Abstract
The overall goal of our research is to establish a preformed molecular guidance pathway to direct the growth of dopaminergic axons from embryonic ventral mesencephalon (VM), tissue placed within the substantia nigra (SN), into the striatum to reconstruct the nigrostriatal pathway in a hemi-Parkinson’s disease rat model. Guidance pathways were prepared by injecting lentivirus encoding either GFP or a combination of glial-cell-line-derived neurotrophic factor (GDNF) with either GDNF family receptor α1 (GFRα1) or netrin1. In another cohort of animals, adeno-associated virus (AAV) encoding brain-derived neurotrophic factor (BDNF) was injected within the striatum after guidance pathway formation. GDNF combined with either GFRα1 or netrin significantly increased growth of dopaminergic axons out of transplants and along the pathway, resulting in a significant reduction in the number of amphetamine-induced rotations. Retrograde tract tracing showed that the dopaminergic axons innervating the striatum were from A9 neurons within the transplant. Increased dopaminergic innervation of the striatum and improved behavioral recovery were observed with the addition of BDNF. Preformed guidance pathways using a combination of GDNF and netrin1 can be used to reconstruct the nigrostriatal pathway and improve motor recovery.
Keywords: Parkinson’s disease, axon guidance, nigral transplantation, netrin1, GDNF, BDNF
Introduction
Loss of nigrostriatal connections is presumed to be the predominant cause of motor symptoms in Parkinson’s disease. The loss of striatal dopamine results in motor dysfunction, including resting tremor, muscular rigidity, bradykinesia, and postural instability. Currently, pharmacologic dopamine supplementation is the most commonly used strategy to reduce the symptoms of Parkinson’s disease. Many experimental strategies have attempted to reduce Parkinson’s disease symptoms by replacing dopamine-secreting neurons.1, 2, 3 Neuronal transplantation has widely been used in laboratory animal models and in several human clinical trials. The most popular procedures have involved ectopic transplantation of healthy dopaminergic neurons into the striatum, but this technique has proven unsatisfactory in clinical trials for multiple reasons.3, 4, 5 Homotopic transplantation of dopaminergic neurons into the substantia nigra (SN) is thought to be better for reconstructing the nigrostriatal circuit.5 However, this approach also has challenges, most prominently, the difficulty of inducing robust axon growth through the inhibitory environment of the mature brain.6, 7 To support long-distance growth between the substantia nigra and the striatum, several studies have used bridging techniques to create a supportive environment for dopaminergic axon growth.8, 9, 10, 11, 12 These techniques failed to either support robust growth and innervation of the striatum or functionally reconstruct the nigrostriatal circuit.
Since the primary difficulty in promoting unidirectional growth of axons is the inhibitory environment and lack of guidance cues within the adult brain, we previously investigated targeting axon growth from transplanted neurons along gradient pathways of guidance molecules.13, 14, 15, 16 We observed that different neuronal populations require different guidance molecules to successfully grow across the corpus callosum in adult animals. With transplanted dorsal root ganglion neurons, we could not only induce unidirectional growth across the corpus callosum but also direct the axons to make a 90° turn into either the striatum or cortex with strategic placement of select guidance cues.15 For dopaminergic neuronal transplants, a pathway composed of glial-cell-line-derived neurotrophic factor (GDNF) combined with either GFRα1 or netrin1 promoted robust, long-distance unidirectional growth of dopaminergic axons across the corpus callosum, at a distance of about 5 mm.14, 16
In this study, we examined whether similar preformed guidance pathways extending along the internal capsule in a hemi-Parkinson’s disease rat model would support partial reconstruction of the nigrostriatal pathway. Guidance pathways were generated using lentiviruses to express GDNF, combined with either GDNF family receptor α1 (GFRα1) or netrin1. We compared the pathway effectiveness for improvements in dopaminergic neurite growth along the pathway and motor recovery. In a separate cohort of animals, we examined whether striatal expression of brain-derived neurotrophic factor (BDNF) could further increase growth into the striatum and enhance recovery of spontaneous motor activity.
Results
Dose Determination to Generate Complete Hemi-Parkinson’s Disease Rat Model
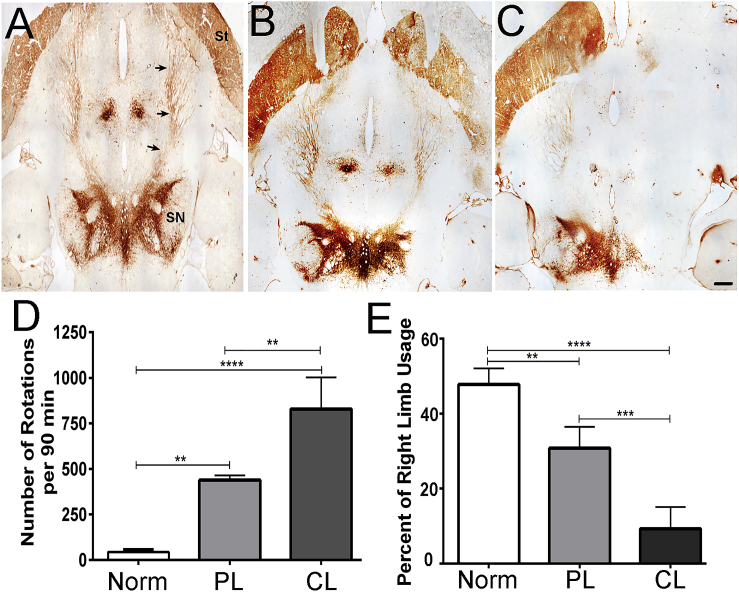
For these studies, we chose to examine reconstruction after complete unilateral loss of dopaminergic neurons within the substantia nigra. To establish this model, we tested 2 doses of 6-OHDA with regard to histopathology and motor movement. We also chose to inject the 6-OHDA into both the medial forebrain bundle and the substantia nigra to ensure complete destruction of A9 dopaminergic neurons. Injection of the lower dose (6 μg) resulted in partial denervation of TH+ axons within the medial forebrain bundle as well as the striatum, which still showed many surviving axons 1 month after lesioning (Figure 1B). The higher dose (12 μg) induced a complete loss of A9 and a partial loss of A10 dopaminergic neurons within midbrain and an almost complete denervation of TH+ axons from the striatum (Figure 1C). Partial lesions resulted in reduced d-amphetamine-induced rotations (<450 rotations per 90 min) when compared to those for complete lesions (>600 rotations per 90 min; Figure 1D; one-way ANOVA, F(2, 9) = 58.67, p < 0.0001). There was also a statistical difference in right or left paw preference (one-way ANOVA, F(2, 9) = 52.22, p < 0.0001) in the glass cylinder test between non-lesioned and partial-lesioned animals that increased with complete lesions. For all remaining studies, the higher dose was used.
Figure 1.
