteidae). This parasite was characterized molecularly and morphologically. This is the first report of a haemosporidian infection in a White-headed woodpecker anywhere in its range, and the first reported suspected mortality from haemoproteosis for a woodpecker (Piciformes, Picidae). The use of radio-tagged birds is an asset in wildlife haemosporidian studies because the effect of the pathogen can be monitored in real time. Additionally, this methodology provides opportunities to collect fresh material for microscopic and histological examination from wild birds that have died from natural causes.
Keywords: Haemoproteus velans, Molecular and morphological characterization, Mortality, Picidae, White-headed woodpecker, Radio-tagging
Graphical abstract
Highlights
-
•
Radio-tagged juvenile White-headed woodpecker found shortly after death.
-
•
Necropsy revealed likely died from complications of an avian haemosporidian, Haemoproteus velans, infection.
-
•
Haemoproteus genus not thought to cause mortality in wild birds.
-
•
First molecular characterization of H. velans and new morphological characterization.
-
•
Avian haemosporidian infections increase in severity as climate change affects imperiled species.
1. Introduction
Prevalent worldwide, avian haemosporidian parasites (Apicomplexa, Haemosporida) belonging to the genera Leucocytozoon, Plasmodium, Haemoproteus, and Fallisia infect a majority of terrestrial avian species (Valkiūnas, 2005). Though morbidity can be high in some populations, mortality is difficult to detect because sick individuals are secretive and/or are rapidly eliminated by predators (Lachish et al., 2011). Current sampling techniques favor capturing wild individuals with light (chronic) parasitemia (Valkiūnas, 2005; Mukhin et al., 2016). These birds have already survived the acute stage of infection and are healthy enough to be mobile in the environment, breed, and migrate (Bennett et al., 1993b; Mukhin et al., 2016). Locating wild haemosporidian caused fatalities presents logistical challenges, leading to biased detection of mortality rates (Holmes, 1982; Bennett et al., 1993b; Valkiūnas, 2005). These sampling biases lead to the assumption that avian haemosporidian infections are relatively benign in wild populations (Bennett et al., 1993b).
Haemosporidian infections of Leucocytozoon and Plasmodium are common and can cause high mortality in domesticated birds in the families Anseriformes, Columbiformes, Galliformes, and Struthioniformes (Bennett et al., 1993a; Valkiūnas, 2005), wild birds kept in captivity (Bennett et al., 1993b; Valkiūnas, 2005; Bueno et al., 2010; Vanstreels et al., 2015), and novel introductions (Warner, 1968; Van Riper et al., 1986; Bennett et al., 1993b; Argilla et al., 2013; Marzal et al., 2015). Haemoproteus infections are also common but are generally not thought to cause mortality in adapted species (Bennett et al., 1993b). However, there have been an increasing number of cases of severe disease and mortality in experimentally infected domesticated birds (Atkinson et al., 1986, 1988; Cardona et al., 2002; Valkiūnas and Iezhova, 2017), naturally infected birds in captivity (Earle et al., 1993; Ferrell et al., 2007; Donovan et al., 2008; Olias et al., 2009; Cannell et al., 2013) and case studies with a few sick individuals (Atkinson and Forrester, 1987; Peirce et al., 2004). Haemoproteus megaloschizonts in skeletal muscle produce severe, acute hemorrhagic myositis which is associated with severe disease and death (Atkinson et al., 1988; Cardona et al., 2002; Olias et al., 2009; Valkiūnas and Iezhova, 2017). The majority of documented Haemoproteus fatalities in wild populations have been represented by single individuals, so it is difficult to determine what factors contribute to lethality.
There are over 150 species in the genus Haemoproteus documented to infect a range of avian hosts (Valkiūnas, 2005; Dimitrov et al., 2014). All haemosporidians use dipteran insect vectors as the definitive host, with haemoproteids of the subgenus Parahaemoproteus utilizing biting midges (Diptera: Ceratopogonidae) (Atkinson et al., 2008). Two species of Haemoproteus have been reported in North American woodpeckers, H. borgesi (Pung et al., 2000) and H. velans (Coatney and Roudabush, 1937; Khan and Fallis, 1971; Greiner et al., 1975, 1977; Pung et al., 2000; Schrader et al., 2003; Valkiūnas, 2005). H. borgesi can be readily distinguished from H. velans primarily due to the shape of its fully-grown gametocytes, which are broadly halteridian in the former species, but are predominantly markedly circumnuclear in the latter parasite (Greiner et al., 1977; Valkiūnas, 2005). H. borgesi remains non-characterized molecularly a detailed re-description of its blood stages is nedded. There are no records of mortality caused by any Haemoproteus species or parasites of other genera of haemosporidians in woodpeckers despite the reports of 11 species harboring infections (Table 1). Most of the unidentified historical Haemoproteus samples are thought to be H. velans (Greiner et al., 1977).
Table 1.
Documented North American haemosporidian infections in Piciformes (1937–2018)a.
| Species | No. examined | No. infected | Leucocytozoon | Infected with Haemoproteus | Plasmodium |
|---|---|---|---|---|---|
| Colaptes auratus | 198 | 108 | 22 | 78 | 8 |
| Picoides arcticus | 9 | 4 | 1 | 3 | 0 |
| Dryobates borealis | 70 | 1 | 0 | 1 | 0 |
| D. pubescens | 140 | 14 | 0 | 14 | 0 |
| D. villosus | 44 | 26 | 3 | 23 | 0 |
| Hylatomus pileatus | 1 | 1 | 0 | 1 | 0 |
| Melanerpes erythrocephalus | 19 | 2 | 0 | 2 | 0 |
| M. formicivorus | 19 | 0 | 0 | 0 | 0 |
| M. carolinus | 243 | 64 | 0 | 63 | 1 |
| Sphyrapicus nuchalis | 10 | 1 | 0 | 1 | 0 |
| S. varius | 39 | 13 | 1 | 11 | 1 |
| Total | 792 | 234 | 27 | 197 | 10 |
| Overall prevalence (%) | 29.5 | 3.4 | 24.9 | 1.2 |
Coatney and Roudabush (1937); Coatney (1938); Huff (1939); Herman (1944); Bennett and Fallis (1960); Collins et al., (1966); Khan and Fallis (1971); Greiner et al., 1975, Greiner et al., 1977; Pung et al., 2000; Ricklefs and Fallon (2002); Schrader et al., (2003); Martinsen et al., (2008); Astudillo et al., (2013); Medeiros et al., (2014), 2015; Ellis et al., (2015); Walther et al., (2016); Smith et al., (2018).
Haemoproteus velans was first described by Coatney and Roudabush (1937) in Northern Flickers (Colaptes auratus). This parasite is readily distinguishable by the morphology of its fully-grown gametocytes, which are circumnuclear and slightly displace laterally the nucleus of infected erythrocytes (Greiner et al., 1977; Valkiūnas, 2005). The gametocytes often possess numerous prominent volutin granules. Additionally, when fully grown, the gametocytes completely encircle the nucleus and occupy the available cytoplasm. Morphometric characterization of gametocytes of this parasite remains incomplete (Coatney and Roudabush, 1937; Greiner et al., 1977; Valkiūnas, 2005). Two biting midge species, Culicoides stilobezzoides and Culicoides sphagnumensis have been identified as vectors in experimental conditions (Khan and Fallis, 1971).
