Abstract
Phosphatidylserine (PS), an anionic phospholipid enriched in the inner leaflet of the plasma membrane, is exposed to the outer leaflet during apoptosis. PS exposure was recently shown to be induced during tumor necrosis factor–induced necroptosis. We herein demonstrated that interferon (IFN)-γ induced necroptosis in Caspase-8–knockout mouse-derived embryonic fibroblasts (C8KO MEFs), as well as in WT MEFs co-treated with the pan-caspase inhibitor, z-VAD-fmk. PS exposure and necroptosis were significant after 6- and 24-h treatments with IFN-γ, respectively. To elucidate the molecular mechanisms underlying IFN-γ–induced PS exposure, we generated C8KO MEF-derived cell lines without the expression of RIPK3 (receptor-interacting protein kinase 3), an essential molecule in tumor necrosis factor–induced necroptosis, and IFN-γ–induced PS exposure and necrotic cell death were shown to be specifically inhibited by the loss of RIPK3 expression. Furthermore, the down-regulated expression of MLKL (mixed lineage kinase domain-like protein), a key molecule for inducing membrane rupture downstream of RIPK3 in necroptosis, abolished IFN-γ–induced PS exposure in C8KO MEFs. In human colorectal adenocarcinoma-derived HT29 cells, PS exposure and necroptosis were similarly induced by treatment with IFN-γ in the presence of Smac mimetics and z-VAD-fmk. The removal of IFN-γ from PS-exposing MEFs after a 6-h treatment completely inhibited necroptotic cell death but not the subsequent increase in the number of PS-exposing cells. Therefore, PS exposure mediated by RIPK3-activated MLKL oligomers was induced by a treatment with IFN-γ for a significant interval of time before the induction of necroptosis by membrane rupture.
Keywords: caspase, cell death, interferon, necrosis (necrotic death), phosphatidylserine, receptor-interacting protein (RIP), interferon-gamma, MLKL, necroptosis, RIPK3
Introduction
Phospholipids are heterogeneously distributed between the inner and outer leaflets of the plasma membrane. Phosphatidylserine (PS)2 is the most abundant negatively charged phospholipid in the plasma membrane of eukaryotic cells and is preferentially distributed in the inner leaflet (1). PS is exposed from the inner to outer leaflet of the plasma membrane during apoptosis and serves as an “eat me” signal that promotes phagocytosis (2, 3). Xkr8 was recently identified as a caspase-3–dependent scramblase responsible for promoting PS exposure in response to apoptosis (4, 5). Furthermore, TMEM16F was shown to function as a calcium-dependent but caspase-independent phospholipid scramblase in the plasma membrane and linked to coagulation reactions (6). In contrast to apoptosis, PS exposure was originally considered to not be induced during necrosis, which is defined as passive cell death (7).
Necroptosis is a nonapoptotic regulated form of necrosis (8, 9). Similar to apoptosis induced by the extrinsic pathway (10), necroptosis is triggered by the binding of several inducers, including tumor necrosis factor (TNF), Fas ligand, TNF-related apoptosis-inducing ligand (TRAIL), and certain pathogens, to their receptors including TNF receptor 1, Fas, TRAIL receptors, and Toll-like receptors, respectively (9). Among the various necroptosis-inducing pathways, the most-characterized pathway is the TNF-induced system. Necroptosis requires the activity of RIPK1 (receptor-interacting protein kinase 1) and its related kinase, RIPK3 (11), and is mediated by the activation of the pseudokinase MLKL (mixed lineage kinase domain-like protein) (12, 13). MLKL binds RIPK3 through its kinase-like domain to form a necrosis-inducing signaling complex, termed necrosome, within which MLKL is activated by phosphorylation by RIPK3. Activated MLKL oligomerizes, translocates to the plasma membrane and eventually induces membrane disruption and subsequent cell death (13). This process requires the inhibition of caspase-8 function, which may be achieved genetically or pharmacologically (14, 15). In recent studies, PS exposure was detected during TNF-induced necroptosis, and PS-exposing cells were shown to rapidly die by necroptosis (16, 17).
Interferon (IFN), which constitutes a family of cytokines with antiviral, cell growth–suppressive, and immunomodulatory activities, is classified into two primary groups: type I (IFN-α/β) and type II (IFN-γ) (18). Both types of IFN activate similar but different JAK/STAT-dependent signaling cascades downstream of their receptors to induce the expression of hundreds of specific genes (19), which collaboratively mediate various biological activities, including cytotoxic and antiproliferative activities (20, 21). However, the collaborative mechanisms of IFN-induced genes in these processes as well as the mechanisms by which IFN induces cell death currently remain unclear.
In the present study, we demonstrated that IFN-γ induced necroptosis when the caspase-8–dependent apoptotic pathway was inhibited, and PS was exposed on the outer plasma membrane of IFN-γ–treated cells for a significant interval of time before the induction of necroptosis by membrane rupture. The activation of RIPK3 and MLKL was shown to be essential for IFN-γ–induced PS exposure. These results provide a novel molecular mechanism for IFN-γ–induced PS exposure, and we propose a new potential function of MLKL in exposing PS on the plasma membrane without inducing membrane rupture.
Results
Long-term PS exposure is induced before the induction of necroptosis in IFN-γ–treated MEFs
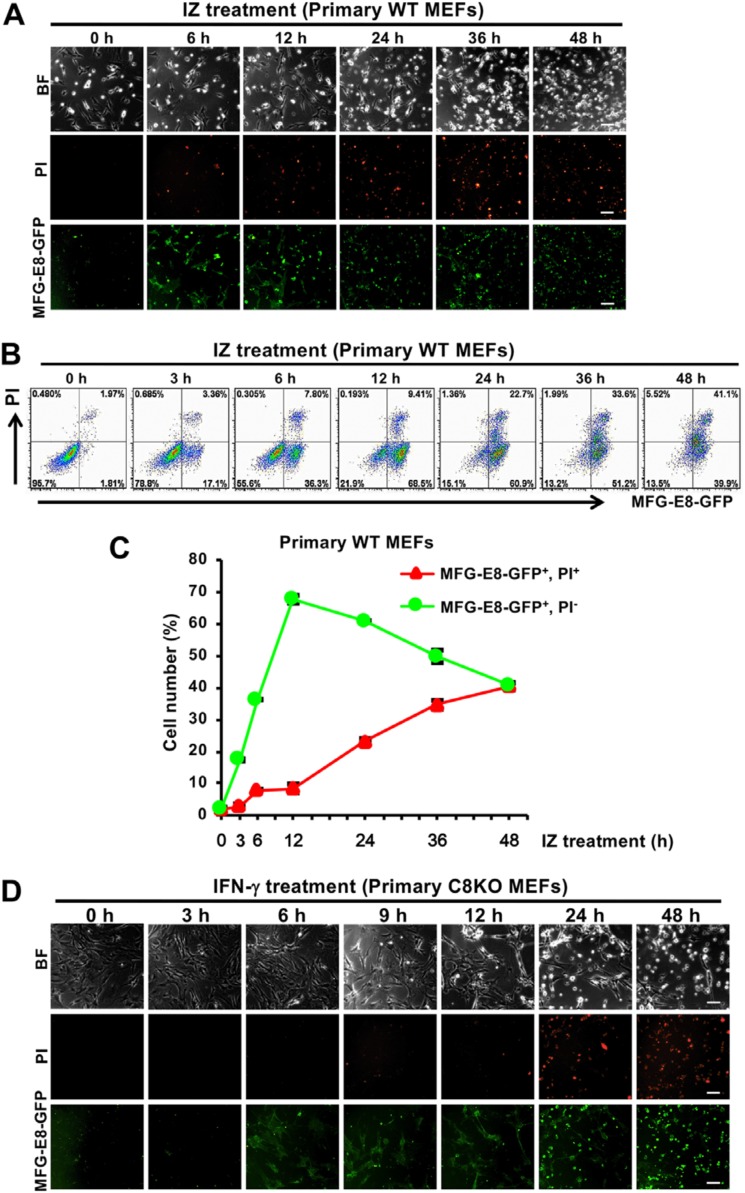
To investigate whether PS is exposed during the IFN-γ–induced necroptotic process, primary WT mouse embryonic fibroblasts (MEFs) with the Balb/c genetical background were treated with IFN-γ and z-VAD-fmk (IZ). z-VAD-fmk is a pan-caspase inhibitor that blocks the protease activities of various caspases, including caspase-8. PS-exposing and dead cells were stained with GFP-conjugated milk fat globule-EGF-factor 8 (MFG-E8) and propidium iodide (PI), respectively; monitored by fluorescent microscopy (Fig. 1A); and quantified by flow cytometry (Fig. 1, B and C). In primary WT MEFs, cell death began to be induced after the IFN-γ treatment for 24 h in the presence of z-VAD-fmk, and cell viability was restored by a treatment with necrostatin-1, an inhibitor of RIPK1 that plays an essential role in the induction of necroptosis (Fig. S1, A and B) (22). The IFN-γ–induced cell death was not associated with the cleavage of caspase-3, which was monitored by Western blotting analyses for caspase-3 (Fig. S1C). The induction of necroptosis but not apoptosis was observed in primary WT MEFs after the IFN-γ treatment for 24 h in the presence of z-VAD-fmk. On the other hand, after 6 h of the IFN-γ treatment in the presence of z-VAD-fmk, PS-exposing cells began to be significantly detectable, whereas dead cells were significantly detected after 24 h (Fig. 1C). Thus, long-term PS exposure was induced before the induction of necroptosis in IFN-γ–treated primary WT MEFs in the presence of z-VAD-fmk.