Partial and Complete Unilateral Nigrostriatal Dopaminergic Lesions
(A–C) TH-stained horizontal sections of non-lesions (A), partial lesions (B), and complete lesions (C) of the nigrostriatal pathway in adult rat brains. (A) In naive brains, dopaminergic neurons residing in the SN project axons into the striatum (St) through the Nigrostriatal bundle (arrows). (B) One month after unilateral stereotaxic injection of 6 μg 6-OHDA into the Nigrostriatal pathway (NSP) and substantia nigra (SN) (3 μg per site), partial survival of dopaminergic axons in the substantia nigra and axons within the striatum is apparent. (C) One month after injection of a total of 12 μg 6-OHDA (6 μg per site), complete loss of dopaminergic neurons and projection axons is apparent. (D) Rats within the completely lesioned group showed significant increases in d-amphetamine-induced rotations when compared to either baseline or partial-lesioned rats; ANOVA, F(2, 9) = 58.67, p < 0.0001. (E) Using the glass cylinder test, rats in the completely lesioned group showed a significant decrease in right forepaw use when compared to either non-lesioned controls or partial-lesioned rats; ANOVA, F(2, 9) = 52.22, p < 0.0001. Scale bar, 800 μm. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Preformed Pathway between the Substantia Nigra and Striatum for Axonal Targeting
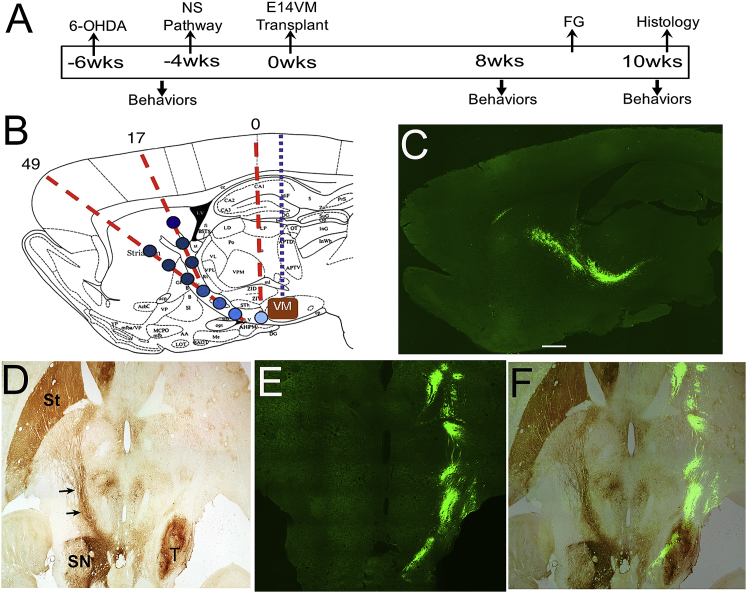
Previously, we showed a gradient of GDNF combined with either GFRα1- or netrin1-induced unidirectional growth of TH+ axons out of E14 ventral mesencephalon (VM) transplants and across the corpus collosum.16 For this study, all pathways were generated from the substantia nigra to the striatum along the internal capsule. All of the studies followed a strict timeline for behavioral assessment, pathway formation, and transplantation (Figure 2A). All expression pathways followed the injection pattern illustrated in Figure 2B. A lentivirus-encoding GFP was injected to verify expression along this pathway (Figure 2C). The lentivirus was used because it has a higher tropism for astrocytes, which we have previously shown to support axonal growth.16, 17 Very nice GFP expression can be observed in sagittal (Figure 2C) and horizontal (Figure 2E) sections extending from the region of the presumptive substantia nigra and into the striatum. In GFP controls (Figure 2D), 10 weeks after transplantation, good survival (Figure S1) and placement of the E14 VM transplant was observed; however, no TH+ axons were observed extending out of the transplant and into host tissue (Figure 2D, right side).
Figure 2.
Experimental Timeline, Injection Parameters, and Transplant Controls
(A) Outline shows time course for 6-OHDA, nigrostriatal preformed growth (NS) pathway, E14 VM transplantation, and behavioral assessments. For these studies, all transplants were done 2 weeks after establishment of guidance pathways and/or 6 weeks after 6-OHDA lesions. Behavioral motor responses were measured at three time points: 4 weeks after 6-OHDA lesion and 8 and 10 weeks after transplantation. In a cohort of animals, Fluoro-Gold (FG) was injected 7 days before euthanizing the rats. (B) Schematic diagram showing the location of lentivirus injection points and transplant location in the sagittal plane, 2.4 mm lateral to bregma. Background map is modified from the stereotaxic rat brain atlas of Paxinos and Watson (2007).48 Red dotted lines represent individual needle paths, and blue circles represent lentivirus injection sites, with darker colors indicating a larger number of viral particles injected at the striatal end of the NS pathway. The blue dotted line represents the injection pathway for E14 ventral mesencephalon tissue pieces. (C) Sagittal brain section showing lentivirus-induced expression of GFP along the injection pathway from substantia nigra to striatum (NS pathway). (D) Horizontal section showing VM placement with no TH+ axonal growth along the GFP control pathway 10 weeks after transplantation. (E) Section adjacent to the one in (D), showing lentivirus-induced GFP expression marking the pathway from substantia nigra to striatum. (F) Merge of images from (E) and (F). Left, normal side; right, lesion side. Scale bar, 1 mm.
Targeting Dopaminergic Axon Growth from Transplant Site to Striatum
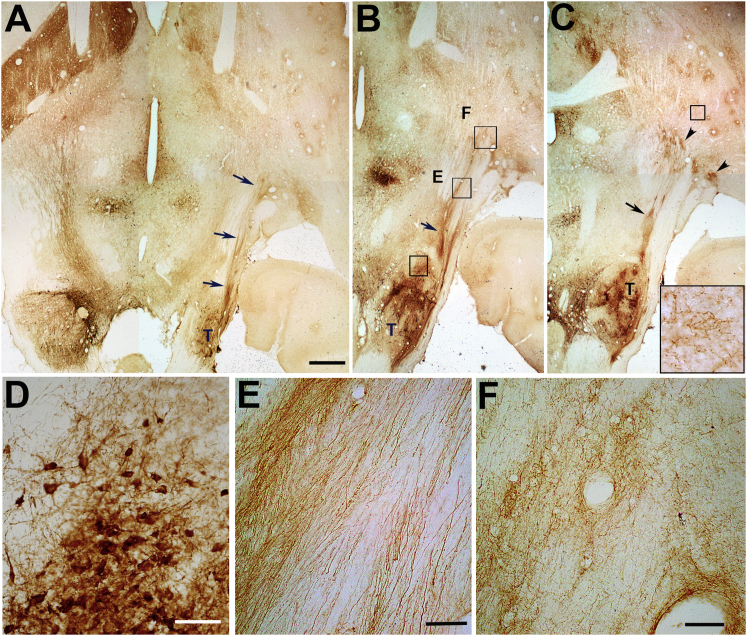
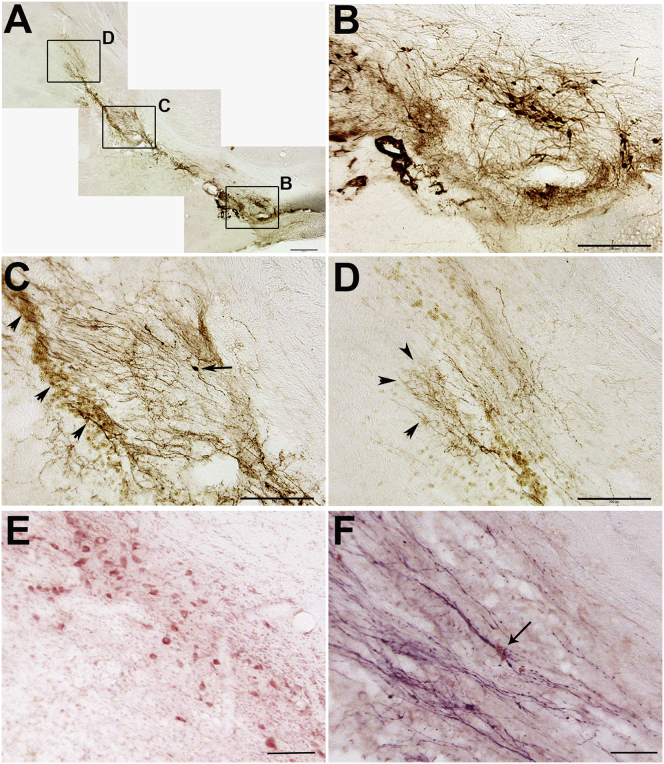
Previously, we observed very little growth of TH+ axons along individual pathways of GDNF or GFRα1; combinations of GDNF with either GFRα1 or netrin showed much better growth.16 For this reason, we chose to examine TH+ axon growth along pathways using these two combinations. Ten weeks after transplantation of E14 VM transplants into the region of the substantia nigra, robust outgrowth of TH+ axons was observed along pathways of GDNF coexpressing either GFRα1 or netrin1 (Figure 3). Tyrosine-hydroxylase-positive axons can be observed extending toward the striatum along the ectopic GDNF/netrin1 pathway adjacent to the internal capsule (Figures 3A–3C). Axons within this pathway were observed in multiple horizontal sections spanning the region of the transplant. This pattern was very different from that observed on the control side (Figure 3, left), which shows TH+ axons confined to the medial forebrain bundle, whereas axons emerging from the transplant mostly grew in tight bundles adjacent to the internal capsule. Many of these axons were observed to accumulate along the border of the striatum just prior to entry (Figure 3C, arrowheads). Higher magnification shows TH+ neurons within the transplant (Figure 3D) and TH+ fibers extending in tight bundles slightly medial to the internal capsule (Figure 3E). Although most fibers abruptly ended near the border of the striatum (Figure 3F), a few grew short distances within the striatum (Figure 3C, inset). Interestingly, higher densities of TH+ axons were often identified near blood vessels both within the striatum and at ectopic striatal border regions (Figure 3F). Regrowing axons are often observed along blood vessels, which are thought to provide a more conducive growth environment.18 To better appreciate axon growth along the pathway from the transplant and into the striatum, sections were also cut in the sagittal plane (Figure 4A). As observed previously, a tightly bundled group of axons was observed growing out of the E14 mesencephalic transplant (Figure 4B). Tyrosine-hydroxylase-positive axons grew near the needle track, identified by a line of macrophages (Figure 4C, arrowheads). Although most of the axons were observed growing into the striatum, several formed small but dense arbors near the location of the distal injection site (Figure 4D, arrowheads). Numerous glial cells in adjacent sections stained positive for netrin1 (Figure 4E), overlapping the region through which axons grew (Figure 4F).