Woodpeckers are part of an important keystone guild of primary cavity excavators (Raphael and White, 1984; Drever et al., 2008). Nest and roost cavities excavated by woodpeckers provide nests and shelter sites for many small-bodied secondary cavity users (Blendinger, 1999; Aitken and Martin, 2007; Tarbill et al., 2015). Woodpeckers have been shown to help control forest insect populations (Fayt et al., 2005) and may help disperse fungi that act as agents of decay (Jusino et al., 2016). Documented woodpecker mortalities attributed to pathogens have been represented by case studies describing single individuals (Gerhold and Yabsley, 2007; Siegel et al., 2012; Garigliany et al., 2014; Jokelainen and Vikøren, 2014), leading to little baseline information on etiological agents of mortality. Health factors, such as haemosporidian infections, impacting population growth rates or the ability of the species to maintain healthy population levels could have a significant impact on forest ecosystem function. The White-headed woodpecker (Dryobates albolarvatus) is believed to be declining in many areas due to habitat loss, particularly in the northern parts of its range (Garret et al., 1996). Listed as an endangered species in Canada and considered a species of special concern in some areas (COSEWIC, 2010; WDFW, 2013; ODFW, 2016), White-headed woodpeckers have not been sampled in past studies of haemosporidians, due to their rarity and restricted geographic range (Altman, 2000). To conserve populations of these important keystone species, more information about mortality factors could be useful for managers. Here, we identify H. velans as the cause of death in a juvenile White-headed woodpecker and as the source of chronic infections in adult Northern Flickers from Eastern Washington, suggesting that avian haemoproteosis caused organ pathologies may be more important in the population dynamics for woodpeckers than previously envisioned.
2. Materials and methods
2.1. Radio-telemetry field methods
During a study on post-fledging dispersal, 54 nestling White-headed woodpeckers were captured and radio-tagged in Yakima County, Washington (approximately 46° 45′ N, 120° 58′ W) from 2014 to 2017. Individuals were tracked every 1–4 days while they were still dependent on their parents, which was defined as starting on the day nestlings fledged and ending three days after the bird was last seen begging for food from a parent. Point locations were recorded when birds moved. We calculated home ranges for the dependence period using the minimum convex polygon method (White and Garrot, 1990; Millspaugh and Marzluff, 2001).
2.2. Blood sampling field methods
We sampled live woodpeckers during the radio-telemetry study in June–July 2016 and May–July 2017 during the breeding season. We searched known territories of White-headed woodpeckers and Black-backed woodpeckers (Picoides arcticus) for nests throughout the nesting season. Northern Flickers and Hairy woodpeckers (Dryobates villosus) nests located during these searches were included to increase our sample size. Adults were captured using targeted mist-nets positioned in front of the nest cavity. We set up nets just after a parent had fed the nestlings to limit the amount of time they were kept away from foraging. Nestlings were sampled from the nest when they were an estimated 1–4 days from fledging using the hole saw method described by Ibarzabal and Tremblay (2006). All birds were removed from the net or nest cavity and were weighed, measured, and banded in compliance with the Ornithological Council Guidelines for the Use of Wild Birds in Research (Fair et al., 2010) and U.S. Department of Agriculture, Forest Service, Institutional Animal Care and Use Committee (Proposal number 2016–007). Adult birds were not aged, and nestlings were between 18 and 25 days old.
We obtained 25–50 μl of blood from each bird via brachial venipuncture with a sterile 27-gauge needle. Blood was then stored in lysis buffer (10 mM Tris-HCL pH 8.0, 100 mM EDTA, 2% SDS) at ambient temperature while in the field and preserved at −20 °C in the laboratory until further processing (Sehgal et al., 2001). Two to three blood smears from each individual were prepared in the field using established techniques (Bennett, 1970; Valkiūnas, 2005), fixed in methanol, and stained with Giemsa and examined microscopically following established protocols in the laboratory (Valkiūnas et al., 2008b). Voucher preparations of H. velans from its vertebrate type host, the Northern Flicker, were deposited in Nature Research Centre, Vilnius, Lithuania (accession nos. 49035 and 49036 NS).
2.3. Post-mortem white-headed woodpecker examination
The Avian Health and Food Safety Laboratory at Washington State University in Payallup, Washington performed a postmortem examination on the dead juvenile White-headed woodpecker. Collected samples of multiple tissues were fixed in 10% neutral buffered formalin for 48 h. All tissues were dehydrated in graded alcohols, cleared in xylene, embedded in paraffin, sectioned at 4 μm, stained with hematoxylin and eosin. The sections were examined under a light microscope from 2x to 100x magnification. In addition, formalin-fixed skeletal muscle, kidney, spleen, liver tissues were processed for transmission electron microscopy (TEM) following (Widáhn and Kindblom (1988). Briefly, selected areas of these tissues were cut from the paraffin block, deparaffinized in xylene and processed into Eponate 12s epoxy resin (Ted Pella Inc., Redding, CA). Thick sections were cut, mounted on glass slides, and stained with Toluidine Blue O and examined by light microscopy. Thin sections were cut, mounted on 150-mesh copper grids, stained with 6% uranyl acetate in 75% ethanol, poststained in Reynold lead citrate, and examined with a transmission electron microscope. Initial PCR testing conducted by the Avian Health and Food Safety Laboratory for a haemosporidian infection was inconclusive. Since macroscopic findings were similar to those found in other Haemoproteus infection fatalities, the case was referred San Francisco State University for further molecular testing.
2.4. Parasite molecular analysis
Molecular analysis took place at San Francisco State University. DNA extraction from muscle tissue from the deceased White-headed woodpecker and blood samples from sampled live birds used the commercial DNA extraction kit Wizard SV Genomic DNA Purification System (Promega Corporation, Madison, WI) and followed the manufacturer's protocol. We confirmed successful DNA extraction using primers that amplified the brain-derived neurotrophic factor (Sehgal and Lovette, 2003). Nested PCR screened for a partial sequence of the mitochondrial cytochrome b (cyt b) gene from Plasmodium and Haemoproteus using the primers HaemNF/HaemNR2-HaemF/HaemR2 (Bensch et al., 2000; Waldenström et al., 2004) and from Leucocytozoon using primers NF/NR3-FL/R2L (Hellgren et al., 2004). All reactions were carried out in 25 μl reactions and accompanied by negative (ddH2O) and positive controls (samples from infected birds previously confirmed by sequencing and microscopy). We visualized the resulting PCR product on a 1.8% agarose gel to check for positive infection. Positive PCR products were sequenced by Elim Biopharmaceuticals Inc. (Hayward, CA).
2.5. Phylogenetic analysis
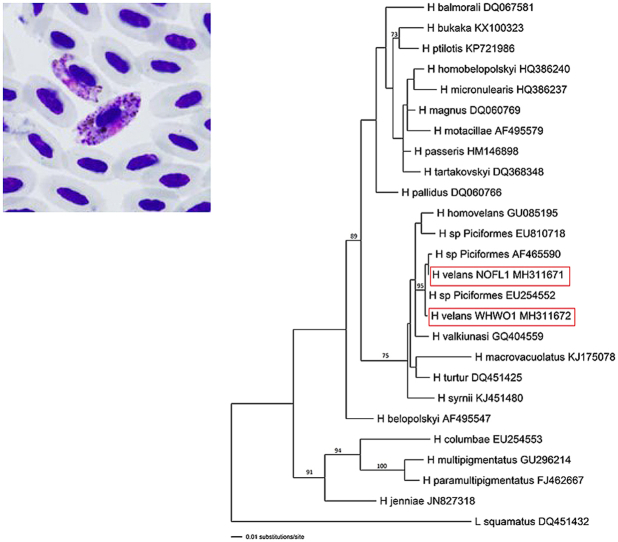
Geneious software was used (v.11.0.4) (https://www.geneious.com, Kearse et al., 2012) to edit and align sequences. After trimming primers, a sequence length of 480 base pairs was obtained. We classified a unique sequence as differing from other sequences by one or more nucleotides (Martinsen et al., 2006; Hellgren et al., 2007). By comparing samples to other haemosporidian sequences available using the National Centre for Biotechnology Information's Basic Local Alignment Search Tool (NCBI Resource Coordinators, 2016) and the MalAvi Database (Bensch et al., 2009), two distinct Haemoproteus lineages were identified and were deposited in GenBank (MH311671, MH311672). We generated a Maximum-likelihood (ML) tree in PAUP* (v.4.0a.b161) (Swofford, 2018) incorporating sequences from this study and 18 reference sequences submitted to GenBank. The analyses only included reference sequences that have well-established morphological species identifications, which were based on microscopic examination of blood films. A sequence from a Downy woodpecker (Dryobates pubsescens) (EU254552) with a suspect morphological identification of H. picae (Dimitrov et al., 2014) was included due its genetic similarity to our lineages. Additionally, we included two unidentified Haemoproteus sequences from a Red-headed woodpecker (Melanerpes erythrocephalus) (AF465590) and a tropical member of Piciformes (Indicator maculatus) (EU810718). A partial cyt b sequence from Leucocytozoon squamatus (DQ4514320) was used as an outgroup. Our ML phylogenetic construction used a GTR + I+Γ model based on an AICc analysis of models and 1000 bootstrap replicates.