Figure 1.
IFN-γ induces PS exposure in WT and C8KO MEFs before the induction of cell death. A and B, primary WT MEFs were treated with 10 ng/ml IFN-γ and 50 μm z-VAD-fmk (IZ) for the indicated periods. The cells were stained with MFG-E8-GFP and PI to monitor PS exposure and cell death, respectively, and then analyzed by fluorescent microscopy (A) and flow cytometry (B). BF, Brightfield. C, the quantified data of the flow cytometric analysis from B are graphically shown. D, primary C8KO MEFs were treated with 10 ng/ml IFN-γ for the indicated periods. The cells were stained with MFG-E8-GFP and PI and then analyzed by fluorescent microscopy. Representative fluorescent images and flow cytometric plots of more than three experiments are shown, and the scale bars represent 100 μm. The quantified data of the flow cytometric analysis are presented as means ± S.D. (n ≥ 3).
To further examine PS exposure in IFN-γ–induced necroptosis, we utilized primary MEFs from previously generated Caspase-8−/− (C8KO) mice with the Balb/c genetical background (23, 24). Primary C8KO MEFs also exposed PS after 6 h of the treatment with IFN-γ, whereas they began to die after 24 h (Fig. 1D). These results were similar to those obtained for primary WT MEFs in the presence of z-VAD-fmk. Further analyses using primary C8KO MEFs were not performed because the amount of available primary C8KO MEFs was too small because of the early embryonic lethality of C8KO mice. The results obtained for primary MEFs indicated that the IFN-γ treatment induced PS exposure for more than 10 h without the induction of necroptosis in the absence of caspase-8 activity.
RIPK3 is essential for PS exposure in IFN-γ–treated immortalized caspase-8 KO MEFs
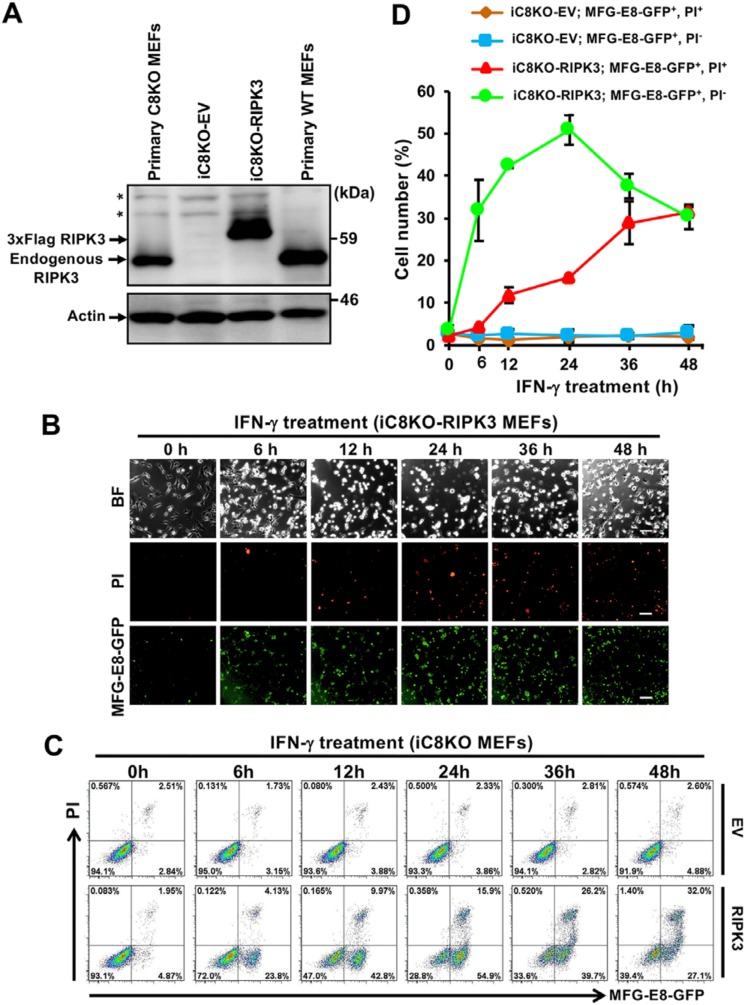
We investigated the mechanisms underlying IFN-γ–induced PS exposure using immortalized C8KO (iC8KO) MEFs with the Balb/c genetical background. Neither cell death nor PS exposure was detected after 24 h of the treatment with IFN-γ by fluorescent microscopic and flow cytometric analyses in our generated iC8KO MEFs (Fig. S2, A and B), indicating that these iC8KO MEFs lost sensitivity to IFN-γ–induced PS exposure, as well as necroptotic cell death. We then examined the expression of RIPK3, an essential molecule for the induction of necroptosis in iC8KO MEFs, and found that RIPK3 expression disappeared (Fig. S2C). We generated more than five iC8KO MEF lines by repeated passage for more than 1 month, and all of the spontaneously generated iC8KO MEFs showed loss or severe down-regulation of RIPK3 expression. To clarify whether the loss of RIPK3 expression caused insensitivity to IFN-γ–induced necroptosis and PS exposure, we exogenously expressed RIPK3 in one of the iC8KO MEF line at a similar expression level to that in primary C8KO MEFs (Fig. 2A). RIPK3-expressing iC8KO (iC8KO-RIPK3) MEFs began to expose PS after 6 h of the IFN-γ treatment before the induction of cell death (Fig. 2, B and C), and exposed PS levels increased in a time-dependent manner (Fig. 2D), similar to that in primary C8KO MEFs (Fig. 1D), indicating that the PS-exposing property was restored in iC8KO MEFs by the introduction of RIPK3. Therefore, RIPK3 is indispensable for IFN-γ–induced PS exposure, as well as necroptosis.
Figure 2.
RIPK3 is necessary for IFN-γ–induced PS exposure in iC8KO MEFs. A, cell lysates from primary WT MEFs, primary C8KO MEFs, and iC8KO MEFs harboring the indicated expression vectors were analyzed by Western blotting with anti-RIPK3 and anti-actin antibodies. EV, an empty vector. *, nonspecific bands. B, iC8KO-RIPK3 MEFs were treated with 10 ng/ml IFN-γ for the indicated periods. The cells were stained with MFG-E8-GFP and PI and then analyzed by fluorescent microscopy. BF, Brightfield. The scale bars represent 100 μm. C, EV-expressing iC8KO (iC8KO-EV) and iC8KO-RIPK3 MEFs were treated with 10 ng/ml IFN-γ for the indicated periods. The cells were stained with MFG-E8-GFP and PI and analyzed by flow cytometry. D, the quantified data of the flow cytometric analysis from C are graphically shown. Representative Western blotting data, fluorescent images, and flow cytometric plots of more than three experiments are shown. The quantified data of the flow cytometric analysis are presented as means ± S.D. (n ≥ 3).
The RIP homotypic interaction motif (RHIM) domains of both RIPK1 and RIPK3, through which RIPK1 and RIPK3 could directly interact, were reported to be necessary for induction of necroptosis (11). Then we analyzed the role of the RHIM domain of RIPK3 in IFN-γ–induced PS exposure. A mutant of RIPK3 (four important amino acid residues, VQIG, of the RHIM domain of RIPK3 were replaced by AAAA as described under “Experimental procedures”) was expressed in iC8KO MEFs, and neither necroptosis nor PS exposure was induced by the IFN-γ treatment in the iC8KO MEFs expressing the RHIM domain mutant of RIPK3 (Fig. S3), indicating that RIPK3 and its interaction with RIPK1 are important for IFN-γ–induced PS exposure as well as necroptosis.