Figure 3.
Representative Pictures Showing TH+ Axons Growing along a Guidance Pathway of GDNF/Netrin1 10 Weeks after Transplantation
(A–C) Low magnification of serial horizontal sections (left to right: ventral to dorsal) showing the placement of the transplant (T) on the right and axons extending in the pathway (arrows) adjacent to the internal capsule toward the striatum. Most of the axons terminate their growth near the striatal border (arrowheads), but a few grew into the striatum (box insert in C). (D) High magnification of TH+ neurons within the transplant from the box in (B). (E and F) In (E), a high magnification is shown of TH+ axons that grew in parallel fiber bundles along the internal capsule; (F) many of these axons displayed a more randomly organized pattern near the striatal border. Interestingly, many of these axons appeared at higher densities along blood vessels. Scale bars: 1 mm in (A) and 100 μm in (D)–(F).
Figure 4.
Dopaminergic Axon Growth along a Netrin1 Pathway
(A) Parasagittal section showing TH+ fibers extending along the Netrin1 guidance pathway between the transplant site in the substantia nigra and the striatum 10 weeks after transplantation. (B) Higher magnification of lower box in (A), showing TH+ cell bodies and dense fiber outgrowth at the transplant site. (C) Higher magnification of middle box in (A), showing TH+ fibers and one TH+ cell that has appeared to migrate (arrow) along the pathway. The needle tract for the pathway is marked by a row of macrophages (arrowheads). (D) Higher magnification of top box in (A), showing TH+ fibers that have grown into the striatum. (E) Section adjacent to that in (A) immunostained for netrin1. Many positive cells can be observed along the pathway. (F) Double immunostaining for netrin1 (light brown) and TH-positive axons (purple), with axons contacting netrin1-positive cells (arrow), Scale bars, 500 μm in (A), 200 μm in (B) and (C), and 100 μm in (E) and (F).
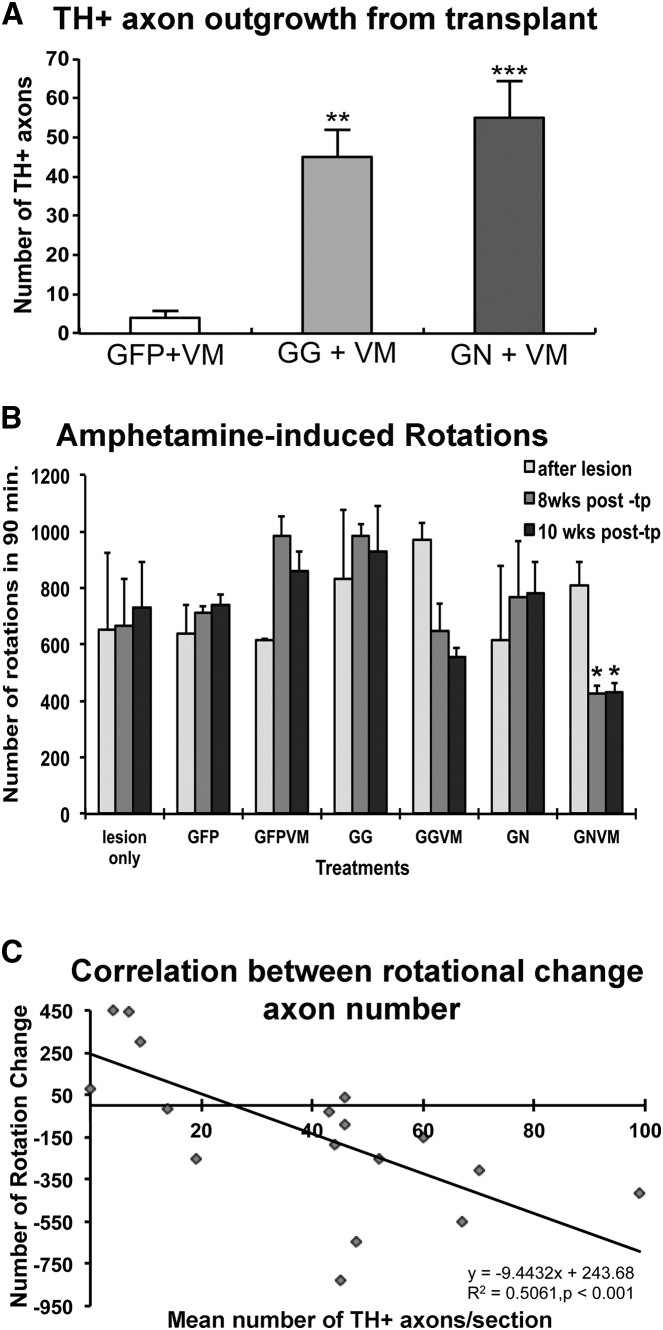
In control animals with a GFP pathway (Figure 2E) or with GDNF/GFRα1 or GDNF/netrin1 pathways not receiving transplants (data not shown), no TH+ fibers were observed growing along the pathway. Quantification of axonal growth along the nigrostriatal path was done by manually counting axons about halfway (box E in Figure 3B) between the striatum and transplant (Figure 5A). Measurements were made at this location because, as axons approached the striatal border, they became diffuse and disorganized, making the counting of individual axons difficult. Axon outgrowth was significantly increased within both treatment groups when compared to GFP controls, F(2, 16) = 13.72 (p = 0.0027). We observed an average of 55 ± 9.4 axons per section for GDNF/netrin1 and 45 ± 7.0 axons per section for lenti-GDNF/GFRα1 when compared to the lenti-GFP transplant group (4 ± 1.8). These data show enhanced axon growth out of transplants and along specific guidance pathways.
Figure 5.
Axon Outgrowth along the Nigrostriatal Pathway
(A) Quantification of TH+ axon growth along the nigrostriatal pathway shows a significant increase in the number of TH+ axons within the pathways of GDNF/GFRα1 (GG) and GDNF/netrin1 (GN) treatment groups compared to the control group injected with GFP. (B) Amphetamine-induced rotational tests for rats with different pathways with (+VM) and without (−VM) E14 VM transplants. Rats from the GDNF/netrin + VM (GN+VM) group showed significant recovery in rotational asymmetry at 8 and 10 weeks after transplantation (tp). Other rats did not display any significant rotational change for up to 10 weeks. (C) Correlation between the number of TH+ fiber growth along the pathways and the rotational change at 10 weeks after transplantation. There is a significant negative correlation between the number of TH+ fibers and the rotational change, indicating that the higher the number of TH+ fibers in the nigrostriatal pathway, the greater the decrease in rotational asymmetry, and the more functional recovery induced by the guidance pathway (Spearman rank correlation [r2] = 0.5061, p = 0.001). *p < 0.05; **p < 0.01; ***p < 0.001.