3. Results
3.1. White-headed woodpecker fatality
The juvenile White-headed woodpecker was radio-tagged at its nest site on 26 June 2015 before released beingback into the nest to fledge naturally. The estimated fledge date was 30 June 2015. We tracked the bird on 12 different days in July before tracking its radio signal led us to locate it dead on the ground, without obvious signs of trauma, on 21 July 2015. It was not observed begging for food from parents in the characteristically loud and vocal manner of recently fledged White-headed woodpeckers. Prior to its death, this juvenile was relatively sedentary compared to other radio-tagged juveniles, the entire area it ranged over during the 22 days before its death was 6.4 ha (ha). This is the smallest range observed for 54 juveniles tracked during their dependence period (mean range = 252.8 ha, SD ± 94.9 ha).
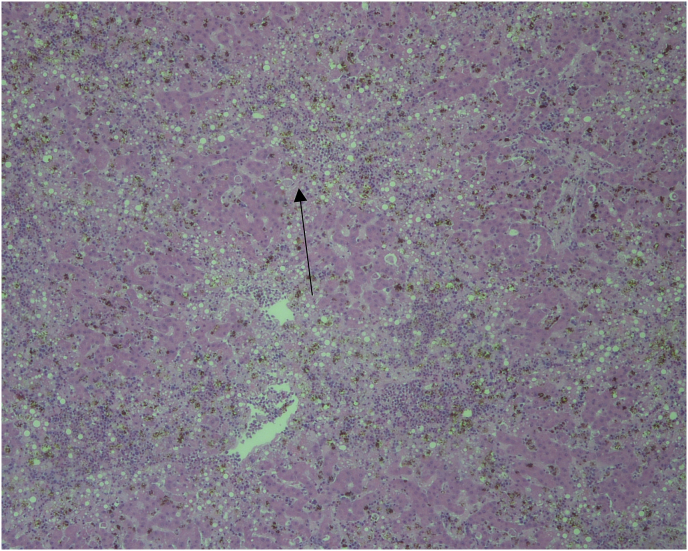
There were several macroscopic findings that pointed to an infectious disease cause of death. The breast muscle was paler than normal with multifocal white streaks throughout and the distal half of both lungs were congested. Lymphatic observations showed the spleen was three to four times the normal size. The liver had green discoloration and was severely enlarged, extending beyond the last rib. Histology revealed acute multifocal necrosis, disseminated moderate to severe microgranulomatosis, lymphohistiocytosis in the liver and spleen as well as in kidney and skeletal muscle. Iron sequestration due to the presence of numerous pigment granules (hemozoin) was seen in the liver (Fig. 1).
Fig. 1.
Histological section of liver tissue of naturally infected White-headed woodpecker (Dryobates albolarvatus). Note clumps of numerous hemozoin granules (arrows), which are remnants of hemozoin developing in gametocytes of Haemoproteus parasites. Magnification x10.
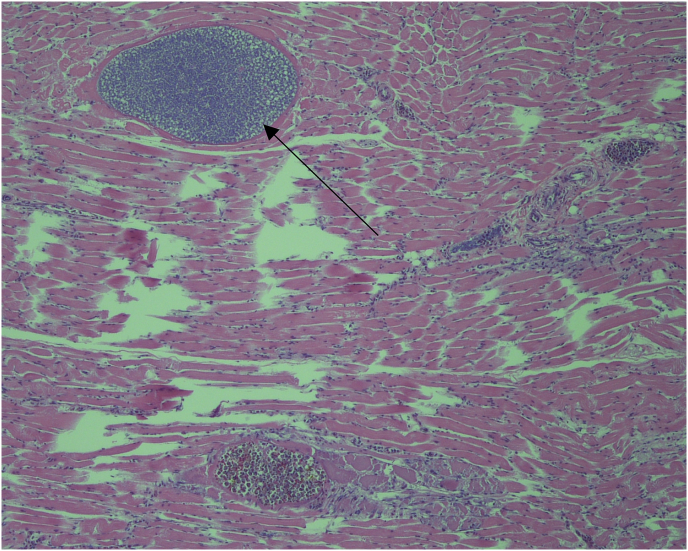
Histological examination revealed that megalomeronts were present within the cytoplasm of capillary endothelial cells, hepatocytes, and myocytes, or free in the tissues (Fig. 2). Electron microscope observations in other organs include numerous large, basophilic granular bodies within the wall of the blood vessels, pulmonary parabronchi, in the hepatic sinusoids, cytoplasm of macrophages, and between the myofibers of the skeletal muscle. Electron microscopy also showed protozoal young gametocytes within the cytoplasm of erythrocytes. Gametocytes had a double cell membrane and were slightly eccentric.
Fig. 2.
Skeletal muscle tissue showing megalomeronts of Haemoproteus velans. Note numerous developing cytomeres (arrowhead) and capsular-like wall around the parasite (arrow). Magnification x20.
3.2. Northern Flicker Haemoproteus velans identification
Sampling from 139 live birds in 2016 and 2017 revealed three adult Northern Flickers with Haemoproteus infections. All three samples had an identical genetic sequences and infections were confirmed with microscopy. The genetic sequence was two base pairs different or 0.417% divergent from the sequence of the White-headed woodpecker. When morphologically comparing gametocytes in blood smears from individuals from this study and from the Downy woodpecker (EU24552), all samples were identified as H. velans. There were no blood smears available from the White-headed woodpecker. Given the genetic similarity Haemoproteus parasites in the Northern Flicker, White-headed woodpecker, and Downy woodpecker samples and also the morphological similarity of gametocytes from the Northern Flicker and Downy woodpecker, it is probable that all records of haemoproteids in these birds were H. velans.