MLKL is a key executive factor in IFN-γ–induced PS exposure
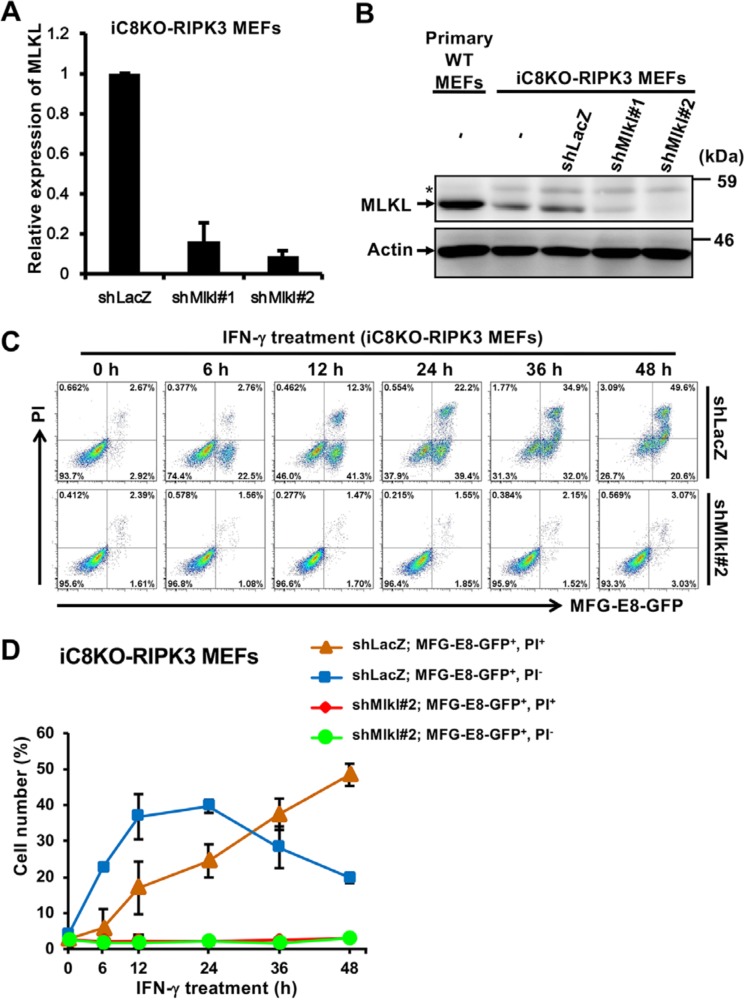
MLKL was recently identified as a molecule involved in the execution of plasma membrane rupture in necroptosis upon the phosphorylation by RIPK3 (12, 13). We investigated whether MLKL contributed to IFN-γ–induced PS exposure. Using short hairpin RNAs (shRNAs) specific for MLKL, the expression of MLKL was significantly down-regulated at both the mRNA and protein levels (Fig. 3, A and B), and shMlkl#2 appeared to be the most effective. Using shMlkl#2-expressing iC8KO MEFs treated with IFN-γ, PS-exposing and dead cells were monitored and quantified by fluorescent microscopy (Fig. S4A) and flow cytometry (Fig. 3, C and D), respectively. In contrast to shLacZ-expressing control cells, shMlkl#2-expressing cells neither exposed PS nor died following treatment with IFN-γ, even after 48 h, indicating that PS exposure was abrogated by the down-regulation of MLKL expression. As well as the iC8KO-RIPK3 MEF knockdown by shMlkl#2, shMlkl#1-expressing iC8KO-RIPK3 MEFs lost their PS-exposing property and sensitivity to necroptosis (Fig. S4, B and C). Collectively, these results indicate that RIPK3-activated MLKL is essential for IFN-γ–induced PS exposure in iC8KO-RIPK3 MEFs.
Figure 3.
PS is exposed in a MLKL-dependent manner in IFN-γ–treated iC8KO-RIPK3 MEFs. A and B, iC8KO-RIPK3 MEFs were infected with lentiviral vectors encoding two different shRNAs for MLKL (shMlkl#1 and shMlkl#2). The expression levels of MLKL were analyzed by qRT-PCR (A) and by Western blotting with anti-MLKL and anti-actin antibodies (B). *, nonspecific bands. C, iC8KO-RIPK3 MEFs expressing shLacZ or shMlkl#2 were treated with 10 ng/ml IFN-γ for the indicated periods. The cells were stained with MFG-E8-GFP and PI and analyzed by flow cytometry. D, the quantified data of the flow cytometric analysis from C are graphically shown. Representative qRT-PCR data, Western blotting data, fluorescent images, and flow cytometric plots of more than three experiments are shown. For qRT-PCR analysis and flow cytometry quantification, the data are presented as means ± S.D. (n ≥ 3).
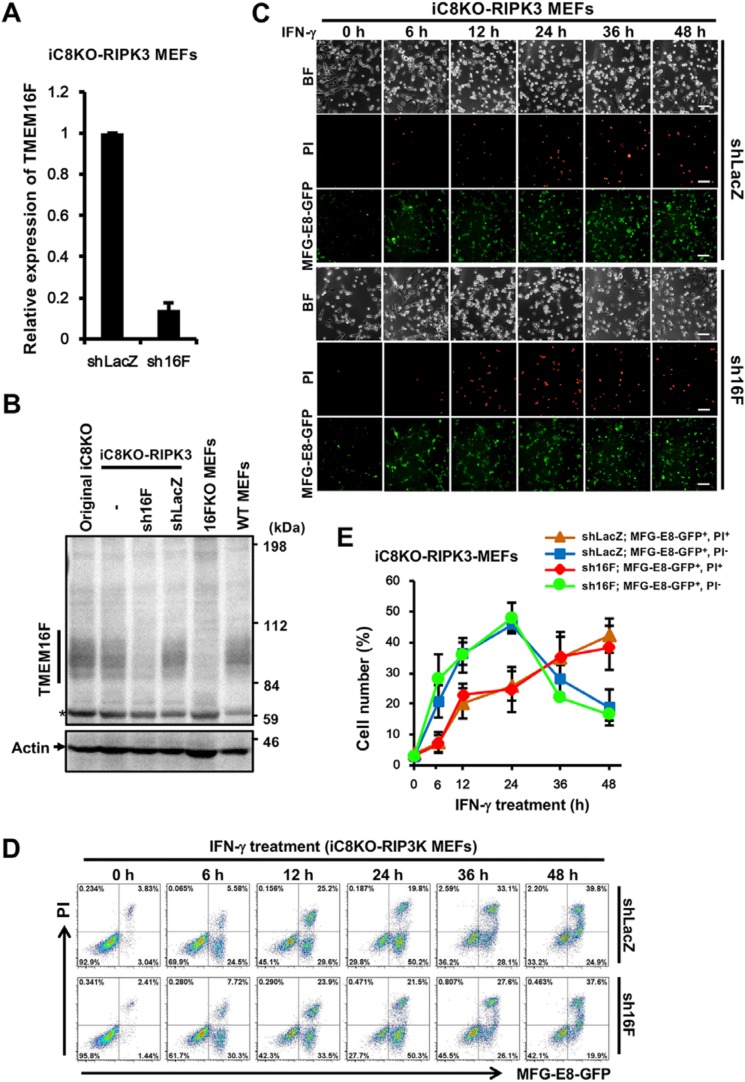
On the other hand, we investigated whether IFN-γ–induced PS exposure is induced by TMEM16F, a calcium-dependent membranous scramblase that has been shown to transport phospholipids bidirectionally in the plasma membrane in a caspase-independent manner (6) and to be activated during necroptosis (25). TMEM16F knockdown cells were generated by the expression of shRNA specific for TMEM16F (sh16F) in iC8KO-RIPK3 MEFs, and sh16F effectively reduced the expression of TMEM16F at both mRNA and protein levels as TMEM16F KO MEFs (Fig. 4, A and B). The down-regulation of TMEM16F expression in iC8KO-RIPK3 MEFs was shown to induce PS exposure before the induction of necroptosis by the treatment with IFN-γ in the same way as control shLacZ-expressing iC8KO-RIPK3 MEFs (Fig. 4, C–E), indicating that TMEM16F was not involved in IFN-γ–induced PS exposure.
Figure 4.