To determine whether axon outgrowth led to functional recovery, rats were tested for amphetamine-induced rotational asymmetry 3 weeks after 6-OHDA lesions and at 8 and 10 weeks after transplantation (Figure 5B). For groups not receiving transplants, the 8- and 10-week time points for behavioral measures were taken at the same time points as those receiving transplants. Control groups either with a GFP pathway or with GDNF/GFRα1 (GG) or GDNF/Netrin1 (GN) pathways not receiving transplants (−VM) showed no recovery in rotational behavior 8 or 10 weeks after pathway formation (Figure 5B). In fact, the GFP group appeared to get worse after injection of GFP viral pathway and transplantation. The overall treatment effect of combining the guidance pathways with E14 VM transplants was significant, as determined by two-way ANOVA of repeated measures, F(6, 42) = 6.025 (p = 0.001). Although there was a decrease in rotational behavior for the GDNF/GFRα1 pathway group 8 and 10 weeks after receiving transplants, it did not reach significance (p = 0.16 and p = 0.064, respectively), as determined by Holm-Sidak post hoc analysis. Rotation scores of rats receiving GDNF/netrin1 pathways and E14 VM transplants showed a significant decline in amphetamine-induced rotation after 8 and 10 weeks: p < 0.01 for both groups versus after lesion for the same group, and p < 0.05 for all other groups except GDNF/GFRα1 with transplant (the GDNF/GFRα1 + VM group) at 8 or 10 weeks. Relative changes in rotational counts between 3 weeks after 6-OHDA lesions and 10 weeks post-treatment (the terminal time point of the study) for the GFP, GDNF/GFRα1, and GDNF/netrin1 groups were compared to the number of TH+ fibers (Figure 5C). A reduction in asymmetric rotation correlated with an increase in TH+ axonal counts, further demonstrating that TH+ fiber growth contributed to behavioral outcome. We did not observe an increase in contralateral forepaw usage or placement in any of the groups (data not shown). This could be due to the relatively low level of striatal innervation observed in these animals (Figure S1).
A9 Dopaminergic Neurons Project Axons into the Striatum from Transplants
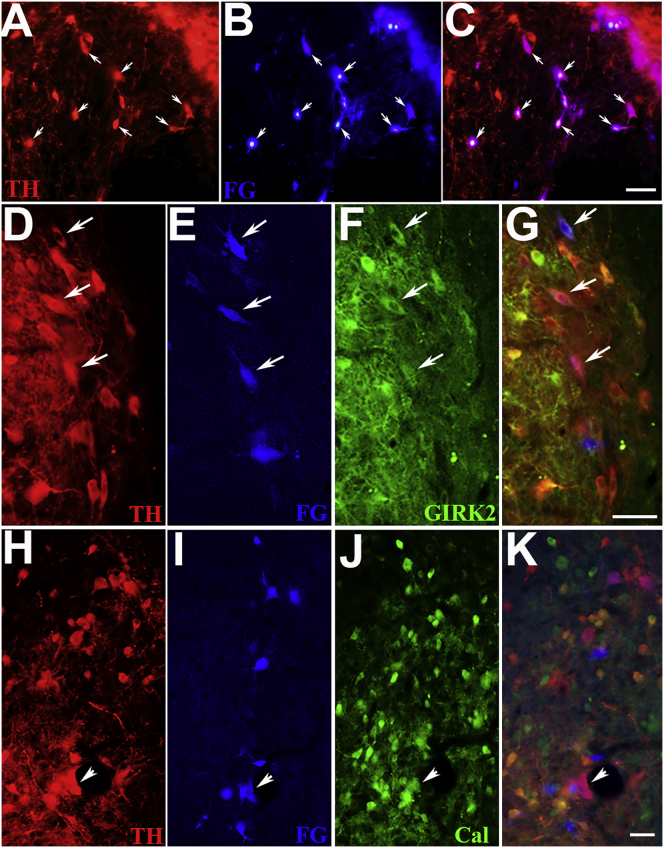
To identify the origin of axons identified in the striatum, we used Fluoro-Gold for retrograde tracing. Fluoro-Gold co-labeled numerous dopaminergic cell bodies within the transplants receiving either GDNF/GFRα1 or GDNF/netrin1 pathways (Figures 6A–6C). Transplants with a GDNF/netrin1 pathway showed the highest average number of FG+/TH+ labeled cells (16 ± 5 per section), whereas those from the GDNF/GFRα1 pathway group showed fewer FG+/TH+ labeled cells (10 ± 4 per section) representing less than 10% of the total TH+ neurons. No retrograde tracer could be identified in any neurons from the GFP-control groups or those not receiving transplants. These data indicate that many of the transplanted TH+ neurons sent axon projections into the striatum along the GDNF/GFRα1 or GDNF/netrin1 pathways. Within the midbrain, there are two separate populations of dopaminergic neurons: A9 from the pars compacta of the substantia nigra and A10 from the ventral tegmental region. Of these neurons, the A9 population exclusively expresses the G-protein-coupled inward rectifying current potassium channel type 2 (Girk2), whereas A10 neurons express the calcium-binding protein calbindin. Only A9 neurons are thought to contribute to motor function both in normal animals and after transplantation.19 To identify which of these neurons from the transplant innervated the striatum, sections from FG-injected rats were co-labeled for mouse anti-TH and rabbit anti-Girk2 or rabbit anti-calbindin. Immunostaining showed that TH+/Girk2+ neurons were also labeled with FG (Figures 6D–6G), while TH+/calbindin+ neurons were rarely labeled by FG (Figures 6H–6K). Thus, A9 dopaminergic neurons are the major neuronal type projecting into the striatum from transplanted VM tissue.
Figure 6.
Retrograde Tracing and Identification of Dopamine Neuron Subtypes within the Transplants
(A–K) FG was injected into the ipsilateral striatum to verify the growth of transplanted TH-positive neurons from the transplant. Numerous FG+ cells (B and C) co-labeled many, but not all, of the TH+ neurons (A and C) within the transplant (small arrows). Since only Girk2+ A9 dopaminergic neurons from the substantia nigra are thought to contribute to recovery, FG (blue in E and I)-labeled sections were stained with antibodies against TH (red in D, G, H, and K) and either Girk2 (GIRK2; green in F and G) or calbindin (Cal; green in J and K). In (D)–(G), white arrows indicate dopamine neurons labeled with FG that were also stained with Girk2; however, few dopaminergic neurons co-labeled for FG and calbindin, as indicated by the arrowheads in (H)–(K). These data identified that the dopamine cells sending axons to the striatum were mostly A9 cells. Scale bars: 100 μm in (C), 50 μm in (G), and 50 μm in (K).
Combining BDNF Expression in the Striatum with a GDNF/Netrin1 Pathway Improves Spontaneous Motor Behavior
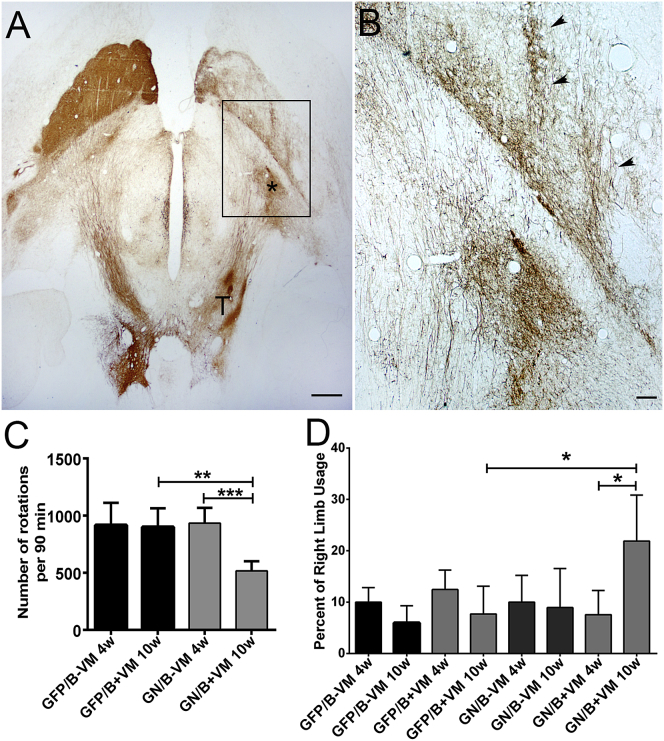
In the preceding experiments, we observed robust growth of TH+ axons along a pathway of GDNF/netrin1; however, the majority of axons failed to grow into the striatum. We hypothesized that expression of BDNF within the striatum at the end of the GDNF/netrin1 pathway would enhance growth into and within the striatum. Previous studies have indicated that striatal expression of BDNF enhances sprouting and functional recovery mediated by E14 VM grafts.20, 21, 22 For this study, we chose to express BDNF using adeno-associated virus, since it has a high tropism for neurons, thus potentially aiding growth and synaptic connectivity of TH+ axons onto striatal neurons. Ten weeks after transplants, extensive innervation within the striatum was observed within rats treated with a GDNF/netrin1 pathway leading to a BDNF striatal target (Figure 7). TH+ axons extending out of the transplant showed some ectopic patterning, with a slight accumulation of fibers at the border of the internal capsule (Figure 7A, asterisk), similar to that observed with pathway expression alone. In some sections, axons were also observed to concentrate along needle tracks within the striatum (Figure 7B, arrowheads); most likely, regions expressing higher levels of BDNF. These animals showed a significant reduction (one-way ANOVA, F(3, 20) = 10.73, p = 0.0002) in rotational behavior for the GDNF/netrin1/BDNF (GN/B) group receiving transplants when compared to controls (Figure 7C). The GN/B group also showed an increase in the percentage of right limb use in a glass cylinder (one-way ANOVA, F(7, 32) = 4.344, p = 0.0018) when compared to the GFP or GN/B no-transplant group (Figure 7D). Striatal injections of adeno-associated virus (AAV)/BDNF alone with or without transplants also had no effect on either d-amphetamine-induced rotational counts or right forelimb use in 6-OHDA-induced lesioned rats.