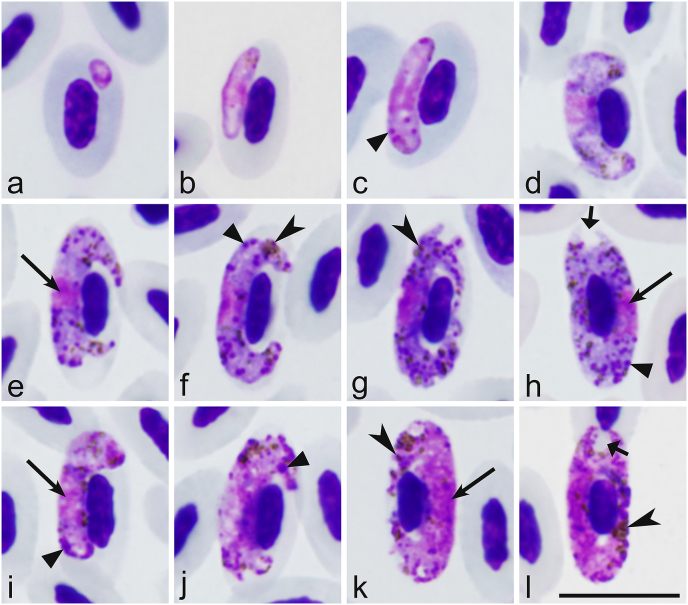
Only one morphotype was present in all three of the positive Northern Flicker slides examined (Fig. 3). The original description of H. velans is fragmentary, and morphometric data are incomplete (Coatney and Roudabush, 1937; Greiner et al., 1977; Valkiūnas, 2005). Here, we provide additional information on the morphology of this parasite (Fig. 3) and morphometrics of its fully-grown gametocytes and their host cells (Table 2) from the type vertebrate host, the Northern Flicker. It should be noted that blood stages were identical in the main morphological features to those described by Coatney and Roudabush (1937) and Valkiūnas (2005), their description is not repeated here. However, several additional features in gametocyte morphology were reported. First, there were more amoeboid gametocyte cells in some individual avian hosts than in previous descriptions (see Fig. 3g, k). Second, volutin was readily visible, but present markedly unequally in individual birds. Third, the number of pigment granules is greater in our material than in the original description. Due to marked volutinization of parasite cytoplasm, it is often difficult to calculate the number of pigment granules in gametocytes of this parasite (Valkiūnas, 2005). Gametocytes in the type material of H. velans are overfilled with prominent volutin, and pigment granules are poorly visible due to marked fading of hemozoin in these old preparations (Dimitrov et al., 2014). We calculated pigment granules in mature gametocytes with a relatively small amount of volutin, and these data show that mature gametocytes contain on average approximately 35 and 28 pigment granules in macro-and microgametocytes, respectively (Table 2). This is greater than was reported in the original description, in which 18 and 21 pigment granules were reported on average in macro- and microgametocytes, respectively (Coatney and Roudabush, 1937; Valkiūnas, 2005).
Fig. 3.
Haemoproteus velans (lineage NOFL1) from the blood of the Northern Flicker (Colaptes auratus): a, b - young gametocytes; c-h - macrogametocytes; i-l - microgametocytes. Long simple arrows – nuclei of parasites. Simple arrowhead – pigment granules. Triangle arrowheads – volutin granules. Short simple wide arrow – unfilled space in poles of erythrocytes. Giemsa-stained thin blood films. Scale bar = 10 μm.
Table 2.
Morphometry of host cells and mature gametocytes of Haemoproteus velans from the blood of the Northern Flicker Colaptes auratus.
| Feature | Measurements (μm)a |
|---|---|
| Uninfected erythrocyte | |
| Length | 12.6–14.6 (13.5 ± 0.6) |
| Width | 6.3–7.5 (6.7 ± 0.3) |
| Area | 65.8–77.9 (71.7 ± 3.3) |
| Uninfected erythrocyte nucleus | |
| Length | 5.3–6.6 (6.1 ± 0.3) |
| Width | 1.9–2.6 (2.2 ± 0.2) |
| Area | 9.0–13.5 (11.5 ± 1.3) |
| Macrogametocyte | |
| Infected erythrocyte | |
| Length | 14.5–17.8 (15.9 ± 0.8) |
| Width | 5.9–7.2 (6.6 ± 0.4) |
| Area | 78.7–97.4 (85.0 ± 5.4) |
| Infected erythrocyte nucleus | |
| Length | 4.9–6.7 (5.9 ± 0.4) |
| Width | 1.9–2.4 (2.2 ± 0.2) |
| Area | 8.1–12.9 (11.2 ± 1.1) |
| Gametocyte | |
| Length | 24.1–27.5 (25.9 ± 1.0) |
| Width | 1.9–3.7 (2.6 ± 0.4) |
| Area | 57.7–77.6 (65.5 ± 5.4) |
| Gametocyte nucleus | |
| Length | 2.8–4.9 (4.0 ± 0.6) |
| Width | 1.7–3.5 (2.3 ± 0.5) |
| Area | 5.9–11.6 (8.1 ± 1.4) |
| Pigment granules | 27.0–42.0 (34.9 ± 4.3) |
| NDRb | 0.4–0.9 (0.8 ± 0.1) |
| Microgametocyte | |
| Infected erythrocyte | |
| Length | 14.4–17.1 (15.4 ± 0.7) |
| Width | 5.8–7.3 (6.6 ± 0.3) |
| Area | 72.6–95.8 (83.8 ± 5.2) |
| Infected erythrocyte nucleus | |
| Length | 4.9–6.6 (5.7 ± 0.4) |
| Width | 1.7–2.6 (2.3 ± 0.2) |
| Area | 9.3–12.7 (11.0 ± 1.0) |
| Gametocyte | |
| Length | 22.3–28.8 (25.0 ± 1.7) |
| Width | 2.3–3.1 (2.8 ± 0.2) |
| Area | 50.7–72.0 (62.1 ± 5.4) |
| Gametocyte nucleus c | |
| Length | 6.7–9.5 (8.3 ± 1.0) |
| Width | 2.3–3.1 (2.6 ± 0.3) |
| Area | 14.5–24.9 (19.6 ± 3.8) |
| Pigment granules | 19.0–36.0 (27.5 ± 4.8) |
| NDR | 0.6–0.8 (0.7 ± 0.1) |
Measurements (n = 21) are given in micrometers. Minimum and maximum values are provided, followed in parentheses by the arithmetic mean and standard deviation.
NDR = nucleus displacement ration according to Bennett and Campbell (1972).
Nuclei of microgametocytes are markedly diffuse and difficult to measure; outline of the nuclei was well seen and measured in 9 microgametocytes.
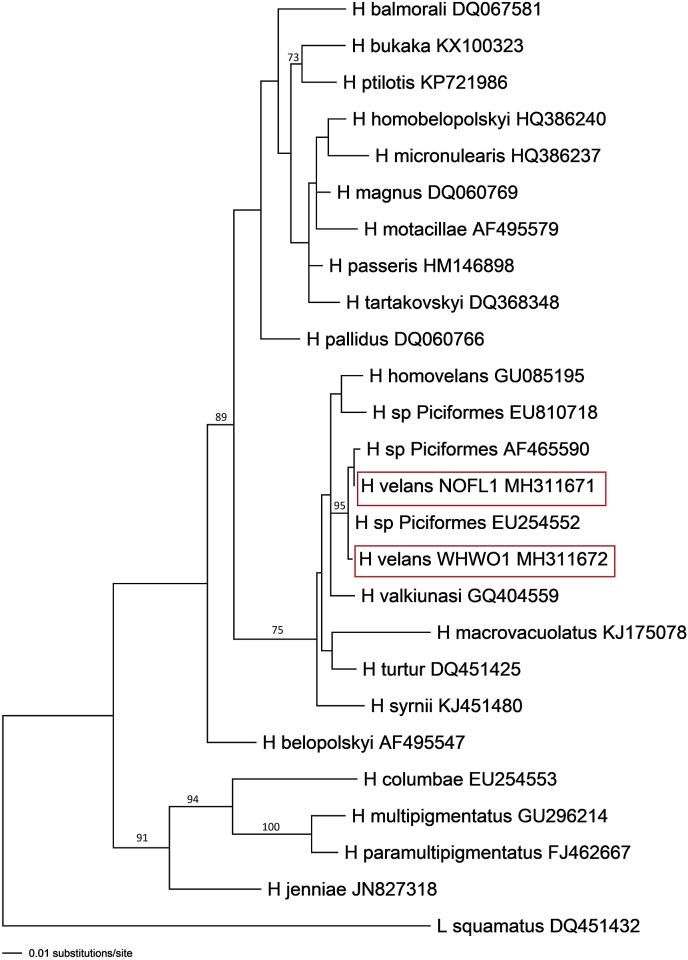
Maximum-likelihood predictions placed H. velans into a clade with Haemoproteus species parasitizing non-passeriform birds belonging to Strigiformes, Ciconiiformes, Columbiformes, and European members of Piciformes with 96% bootstrap support (Fig. 4). However, the relationships between these species did not have significant bootstrap support. The NOFL1 (Northern Flicker) and WHWO1 (White-headed woodpecker) lineages of H. velans formed a clade with 100% bootstrap support with the likely misidentified sequence from the Downy woodpecker (EU254552, H. picae) and the unidentified Haemoproteus sequence from the Red-headed woodpecker (AF465590).