TMEM16F is dispensable for IFN-γ–induced PS exposure. A and B, iC8KO-RIPK3 MEFs were infected with a lentiviral vector encoding shRNA for TMEM16F (sh16F). The expression of TMEM16F was quantified by qRT-PCR (A) and Western blotting with anti-TMEM16F and anti-actin antibodies (B). *, nonspecific bands. C and D, iC8KO-RIPK3 MEFs expressing shLacZ or sh16F were treated with 10 ng/ml IFN-γ for the indicated periods. The cells were stained with MFG-E8-GFP and PI and analyzed by fluorescent microscopy (C) and flow cytometry (D). BF, Brightfield. The scale bar represents 100 μm. E, the quantified data of the flow cytometric analysis from D are graphically shown. Representative qRT-PCR data, Western blotting data, fluorescent images, and flow cytometric plots of more than three experiments are shown. For qRT-PCR analysis and flow cytometry quantification, the data are presented as means ± S.D. (n ≥ 3).
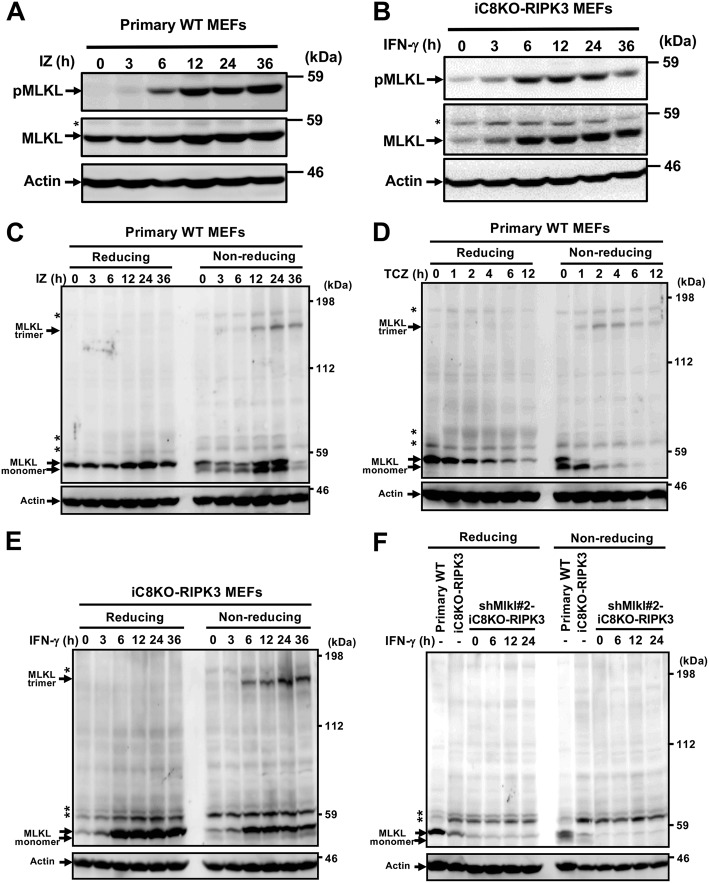
MLKL was phosphorylated and oligomerized in IFN-γ–treated MEFs exposing PS before necroptosis
In TNF-induced necroptosis, RIPK3-activated MLKL was reported to be phosphorylated and to form a trimer, which induced membrane rupture (13, 26, 27). The phosphorylation of MLKL was detected after 3–6 h of the treatment with IFN-γ or IFN-γ plus z-VAD-fmk in iC8KO-RIPK3 or primary WT MEFs, respectively, and then the amounts of phosphorylated MLKL increased (Fig. 5, A and B). To establish whether the oligomerization of the MLKL protein was induced in PS-exposing MEFs, primary WT MEFs were treated with IZ, and the expression levels and trimerization of MLKL were analyzed by SDS-PAGE. The MLKL protein was detected as a monomer with a molecular mass (Mr) of ∼52 kDa under reducing conditions, and as an oligomer with Mr of ∼160 kDa, roughly 3-fold higher than that of the monomer of MLKL under nonreducing conditions (Fig. 5C). Similar monomer and trimer bands of MLKL were observed in primary WT MEFs treated with TNFα, cycloheximide, and z-VAD-fmk (TCZ) (Fig. 5D) and in iC8KO-RIPK3 MEFs treated with IFN-γ (Fig. 5E). These monomer and trimer MLKL proteins were not detected in shMlkl#2-expressing iC8KO-RIPK3 MEFs, even those treated with IFN-γ (Fig. 5F). Between 3 and 24 h after the treatment with IFN-γ, expression levels of MLKL increased, whereas the treatment with TCZ rapidly decreased the expression levels of MLKL, indicating that IFN-γ induces the expression of MLKL in MEFs. The phosphorylated MLKL was similarly oligomerized in these IFN-γ–treated MEFs (Fig. S5, A and B), and the oligomerization was not observed in iC8KO MEFs expressing the RHIM domain mutant of RIPK3 (Fig. S5C).
Figure 5.
The MLKL protein forms a trimer in PS-exposing MEFs treated with IFN-γ. A and B, primary WT MEFs (A) and iC8KO-RIPK3 MEFs (B) were treated with 10 ng/ml IFN-γ and 50 μm z-VAD-fmk (IZ) and with 10 ng/ml IFN-γ, respectively, for the indicated periods. Cell lysates were resolved on reducing SDS-PAGE and analyzed by Western blotting with anti-phosphorylated MLKL (pMLKL), anti-MLKL, and anti-actin antibodies. C and D, cell lysates of primary WT MEFs treated with IZ (C) or with 10 ng/ml TNFα, 1 μg/ml cycloheximide, and 50 μm z-VAD-fmk (TCZ) (D) for the indicated periods were resolved on reducing or nonreducing SDS-PAGE and analyzed by Western blotting with anti-MLKL and anti-actin antibodies. E and F, iC8KO-RIPK3 MEFs (E) or primary WT MEFs, iC8KO-RIPK3 MEFs, and iC8KO-RIPK3 MEFs expressing shMlkl#2 (F) were treated with IFN-γ for the indicated periods. Cell lysates were resolved on reducing or nonreducing SDS-PAGE and analyzed by Western blotting with anti-MLKL and anti-actin antibodies. *, nonspecific bands. Representative Western blotting data of more than three experiments are shown.
The trimer of MLKL was detected after 3–6 h of the treatment with IZ in primary WT and after 6 h of the treatment with IFN-γ in iC8KO-RIPK3 MEFs. These time courses for the appearance of the MLKL trimer were similar to those for PS exposure (Figs. 1 and 2) and phosphorylation of MLKL (Fig. 5, A and B). The MLKL protein was shown to be phosphorylated and to trimerize in PS-exposing MEFs treated with IFN-γ for more than 10 h before the execution of necroptosis. In primary WT MEFs, the MLKL trimer, PS exposure, and necroptosis began to be detected after 2, 2, and 4 h of the treatment with TCZ, respectively (Fig. 5D and Fig. S6), indicating that the time courses for the appearance of the MLKL trimer were similar to those for PS exposure, and PS was exposed for ∼2 h before the execution of necroptosis. Collectively, these results indicate that the MLKL trimer induces PS exposure and the execution of necroptosis, and the amount of trimerized MLKL may influence the fate of cells to expose PS without disrupting the plasma membrane or to execute necroptosis.
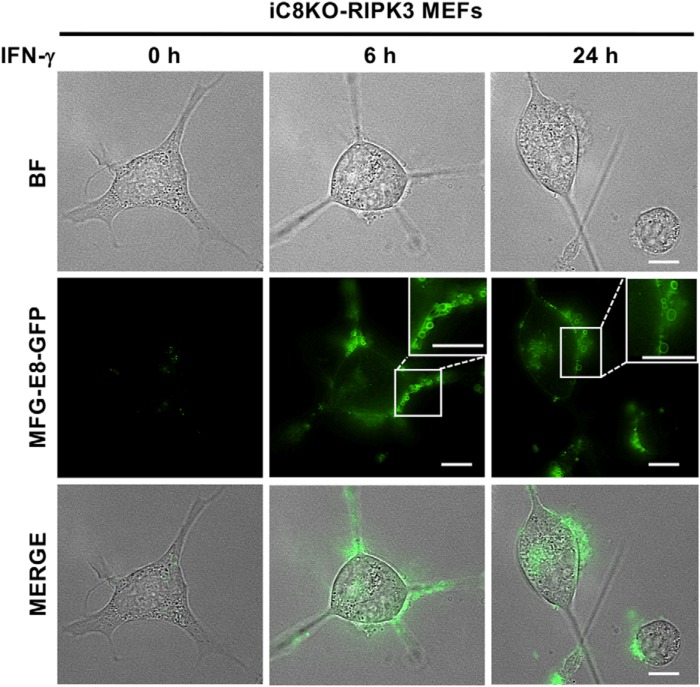
We then investigated the behavior of activated MLKL before the loss of membrane integrity in our IFN-γ–induced PS exposure model. After 6 h of the treatment with IFN-γ, we found not only PS exposure, but also MFG-E8-GFP+ bubble formation on the PS-exposing plasma membrane of iC8KO-RIPK3 MEFs, and bubbles were still observed but appeared to be shed from the cell surface after 24 h of the treatment with IFN-γ (Fig. 6). The IFN-γ–induced formation and shedding of PS-exposing bubbles were similar to recently reported bubble formation during TNF-induced necroptosis (13, 28). We speculated that IFN-γ–induced bubbles, which may contain activated MLKL, may contribute to maintaining the amount of activated MLKL at a level that is sufficient to induce PS exposure but inadequate for membrane rupture.