Figure 7.
Combination of GDNF/Netrin1 Pathway and BDNF Expression in Striatum Promote TH+ Fiber Growth and Increased Innervation in Striatum
(A) Horizontal brain section immunostained with tyrosine hydroxylase. TH+ fibers grew out of the transplant (T) on the right and extended along the guidance pathway to the striatum, where many ectopically concentrated adjacent to the striatum (asterisk). (B) High magnification of TH+ axons adjacent to and within the striatum. In the striatum, axons appear, extending in 2 lines (arrowheads) associated with BDNF injection tracts. (C) A significant (p < 0.05) decrease in amphetamine-induced rotational number was observed with GDNF/netrin1 pathways + BDNF (GN/B) at 10 weeks post-transplantation (GN/B+VM 10w), compared to the GFP/BDNF (GFP/B) groups at 4 weeks (GFP/B 4w), with transplants at 10 weeks (GFP/B+VM 10w), or GN/B-VM 4w groups (pre-transplant). ANOVA, F(3, 20) = 10.73, p = 0.0002, Bonferroni post hoc. (D) Spontaneous forelimb use asymmetry was tested using the modified cylinder test for rats 4 weeks after lesion and before transplantation (4w) and 10 weeks post-transplantation (10w). A significant (p < 0.05) increase in the percentage of right limb usage was observed with GDNF/netrin1 pathways + BDNF (GN/B) at 10 weeks post-transplantation (GN/B+VM 10w), compared to GFP/BDNF groups without (−VM) or with (+VM) transplants at 10 weeks and GN/B 4w groups. ANOVA, F(7, 32) = 4.344, p = 0.0018, Bonferroni post hoc. Scale bars, 1 mm in (A) and 200 μm in (B). *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The aim of this study was to investigate whether we could reconstruct the nigrostriatal pathway in an adult rat hemi-Parkinson’s disease model by establishing a molecular guidance pathway between an E14 VM transplant and the striatum. To create the guidance pathway between the substantia nigra and the striatum, we chose to follow the course along the internal capsule rather than making a straight line between these two regions. This approach was chosen because several studies indicate good long-distance axon growth along axon tracts, due to their physical properties.15, 16, 23 In a previous study, we demonstrated very good growth of dopaminergic axons along a guidance pathway using the combined expression of GDNF with either GFRα1 or netrin1 within the corpus callosum.14, 16
Embryonic ventral mesencephalon (VM) tissue has widely been used for transplantation studies associated with rodent models of Parkinson’s disease (PD).2, 11, 24 Numerous studies have indicated that embryonic tissue transplanted directly into striatal targets greatly reduced amphetamine-induced rotational behaviors in animal models, although the recovery was suboptimal.1, 3, 24 Likewise, clinical trials using ectopic injections of human mesencephalic dopaminergic transplants resulted in some clinical benefit in younger, but not older, patients.4, 5 However, a cohort of these patients developed debilitating dyskinesia. Dyskinesia is thought to be due to either dopamine “hotspots” or the presence of contaminating serotonergic neurons within the transplants.4, 25 Thus, although these ectopic transplants resulted in some benefit, they failed to meet their primary clinical endpoints. To truly reconstruct the neural circuitry in PD, tissue needs to be transplanted into the substantia nigra, and axons need to be guided to striatal targets to support activity regulating striatal dopamine release. Several studies have attempted to reconstruct the nigrostriatal pathway using different strategies to create a growth supportive conduit extending from the substantia nigra to the striatum.8, 10, 11, 12 Of these studies, some show low numbers of axons growing within the pathway or bridge, but the extent of striatal reinnervation was still too low to reach clinically relevant behavioral improvements in contralateral forelimb use, despite improvements in rotational scores.
To identify the molecular determinants involved in mediating axon growth and targeting from transplants, we previously established molecular guidance pathways to support unidirectional growth of axons along a specific trajectory13, 14, 15, 16 that included a turn into either the striatum or cortex.15 These studies targeted expression to astrocytes within the white matter tracts using adenovirus or lentivirus. Furthermore, we identified that the guidance factors GDNF, GFRα1, and netrin1 could direct the growth of dopaminergic axons when used in combination. Of these factors, GDNF has widely been used in animal models and in clinical trials for PD.26, 27 It has been shown to increase differentiation, fiber outgrowth, and dopamine release of fetal midbrain dopaminergic neurons both in vitro and in vivo.28 GDNF also increases the survival of fetal dopaminergic cell transplants in the 6-OHDA-lesioned rat striatum.29, 30 After partial lesions of the substantia nigra in either mouse or non-human primate models, viral mediated overexpression of GDNF within the striatum induces axon growth into the striatum from transplanted dopaminergic neurons within the substantia nigra.9, 11 In completely lesioned rodent models, no growth of transplanted axons toward the striatum was observed, indicating that preserved axons acted as a growth-supportive scaffold.11 Although there is little evidence of GDNF as a chemoattractive factor, when combined with the glycosylphosphatidylinisotol (GPI)-linked GFRα1, it can elicit a strong attractive guidance effect.16, 31 Since dopaminergic axons express GFRα1, surviving axons after a partial lesion could act to guide axons from transplanted neurons to their striatal targets. With complete lesions, the absence of this guidance cue prevents axon growth, even in the presence of GDNF.
Another molecule with known chemoattractant properties is netrin1, which also provides outgrowth and directional cues to cultured rat and human dopaminergic neurons.32, 33, 34 Interestingly, netrin1 is a bi-functional guidance factor, having either attractive or repulsive effects on developing axons, depending on their receptor expression.35, 36 Attraction occurs via binding to the receptor deleted in colorectal cancer (DCC), whereas expression of the UNC5 homologs (UNC5Hs) induces chemorepulsion.37 The receptor DCC is widely expressed in the developing brain, and DCC knockout mice show medial forebrain bundle guidance abnormalities that significantly reduced growth of substantia nigral axons into the dorsomedial striatum.33, 38 In the adult brain, DCC is only expressed in limited populations of neurons, the majority of them being ventral A9 dopamine neurons in the substantia nigra.39 There is a dramatic decrease in the ratio of DCC:UNC5H receptor expression in the dopamine (DA) neurons after puberty, with UNC5H receptor predominance.40 When UNC5H receptor signaling predominates, axons fail to extend. The expression of UNC5H receptors within the vast majority of adult neurons would reduce the potential of endogenous axons to sprout along the pathway, thus reducing the probability of endogenous circuit reformation.
