Abstract
Ribavirin is effective for treating immunocompromised patients with chronic hepatitis E virus infection. However, ribavirin treatment is not always successful. We describe 3 solid organ transplant recipients treated with sofosbuvir and ribavirin after failing ribavirin monotherapy. Complete elimination of hepatitis E virus could not be achieved.
Keywords: chronic hepatitis E, sofosbuvir, solid organ transplant recipients
Hepatitis E virus (HEV) genotype 3 is a common cause of acute viral hepatitis in the Netherlands [1]. In general, immunocompetent patients with HEV infection are able to clear the virus spontaneously. However, immunocompromised patients develop chronic infection in approximately 60% of cases (defined as HEV ribonucleic acid [RNA] detectable in plasma for more than 3 months), which may lead to liver fibrosis and cirrhosis. Reduction of immunosuppression, if possible, is the first step in the treatment of chronic HEV infection and results in viral clearance in approximately one third of patients. The second-line treatment, with ribavirin monotherapy, can lead to a sustained virological response (SVR) in 78% of patients and 85% after retreatment. For solid organ transplant (SOT) recipients who fail to achieve SVR on ribavirin therapy, alternative treatment options are scarce [2]. Liver transplant patients may benefit from pegylated-interferon (PEG-IFN) therapy, but PEG-IFN is contraindicated after other SOTs, because of the increased risk of allograft rejection [3, 4].
Sofosbuvir in combination with other antiviral agents is approved by the US Food and Drug Administration to treat chronic hepatitis C virus (HCV) infection [5]. It has been shown that sofosbuvir can inhibit HEV genotype 3 replication in vitro and has an additive effect when combined with ribavirin [6]. Thus far, the clinical effect of sofosbuvir in chronic HEV infections is unclear. A multicenter trial for the treatment of chronic HEV with sofosbuvir is ongoing (EudraCT Number 2017-000403-24), but results have not yet been reported. Until now, sofosbuvir treatment for chronic HEV infection has been described in 6 patients. Two liver transplant recipients with a HCV and HEV coinfection were treated with sofosbuvir and daclatasvir. In 1 patient, this therapy regimen was not effective in clearing the chronic HEV infection, whereas in the other patient both HCV and HEV were cleared after 12 weeks of treatment; this latter patient was also treated with ribavirin [7, 8]. Four additional cases of ribavirin plus sofosbuvir therapy for chronic HEV infections have been described. In 3 of these 4 cases an antiviral effect was observed; however, SVR was not achieved [9–11]. In the last, most recently described case report, HEV was cleared after 3 months of ribavirin plus sofosbuvir treatment [12].
We describe 3 SOT recipients with chronic HEV infection, who initially failed to respond to ribavirin monotherapy and were retreated with a combination of sofosbuvir and ribavirin. Informed consent for publication was obtained for all patients.