Fig. 4.
Consensus tree displaying Haemoproteus velans phylogenetic relationships as predicted by Maximum-likelihood inference, using GTR + I+Γ substitution model in PAUP* v.4.0a.b161. Maximum-likelihood bootstrap values > 70 are indicated. Lineages detected in the current study are indicated by red boxes. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Haemoproteus velans infection can result in fatalities in woodpeckers, but the frequency of mortality remains unclear. Haemosporidian infections of any kind have not been described as a source of mortality in White-headed woodpeckers in past publications. Due to their sensitive status and concerns about impacting nest success, no blood samples have previously been taken from this species. The three positive adult Northern Flickers detected during sampling in 2016 and 2017 coupled with a likely historical prevalence of 24.9% (Table 1), indicates that H. velans can persist chronically in adult woodpecker populations. Work by Schrader et al. (2003) found a prevalence of 25% of H. velans in Red-bellied woodpeckers (Melanerpes carolinus) and showed a seasonal variation in the proportion of individuals infected. Results from monitoring infected adults suggests that infection with H. velans may not directly affect adult host survival but can negatively affect host condition (Schrader et al., 2003). Experimental observations indicate that juvenile chaffinches (Fringilla coelebs) are less mobile during peak parasitemia in comparison to non-infected individuals and are thus more attractive to predators (Valkiūnas, 2005). Additionally, Haemoproteus spp. infected Blackcap (Sylvia atricalilla) nestlings lost weight during peak parasitemia (Valkiūnas et al., 2006). Nestlings sampled in this study were 20–24 days old may not have had infections detected due to the infection being in the prepatent period and not detectable yet in the blood. These data and the observations in this study indicate that Haemoproteus infections may be more virulent than formerly believed (Bennett et al., 1993b). However, more sensitive epidemiology approaches than microscopic examination or PCR-based blood testing are needed to recognize true host-parasite relationships in wildlife. This study shows how the application of radio-tagging can be a useful methodology in such research.
Necropsy results of the White-headed woodpecker attributed the cause of death to decreased liver and spleen function and a high number of infected erythrocytes which lead to hypoxia. The streaking patterns seen in the muscle tissue are similar to findings by Cardona et al. (2002) in captive Bobwhite Quail (Colinius virginsius) infected with H. lophortyx and in experimentally infected turkeys (Meleagris gallapavo) infected with H. mansoni (syn. H. meleagridis) (Atkinson et al., 2008). The prepatent period of tissue stage development of the majority of Haemoproteus species studied varies from 11 days to three weeks (Valkiūnas, 2005; Atkinson et al., 2008). Since the juvenile was found dead 22 days after fledgling, it was probably infected after it left the nest. It was not exhibiting normal juvenile behavior. Coupled with the significantly lower home range (mean range = 252.8 ha, SD ± 94.9 ha) than other juveniles, these observations suggest this infection altered its behavior prior to death.
Other eukaryotic parasites were not reported in the dead juvenile. It is conceivable that H. velans may only cause mortality when a bird is already subject to other health impacts. It is possible the White-headed woodpecker juvenile had a viral or bacterial infection not detected during necropsy. Testing PCR material for additional pathogens was beyond the scope of this study. Environmental conditions could also have affected the juvenile's overall health. The mortality occurred on 20 July 2015 and followed the warmest June and January–June period for the eastern Washington Cascades ever reported, as determined by climatological data from 1895 to 2015 (NOAA, 2015). An increase in temperature may have resulted in decreased or shifted foraging opportunities for adults, leading to decreased overall health of their chicks (Walther et al., 2002). These factors might have contributed to the death of the individual. It would be important to study how the accumulation of several health factors simultaneously affect overall immune health and contribute to the pathogenicity of haemosporidians.
This study provides the first molecular characterization of H. velans, providing opportunities for better disease diagnostics in the wild. The genetic similarity between the White-headed woodpecker and Norther Flicker sequences (0.417%) indicates they are potentially different lineages from the same species of parasite (Martinsen et al., 2006; Hellgren et al., 2007). Morphological characterization (Fig. 3, Table 2) is valuable for easier identification of this infection during microscopic examination of blood films. Morphological studies combined with molecular characterization of parasite species in different host individuals from a variety of locales can help better understand intraspecies variation. The higher frequency of amoeboid gametocyte cells than in previous descriptions (see Fig. 3g, k) shows that this character should be used carefully during species identification as the frequency depends on the stage of gametocytemia. The amount of volutin in gametocytes of H. velans also likely depends on the stage of gametocyte development and probably on the individual host thus, this character is not as dependable in this parasite species identification as previously though (Coatney and Roudabush, 1937; Valkiūnas, 2005). This is important for distinguishing abortive haemosporidian infections, which might cause severe diseases in birds (Valkiūnas and Iezhova, 2017).
The phylogenetic relationship of H. velans to other Haemoproteus species has low bootstrap support in our maximum-likelihood analysis. This is likely due the lack of sufficient informative information associated with the 480 bp cyt b fragment used in our analysis. Despite the low bootstrap support, the relationships between Haemoproteus species in this study are generally the same as those found by Pacheco et al. (2018) in their analysis of the whole mitochondrial genome. Though longer sequences may be necessary to understand complex phylogenetic relationships, the partial cyt b sequence used appears to be sufficient for ecological investigations (Pacheco et al., 2018). There is extensive data available on this fragment and it is the current standard in avian haemosporidian studies (Bensch et al., 2009; Outlaw and Ricklefs, 2014).
Our data led to a likely species identification of the unidentified Haemoproteus sequence from the Red-headed woodpecker (AF465590) as H. velans due to its clustering in a clade with lineages from this study as well as a likely misidentified parasite from a Downy woodpecker (EU254552). This indicates that they likely all belong to H. velans and that H. velans is distributed across North America. An analysis by Valkiūnas et al. (2008a, b) showed that most haemosporidian lineages deposited in GenBank only have a genus level identification. These are not useful when investigating the life histories and phylogenetic relationships (Valkiūnas et al., 2008a), particularly with the broad distribution of abortive haemosporidian infections (Moens et al., 2016; Valkiūnas and Iezhova, 2017). There are also a number of incorrectly identified haemosporidian parasite DNA sequences in GenBank (Valkiūnas et al., 2008a). The genetic sequence from the White-headed woodpecker fatality matched the genetic sequence of parasites morphologically identified as H. picae in a Downy woodpecker (EU254552) (Martinsen et al., 2008). Dimitrov et al. (2014) suspected it to be incorrectly identified since H. picae previously only infected species in the passerine family Corvidae (Valkiūnas, 2005), and it is unlikely it can complete its life cycle and produce gametocytes in birds of Piciformes, as suggested by Martinsen et al. (2008). Mature H. velans gametocytes (Fig. 3h, l) completely surround the nucleus of erythrocytes occupying all available cytoplasmic space (Greiner et al., 1977; Valkiūnas, 2005) and this is not found in H. picae (Valkiūnas, 2005). Molecular data (Fig. 4) supported the conclusion the cyt b sequence EU254552 likely belongs to H. velans. This misidentification created confusion on the part of the authors of this paper when trying to identify the parasites from this study.
