Abstract
Photodynamic therapy (PDT) involves light activation of the photosensitizer to generate reactive molecular species that induce cell modulation or death. Based on earlier findings showing that the photosensitizer benzoporphyrin derivative (BPD) is a breast cancer resistance protein (ABCG2) substrate, we investigated the ability of the P-glycoprotein (P-gp) and multidrug resistance-associated protein 1 (MRP1) to transport BPD. In a panel of breast cancer cell lines overexpressing P-gp, MRP1, or ABCG2, BPD transport occurs only in cells overexpressing P-gp and ABCG2. Intracellular BPD fluorescence is not affected by MRP1 as determined by flow cytometry. To bypass P-gp- and ABCG2-mediated efflux of BPD, we introduce a lipidation strategy to create BPD derivatives that are no longer P-gp and ABCG2 substrates. The phospholipid-conjugated BPD and its nanoliposomal formulation evade both P-gp- and ABCG2-mediated transport. In cytotoxicity assays, lipidated BPD and its nanoliposomal formulation abrogate P-gp- and ABCG2-mediated PDT resistance. We verify that P-gp, like ABCG2, plays a role in BPD transport and BPD-PDT resistance. Furthermore, we introduce porphyrin-lipid nanovesicles as a new strategy to escape P-gp and ABCG2-mediated efflux of BPD for improved PDT outcomes in two breast cancer cell lines.
Keywords: Multiple drug resistance, ATP-binding cassette transporters, Benzoporphyrin derivative, Porphysome nanotechnology, Photodynamic therapy
1. Introduction
One of the major problems encountered in cancer treatment is the development of multidrug resistance, a process mediated by the ATP-binding cassette (ABC) transmembrane transporters that utilize energy from ATP hydrolysis to actively pump anti-cancer agents and pro-tumorigenic molecules out of cells.[1] Among the 48 human ABC transporters identified, multidrug resistance protein 1 (MDR1, also known as P-glycoprotein or P-gp; encoded by ABCB1), breast cancer resistance protein (BCRP; encoded by ABCG2), and multidrug resistance-associated protein 1 (MRP1, encoded by ABCC1) are known to export hundreds of chemically unrelated anti-cancer compounds.[2–4] Over the past 30 years, the focus on developing transporter inhibitors to reverse multidrug resistance has provided little to no benefits to cancer patients.[5, 6] Some transporter inhibitors are simply too toxic, while others induce pharmacokinetic changes owing to drug-drug interactions.[7] In addition, different ABC transporters are co-expressed in tumors and show high interpatient and intertumor variability with only a subset of resistant cancers expressing these transporters.[6, 8] With new analytical, optical, and nanotechnology tools being developed for advancing personalized medicine, there is a surge of research interest towards the development of therapeutic agents that are poor substrates for these efflux transporter proteins.[6] This paradigm-shifting approach may result in the discovery of new agents that overcome ABC transporter-mediated resistance entirely.
Photodynamic therapy (PDT) is a mechanistically-distinct, cytotoxic modality that is effective on chemo- and radio- resistant tumors.[9] PDT uses near-infrared (NIR) light to excite a light-absorbing molecule called a photosensitizer.[10, 11] Upon activation, the photosensitizer induces the production of highly reactive molecular species (RMS; e.g., 1O2, H2O2, O2•−, •OH) that confer toxicity to nearby targets.[10, 11] PDT is used clinically as a salvage therapy to treat a wide range of cancers, including metastatic breast,[12] esophageal,[13] lung,[14] basal cell,[15] gastric,[16] cervical,[17] prostate,[18] head and neck,[19] brain,[20] and pancreatic carcinoma[21, 22]. Light delivery to confined areas such as the chest, abdomen, and brain can be done intra-operatively or laparoscopically.[22–25] Cytotoxicity from PDT is governed by intracellular photosensitizer accumulation, subcellular localization of the photosensitizer, spatial confinement of light, and the short distances over which the RMS remain active.[9–11] Combination of targeted delivery of photosensitizer and confined light exposure often lead to a significant improvement in therapeutic efficacy.[26, 27] It is well established that ABCG2 expression can limit the intracellular retention of a number of clinically used photosensitizing agents, i.e., the benzoporphyrin derivative (BPD), chlorin-based drugs, aminolevulinic acid (ALA)-induced protoporphyrin IX (PpIX), as well as 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH).[28] While it has been confirmed that chlorin e6, PpIX, and HPPH are not substrates for P-gp and MRP1,[28] the role of P-gp and MRP1 in BPD transport remains unclear.