CASE DESCRIPTION
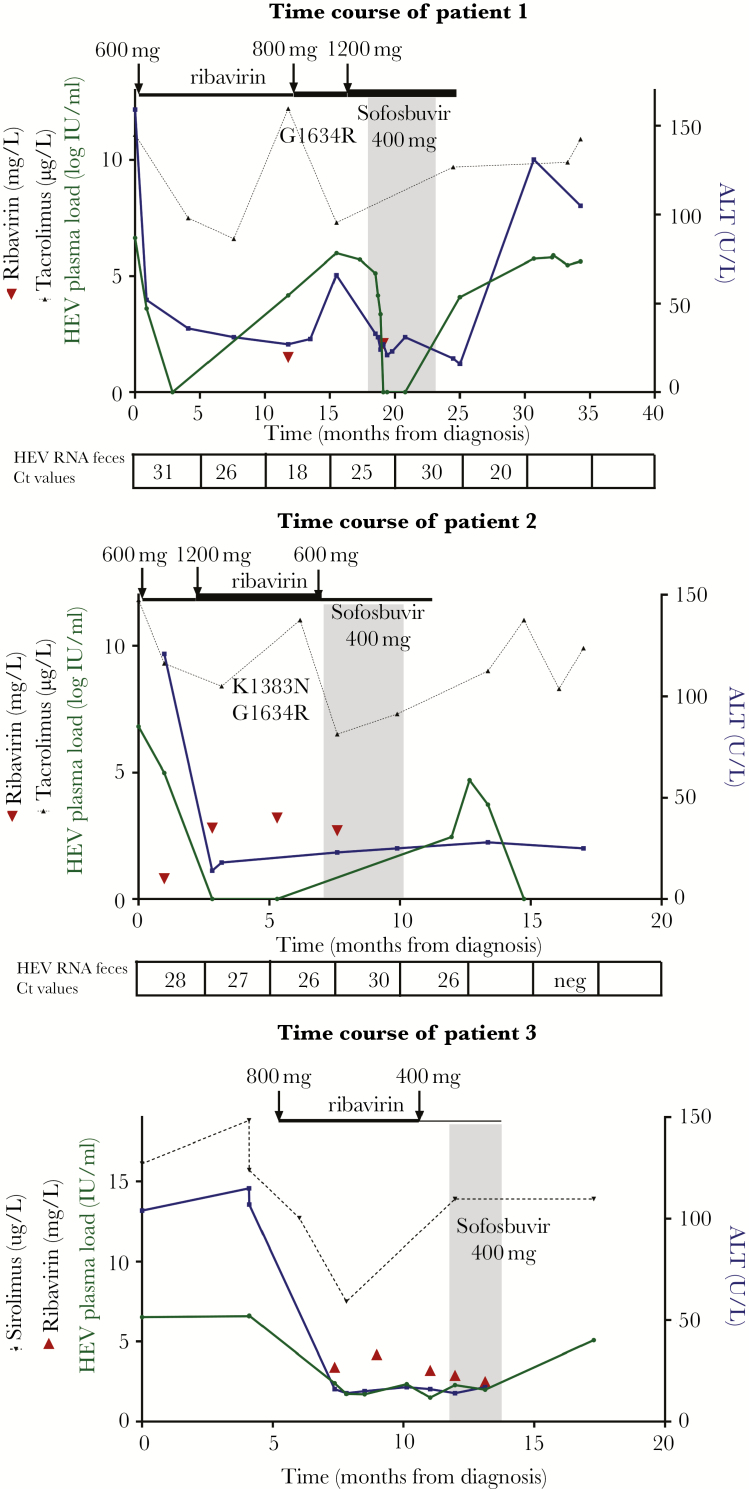
Patient 1, a 25-year-old male, required a heart transplantation in 2012 because of end-stage heart failure caused by a cardiomyopathy. In 2015, he was diagnosed with a HEV genotype 3c infection with elevated alanine aminotransferase (ALT) levels (Figure 1A). Reduction of immunosuppression (consisting of mycophenolate mofetil bid 1000 mg and tacrolimus bid 5 mg) was not possible because of the risk of allograft rejection in this patient who had suffered a previous episode of threatened rejection. Therefore, treatment with ribavirin 600 mg qd was initiated. After 3 months of treatment, ALT levels returned to normal and HEV RNA became undetectable in plasma, but not in feces. Therefore, ribavirin therapy was continued. One year after diagnosis, HEV RNA relapsed in plasma. The ribavirin dose was increased to 400 mg bid and subsequently to 600 mg bid (ribavirin plasma levels varied from 1.5 to 2.1 mg/L). Despite this intensification of therapy, HEV RNA remained detectable in plasma. By means of sequence analysis of ORF1, a previously described mutation [13, 14] was detected (G1634R). Fibroscan showed no signs of liver fibrosis. Sofosbuvir 400 mg qd was added to the treatment in May 2017. After 1 month of treatment, HEV RNA in plasma became undetectable. Hepatitis E virus RNA remained detectable in feces, but cycle threshold values increased from 25 to 30, possibly suggesting a response to treatment. After 24 weeks of combined treatment, HEV RNA was still detected in feces without further improvement, and therefore sofosbuvir and ribavirin were stopped. Two weeks later, HEV RNA load in plasma and feces relapsed to baseline levels as expected [15]. Currently, the patient still has a detectable HEV load in plasma and elevated ALT levels. Fibroscan shows no signs of fibrosis.