Although GDNF in combination with either GFRα1 or netrin1 promoted good outgrowth along the pathways, we observed much less growth into the striatum in these animals. Most of these axons appeared ectopically to terminate at the border of the internal capsule just prior to entry into the striatum, often adjacent to blood vessels. At first, we thought that this growth restriction could be due to the inhibitory effects of chondroitin sulfate proteoglycans (CSPGs) within perineuronal nets of striatal neurons.41 The enzyme chondroitinase ABC digests CSPGs within perineuronal nets and increases axon growth and functional synaptic connections in adult rodents.17, 42 However, the injection of lentivirus encoding chondroitinase17 into the striatum did not improve TH+ axon growth into the striatum in either GDNF/GFRα1- or GDNF/netrin1-treated animals (data not shown). Previous studies also identified that infusion of BDNF into the striatum for 28 days significantly increased TH+ axon outgrowth throughout the striatum and functional outcome after ectopic transplantation.21, 22 When BDNF was expressed within the striatum, we also observed increases in striatal innervation in animals treated with GDNF/netrin1. This increased striatal innervation from nigral transplants supported much better behavioral recovery, including increased contralateral forelimb use. However, we still observed relatively robust ectopic termination of TH+ axons along the internal capsule. Further reducing this barrier could support even higher levels of growth into the striatum.
Fluoro-Gold was injected into the striatum to examine the transplant origin and dopaminergic neuronal phenotypes. Since FG is known to diffuse more than other tracers of larger molecular weight, we injected a very small volume deep within the striatum to reduce the probability of tracer diffusion out of the basal ganglion. Within the transplant, many TH+ cells were labeled by FG, indicating that dopaminergic cells from the transplant sent fibers into the striatum. Moreover, these cells were identified as A9 neurons expressing GIRK2 and not A10 neurons expressing calbindin. Interestingly, GIRK2-expressing neurons were previously identified in ectopic transplants showing behavioral improvements,43 and more recently, these A9 cells have been considered highly important for behavioral recovery after transplantation of dopamine-expressing neurons.44
In summary, this study demonstrates that a functional nigrostriatal circuit can be reconstructed by intranigral transplantation of dopaminergic cells after the formation of a virally mediated, growth-supportive pathway along the internal capsule from the substantia nigra to striatum. With continuing refinements, our transplant and pathway method might be used to restore dopaminergic input in an anatomically correct manner to provide a therapeutic option for patients with PD.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (200–225 g) were obtained from Harlan Laboratories (Indianapolis, IN, USA). Rats were housed and cared for in either the University of Kentucky’s Division of Laboratory Animal Resources facility or Temple University’s Division of Laboratory Animal Resources facility. All procedures were conducted with the approval of the institutional animal care and use committees and in accordance with all provisions described in the Guide for the Care and Use of Laboratory Animals.
6-Hydroxydopamine-Induced Rat Model of PD
Rats were given unilateral 6-hydroxydopamine (6-OHDA; Sigma) stereotactic injections on the left side under ketamine (67 mg/kg) and xylazine (6.7 mg/kg) anesthesia. A total of either 6 μg or 12 μg of 6-OHDA was injected at two separate sites for either partial or complete lesions, respectively. Injections of 3.0 μg/μL 6-OHDA in 0.9% sterile saline with 0.2% ascorbic acid were made at a rate of 1.0 μL/min into the left medial forebrain bundle (anterior/posterior [AP], −4.4 mm; medial/lateral [ML], 1.2 mm, 8.2 mm deep) and the left rostral substantia nigra pars compacta (AP, −5.3 mm; ML, 2.0 mm, 8.2 mm deep). The needle remained in place for 5 min before removal.
Amphetamine-Induced Rotational Motor Behavior Test
For motor behavioral assessment of lesioned rats, d-amphetamine (2.5 mg/kg in 0.9% saline) was injected intraperitoneally (i.p.). The total number of complete counterclockwise rotations was measured during a 90-min test session in a computer-system-operated rota system (Columbus Instruments, Columbus, OH, USA). Only animals that made at least 600 complete counterclockwise turns were used for the experiment. A detailed time course of amphetamine-induced rotational tests is shown in Figure 2A.
Spontaneous Motor Behavior Test (Cylinder Test)
Rats were tested for limb-use asymmetry during spontaneous motor behavior.45 Rats were videotaped for 5 min as they moved freely in a 20-cm-wide clear glass cylinder. A minimum of 20 rearings and placements of a forepaw onto the cylinder wall were counted per time point per animal. A person blinded to the experimental design analyzed videotapes and counted the total number of forepaw touches. The percent usage of the right (affected) forepaw was calculated by dividing the counts for individual right forepaw touches by the total number of forepaw touches (left, right, and both paws together). A detailed time course of cylinder tests is shown in Figure 2A.
Preparation of Lentiviruses for GDNF, GFRα1, Netrin1, and GFP
Lentivirus was constructed as described in Zhang et al., 2013.16 Briefly, a four-plasmid system was used to package the virus ensuring that resultant virons were replication incompetent. The packaged virus was collected 48 h after transfection and concentration using sucrose gradient ultracentrifugation. Viral titers were estimated using a commercially available p24 ELISA kit (HIV-1 p24 antigen ELISA, Aalto Bio Reagents). Lentivirus-induced expression and function of transgene proteins were tested after each preparation according to the method as described in our previous publication.16
Preparation of AAV BDNF and AAV GFP
Serotype 2 AAV vector was generated with a three-plasmid helper-virus free system, as described by Liu et al. (2012).46 The coding regions of an alternatively spliced mature form of rat BDNF was cloned into our standard adeno-associated viral vector plasmid and co-transfected into 293T cells with a plasmid encoding the AAV rep and cap (replication and Capsid) genes and another plasmid carrying the adenovirus helper function. Three days after transfection, cell lysates and supernatants were harvested and purified by double centrifugation with cesium chloride (CsCl).47 The titer of AAV-GFP was determined by infecting a monolayer of fibroblasts with serial dilutions of AAV-GFP in a 24-well plate.47 The titer of AAV-BDNF was determined against a known titer by ELISA method using the Emax ImmunoAssay Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. AAV-GFP and AAV-BDNF titers were 1.9 × 1012 and 2.6 × 1012 infectious units per milliliter, respectively.
Formation of Guidance Pathway
Lesioned rats were randomly assigned into three different groups for lentiviral injections to generate a pathway between the substantia nigra and the striatum: lenti-GDNF + lenti-GFRα1 (transplant, n = 7; no-transplant control, n = 4), lenti-GDNF + lenti-netrin1 (transplant, n = 8; no-transplant control, n = 6), and lenti-GFP (transplant, n = 6; no- transplant control, n = 4). Each rat was anesthetized and placed in a stereotaxic frame, with skull exposed, and holes were drilled at three AP locations along a line 2.4 mm lateral to bregma on the animal’s left side: −4.0 mm, +0.3 mm, and +5.8 mm relative to bregma.48 To generate the pathway, the needle was inserted at different angles: 0° at −4.0 mm AP, 17° at +0.3 mm AP, and 49° at +5.8 mm AP (Figure 2B). The depth (relative to the dura) and volume of virus injections were as follows: at −4.0 AP (0°), one injection at −8.2 mm deep; at +0.3 mm AP (17°), 1 μL at −5.3 mm, 1.2 μL at −4.3 mm, and 1.4 μL at −3.3mm; and at +5.8 mm AP (49°), 0.6 μL at −9.3 mm, 0.8 μL at −8.3 mm, 1.0 μL at −7.3 mm, 1.2 μL at −6.3 mm, 1.4 μL at −5.3 mm, and 2.0 μL at −4.3 mm. A schematic of this injection protocol is shown in Figure 2B.
Preparation of Guidance Pathway with Striatal BDNF Injections
Lesioned rats were randomly assigned into two groups: (1) lenti-GDNF + lenti-netrin1 plus striatal injections with AAV-BDNF (transplant, n = 8; no-transplant control, n = 6) and (2) lenti-GFP pathway plus striatal injections of AAV-BDNF (transplant, n = 8; no-transplant control, n = 6). Three additional holes were drilled at three AP locations (either 2.4 mm or 3.5 mm lateral to bregma) on the animal’s left side: −1.0 mm, +0.2 mm, and +1.0 mm relative to bregma. The needle was lowered to a depth of −3.5 mm, −4.5 mm, and −5.5 mm from the surface of the dura and 1 μL virus injected at each depth.