Figure 6.
Formation and shedding of membrane bubbles in IFN-γ–treated MEFs exposing PS. iC8KO-RIPK3 MEFs were treated with 10 ng/ml IFN-γ for the indicated periods and then directly stained with MFG-E8-GFP. The cells were then observed under fluorescent microscopy. The scale bars represent 10 μm. Representative fluorescent images of more than three experiments are shown. BF, Brightfield.
IFN-γ induces PS exposure in various cells
IFN-γ was shown to induce PS exposure and necroptosis dependently on RIPK1, RIPK3, and MLKL, which mechanism was similar to that of TNFα (11–13). Because there remains a possibility that IFN-γ may only sensitize cells to PS exposure and necroptosis, which are induced by autocrine TNFα, we analyzed the effect of anti-TNFα antibody with neutralizing activity to the IFN-γ–induced PS exposure and necroptosis. Treatment with anti-TNFα antibody, which clearly inhibited TNFα-induced necroptosis in NIH3T3 cells, inhibited neither IFN-γ–induced PS-exposure nor necroptosis in iC8KO-RIPK3 MEFs (Fig. S7), indicating that IFN-γ induces PS exposure and necroptosis without the help of autocrine TNFα.
Recently, combination of IFN-γ and Smac mimetics (synthetic antagonists to inhibitor of apoptosis protein/IAP) was reported to induce novel caspase-10– and RIPK1-dependent cell death pathways in human colorectal adenocarcinoma-derived HT29 cells (29). Although PS exposure and cell death were observed in IFN-γ–treated HT29 cells in the presence of Smac mimetics (Fig. S8A), they were not significantly inhibited by co-treatment with a human MLKL-specific inhibitor necrosulfonamide (NSA) (27) (data not shown). The mechanism by which IFN-γ induced PS exposure in HT29 cells in the presence of Smac mimetics seemed to be different from that observed in IFN-γ–treated MEFs in the absence of caspase-8 activity. Then we analyzed the effect of IFN-γ on human HT29 cells in the presence of Smac mimetics and z-VAD-fmk (Fig. S8B). IFN-γ treatment of human HT29 cells together with Smac mimetics and z-VAD-fmk induced PS exposure and cell death, both of which were inhibited by co-treatment with NSA. Thus, IFN-γ treatment can induce MLKL-dependent PS exposure in not only MEFs but also human colorectal adenocarcinoma-derived cells in the absence of caspase-8 activity.
Then we examined another immortalized caspase-8 knockout MEFs, iC8KO17e, with the C57BL/6 genetic background. iC8KO17e still expressed little amount of RIPK3, and we generated iC8KO17e expressing shRipk3 in which RIPK3 expression was clearly inhibited (Fig. S9, A and B). After a 24-h treatment with IFN-γ, only ∼20% and less than 10% of parental iC8KO17e were shown to expose PS and die, respectively, and both PS exposure and cell death were inhibited by knockdown of RIPK3 expression using shRipk3 (Fig. S9C). We then generated iC8KO17e expressing exogenous RIPK3 (17e-RIPK3) at a similar level to that in WT MEFs (Fig. S9A). Between 12 and 36 h after treatment of 17e-RIPK3 with IFN-γ, PS exposure and necroptosis were observed in approximately half and only less than 10% of cells, respectively (Fig. S9D). Taken together, IFN-γ treatment mainly induces PS exposure dependently on RIPK3 but hardly induces necroptosis in iC8KO17e MEFs.
IFN-γ–induced PS-exposing MEFs are not committed to die by necroptosis
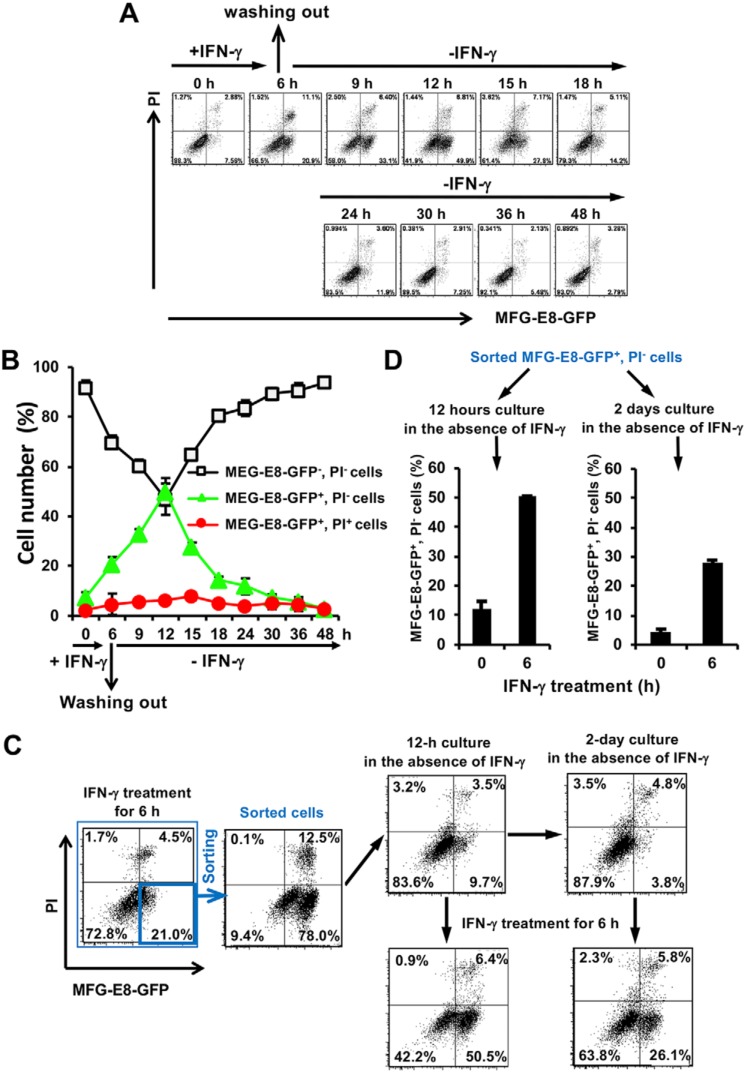
The oligomerization of activated MLKL is essential for PS exposure and the execution of necroptotic cell death in MEFs. We investigated whether IFN-γ–induced PS-exposing MEFs with activated MLKL are fated to die by necroptosis or remain alive after the removal of IFN-γ. PS exposure was induced in iC8KO-RIPK3 MEFs after 6 h of the treatment with IFN-γ, and IFN-γ was then removed by washing wells with prewarmed PBS to clear the remaining IFN-γ interacting with IFN-γ receptors. Thereafter, PS-exposing cells were cultured with fresh culture medium for 48 h without IFN-γ. As expected, PS-exposing cells without the removal of IFN-γ did not grow and died, whereas PS-exposing cells cultured without IFN-γ resumed growth and did not die (Fig. 7, A and B, and Fig. S10). The number of PS-exposing cells (MFG-E8-GFP+PI− cells) continued to increase in a further 6-h cultivation without IFN-γ, whereas the number of PS-negative living cells (MFG-E8-GFP−PI− cells) began to increase 6 h after the removal of IFN-γ (Fig. 7, A and B). The PS exposure-inducing signal through the oligomerization of MLKL, which remained active for at least 6 h after the removal of IFN-γ, did not induce necroptotic cell death.
Figure 7.
IFN-γ induces PS exposure without the induction of necroptotic cell death. A, after a 6-h treatment of iC8KO-RIPK3 MEFs with 10 ng/ml IFN-γ, IFN-γ was removed, and the cells were washed with prewarmed PBS twice and then cultured with fresh culture medium for the indicated periods. The cells at the indicated time points were stained with MFG-E8-GFP and PI and then analyzed by flow cytometry. B, the quantified data of the flow cytometric analysis from A are graphically shown. C, after a 6-h treatment of iC8KO-RIPK3 MEFs with 10 ng/ml IFN-γ, the cells were stained with MFG-E8-GFP and PI, and the MFG-E8-GFP+PI− cells were sorted and immediately analyzed by flow cytometry. Sorted cells were cultured without IFN-γ for 12 h or 2 days and analyzed by flow cytometry after staining with MFG-E8-GFP and PI. PS exposure by the sorted cells after cultivation without IFN-γ for 12 h and 2 days was again assessed by flow cytometry after a treatment with 10 ng/ml IFN-γ for 6 h. D, the quantified data of the flow cytometric analysis from C are graphically shown. Representative flow cytometric plots of more than three experiments are shown. For flow cytometry quantification, the data are presented as means ± S.D. (n ≥ 3).