Figure 1.
Graphs indicate the following: therapeutic interventions, hepatitis E virus (HEV) plasma and fecal load, alanine aminotransferase (ALT) levels, ribavirin levels, sirolimus/tacrolimus levels, and presence of mutations. (x-axis) Time from diagnosis of HEV infection. (y-axis, left) Hepatitis E virus plasma load (green), ribavirin levels (red), and sirolimus/tacrolimus levels (black). (y-axis, right) Alanine aminotransferase level (blue). Sofosbuvir treatment is indicated by the gray box. Ribavirin dosing changes are shown by the black arrows and the thickness of the line. In the boxes below the graphs, the presence of HEV RNA in feces is depicted, expressed in cycle threshold (Ct) values. The x-axis of the graph indicates the time point. For patient 3, HEV loads in feces were not measured.
Patient 2, a 64-year-old woman, underwent a bilateral lung transplantation in January 2000 because of severe emphysema caused by alpha-1-antitripsin deficiency. In 2017, she had elevated ALT levels and was diagnosed with a HEV infection, genotype 3c (Figure 1B). The intensity of immunosuppressive therapy (consisting of mycophenolate mofetil 500 mg bid, tacrolimus 2.5 mg bid, and prednisolone 5 mg qd) could only be modestly reduced (tacrolimus decreased to 2 mg bid) because of prior signs of rejection. Treatment with ribavirin 600 mg qd was initiated. After 3 months of treatment, HEV RNA load in plasma was undetectable, but feces remained positive. Therefore, the ribavirin dose was increased to 600 mg bid, which did not result in fecal clearance of the virus either (ribavirin plasma levels ranged from 0.8 to 3.8 mg/L). Two previously described mutations [13, 14] were detected (K1383N and G1634R). After more than 7 months of ribavirin treatment, sofosbuvir 400 mg qd was added and the ribavirin dose was decreased to 600 mg qd because of side effects (hemoglobin [Hb] decreased by 2 mmol/L to a nadir of 7.2 mmol/L). Feces remained HEV-RNA positive after 8 weeks of combined treatment, and sofosbuvir was stopped after 12 weeks of therapy. Ribavirin was stopped 2 months later. Subsequently, as expected, HEV RNA relapsed in plasma. Four months later, HEV RNA spontaneously became undetectable in plasma and feces, without any modulation of immunosuppressive therapy, and remained as such up until today (9 months later).
Patient 3, a 52-year-old woman, underwent a bilateral lung transplantation in 2011 because of restrictive lung disease caused by chemoradiation therapy in childhood. In February 2017, elevated ALT levels were observed and HEV RNA was detected in plasma (Figure 1C). In hindsight, ALT levels had been elevated since transplantation, and a plasma sample 6 months earlier (September 2016) was tested positive for HEV RNA, which confirmed the diagnosis of chronic HEV gt3 infection. Fibroscan revealed stage F4 fibrosis. Immunosuppressive treatment consisted of sirolimus 2 mg qd, mycophenolate mofetil 750 mg bid, and prednisolone 7.5 mg qd. Immunosuppression was not reduced at first due to fear of rejection; the restricted anatomical space only allowed transplantation of the lower lobes resulting in a total lung capacity of 2.2 liters of air, and she remained dependent on intermittent noninvasive ventilation after lung transplantation. Ribavirin treatment was initiated (400 mg bid). Hepatitis E virus RNA load in plasma decreased but remained detectable at low levels. The ALT levels returned to normal range. Five months after start of treatment, the dose was reduced to 200 mg bid, because of high ribavirin levels (up to 4.2 mg/L) and significant anemia (Hb decreased by 2.5 mmol/L to a nadir of 6.3 mmol/L). One month later, immunosuppressive treatment could be reduced (mycophenolate mofetil was lowered to 500 mg bid). After 7 months of ribavirin therapy, HEV RNA remained detectable in plasma. As salvage treatment, sofosbuvir was added to the ribavirin regimen. After 8 weeks of combination therapy, no antiviral response was observed and the treatment with ribavirin and sofosbuvir was stopped. Three months later, HEV RNA was detected in plasma at pretreatment levels. After 6 months, ALT levels increased up to 146 U/L and the patient developed decompensated liver cirrhosis.