Since the discovery of P-gp over four decades ago in drug resistant cells,[29–31] a number of strategies have been explored to target ABC transporters and overcome PDT resistance. Ample preclinical studies have shown that using tyrosine kinase inhibitors (TKIs; e.g., imatinib mesylate, gefitinib and erlotinib) to block drug efflux by ABC transporters could augment photosensitizer retention in cancer cells and increase PDT cytotoxicity.[32–35] Studies by Sun et al. provide evidence that gefitinib could decrease the mRNA and protein expression of ABCG2, thereby enhancing intracellular PpIX levels in a dose-dependent manner, yielding superior PDT toxicity against human glioma cell lines.[33] However, oral administration of gefitinib at 100 mg/kg was not adequate to inhibit the activity of ABCG2 in a xenograft model. This is possibly due to suboptimal experimental conditions, as well as PpIX’s high affinity to human ABCG2.[32] Using endothelial cells, Gallagher-Colombo et al. show that pretreatment with erlotinib, a potent ABCG2 inhibitor, significantly increases the intracellular BPD level and the cytotoxic effect of PDT.[34] Liu et al. suggest that imatinib mesylate increased accumulation of HPPH, PpIX, and BPD in ABCG2-overexpressing cancer cells (i.e., Colo 26, RIF-1, BCC-1), but not in ABCG2 negative cells, and enhanced PDT efficacy in vitro.[35] A combination of imatinib mesylate and HPPH-PDT prolonged the survival of C3H/HeJCr mice bearing RIF-1 tumors compared to monotherapies. Despite convincing laboratory data showing that photosensitizer transport can be inhibited by TKIs, the clinical translatability of the TKI approach has yet to be confirmed.
As it is still unclear whether FDA-approved BPD is a substrate of P-gp and MRP1 or is a substrate of neither, we investigated the effect of P-gp and MRP1 on the intracellular accumulation of BPD in human breast cancer cell lines. Our results suggest that P-gp, like ABCG2, impairs the effectiveness of PDT in breast cancer cells by decreasing intracellular levels of BPD. While continued efforts are needed to analyze the structure-activity relationships between photosensitizers and ABC transporters, several studies indicate that the variation in photosensitizer transport correlates with the chemical structure of the photosensitizer.[28, 36] Thus, we hypothesize that a tetrapyrrole-type photosensitizer with a more complex biomolecular structure will be less effectively effluxed by a variety of ABC transporters. Here, we introduce a TKI-free lipidation strategy—by conjugating photosensitizer (BPD) to lysophosphocholine (16:0)LysoPC—to escape ABCG2- and P-gp-mediated photosensitizer efflux in cancer cells. The ability of (16:0)LysoPC-BPD assemblies and its nanoformulation to improve the effectiveness of the PDT is also examined in two human breast cancer cell lines.
2. Materials and methods
2.1. Cell culture
Four human breast cancer cell lines including the MCF-7 parental cell line, P-gp-overexpressing MCF-7 TX400 subline (selected in 400 ng/ml paclitaxel), ABCG2-overexpressing MCF-7 MX100 subline (selected in 100 nM mitoxantrone), and MRP1-overexpressing MCF-7/VP subline (selected in 4 µM etoposide) were maintained in Eagle’s Minimum Essential Medium (EMEM) growth medium (Cellgro) supplemented with 10 % (v/v) fetal bovine serum (FBS; Gibco), 100 U/mL penicillin and 100 µg/mL streptomycin (Lonza), and 0.01 mg/ml insulin (Sigma). All cell lines were characterized previously,[28, 36] confirmed to be free of mycoplasma, and cultured in 5% CO2 and at 37°C.