To further confirm these results, MFG-E8-GFP+PI− cells were sorted 6 h after the IFN-γ treatment by FACS and were then cultured without IFN-γ. Approximately 80% of the sorted cells were shown to expose PS, and more than 80% of cells became MFG-E8-GFP−PI− cells after a 12-h or 2-day cultivation without IFN-γ (Fig. 7C). In addition, recovered MFG-E8-GFP−PI− cells were still sensitive to IFN-γ–induced PS exposure (Fig. 7, C and D). Collectively, these results indicate that PS-exposing (MFG-E8-GFP+PI−) iC8KO-RIPK3 MEFs may be resuscitated to normal MFG-E8-GFP−PI− MEFs after the removal of the IFN-γ–induced signal responsible for the activation of MLKL. The present results also suggest that caspase-8–inactivated MEFs treated with IFN-γ for at least 6 h finally expose PS on their cell surface through the activation of MLKL after the removal of IFN-γ, do not die by MLKL-mediated necroptosis, and may be resuscitated to normal PS-negative cells.
Discussion
The mechanisms underlying PS exposure during the process of apoptosis have been extensively examined. In the case of necrosis, PS exposure is not induced; however, recent studies showed that PS was exposed during TNF-induced necroptosis (16, 17). We demonstrated that IFN-γ not only induced necroptosis but also PS exposure in MEFs. In IFN-γ–induced necroptosis, PS exposure was observed prior to cell death, as with PS exposure during the induction of apoptosis (3, 30); however, PS exposure during IFN-γ–induced necroptosis was caspase-independent and MLKL-dependent, which is in contrast to PS exposure during apoptosis. Furthermore, IFN-γ induced PS exposure for more than 10 h before the induction of necroptotic cell death, and the majority of cells treated with IFN-γ for 6 h exposed PS without dying by necroptosis after the removal of IFN-γ. Short-term treatments (less than ∼10 h) with IFN-γ may only induce PS exposure, not necroptosis, indicating that the main activity of IFN-γ may be the induction of PS exposure, not necroptosis. PS-exposing cells may be engulfed by phagocytic macrophages or enhance the inflammatory responses of PS receptor-positive cells. The fate of PS-exposing cells warrants further study.
The MLKL trimer, an active form of MLKL, began to be detected after 6 h of the treatment with IFN-γ, and its amount increased in a time-dependent manner (Fig. 5, C and E). This time course is in accordance with that of PS-exposing cells (Fig. 2, B and C). Because 1) activated MLKL has been reported to oligomerize and translocate to the plasma membrane (26, 31, 32), 2) MLKL itself actually has binding affinity for PS to some extent (13), and 3) another scramblase, TMEM16F, was not involved in IFN-γ–induced PS exposure (Fig. 4), the activated MLKL trimer appears to be essential for PS exposure and may function as a scramblase to directly scramble PS to the outer leaflet of the plasma membrane after a treatment with IFN-γ.
The amount of MLKL was previously shown to rapidly decrease within 4 h after the treatment with TNF in MEFs (26, 33). This rapid reduction in MLKL expression was confirmed in the present study (Fig. 5D). In contrast to the TNF stimulation, the level of MLKL increased in a time-dependent manner between 3 and 24 h of the IFN-γ treatment (Fig. 5). Previous studies showed that classical JAK/STAT signaling was utilized by IFN-γ to induce the expression of pro-necrotic genes (e.g. RIPK1, PKR, and MLKL) and activate RIPK1/RIPK3-dependent necroptosis (34, 35). Therefore, IFN-γ directly induces the expression of MLKL, and the IFN-γ-activated pro-necrotic signaling pathway may affect not only the induction of necroptosis but also PS exposure.
In TNF-induced necroptosis, PS-exposing bubbles were released from the plasma membrane by a budding mechanism that depended on MLKL and the ESCRT machinery (16, 28). The present results showed that IFN-γ–treated MEFs also released PS-exposing bubbles; however, we have not yet demonstrated that the same mechanism as TNF-induced necroptosis is involved in the formation and release of PS-exposing bubbles described herein. Therefore, further studies are needed to elucidate the underlying mechanisms and physiological significance of these results.
In IFN-γ–induced PS exposure, the activated MLKL trimer appears to scramble PS for at least 10 h without inducing membrane rupture. A low level of the activated MLKL trimer may act as a scramblase to expose PS, and sufficiently increased amounts of the activated MLKL trimer may stimulate membrane rupture to induce necroptosis. IFN-γ–induced MLKL expression may be partly involved in the conversion of the activity of the MLKL trimer from scrambling PS in the plasma membrane to disrupt the plasma membrane. Furthermore, the formation and shedding of membrane bubbles induced by the IFN-γ treatment, which may contain the activated MLKL timer, may also be involved in the conversion from necroptotic membrane rupture to only PS exposure by decreasing the amounts of the activated MLKL trimer under the plasma membrane.
The result showing that PS exposure and necroptosis induced by IFN-γ has provided insights into the underlying mechanisms and physiological roles of PS exposure in nonapoptotic cell death. Further studies are needed to clarify whether these “eat me” and/or “find me” signals from IFN-γ–induced PS-exposing cells mediate phagocytosis and/or modulate inflammatory responses.
Experimental procedures
Cell lines
WT and C8KO MEFs with the Balb/c and C57BL/6 genetical backgrounds (23, 24) were generated in our laboratory. Immortalized MEF lines were generated by repeated passage for more than 1 month. TMEM16F KO MEFs (6) were kindly provided by S. Nagata (Osaka University). These MEFs, human embryonic kidney-derived 293T cells, and colorectal adenocarcinoma-derived HT29 cells were cultured in Dulbecco's modified Eagle's medium (Nacalai Tesque) supplemented with 10% fetal bovine serum (FBS, BioWest), 100 units/ml penicillin, and 100 μg/ml streptomycin (Nacalai Tesque). Chinese hamster ovary (CHO) cells were cultured in Dulbecco's modified Eagle's medium and Ham's F-12 medium (Nissui) mixture (1/1) supplemented with 5% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin.
Lentiviral vectors
Lentiviral vectors, provided by H. Miyoshi (RIKEN, Wako, Japan), were prepared as described earlier (36). Regarding the expression of MFG-E8-GFP (37), the original plasmid, pEF-BOS-MFG-E8 (D89E) (38, 39), was kindly provided by S. Nagata (Osaka University, Osaka, Japan), and C-terminal GFP-fused MFG-E8 cDNA was subcloned from the original plasmid and inserted into the lentiviral expression vector, pCSII-EF-MCS-IRES-puro. Concerning the expression of mouse RIPK3, its cDNA (GenBankTM accession number NM_019955.2) was subcloned and inserted into pCSII-PGK-3×FLAG-MCS-IRES-puro. The RHIM domain mutant of RIPK3 was generated by replacing the amino acid sequence of the RHIM domain of mouse RIPK3, 422GTPWYPWTPPNPMTGPPALVFNNCSEVQIGNYNSLVAPPR461 with 422GTPWYPWTPPNPMTGPPALVFNNCSEAAAANYNSLVAPPR461. All generated constructs were verified by DNA sequencing (Applied Biosystems).
shRNA expression system
To achieve the specific knockdown of mouse TMEM16F, MLKL, and RIPK3, the shRNA expression system with lentivirus-based vectors was utilized as described previously (40, 41). The DNA oligonucleotide sequence encoding shRNA for TMEM16F (sh16F, GenBankTM accession number NM_175344.4) was 5′-GAGAATCAACATGATTAATCA-3′ (sh16F). The DNA oligonucleotide sequences encoding shRNAs for MLKL (shMlkl, GenBankTM accession number NM_029005.2) were 5′-TCCCAACATCTTGCGTATATT-3′ (shMlkl#1) and 5′-AGATCCAGTTCAACGATATAT-3′ (shMlkl#2). The DNA oligonucleotide sequence encoding shRNA for RIPK3 (GenBankTM accession number NM_019955.2) was 5′-TATGGTTATTCTTCGTAATGA-3′ (shRipk3). The DNA oligonucleotide sequence encoding shRNA for LacZ was described previously (41).