DISCUSSION
We describe 3 SOT recipients who were treated with a combination of sofosbuvir and ribavirin for chronic HEV infection, because SVR was not achieved with ribavirin monotherapy. Two of 3 patients showed partial antiviral response to treatment, but complete clearance of the virus was not observed. After treatment was stopped, HEV-RNA plasma levels returned to pretreatment levels, suggesting an antiviral activity of the sofosbuvir plus ribavirin combination.
The sofosbuvir treatment length varied in these patients, ranging from 8 to 24 weeks. Even after a long treatment period of 24 weeks, HEV remained detectable at low levels and was not cleared from the feces. This is in line with the findings of Valk et al [9] and Fraga et al [11], by whom an antiviral effect of sofosbuvir was observed; however, SVR was not achieved.
All patients were treated with a sofosbuvir dose of 400 mg qd, in analogy to treatment of HCV infections. Because the anti-HEV activity of sofosbuvir has been shown to be less pronounced than the anti-HCV activity in vitro [6], a higher dose might be needed to achieve complete HEV elimination. However, higher dosing is not registered and the safety of this is unknown.
The success of sofosbuvir treatment may also be dependent on the combined effect with ribavirin [6]. In our patients, ribavirin monotherapy failed, which may have compromised the effectiveness of the combination therapy. Nevertheless, ribavirin will remain the first-line therapy, and sofosbuvir would only be indicated in those who fail ribavirin monotherapy.
In all 3 patients, ribavirin plasma levels were measured; however, the optimal ribavirin plasma level and effect on SVR in HEV is unclear [16]. Two patients showed mutations that have been found in other patients with ribavirin treatment failure [13, 14], but the impact of these variants on HEV treatment outcome is yet debatable [4, 15].
Although the antiviral effect of sofosbuvir in vitro was promising, it seems that in patients with chronic HEV infection who failed on ribavirin monotherapy and for whom reduction of immunosuppressive medication or treatment with PEG-IFN is not an option, the above-mentioned addition of sofosbuvir to ribavirin treatment may not be the salvage therapy we hoped for, in spite of the 2 case reports with successful combination treatment [8, 12]. The liver transplant recipient with clearance of HEV under sofosbuvir plus ribavirin treatment had a concurrent HCV infection [8]. In theory, the treatment of HCV may have triggered an immunological response that induced HEV clearance as well. Another explanation may be that the ribavirin was the effective agent in clearing the HEV infection in this patient, because he had not been treated with ribavirin monotherapy before.
Methods
Our methods for quantitative real-time polymerase chain reaction and sequencing analysis for HEV genotyping are based on Zhao et al [17], Wang et al [18], van der Poel et al [19], and Baylis et al [20].
CONCLUSIONS
In conclusion, sofosbuvir showed variable antiviral activity against HEV but did not result in SVR in our patients. Alternative treatment options are urgently needed for SOT recipients with chronic HEV infection failing ribavirin treatment and being at risk for progression of liver fibrosis leading to cirrhosis and, in some cases, decompensation and death.