2.2. Western blot
Protein expression was analyzed using western blots. Briefly, 20µg of cell lysates were separated on 4–12% precast Bis-Tris protein gels (NuPage) and transferred onto polyvinylidene difluoride (PVDF) membranes (Thermo). After blocking with 5% bovine serum albumin (BSA)-containing tris-buffered saline and Polysorbate 20 (TBST) solution, proteins were detected using antibodies against ABCG2 (1:500, Kamiya BioMedical MC-177), MRP1 (1:500, Kamiya BioMedical MC-162), and P-gp (1:500, ThermoFisher MA1–26528). Anti β-actin antibodies (1:5000, Cell Signaling 3700) were used for loading control. Visualization of protein bands was developed by chemiluminescence (SuperSignal, ThermoFisher 34577) with exposure to a Gel Imager (FluorChem E System, ProteinSimple).
2.3. Preparation and characterization of (16:0)LysoPC-BPD conjugates
The (16:0)LysoPC-BPD conjugate was synthesized by crosslinking the carboxylic acid group of BPD (U.S. Pharmacopeial Convention) to the hydroxyl functional group of (16:0)LysoPC via the esterification reaction. Briefly, 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine ((16:0)LysoPC), BPD, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), 4(dimethylamino) pyridine (DMAP), and N,N-diisopropylethylamine (DIPEA) were mixed in dichloromethane at a fixed molar ratio of 1:5:50:25:60 for 24 hours at room temperature. Dichloromethane was removed via rotary evaporation, and the residue was subjected to Sephadex® LH-20 gel chromatography column purification in methanol, following which methanol was removed via rotary evaporation and the purified (16:0)LysoPC-BPD was stored at −20 °C. The purified (16:0)LysoPC-BPD conjugates were analyzed using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS; Bruker).
2.4. Synthesis and purification of nanoliposomal formulations of BPD (L-BPD) and (16:0)LysoPC-BPD (L-LysoPC-BPD)
The two types of nanoliposomes: (1) L-BPD; and (2) L-LysoPC-BPD were prepared by following our established protocol.[37, 38] Briefly, dipalmitoylphosphatidylcholine (DPPC), cholesterol, distearoylphosphatidylethanolamine-methoxy polyethylene glycol (DSPE-PEG), and dioleoyltrimethylammoniumpropane (DOTAP) (Avanti Polar Lipids) were mixed in chloroform at a fixed molar ratio of 20:10:1:2.5. For the L-BPD formulation, 50 nmoles of BPD were co-dissolved with lipids at BPD-to-total lipid ratio of ~0.6 mol%. For L-LysoPC-BPD formulation, 50 nmoles of (16:0)LysoPC-BPD was co-dissolved with lipids. Chloroform was removed by rotary evaporation overnight to afford a thin lipid film. The resulting lipid film was rehydrated with 1 mL of phosphate-buffered saline (PBS) at 45°C, and then subjected to freeze-thaw cycles (4°C-45°C) for 2 hours. The dispersion was then extruded 10 times through two stacked polycarbonate membranes (0.1 µm pore size; Nuclepore, Whatman, Ltd.) at 42°C using a mini-extruder system (Avanti Polar Lipids, Inc.) to form unilamellar vesicles. Un-encapsulated photosensitizers or drugs were removed by dialysis (Spectra/Por, MWCO 300kD, Spectrum Laboratories, Inc.) against PBS. Zetasizer NanoZS (Malvern Instruments) was used to measure the size of nanoliposomes. Concentration of BPD was determined by UV-Vis spectroscopy with an appropriate standard curve (ε= 34,895 M−1 cm−1, at 687 nm in dimethyl sulfoxide, DMSO).