Radio-tagging provides valuable information about the fate of infected vertebrates. It is worth a broader use in parasitology and ecology research where it is difficult to access sick individuals. Monitoring radio-tagged individuals can show how haemosporidians and other parasites affect activity levels and behavior in real time. Importantly, fresh tissue from dead birds can be collected using this methodology which is essential for histopathology research. This study is the first to document what happens to a sick radio-tagged bird and shows that radio-tagging can be a useful methodology in future research on pathogenicity of haemosporidians.
As climate and land use change continues to shift suitable habitat ranges for many bird species (Langham et al., 2015), the risk and severity of infectious diseases are projected to increase (Patz et al., 2000; Garamszegi, 2011). Future models of temperature and precipitation patterns reveal potential dramatic changes in coming years (Langham et al., 2015), a particular concern for species that are already rare, declining, or otherwise of conservation concern. The population health of indicator species such as woodpeckers, which have a disproportionate effect on ecosystems they inhabit (Virkkala, 2006), becomes an important measure of how the rest of the ecosystem is reacting to these changes (Lindenmayer et al., 2000). Haemoproteosis remains a neglected avian parasitosis. Due to pathology associated with tissue stages, non-adapted avian species already experiencing population declines may be at risk (Valkiūnas and Iezhova, 2017). Identifying the effects of haemosporidian infections on woodpecker population health could contribute to stronger management of an important keystone guild.
Declaration of interest
None.
Acknowledgements
This work was supported under a joint venture agreement between the U.S. Forest Service, Pacific Northwest Research Station, Olympia, WA and San Francisco State University, San Francisco, CA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.07.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aitken K.E.H., Martin K. The importance of excavators in hole-nesting communities: availability and use of natural tree holes in old mixed forests of western Canada. J. Ornithol. 2007;148:425–434. [Google Scholar]
- Altman B. 2000. Conservation Strategy for Landbirds of the East-Slope of the Cascade Mountains in Oregon and Washington. Oregon-Washington Partners in Flight. [Google Scholar]
- Argilla L.S., Howe L., D G.B., Alley M.R. High prevalence of Leucocytozoon spp. in the endangered Yellow-eyed Penguin (Megadyptes antipodes) in the sub-Antarctic regions of New Zealand. Parasitology. 2013;140:672–682. doi: 10.1017/S0031182012002089. [DOI] [PubMed] [Google Scholar]
- Astudillo V.G., Hernandez S.M., Kistler W.M., Boone S.L., Lipp E.K., Shrestha S., Yabsley M.J. Spatial, temporal, molecular, and intraspecific differences of haemoparasite infection and relevant selected physiological parameters of wild birds in Georgia, USA. Int. J. Parasitol. Parasites Wildl. 2013;2:178–189. doi: 10.1016/j.ijppaw.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson C.T., Forrester D.J. Myopathy associated with megaloschizonts of Haemoproteus meleagridis in a Wild Turkey from Florida. J. Wildl. Dis. 1987;23:495–498. doi: 10.7589/0090-3558-23.3.495. [DOI] [PubMed] [Google Scholar]
- Atkinson C.T., Forrester D.J., Greiner E.C. Pathogenicity of Haemoproteus meleagridis (Haemosporina: haemoproteidae) in experimentally infected domestic turkeys. J. Parasitol. 1988;74:228–239. [PubMed] [Google Scholar]
- Atkinson C.T., Greiner E.C., Forrester D.J. Pre‐erythrocytic development and associated host responses to Haemoproteus meleagridis (Haemosporina: haemoproteidae) in experimentally infected domestic turkeys. J. Protozool. 1986;33:375–381. doi: 10.1111/j.1550-7408.1986.tb05626.x. [DOI] [PubMed] [Google Scholar]
- Atkinson C.T., Thomas N.J., Hunter D.B. Wiley-Blackwell; Oxford: 2008. Parasitic Diseases of Wild Birds. [Google Scholar]
- Bennett F. Simple techniques for making avian blood smears. Can. J. Zool. 1970;48:585–586. [Google Scholar]
- Bennett G.F., Campbell A.G. Avian Haemoproteidae. I. Description of Haemoproteus fallisi n. sp. and a review of the haemoproteids of the family Turdidae. Can. J. Zool. 1972;50:1269–1275. doi: 10.1139/z72-172. [DOI] [PubMed] [Google Scholar]
- Bennett G.F., Earle R.A., Pierce M.A. The leucocytozoidae of South African birds: musophagiformes, cuculiformes, and Piciformes. Ostrich. 1993;64:73–78. [Google Scholar]
- Bennett G.F., Fallis A.M. Blood parasite of birds in algonquin park, Canada, and a discussion of their transmission. Can. J. Zool. 1960;38:261–273. [Google Scholar]
- Bennett G.F., Peirce M.A., Ashford R.W. Avian Haemotozoa: mortality and pathogenicity. J. Nat. Hist. 1993;27:993–1001. [Google Scholar]
- Bensch S., Hellgren O., Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Bensch S., Stjernman M., Hasselquist D., Ostman O., Hansson B., Westerdahl H., Pinheiro R.T. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. Biol. Sci. 2000;267:1583–1589. doi: 10.1098/rspb.2000.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendinger P.G. Facilitation of sap-feeding birds by the white-fronted woodpecker in the monte desert, Argentina. Condor. 1999;101:402–407. [Google Scholar]
- Bueno M.G., Lopez R.P.G., Menezes de R.M.T., Costa-Nascimento de M.J., Lima de G.F.M.C., Araújo de R.A.S., Guida F.J.V., Kirchgatter K. Identification of Plasmodium relictum causing mortality in penguins (Spheniscus magellanicus) from São Paulo Zoo, Brazil. Vet. Parasitol. 2010;173:123–127. doi: 10.1016/j.vetpar.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Cannell B.L., Krasnec K.V., Campbell K., Jones H.I., Miller R.D., Stephens N. The pathology and pathogenicity of a novel Haemoproteus spp. infection in wild Little Penguins (Eudyptula minor) Vet. Parasitol. 2013;197:74–84. doi: 10.1016/j.vetpar.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Cardona C.J., Ihejirika A., Mcclellan L. Haemoproteus lophortyx infection in bobwhite Quail. Avian Dis. 2002;46:249–255. doi: 10.1637/0005-2086(2002)046[0249:HLIIBQ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Coatney G.R. Some blood parasites from birds of the lake okaboji region. Am. Midl. Nat. 1938;20:336–340. [Google Scholar]
- Coatney G.R., Roudabush R.L. Some blood parasites from Nebraska birds. Am. Midl. Nat. 1937;18:1005–1030. [Google Scholar]
- Collins W.E., Jeffery G.M., Skinner J.C., Harrison A.J., Arnold F. Blood parasites of birds at wateree, South Carolina. J. Parasitol. 1966;52:671–673. [PubMed] [Google Scholar]
- COSEWIC, C. on the S. of E.W. in C. 2010. COSEWIC Assessment and Status Report on the White-headed Woodpecker Picoides Albolvartus in Canada. (Ottawa, Canada) [Google Scholar]
- Dimitrov D., Zehtindjiev P., Bensch S., Ilieva M., Iezhova T., Valkiūnas G. Two new species of Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) from European birds, with emphasis on DNA barcoding for detection of haemosporidians in wildlife. Syst. Parasitol. 2014;87:135–151. doi: 10.1007/s11230-013-9464-1. [DOI] [PubMed] [Google Scholar]