Detection of dead and PS-positive cells
To detect dead and PS-exposing cells, solutions containing 50 ng/ml of PI (Nacalai Tesque) and MFG-E8-GFP, respectively, were used to stain cells. To prepare MFG-E8-GFP–containing solution, CHO cells stably expressing MFG-E8-GFP were generated as follows. pCSII-EF-MFG-E8-GFP-IRES-puro was introduced into CHO cells by lentiviral infection, and highly MFG-E8-GFP-expressing CHO cells were sorted by FACSAria III (BD Biosciences) after 2 μg/ml puromycin (InvivoGen) selection for 2 days. Sorted cells were cultured until 80% confluency, washed with PBS twice, and then cultured with ASF104 medium (AJINOMOTO) supplemented with 1% FBS (BioWest) at 37 °C in a 5% (v/v) CO2 humidified incubator for 2 days to produce the MFG-E8-GFP protein. The culture supernatant was then collected, filtered with a 0.45-μm filter (Millipore), and utilized as MFG-E8-GFP–containing solution, which was directly used for cell staining. We used an MFG-E8 cDNA, carrying a point mutation (D89E) in its RGD motif that can bind to PS but not to bind integrins (β5 and β3) (38, 39). The MFG-E8-GFP showed essentially the same binding pattern to PS-exposing cells as FITC-conjugated annexin V (Fig. S11).
Western blotting analysis
Cells were suspended in ice-cold lysis buffer (20 mm Tris-HCl, pH 7.4, 10% glycerol, 1% Triton X-100, 0.5% Nonidet P-40, 150 mm NaCl, and 1 mm EDTA) supplemented with a protease inhibitor mixture (Nacalai Tesque), resolved by SDS-PAGE, and analyzed by Western blotting as described previously (42). To detect MLKL oligomers, cells were lysed in SDS-PAGE sample buffer without DTT (nonreducing conditions) before performing SDS-PAGE. The antibodies used in the present study were those for mouse RIPK3 (1:1000; 2283, ProSci), mouse MLKL (1:1000; MABC604, Millipore), mouse phosphorylated MLKL (1:1000; ab196436, Abcam), mouse Caspase-3 (1:1000; 611049, BD Transduction Laboratories), mouse cleaved caspase-3 (1:1000; 9664, Cell Signaling), mouse TMEM16F (1: 5000; a kind gift from S. Nagata), and actin (1:5000; MAB1501, Millipore). The bands of Western blotting analyses were quantified using MultiGauge V3.0 software (FUJIFILM).
qRT-PCR
Total RNA was isolated using Sepasol RNA I Super G (Nacalai Tesque) according to the manufacturer's instructions and subjected to a RT (reverse transcription) reaction using the ReverTra Ace qPCR RT Master Mix (TOYOBO). After mixing 30 ng of the RT products with the THUNDERBIRD SYBR qPCR Mix (TOYOBO), real-time RT-PCR was performed using the StepOne Real-Time PCR System (Applied Biosystems) with the following primer sets: 5′-CACGGACTTCAAGAACACAGACA-3′ (forward) and 5′-GTCACGGTACCTGCACAAGGT-3′ (reverse) for mouse Tmem16F, 5′-GACCAAACTGAAGACAAGTA-3′ (forward) and 5′-CTCACTATTCCAACACTTTC-3′ (reverse) for mouse Mlkl, and 5′-GATGACATCAAGAAGGTGGTGA-3′ (forward) and 5′-TGCTGTAGCCGTATTCATTGTC-3′ (reverse) for mouse Gapdh. Expression levels of mRNAs were normalized to that of GAPDH and expressed as ratios to the expression level in control cells.
Flow cytometric analysis
To quantify the numbers of PS-exposing and dead cells, the cells were trypsinized, resuspended in solution containing 0.2 μg/ml PI and MFG-E8-GFP, and maintained at room temperature for 5 min. The cells were then analyzed by flow cytometry using the FACSAria III system (BD Biosciences) and FACSDiva software (BD Biosciences) as previously described (36). The percentages of PS-exposing and dead cells were calculated using FlowJo software (version 7.6.5, Tree Star).
Fluorescence imaging
To visualize PS exposure and cell death in IFN-γ–treated cells or observe MGF-E8-GFP+ bubble formation on PS-exposing cells, MEFs were seeded into a plastic sterile 24-well culture plate (Greiner Bio-One) or a 24-well SensoPlate, PS, glass bottom (Greiner Bio-One), respectively; cultured overnight; and treated with IFN-γ for the indicated periods. After removing culture medium, MFG-E8-GFP–containing solution with or without 50 ng/ml PI was directly added to cells and incubated at 37 °C for 20 min. The cells were observed under a fluorescence microscope (DMIRE2, Leica), and the data were analyzed by MetaMorph software (Molecular Devices).
Reagents
z-VAD-fmk (Peptide Institute), mouse and human IFN-γ (Pepro Tech), mouse TNFα (ProSpec), Staurosporine (Alomone Labs), Necrostatin-1 (Santa Cruz Biotechnology), anti-mouse TNFα antibody (506310, Biolegend), Smac mimetics (A11928, AdooQ Bioscience), and NSA (Toronto Research Chemicals) were used as a pan-caspase inhibitor, necroptosis inducer, necroptosis inducer, apoptosis inducer, RIPK1 inhibitor, TNFα inhibitor, IAP inhibitor, and human MLKL inhibitor, respectively.
Author contributions
J. C., S. K., and S. Y. conceptualization; J. C. data curation; J. C. and S. Y. formal analysis; J. C. and S. Y. validation; J. C., S. K., M. S., and S. Y. investigation; J. C. and S. Y. visualization; J. C., S. K., M. S., and S. Y. methodology; J. C. and S. Y. writing-original draft; J. C., S. K., M. S., and S. Y. writing-review and editing; S. K. and M. S. resources; S. Y. supervision; S. Y. funding acquisition; S. Y. project administration.
Supplementary Material
Acknowledgments
We thank to all members of the Yonehara laboratory for helpful discussions. We are grateful to S. Nagata for providing cDNA of mouse MFG-E8 (D89E), TMEM16F KO MEFs, and anti-mouse TMEM16F antibody.
1 This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas (homeostatic regulation by various types of cell death) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15H01376 to S. Y.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S11.
- PS
- phosphatidylserine
- TNF
- tumor necrosis factor
- TRAIL
- TNF-related apoptosis-inducing ligand
- RIPK
- receptor-interacting protein kinase
- MLKL
- mixed lineage kinase domain-like protein
- IFN
- interferon
- MEF
- mouse embryonic fibroblast
- IZ
- IFN-γ and z-VAD-fmk
- MFG-E8
- milk fat globule-EGF-factor 8
- PI
- propidium iodide
- C8KO
- caspase-8 knockout
- iC8KO
- immortalized C8KO
- shRNA
- short hairpin RNA
- z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- TCZ
- TNF-α, cycloheximide, and z-VAD-fmk
- RHIM
- RIP homotypic interaction motif
- NSA
- necrosulfonamide
- FBS
- fetal bovine serum
- CHO
- Chinese hamster ovary
- qRT-PCR
- quantitative Reverse Transcription PCR.