2.5. Flow cytometry
Flow cytometry studies were performed by adapting the procedure described previously.[28, 36] Briefly, cells (MCF-7, MCF-7 MX100, MCF-7/VP, or MCF-7 TX400; 300k per 35-mm dish) were incubated with desired photosensitizing agents (i.e., free BPD, (16:0)LysoPC-BPD, L-BPD or L-LysoPC-BPD; 0.25 and 1 µM) or known ABC transporter substrates (BODIPY-prazosin, Calcein AM, or Rhodamine 123; Table S1) for 1 hour at 37°C in 5% CO2. Subsequently, cells were washed with cold PBS and then incubated with photosensitizer-free complete medium with or without known ABC transporter inhibitors (Fumitremorgin C or FTC, MK571, or valspodar; Table S1) for 1 hour at 37°C. Trypsinized cells were subsequently washed with cold PBS prior to flow cytometry analyses. Functional ABCG2 expression was confirmed by incubating MCF-7 MX100 cells in 0.25 µM BODIPY-prazosin with or without 10 µM ABCG2 inhibitor FTC. Functional MRP1 expression was determined by incubating MCF-7/VP cells in 3 µg/mL calcein-AM with or without 25 µM MRP1 inhibitor MK571. Functional P-gp was confirmed by incubating MCF-7 TX400 cells in 1 µg/ml Rhodamine 123 with or without 3 µg/mL P-gp inhibitor valspodar (Table S1). Samples were analyzed on a fluorescence-activated cell sorting (FACS) flow cytometer (BD FACScanto II). Calcein (Ex/Em: 494/517 nm) and Rhodamine 123 (Ex/Em: 488/515 nm) fluorescence were detected with a 488-nm laser and an AmCyan filter (525/50 nm). BODIPY-prazosin (Ex/Em: 503/512 nm) was detected using a 488-nm laser and a FITC filter (530/30 nm). BPD (Ex/Em: 435/690 nm) fluorescence was detected with a 405-nm laser and a PerCP-Cy5–5 filter (LP670 nm). At least 50,000 events were collected for all of the flow cytometry studies. The gated single cell populations were analyzed using Flowjo V10.
2.6. Photodynamic therapy (PDT) of MCF-7 parental and sub lines
Cells cultured overnight in 35-mm Petri dishes (300k cells per dish) were incubated with photosensitizing agents (i.e., free BPD or (16:0)LysoPC-BPD; 0.25 µM) for 4 hours. Subsequently, cells were washed three times with PBS and then incubated with photosensitizer-free complete medium for different periods of time (1, 16, or 24 hours) prior to light activation. Photosensitizer uptake in cancer cells was determined using extraction methods as described previously.[26] PDT was performed by exposing the cells to near-infrared light (690-nm, 0–20 J/cm2, 10 W/cm2, bottom illumination; Modulight). Cell viability was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay at 24 hours after PDT following the vendor’s protocol.
3. Results
3.1. BPD is an ABCG2- and P-gp-specific substrate
To determine the effects of ABC transporters on the intracellular retention of BPD photosensitizers, efflux studies were performed on a panel of human breast cancer cell lines overexpressing ABCG2, MRP1, or P-gp. Immunoblotting was used to verify high levels of ABCG2, MRP1, and P-gp in MCF-7 MX100, MCF-7/VP, and MCF-7 TX400 cell lines, respectively, compared to their parental MCF-7 cell line (Figure 1). Cells were incubated with BPD photosensitizers or known substrates of ABC transporters (0.25 µM BODIPY-prazosin for ABCG2; 3 µg/mL Calcein AM for MRP1; 1 µg/mL Rhodamine 123 for P-gp; Table S1) for 1 hour, washed with PBS, then incubated for one hour in photosensitizer-free or substrate-free media with or without known transporter inhibitors (10 µM FTC for ABCG2; 25 µM MK571 for MRP1; 3 µg/mL Valspodar for P-gp; Table S1). The intracellular fluorescence of photosensitizers or substrates were determined by flow cytometry. In ABCG2-overexpressing MCF-7 MX100 cells, the addition of ABCG2 inhibitor FTC increased both the intracellular fluorescence of BPD (Figure 2A) and ABCG2 substrate BODIPY-prazosin (Figure S2A). When MRP1-overexpressing MCF-7/VP cells were incubated in medium containing MRP1 substrate calcein AM with or without MRP1 inhibitor MK571, high levels of MK571-inhibitable calcein AM efflux were observed (Figure S2B). However, when MCF-7/VP cells were incubated with the BPD in the presence or absence of MK571, no change in the intracellular levels of BPD was observed (Figure 2B). The P-gp-overexpressing MCF-TX400 cells demonstrated high levels of valspodar-inhibitable BPD efflux (Figure 2C) and valspodar-inhibitable Rhodamine 123 efflux (Figure S2C). In the MCF-7 parent cell line, FTC-inhibitable BPD efflux, MK571-inhibitable BPD efflux, and valspodar-inhibitable BPD efflux were not detected (Figures 2D–F). These results suggest that BPD is a substrate of ABCG2 and P-gp, but not MRP1.