- Donovan T.A., Schrenzel M., Tucker T.A., Pessier A.P., Stalis I.H. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. J. Vet. Diagn. Investig. 2008;20:304–313. doi: 10.1177/104063870802000307. [DOI] [PubMed] [Google Scholar]
- Drever M.C., Aitken K.E.H., Norris A.R., Martin K. Woodpeckers as reliable indicators of bird richness, forest health and harvest. Biol. Conserv. 2008;141:624–634. [Google Scholar]
- Earle R.A., Bastianello S.S., Bennett G.F., Krecek R.C. Histopathology and morphology of the tissue stages of Haemoproteus columbae causing mortality in Columbiformes. Avian Pathol. 1993;22:67–80. doi: 10.1080/03079459308418901. [DOI] [PubMed] [Google Scholar]
- Ellis V.A., Collins M.D., Medeiros M.C.I., Sari E.H.R., Coffey E.D., Dickerson R.C., Lugarini C., Stratford J.A., Henry D.R., Merrill L., Matthews A.E., Hanson A.A., Roberts J.R., Joyce M., Kunkel M.R., Ricklefs R.E. Local host specialization, host-switching, and dispersal shape the regional distributions of avian haemosporidian parasites. Proc. Natl. Acad. Sci. 2015;112:11294–11299. doi: 10.1073/pnas.1515309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair J.M., Paul E., Jones J., Davie C., Kaiser G. Guidelines to the use of wild birds in research. Ornithol. Counc. 2010:1–215. [Google Scholar]
- Fayt P., Machmer M.M., Steeger C. Regulation of spruce bark beetles by woodpeckers - a literature review. For. Ecol. Manage. 2005;206:1–14. [Google Scholar]
- Ferrell S.T., Snowden K., Marlar A.B., Garner M., Lung N.P. Fatal hemoprotozoal infections in multiple avian species in a zoological park. J. Zoo Wildl. Med. 2007;38:309–316. doi: 10.1638/1042-7260(2007)038[0309:FHIIMA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Garamszegi L.Z. Climate change increases the risk of malaria in birds. Glob. Chang. Biol. 2011;17:1751–1759. [Google Scholar]
- Garigliany M.M., Marlier D., Tenner-Racz K., Eiden M., Cassart D., Gandar F., Beer M., Schmidt-Chanasit J., Desmecht D. Detection of usutu virus in a bullfinch (Pyrrhula pyrrhula) and a great-spotted woodpecker (Dendrocopos major) in North-west europe. Vet. J. 2014;199:191–193. doi: 10.1016/j.tvjl.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Garret K.L., Raphael M.G., Dixon R.D. Birds of North America. Cornell Lab of Ornithology; 1996. White-headed woodpecker (Picoides albolarvatus) [Google Scholar]
- Gerhold R.W., Yabsley M.J. Toxoplasmosis in a red-bellied woodpecker (Melanerpes carolinus) Avian Dis. 2007;51:992–994. doi: 10.1637/7978-040407-CASER.1. [DOI] [PubMed] [Google Scholar]
- Greiner E.C., Bennett G.F., White E.M., Coombs R.F. Distribution of the avian haematozoa of North America. Can. J. Zool. 1975;53:1762–1787. doi: 10.1139/z75-211. [DOI] [PubMed] [Google Scholar]
- Greiner E.C., Mandal A.K., Nandi N.C. Haemoproteus bennetti sp. n. and a review of the haemoproteids from the picidae (woodpeckers) J. Parasitol. 1977;63:651–656. [PubMed] [Google Scholar]
- Hellgren O., Kriz A., Valkiūnas G., Bensch S. Diversity and phylogeny of mitochondrial cytochrome b lineages from six morphospecies of avian Haemoproteus. Haemosporida: Haemoproteidae) 2007;93:889–896. doi: 10.1645/GE-1051R1.1. [DOI] [PubMed] [Google Scholar]
- Hellgren O., Waldenström J., Bensch S. vol. 90. 2004. pp. 797–802. (A New PCR Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian Blood). [DOI] [PubMed] [Google Scholar]
- Herman C.M. The blood Protozoa of north American birds. Bird-Banding. 1944;15:89–112. [Google Scholar]
- Holmes J.C. Population Biology of Infectious Diseases: Report of the Dahlem Workshop on Population Biology of Infectious Disease Agents, Berlin 1982, March 14-19. 1982. Impact of infectious disease agents on the population growth and geographical distribution of animals; pp. 37–51. [Google Scholar]
- Huff C.G. A survey of the blood parasite of birds caught for banding purposes. J. Am. Vet. Med. Assoc. 1939;47:615–620. [Google Scholar]
- Ibarzabal J., Tremblay J. a. The hole saw method for accessing woodpecker nestlings during developmental studies. Ann. Zool. Fenn. 2006;43:235–238. [Google Scholar]
- Jokelainen P., Vikøren T. Acute fatal toxoplasmosis in a great-spotted woodpecker (Dendrocopos major) J. Wildl. Dis. 2014;50:117–120. doi: 10.7589/2013-03-057. [DOI] [PubMed] [Google Scholar]
- Jusino M.A., Lindner D.L., Banik M.T., Rose K.R., Walters J.R. 2016. Experimental Evidence of a Symbiosis between Red-Cockaded Woodpeckers and Fungi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.A., Fallis A.M. A note on the sporogony of Parahaemoproteus velans (=Haemoproteus velans Coatney and Roudabush) (haemosporidia: haemoproteidae) in species of culicoid. Can. J. Zool. 1971;49:420–421. doi: 10.1139/z71-062. [DOI] [PubMed] [Google Scholar]
- Lachish S., Knowles S.C.L., Alves R., Wood M.J., Sheldon B.C. Fitness effects of endemic malaria infections in a wild bird population: the importance of ecological structure. J. Anim. Ecol. 2011;80:1196–1206. doi: 10.1111/j.1365-2656.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- Langham G., Schuetz J., Soykan C., Chad W., Auer T., LeBaron G., Sanchez C., Distler T. 2015. Audubon's Birds and Climate Change Report: A Primer for Practitioners. (New York) [Google Scholar]
- Lindenmayer D.B., Margules C.R., Botkin D.B. Indicators of biodiversity for ecologically sustainable forest management. Conserv. Biol. 2000;14:941–950. [Google Scholar]
- Martinsen E.S., Paperna I., Schall J.J. Morphological versus molecular identification of avian Haemosporidia: an exploration of three species concepts. Parasitology. 2006;133:279–288. doi: 10.1017/S0031182006000424. [DOI] [PubMed] [Google Scholar]
- Martinsen E.S., Perkins S.L., Schall J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol. Phylogenetics Evol. 2008;47:261–273. doi: 10.1016/j.ympev.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Marzal A., García-Longoria L., Cárdenas Callirgos J.M., Sehgal R.N.M. Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol. Invasions. 2015;17:39–45. [Google Scholar]
- Medeiros M.C., Anderson T.K., Higashiguchi J.M., Kitron U.D., Walker E.D., Brawn J.D., Krebs B.L., Ruiz M.O., Goldberg T.L., Ricklefs R.E., Hamer G.L. An inverse association between West Nile virus serostatus and avian malaria infection status. Parasites Vectors. 2014;7:415. doi: 10.1186/1756-3305-7-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millspaugh J.J., Marzluff J.M., editors. Radio Tracking and Animal Populations. Academic Press; San Diego: 2001. [Google Scholar]
- Moens M.A.J., Valkiūnas G., Paca A., Bonaccorso E., Aguirre N., Pérez-Tris J. Parasite specialization in a unique habitat: hummingbirds as reservoirs of generalist blood parasites of Andean birds. J. Anim. Ecol. 2016;85:1234–1245. doi: 10.1111/1365-2656.12550. [DOI] [PubMed] [Google Scholar]