References
- 1. Leventis P. A., and Grinstein S. (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 39, 407–427 10.1146/annurev.biophys.093008.131234 [DOI] [PubMed] [Google Scholar]
- 2. Segawa K., and Nagata S. (2015) An apoptotic “eat me” signal: phosphatidylserine exposure. Trends Cell Biol. 25, 639–650 10.1016/j.tcb.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 3. Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., and Henson P. M. (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 [PubMed] [Google Scholar]
- 4. Suzuki J., Imanishi E., and Nagata S. (2014) Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. J. Biol. Chem. 289, 30257–30267 10.1074/jbc.M114.583419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki J., Denning D. P., Imanishi E., Horvitz H. R., and Nagata S. (2013) Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341, 403–406 10.1126/science.1236758 [DOI] [PubMed] [Google Scholar]
- 6. Suzuki J., Umeda M., Sims P. J., and Nagata S. (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838 10.1038/nature09583 [DOI] [PubMed] [Google Scholar]
- 7. Krysko D. V., Vanden Berghe T., D'Herde K., and Vandenabeele P. (2008) Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44, 205–221 10.1016/j.ymeth.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 8. Hitomi J., Christofferson D. E., Ng A., Yao J., Degterev A., Xavier R. J., and Yuan J. (2008) Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135, 1311–1323 10.1016/j.cell.2008.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dondelinger Y., Hulpiau P., Saeys Y., Bertrand M. J. M., and Vandenabeele P. (2016) An evolutionary perspective on the necroptotic pathway. Trends Cell Biol. 26, 721–732 10.1016/j.tcb.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 10. Green D. R. (2005) Apoptotic pathways: ten minutes to dead. Cell 121, 671–674 10.1016/j.cell.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 11. Cho Y. S., Challa S., Moquin D., Genga R., Ray T. D., Guildford M., and Chan F. K. (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 10.1016/j.cell.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun L., Wang H., Wang Z., He S., Chen S., Liao D., Wang L., Yan J., Liu W., Lei X., and Wang X. (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 10.1016/j.cell.2011.11.031 [DOI] [PubMed] [Google Scholar]
- 13. Wang H., Sun L., Su L., Rizo J., Liu L., Wang L. F., Wang F. S., and Wang X. (2014) Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 10.1016/j.molcel.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 14. Varfolomeev E. E., Schuchmann M., Luria V., Chiannilkulchai N., Beckmann J. S., Mett I. L., Rebrikov D., Brodianski V. M., Kemper O. C., Kollet O., Lapidot T., Soffer D., Sobe T., Avraham K. B., Goncharov T., et al. (1998) Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 10.1016/S1074-7613(00)80609-3 [DOI] [PubMed] [Google Scholar]
- 15. Oberst A., Dillon C. P., Weinlich R., McCormick L. L., Fitzgerald P., Pop C., Hakem R., Salvesen G. S., and Green D. R. (2011) Catalytic activity of the caspase-8-FLIP (L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 10.1038/nature09852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gong Y. N., Guy C., Olauson H., Becker J. U., Yang M., Fitzgerald P., Linkermann A., and Green D. R. (2017) ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169, 286–300.e16 10.1016/j.cell.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zargarian S., Shlomovitz I., Erlich Z., Hourizadeh A., Ofir-Birin Y., Croker B. A., Regev-Rudzki N., Edry-Botzer L., and Gerlic M. (2017) Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PLoS Biol. 15, e2002711 10.1371/journal.pbio.2002711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pestka S., Krause C. D., and Walter M. R. (2004) Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32 10.1111/j.0105-2896.2004.00204.x [DOI] [PubMed] [Google Scholar]
- 19. Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., and Schreiber R. D. (1998) How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 10.1146/annurev.biochem.67.1.227 [DOI] [PubMed] [Google Scholar]
- 20. Borden E. C., Sen G. C., Uze G., Silverman R. H., Ransohoff R. M., Foster G. R., and Stark G. R. (2007) Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug. Discov. 6, 975–990 10.1038/nrd2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cekay M. J., Roesler S., Frank T., Knuth A. K., Eckhardt I., and Fulda S. (2017) Smac mimetics and type II interferon synergistically induce necroptosis in various cancer cell lines. Cancer Lett. 410, 228–237 10.1016/j.canlet.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 22. Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G. D., Mitchison T. J., Moskowitz M. A., and Yuan J. (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 10.1038/nchembio711 [DOI] [PubMed] [Google Scholar]
- 23. Sakamaki K., Inoue T., Asano M., Sudo K., Kazama H., Sakagami J., Sakata S., Ozaki M., Nakamura S., Toyokuni S., Osumi N., Iwakura Y., and Yonehara S. (2002) Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 9, 1196–1206 10.1038/sj.cdd.4401090 [DOI] [PubMed] [Google Scholar]
- 24. Takahashi S., Futatsugi-Yumikura S., Fukuoka A., Yoshimoto T., Nakanishi K., and Yonehara S. (2013) Fas deficiency in mice with the Balb/c background induces blepharitis with allergic inflammation and hyper-IgE production in conjunction with severe autoimmune disease. Int. Immunol. 25, 287–293 10.1093/intimm/dxs109 [DOI] [PubMed] [Google Scholar]
- 25. Ousingsawat J., Cabrita I., Wanitchakool P., Sirianant L., Krautwald S., Linkermann A., Schreiber R., and Kunzelmann K. (2017) Ca2+ signals, cell membrane disintegration, and activation of TMEM16F during necroptosis. Cell Mol. Life Sci. 74, 173–181 10.1007/s00018-016-2338-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai Z., Jitkaew S., Zhao J., Chiang H. C., Choksi S., Liu J., Ward Y., Wu L. G., and Liu Z. G. (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 16, 55–65 10.1038/ncb2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu S., Liu H., Johnston A., Hanna-Addams S., Reynoso E., Xiang Y., and Wang Z. (2017) MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc. Natl. Acad. Sci. U.S.A. 114, E7450–E7459 10.1073/pnas.1707531114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon S., Kovalenko A., Bogdanov K., and Wallach D. (2017) MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity 47, 51–65.e7 10.1016/j.immuni.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 29. Tanzer M. C., Khan N., Rickard J. A., Etemadi N., Lalaoui N., Spall S. K., Hildebrand J. M., Segal D., Miasari M., Chau D., Wong W. L., McKinlay M., Chunduru S. K., Benetatos C. A., Condon S. M., et al. (2017) Combination of IAP antagonist and IFNγ activates novel caspase-10- and RIPK1-dependent cell death pathways. Cell Death Differ. 24, 481–491 10.1038/cdd.2016.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vermes I., Haanen C., Steffens-Nakken H., and Reutelingsperger C. (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 184, 39–51 10.1016/0022-1759(95)00072-I [DOI] [PubMed] [Google Scholar]
- 31. Chen X., Li W., Ren J., Huang D., He W. T., Song Y., Yang C., Li W., Zheng X., Chen P., and Han J. (2014) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 24, 105–121 10.1038/cr.2013.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hildebrand J. M., Tanzer M. C., Lucet I. S., Young S. N., Spall S. K., Sharma P., Pierotti C., Garnier J. M., Dobson R. C., Webb A. I., Tripaydonis A., Babon J. J., Mulcair M. D., Scanlon M. J., Alexander W. S., et al. (2014) Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl. Acad. Sci. U.S.A. 111, 15072–15077 10.1073/pnas.1408987111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang H., Meng H., Li X., Zhu K., Dong K., Mookhtiar A. K., Wei H., Li Y., Sun S. C., and Yuan J. (2017) PELI1 functions as a dual modulator of necroptosis and apoptosis by regulating ubiquitination of RIPK1 and mRNA levels of c-FLIP. Proc. Natl. Acad. Sci. U.S.A. 114, 11944–11949 10.1073/pnas.1715742114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balachandran S., and Adams G. P. (2013) Interferon-γ–induced necrosis: an antitumor biotherapeutic perspective. J. Interferon Cytokine Res. 33, 171–180 10.1089/jir.2012.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thapa R. J., Nogusa S., Chen P., Maki J. L., Lerro A., Andrake M., Rall G. F., Degterev A., and Balachandran S. (2013) Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl. Acad. Sci. U.S.A. 110, E3109–E3118 10.1073/pnas.1301218110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okamoto K., Fujisawa J., Reth M., and Yonehara S. (2006) Human T-cell leukemia virus type-I oncoprotein Tax inhibits Fas-mediated apoptosis by including cellular FLIP through activation of NF-κB. Genes Cells 11, 177–191 10.1111/j.1365-2443.2006.00927.x [DOI] [PubMed] [Google Scholar]
- 37. Hanayama R., Tanaka M., Miwa K., Shinohara A., Iwamatsu A., and Nagata S. (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 10.1038/417182a [DOI] [PubMed] [Google Scholar]
- 38. Miyasaka K., Hanayama R., Tanaka M., and Nagata S. (2004) Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur. J. Immunol. 34, 1414–1422 10.1002/eji.200424930 [DOI] [PubMed] [Google Scholar]
- 39. Toda S., Hanayama R., and Nagata S. (2012) Two-step engulfment of apoptotic cells. Mol. Cell Biol. 32, 118–125 10.1128/MCB.05993-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kobayashi Y., and Yonehara S. (2009) Novel cell death by downregulation of eEF1A1 expression in tetraploids. Cell Death Differ. 16, 139–150 10.1038/cdd.2008.136 [DOI] [PubMed] [Google Scholar]
- 41. Kiriyama M., Kobayashi Y., Saito M., Ishikawa F., and Yonehara S. (2009) Interaction of FLASH with arsenite resistance protein 2 is involved in cell cycle progression at S phase. Mol. Cell Biol. 29, 4729–4741 10.1128/MCB.00289-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee K. K., and Yonehara S. (2002) Phosphorylation and dimerization regulate nucleocytoplasmic shuttling of mammalian STE20-like kinase (MST). J. Biol. Chem. 277, 12351–12358 10.1074/jbc.M108138200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.