Figure 1.

Western blot analysis of ABCG2, MRP1, and P-gp expression in MCF-7, MCF-7 MX100, MCF-7/VP, and MCF-7 TX400 cells. β-Actin was used as a loading control. Whole cell extracts (20 µg) were loaded in each lane.
Figure 2.
BPD is transported by ABCG2 and P-gp, but not MRP1. Selected cell lines overexpressing (A) ABCG2 (MCF-7 MX100), (B) MRP1 (MCF-7/VP), or (C) P-gp (MCF-7 TX400), and (D-F) parental MCF-7 cells were incubated with 0.25 µM BPD for 1 hr at 37°C. Subsequently, cells were washed and incubated in BPD-free complete medium with or without the desired inhibitor (10 µM FTC for ABCG2, 25 µM MK571 for MRP1, or 3 µg/ml valspodar for P-gp) for 1 hr at 37°C. Intracellular fluorescence of BPD was determined using a flow cytometer equipped with the appropriate filter. Representative data from at least three independent experiments are shown. NT: not treated, control. (16:0)LysoPC-BPD is not transported by ABCG2 and MRP1, and becomes a weaker substrate of P-gp compared to BPD. Selected cell lines overexpressing (G) ABCG2 (MCF-7 MX100), (H) MRP1 (MCF-7/VP), or (I) P-gp (MCF-7 TX400) were incubated with (16:0)LysoPC-BPD for 1 hr at 37°C. Subsequently, cells were washed and incubated in (16:0)LysoPC-BPD-free complete medium with or without the desired inhibitor (10 µM FTC for ABCG2, 25 µM MK571 for MRP1, or 3 µg/ml valspodar for P-gp) for 1 hr at 37°C. Intracellular fluorescence of BPD was determined using a flow cytometer equipped with appropriate filter. (J-L) The mean intracellular fluorescence signal of BPD and (16:0)LysoPC-BPD with or without ABC transporter inhibitors in MCF-7 MX100, MCF-7/VP and MCF-7 TX400 cell lines. (n=3, *P< 0.05, **P< 0.01, ***P< 0.001, n.s.: nonsignificant, two-tailed t-test). NT: not treated, control.
3.2. Lipidation of BPD affects the transport of BPD by ABCG2 and P-gp
Covalent conjugation or non-covalent association of photosensitizers and phospholipids is an expedient strategy to improve the photoactivity of photosensitizers under biologically relevant conditions and in clinical settings. Next, we investigated whether a more complex lipid-photosensitizer structure would be less effectively transported by ABCG2 and P-gp. The phospholipid-photosensitizer conjugate, (16:0) LysoPC-BPD, was synthesized by an esterification reaction between the carboxyl groups of BPD photosensitizer and the alcohol groups of (16:0)LysoPC phospholipids (Figure S2 and Figure S3). To evaluate the specificity of (16:0)LysoPC-BPD for ABCG2, MRP1 and P-gp, flow cytometry studies were performed on MCF-7 MX100, MCF-7/VP, and MCF-7 TX400 cells, respectively (Figure 2G–L). When MCF-7 MX100 cells were incubated in (16:0)LysoPC-BPD with or without ABCG2 inhibitor FTC, no FTC-inhibitable (16:0)LysoPC-BPD efflux was observed (Figure 2G). Similarly, in MCF-7/VP cells incubated in (16:0)LysoPC-BPD with or without MRP1 inhibitor MK571, no MK571-inhibitable (16:0)LysoPC-BPD efflux was observed (Figure 2H). When MCF-7/VP cells were incubated in (16:0)LysoPC-BPD with or without P-gp inhibitor valspodar, only minimal valspodar-inhibitable (16:0)LysoPC-BPD efflux was seen (Figure 2I). This data indicates that (16:0)LysoPC-BPD is not an ABCG2 or MRP1 substrate, but remains a P-gp substrate.