E14 VM Tissue Isolation and Transplantation
Two weeks after virus injection, animals received transplants of embryonic-day-14 (E14) VM tissue. Immediately before transplantation, embryos at E14 were removed from a pregnant Sprague-Dawley rat, and the VM brain region (approximately 1 mm × 1.5 mm × 1 mm) was dissected out of each fetus and kept in ice-cold, sterile, calcium- and magnesium-free buffer until transplant. Once each rat was anesthetized, a hole was drilled at −5.2 mm AP and 2.4 mm ML on the left side relative to bregma, and VM was implanted as a whole-tissue chunk using a modified 22G spinal needle lowered to −8.6 mm from skull level and then pulled up to −8.4 mm before the tissue was ejected. Each tissue chunk was ejected slowly by depressing the needle’s plunger ∼1 mm every 20 s, for a total distance of ∼10 cm (∼3 min total). The needle was kept in place for 5 min after ejection, raised 0.4 mm, and kept there for another 5 min before we slowly raised it all the way out of the brain to be sure that the transplant remained in place.13, 15, 16
Fluoro-Gold Labeling
Nine weeks after transplantation, 2 randomly selected rats from each transplanted group received stereotaxic injections of the retrograde neuronal tracer, Fluoro-Gold (FG; 2% in distilled water; Molecular Probes), into the striatum. The tracer was injected (0.2 μL per injection) into the dorsal-lateral striatum using a 10-μL Hamilton syringe at the following coordinates in millimeters with respect to the bregma AP: +1.0, lateral; 3.0, ventral: 5.0.48 Seven days after the injection, the animals were anesthetized and perfused, and the brains were sectioned.
Immunohistochemistry
After the final 10-week behavior tests, rats were anesthetized with pentobarbital and perfused with saline followed by 4% paraformaldehyde (PFA) in phosphate buffer. Brains were carefully removed and post-fixed overnight in 4% PFA at 4°C and then placed in a 30% sucrose solution at 4°C until sinking to the bottom of the vial (2–3 days). Brains were then embedded in optimal cutting temperature (OCT) compound, frozen on dry ice, and sliced at 30 μm in either the sagittal (n = 2 per group) or horizontal (n = 5–6 per group) plane with a cryostat.
Immunohistochemistry procedures were carried out at room temperature following our previous protocols.13, 15, 16 Sections were incubated overnight with a mouse primary antibody against tyrosine hydroxylase (TH; 1:2,000 dilution; Millipore), followed by biotinylated secondary antibody (1:500, Jackson Laboratory) and streptavidin-horseradish peroxidase complex (Vectastain, Vector Laboratories). Sections were developed, dehydrated, and coverslipped.
For fluorescent immunohistochemistry, antibodies and dilution factors were as follows: mouse anti-GFP (1:200, Molecular Probes) followed by goat-anti-mouse fluorescein isothiocyanate (FITC) (1:500, Jackson ImmunoResearch); mouse anti-TH (1:400, Millipore) followed by goat anti-mouse TexRed (1:500, Jackson ImmunoResearch), rabbit anti-GirK2 (1:100, Alomone Labs, Jerusalem, Israel) or rabbit anti-calbindin (1:100, Sigma) followed by goat anti-rabbit FITC (1:500, Jackson ImmunoResearch).
Quantification
All quantification was carried out by an observer blinded to treatment. Sections were chosen based on the following criteria: Sections (1) contained transplanted TH+ cell bodies and (2) either had visible needle tracts from lentiviral pathway injections or were located in sections between 4 and 8.0 mm ventral to Bregma according to the rat brain atlas.48 Only horizontal sections were used for analyses of axon growth along the pathway. Sections without striatum (too dorsal) or substantia nigra (too ventral) were not included in the analysis. To quantify TH+ axonal growth, the number of identifiable TH+ fibers was counted manually with a 0.5-mm2 grid at 200× at a distance of 2–3 mm from the rostral edge of the transplant (near box E in Figure 3B) from 6 sections per animal spaced 100 μm apart and an average of axon counts used per rat (5–6 rats per group). For counting the number of cells labeled with TH, GirK2, or calbindin, FG immunofluorescence-treated sections were examined with an Olympus AX80 microscope equipped with fluorescein and UV filters. Sections containing triple-labeled cells were sampled using a confocal microscope, and co-localization was confirmed by z axis analysis. Quantification was performed on 6 evenly spaced sections (80 μm apart) within the transplanted region.
Statistical Analyses
The level of significance was set at p < 0.05. ANOVAs or t tests were used for statistical analyses; the choice of test was dependent on the experimental design. Means are reported with their corresponding SEM. For comparison of TH+ fibers between different treatments, one-way ANOVA was used. For rotational behavior and cylinder test scores, data were analyzed using two-way ANOVA of repeated measures: the time point (after lesion, 8 weeks post-transplantation, and 10 weeks post-transplantation) was one factor, and treatment was the second factor.
Author Contributions
B.G., C.Z., K.S.Z., A.M.F., D.M.Y., and G.M.S. designed research; B.G., C.Z., K.S.Z., and A.M.F. performed research; B.G., C.Z., K.S.Z., D.M.Y., and G.M.S. analyzed data; and B.G., K.S.Z., D.M.Y., and G.M.S. wrote the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was funded by grants from the National Institute of Neurological Disorders and Stroke (R01 NS060784 to G.M.S. and R01 NS075871 and R01 NS050311 to D.M.Y.) and the Shriners Hospital for Pediatric Research grant SHC 84051.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.06.008.
Supplemental Information
References
- 1.Björklund A., Dunnett S.B., Stenevi U., Lewis M.E., Iversen S.D. Reinnervation of the denervated striatum by substantia nigra transplants: functional consequences as revealed by pharmacological and sensorimotor testing. Brain Res. 1980;199:307–333. doi: 10.1016/0006-8993(80)90692-7. [DOI] [PubMed] [Google Scholar]
- 2.Braak H., Del Tredici K. Assessing fetal nerve cell grafts in Parkinson’s disease. Nat. Med. 2008;14:483–485. doi: 10.1038/nm0508-483. [DOI] [PubMed] [Google Scholar]
- 3.Brundin P., Barker R.A., Parmar M. Neural grafting in Parkinson’s disease: problems and possibilities. Prog. Brain Res. 2010;184:265–294. doi: 10.1016/S0079-6123(10)84014-2. [DOI] [PubMed] [Google Scholar]
- 4.Freed C.R., Greene P.E., Breeze R.E., Tsai W.Y., DuMouchel W., Kao R., Dillon S., Winfield H., Culver S., Trojanowski J.Q. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 5.Olanow C.W., Goetz C.G., Kordower J.H., Stoessl A.J., Sossi V., Brin M.F., Shannon K.M., Nauert G.M., Perl D.P., Godbold J., Freeman T.B. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 6.Caroni P., Schwab M.E. Oligodendrocyte- and myelin-associated inhibitors of neurite growth in the adult nervous system. Adv. Neurol. 1993;61:175–179. [PubMed] [Google Scholar]
- 7.Schwab M.E., Kapfhammer J.P., Bandtlow C.E. Inhibitors of neurite growth. Annu. Rev. Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 8.Dunnett S.B., Rogers D.C., Richards S.J. Nigrostriatal reconstruction after 6-OHDA lesions in rats: combination of dopamine-rich nigral grafts and nigrostriatal “bridge” grafts. Exp. Brain Res. 1989;75:523–535. doi: 10.1007/BF00249903. [DOI] [PubMed] [Google Scholar]