Acknowledgments
We thank Dr. K. van Erpecum for clinical input and for proofreading the manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Hogema BM, Molier M, Sjerps M, et al. Incidence and duration of hepatitis E virus infection in Dutch blood donors. Transfusion 2016; 56:722–8. [DOI] [PubMed] [Google Scholar]
- 2. Kamar N, Abravanel F, Lhomme S, et al. Hepatitis E virus: chronic infection, extra-hepatic manifestations, and treatment. Clin Res Hepatol Gastroenterol 2015; 39:20–7. [DOI] [PubMed] [Google Scholar]
- 3. Haagsma EB, Riezebos-Brilman A, van den Berg AP, et al. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl 2010; 16:474–7. [DOI] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver. EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol 2018; 68:1256–71. [DOI] [PubMed] [Google Scholar]
- 5. Mangia A, Piazzolla V. Overall efficacy and safety results of sofosbuvir-based therapies in phase II and III studies. Dig Liver Dis 2014; 46(Suppl 5):S179–85. [DOI] [PubMed] [Google Scholar]
- 6. Dao Thi VL, Debing Y, Wu X, et al. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology 2016; 150:82–5.e4. [DOI] [PubMed] [Google Scholar]
- 7. Donnelly MC, Imlach SN, Abravanel F, et al. Sofosbuvir and daclatasvir anti-viral therapy fails to clear HEV viremia and restore reactive T cells in a HEV/HCV co-infected liver transplant recipient. Gastroenterology 2017; 152:300–1. [DOI] [PubMed] [Google Scholar]
- 8. De Martin E, Antonini TM, Coilly A, et al. HCV and HEV recurrence after liver transplantation: one antiviral therapy for two viruses. Transpl Int 2017; 30:318–9. [DOI] [PubMed] [Google Scholar]
- 9. van der Valk M, Zaaijer HL, Kater AP, Schinkel J. Sofosbuvir shows antiviral activity in a patient with chronic hepatitis E virus infection. J Hepatol 2017; 66:242–3. [DOI] [PubMed] [Google Scholar]
- 10. Todesco E, Mazzola A, Akhavan S, et al. Chronic hepatitis E in a heart transplant patient: sofosbuvir and ribavirin regimen not fully effective. Antivir Ther 2018; 23:463–5. [DOI] [PubMed] [Google Scholar]
- 11. Fraga M, Gouttenoire J, Sahli R, et al. Sofosbuvir add-on to ribavirin for chronic hepatitis E in a cirrhotic liver transplant recipient: a case report. BMC Gastroenterol 2019; 19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drinane M, Jing Wang X, Watt K. Sofosbuvir and ribavirin eradication of refractory hepatitis E in an immunosuppressed kidney transplant recipient. Hepatology 2019; 69:2297–9. [DOI] [PubMed] [Google Scholar]
- 13. Todt D, Walter S, Brown RJ, Steinmann E. Mutagenic effects of ribavirin on hepatitis E virus-viral extinction versus selection of fitness-enhancing mutations. Viruses 2016; 8:10.3390/v8100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Debing Y, Gisa A, Dallmeier K, et al. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology 2014; 147:1008–11.e7; quiz e15–6. [DOI] [PubMed] [Google Scholar]
- 15. Marion O, Lhomme S, Del Bello A, et al. Monitoring hepatitis E virus fecal shedding to optimize ribavirin treatment duration in chronically infected transplant patients. J Hepatol 2019; 70:206–9. [DOI] [PubMed] [Google Scholar]
- 16. Kamar N, Lhomme S, Abravanel F, et al. An early viral response predicts the virological response to ribavirin in hepatitis E virus organ transplant patients. Transplantation 2015; 99:2124–31. [DOI] [PubMed] [Google Scholar]
- 17. Zhao C, Li Z, Yan B, et al. Comparison of real-time fluorescent RT-PCR and conventional RT-PCR for the detection of hepatitis E virus genotypes prevalent in China. J Med Virol 2007; 79:1966–73. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Ling R, Erker JC, et al. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J Gen Virol 1999; 80(Pt 1):169–77. [DOI] [PubMed] [Google Scholar]
- 19. van der Poel WH, Verschoor F, van der Heide R, et al. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg Infect Dis 2001; 7:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baylis SA, Hanschmann KM, Blümel J, Nübling CM; HEV Collaborative Study Group Standardization of hepatitis E virus (HEV) nucleic acid amplification technique-based assays: an initial study to evaluate a panel of HEV strains and investigate laboratory performance. J Clin Microbiol 2011; 49:1234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]