- Mukhin A., Palinauskas V., Platonova E., Kobylkov D., Vakoliuk I., Valkiūnas G. The strategy to survive primary malaria infection: an experimental study on behavioral changes in parasitized birds. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0159216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCBI Resource Coordinators Database resources of the national center for Biotechnology information. Nucleic Acids Res. 2016;44 doi: 10.1093/nar/gkv1290. Databa, D7–D19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA . National Centers for Environmental Information; 2015. Climate Monitoring: Temperature, Precipitation, and Drought.http://www.ncdc.noaa.gov/ [WWW Document]. URL. accessed 1.10.16. [Google Scholar]
- ODFW O.C.S. Oregon Department of Fish and Wildlife. 2016. http://www.oregonconservationstrategy.org/ [WWW Document]. URL. accessed 11.25.16)
- Olias P., Wegelin M., Zenker W., Freter S., Gruber A.D., Klopfleisch R. Avian malaria deaths in parrots, Europe. J. Infect. Dis. 2009;200(Suppl. l):950–952. doi: 10.3201/eid1705.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw D.C., Ricklefs R.E. Species limits in avian malaria parasites (Haemosporida): how to move forward in the molecular era. Parasitology. 2014;141:1223–1232. doi: 10.1017/S0031182014000560. [DOI] [PubMed] [Google Scholar]
- Pacheco M.A., Matta N.E., Valkiūnas G., Parker P.G., Mello B., Stanley C.E., Lentino M., Garcia-Amado M.A., Cranfield M., Kosakovsky Pond S.L., Escalante A.A. Mode and rate of evolution of Haemosporidian mitochondrial genomes: timing the radiation of avian parasites. Mol. Biol. Evol. 2018;35:383–403. doi: 10.1093/molbev/msx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz J.A., Graczyk T.K., Geller N., Vittor A.Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Peirce M.A., Lederer R., Adlard R.D., O'Donoghue P.J. Pathology associated with endogenous development of haematozoa in birds from southeast Queensland. Avian Pathol. 2004;33:445–450. doi: 10.1080/03079450410001724076. [DOI] [PubMed] [Google Scholar]
- Pung O.J., Carlile L.D., Whitlock J., Vives S.P., Durden L. a, Spadgenske E. Survey and host fitness effects of Red-cockaded woodpecker blood parasites and nest cavity arthropods. J. Parasitol. 2000;86:506–510. doi: 10.1645/0022-3395(2000)086[0506:SAHFEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Raphael M.G., White M. Use of snags by cavity-nesting birds in the Sierra Nevada. Wildl. Monogr. 1984;86:3–66. [Google Scholar]
- Ricklefs R.E., Fallon S.M. Diversification and host switching in avian malaria parasites. Proc. R. Soc. Biol. Sci. 2002;269:885–892. doi: 10.1098/rspb.2001.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M.S., Walters E.L., James F.C., Greiner E.C., Auk S.T., Jan N. Seasonal prevalence of a Haematozoan parasite of Red-bellied Woodpeckers (Melanerpes carolinus) and its association with host condition and overwinter survival. Auk. 2003;120:130–137. [Google Scholar]
- Sehgal R.N.M., Jones H.I., Smith T.B. Host specificity and incidence of Trypanosoma in some African rainforest birds: a molecular approach. Mol. Ecol. 2001;10:2319–2327. doi: 10.1046/j.1365-294x.2001.01339.x. [DOI] [PubMed] [Google Scholar]
- Sehgal R.N.M., Lovette I.J. Molecular evolution of three avian neurotrophin genes: implications for proregion functional constraints. J. Mol. Evol. 2003;57:335–342. doi: 10.1007/s00239-003-2484-8. [DOI] [PubMed] [Google Scholar]
- Siegel R.B., Bond M.L., Wilkerson R.L., Barr B.C., Gardiner C.H., Kinsella J.M. Lethal procyrnea infection in a black-backed woodpecker (Picoides arcticus) from California. J. Zoo Wildl. Med. 2012;43:421–424. doi: 10.1638/2011-0226.1. [DOI] [PubMed] [Google Scholar]
- Smith J.D., Gill S.A., Baker K.M., Vonhof M.J. Prevalence and diversity of avian Haemosporida infecting songbirds in southwest Michigan. Parasitol. Res. 2018;117:471–489. doi: 10.1007/s00436-017-5724-3. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. 2018. Paup*. Phylogenetic Analysis Using Parsimony (*and Other Methods) [Google Scholar]
- Tarbill G.L., Manley P.N., White A.M. Drill, baby, drill: the influence of woodpeckers on post-fire vertebrate communities through cavity excavation. J. Zool. 2015;296:95–103. [Google Scholar]
- Valkiūnas G. CRC Press; 2005. Avian Malaria Parasites and Other Haemosporidia. [Google Scholar]
- Valkiūnas G., Atkinson C.T., Bensch S., Sehgal R.N.M., Ricklefs R.E. Parasite misidentifications in GenBank: how to minimize their number? Trends Parasitol. 2008;24:247–248. doi: 10.1016/j.pt.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G., Iezhova T.A. Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malar. J. 2017;16:1–24. doi: 10.1186/s12936-017-1746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiūnas G., Lezhova T.A., Križanauskienė A., Palinauskas V., Sehgal R.N.M., Bensch S. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J. Parasitol. 2008;94:1395–1401. doi: 10.1645/GE-1570.1. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G., Z̆ic̆kus T., Shapoval A.P., Iezhova T.A. Effect of Haemoproteus belopolskyi (Haemosporida: haemoproteidae) on body mass of the Blackcap Sylvia atricapilla. J. Parasitol. 2006;92:1123–1125. doi: 10.1645/GE-3564-RN.1. [DOI] [PubMed] [Google Scholar]
- Van Riper C., Van Riper S., Goff L.M., Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 1986;56:327–344. [Google Scholar]
- Vanstreels R.E.T., Da Silva-Filho R.P., Kolesnikovas C.K.M., Bhering R.C.C., Ruoppolo V., Epiphanio S., Amaku M., Junior F.C.F., Braga É.M., Catão-Dias J.L. Epidemiology and pathology of avian malaria in penguins undergoing rehabilitation in Brazil. Vet. Res. 2015;46:1–12. doi: 10.1186/s13567-015-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkkala R. Why study woodpeckers? The significance of woodpeckers in forest ecosystems. Ann. Zool. Fenn. 2006;43:82–85. [Google Scholar]
- Waldenström A.J., Bensch S., Hasselquist D., Östman Ö., Waldenström J., Bensch S., Hasselquist D., Östman Ö. A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J. Parasitol. 2004;90:191–194. doi: 10.1645/GE-3221RN. [DOI] [PubMed] [Google Scholar]
- Walther E.L., Carlson J.S., Cornel A., Morris B.K., Sehgal R.N.M. First molecular study of prevalence and diversity of avian haemosporidia in a central California songbird community. J. Ornithol. 2016;157:549–564. [Google Scholar]
- Walther G., Post E., Convey P., Menzel A., Parmesan C., Beebee T.J.C., Fromentin J., I O.H., Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Warner R.E. The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor. 1968;70:101–120. [Google Scholar]
- WDFW, W.D. of F. and W. Wildlife Program; Olympia: 2013. Threatened and Endangered Wildlife in Washington: 2012 Annual Recovery Section. [Google Scholar]
- White G.C., Garrot R.A. Academic Press; San Diego: 1990. Analysis of Wildlife Radio-Tracking Data. [Google Scholar]
- Widáhn S., Kindblom L.-G. A rapid and simple method for electron microscopy of paraffin-embedded tissue. Ultrastruct. Pathol. 1988;12:131–136. doi: 10.3109/01913128809048481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.