- 9.Gaillard A., Decressac M., Frappé I., Fernagut P.O., Prestoz L., Besnard S., Jaber M. Anatomical and functional reconstruction of the nigrostriatal pathway by intranigral transplants. Neurobiol. Dis. 2009;35:477–488. doi: 10.1016/j.nbd.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Redmond D.E., Jr., Elsworth J.D., Roth R.H., Leranth C., Collier T.J., Blanchard B., Bjugstad K.B., Samulski R.J., Aebischer P., Sladek J.R., Jr. Embryonic substantia nigra grafts in the mesencephalon send neurites to the host striatum in non-human primate after overexpression of GDNF. J. Comp. Neurol. 2009;515:31–40. doi: 10.1002/cne.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson L.H., Grealish S., Kirik D., Björklund A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur. J. Neurosci. 2009;30:625–638. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- 12.Wilby M.J., Sinclair S.R., Muir E.M., Zietlow R., Adcock K.H., Horellou P., Rogers J.H., Dunnett S.B., Fawcett J.W. A glial cell line-derived neurotrophic factor-secreting clone of the Schwann cell line SCTM41 enhances survival and fiber outgrowth from embryonic nigral neurons grafted to the striatum and to the lesioned substantia nigra. J. Neurosci. 1999;19:2301–2312. doi: 10.1523/JNEUROSCI.19-06-02301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Y., Ziemba K.S., Smith G.M. Axon growth across a lesion site along a preformed guidance pathway in the brain. Exp. Neurol. 2008;210:521–530. doi: 10.1016/j.expneurol.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y., Zhang C., Ziemba K.S., Goldstein G.A., Sullivan P.G., Smith G.M. Directing dopaminergic fiber growth along a preformed molecular pathway from embryonic ventral mesencephalon transplants in the rat brain. J. Neurosci. Res. 2011;89:619–627. doi: 10.1002/jnr.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziemba K.S., Chaudhry N., Rabchevsky A.G., Jin Y., Smith G.M. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J. Neurosci. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C., Jin Y., Ziemba K.S., Fletcher A.M., Ghosh B., Truit E., Yurek D.M., Smith G.M. Long distance directional growth of dopaminergic axons along pathways of netrin-1 and GDNF. Exp. Neurol. 2013;250:156–164. doi: 10.1016/j.expneurol.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y., Ketschek A., Jiang Z., Smith G., Fischer I. Chondroitinase activity can be transduced by a lentiviral vector in vitro and in vivo. J. Neurosci. Methods. 2011;199:208–213. doi: 10.1016/j.jneumeth.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong M.S., Woolf C.J., Haque N.S., Anderson P.N. Axonal regeneration from injured dorsal roots into the spinal cord of adult rats. J. Comp. Neurol. 1999;410:42–54. [PubMed] [Google Scholar]
- 19.Grealish S., Jönsson M.E., Li M., Kirik D., Björklund A., Thompson L.H. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson’s disease. Brain. 2010;133:482–495. doi: 10.1093/brain/awp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucidi-Phillipi C.A., Gage F.H., Shults C.W., Jones K.R., Reichardt L.F., Kang U.J. Brain-derived neurotrophic factor-transduced fibroblasts: production of BDNF and effects of grafting to the adult rat brain. J. Comp. Neurol. 1995;354:361–376. doi: 10.1002/cne.903540306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauer H., Fischer W., Nikkhah G., Wiegand S.J., Brundin P., Lindsay R.M., Björklund A. Brain-derived neurotrophic factor enhances function rather than survival of intrastriatal dopamine cell-rich grafts. Brain Res. 1993;626:37–44. doi: 10.1016/0006-8993(93)90560-a. [DOI] [PubMed] [Google Scholar]
- 22.Yurek D.M., Lu W., Hipkens S., Wiegand S.J. BDNF enhances the functional reinnervation of the striatum by grafted fetal dopamine neurons. Exp. Neurol. 1996;137:105–118. doi: 10.1006/exnr.1996.0011. [DOI] [PubMed] [Google Scholar]
- 23.Davies S.J., Fitch M.T., Memberg S.P., Hall A.K., Raisman G., Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 24.Yurek D.M. Intranigral transplants of fetal ventral mesencephalic tissue attenuate D1-agonist-induced rotational behavior. Exp. Neurol. 1997;143:1–9. doi: 10.1006/exnr.1996.6359. [DOI] [PubMed] [Google Scholar]
- 25.Politis M., Wu K., Loane C., Quinn N.P., Brooks D.J., Rehncrona S., Bjorklund A., Lindvall O., Piccini P. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci. Transl. Med. 2010;2:38ra46. doi: 10.1126/scitranslmed.3000976. [DOI] [PubMed] [Google Scholar]
- 26.Gash D.M., Zhang Z., Ovadia A., Cass W.A., Yi A., Simmerman L., Russell D., Martin D., Lapchak P.A., Collins F. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 27.Kirik D., Georgievska B., Björklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat. Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- 28.Lin L.F., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 29.Kordower J.H., Emborg M.E., Bloch J., Ma S.Y., Chu Y., Leventhal L., McBride J., Chen E.Y., Palfi S., Roitberg B.Z. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 30.Tomac A., Lindqvist E., Lin L.F., Ogren S.O., Young D., Hoffer B.J., Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 31.Ledda F., Paratcha G., Ibáñez C.F. Target-derived GFRalpha1 as an attractive guidance signal for developing sensory and sympathetic axons via activation of Cdk5. Neuron. 2002;36:387–401. doi: 10.1016/s0896-6273(02)01002-4. [DOI] [PubMed] [Google Scholar]
- 32.Cord B.J., Li J., Works M., McConnell S.K., Palmer T., Hynes M.A. Characterization of axon guidance cue sensitivity of human embryonic stem cell-derived dopaminergic neurons. Mol. Cell. Neurosci. 2010;45:324–334. doi: 10.1016/j.mcn.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Duarte T., Kocabas A., Works M., McConnell S.K., Hynes M.A. Evidence for topographic guidance of dopaminergic axons by differential Netrin-1 expression in the striatum. Mol. Cell. Neurosci. 2014;61:85–96. doi: 10.1016/j.mcn.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Lin L., Rao Y., Isacson O. Netrin-1 and slit-2 regulate and direct neurite growth of ventral midbrain dopaminergic neurons. Mol. Cell. Neurosci. 2005;28:547–555. doi: 10.1016/j.mcn.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Dent E.W., Barnes A.M., Tang F., Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J. Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy T.E., Serafini T., de la Torre J.R., Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 37.Huber A.B., Kolodkin A.L., Ginty D.D., Cloutier J.F. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 38.Xu B., Goldman J.S., Rymar V.V., Forget C., Lo P.S., Bull S.J., Vereker E., Barker P.A., Trudeau L.E., Sadikot A.F., Kennedy T.E. Critical roles for the netrin receptor deleted in colorectal cancer in dopaminergic neuronal precursor migration, axon guidance, and axon arborization. Neuroscience. 2010;169:932–949. doi: 10.1016/j.neuroscience.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Osborne P.B., Halliday G.M., Cooper H.M., Keast J.R. Localization of immunoreactivity for deleted in colorectal cancer (DCC), the receptor for the guidance factor netrin-1, in ventral tier dopamine projection pathways in adult rodents. Neuroscience. 2005;131:671–681. doi: 10.1016/j.neuroscience.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 40.Manitt C., Labelle-Dumais C., Eng C., Grant A., Mimee A., Stroh T., Flores C. Peri-pubertal emergence of UNC-5 homologue expression by dopamine neurons in rodents. PLoS ONE. 2010;5:e11463. doi: 10.1371/journal.pone.0011463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charvet I., Hemming F.J., Feuerstein C., Saxod R. Mosaic distribution of chondroitin and keratan sulphate in the developing rat striatum: possible involvement of proteoglycans in the organization of the nigrostriatal system. Brain Res. Dev. Brain Res. 1998;109:229–244. doi: 10.1016/s0165-3806(98)00088-1. [DOI] [PubMed] [Google Scholar]
- 42.Massey J.M., Hubscher C.H., Wagoner M.R., Decker J.A., Amps J., Silver J., Onifer S.M. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J. Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson L., Barraud P., Andersson E., Kirik D., Björklund A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J. Neurosci. 2005;25:6467–6477. doi: 10.1523/JNEUROSCI.1676-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Keeffe F.E., Scott S.A., Tyers P., O’Keeffe G.W., Dalley J.W., Zufferey R., Caldwell M.A. Induction of A9 dopaminergic neurons from neural stem cells improves motor function in an animal model of Parkinson’s disease. Brain. 2008;131:630–641. doi: 10.1093/brain/awm340. [DOI] [PubMed] [Google Scholar]
- 45.Schallert T., Fleming S.M., Leasure J.L., Tillerson J.L., Bland S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y., Keefe K., Tang X., Lin S., Smith G.M. Use of self-complementary adeno-associated virus serotype 2 as a tracer for labeling axons: implications for axon regeneration. PLoS ONE. 2014;9:e87447. doi: 10.1371/journal.pone.0087447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayuso E., Mingozzi F., Montane J., Leon X., Anguela X.M., Haurigot V., Edmonson S.A., Africa L., Zhou S., High K.A. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17:503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G., Watson C. Sixth Edition. Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.