We then compared the intracellular levels of BPD and (16:0)LysoPC-BPD with or without ABC transporter inhibitors in the selected cell lines (Figure 2J–L). In MCF-7 MX100 cells, the addition of FTC resulted in an approximately 3.6±0.9 fold increase in intracellular fluorescence of BPD, but no change in the intracellular fluorescence of (16:0)LysoPC-BPD was observed in the presence of FTC (Figure 2J). In MCF-7/VP cells, the addition of MK571 did not alter the intracellular fluorescence of BPD or (16:0)LysoPC-BPD (Figure 2K). In MCF-7 TX400 cells, the addition of valspodar resulted in a 3±0.3 fold increase in the intracellular fluorescence of BPD. Interestingly, a significantly less pronounced 1.98±0.5 fold increase in intracellular fluorescence of (16:0)LysoPC-BPD was observed in the presence of valspodar. This observation implies that (16:0)LysoPC-BPD could be a weaker P-gp substrate compared to BPD (Figure 2L). We further confirmed that the escape of (16:0)LysoPC-BPD from P-gp and ABCG2-mediated efflux relies on the successful click chemistry coupling between (16:0)LysoPC and BPD (Figure S4). Using mixtures of unconjugated (16:0)LysoPC and BPD, we showed that BPD remains a substrate of P-gp and ABCG2 (Figure S4).
3.3. Design, preparation, and characterization of L-BPD and L-LysoPC-BPD
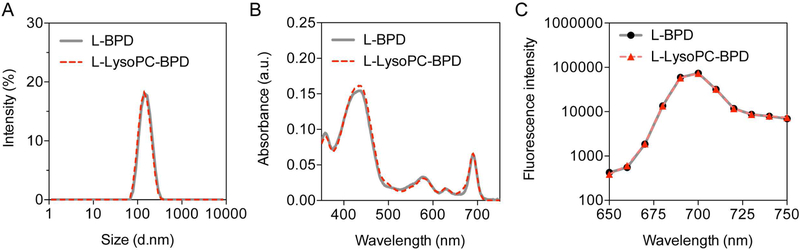
Hydrophobic BPD photosensitizers benefit from nanoliposomal formulation through an improvement in their pharmacokinetic profile and PDT efficacy in clinic. Here, we prepared two nanoliposomal formulations stably entrapping BPD or (16:0)Lyso-BPD. Nanoliposomal BPD (L-BPD) and nanoliposomal (16:0)Lyso-BPD (L-LysoPC-BPD) (Figure 3) were reproducibly synthesized via the freeze-thaw extrusion method as described previously.[37, 38] Both L-BPD and L-LysoPC-BPD were grafted with ~3mol% of PEG and formed in the size range of 140–150 nm with a narrow size distribution (polydispersity index, PdI ≤ 0.1) (Figure 3A). The entrapment efficiency and the loading capacity of BPD or (16:0)Lyso-BPD in the nanoliposomes were determined by UV-visible spectroscopy after complete dissolution of the nanoliposomes in dimethyl sulfoxide (DMSO) (Figure 3B). Conjugation of BPD to (16:0)LysoPC did not alter the Q band (690 nm) or the Soret peak (435 nm) of BPD, as lipidation does not reduce the number of double bonds in the pyrrole rings of BPD (Figure 3B).[39] For L-BPD, BPD molecules were embedded within the liposomal lipid-bilayer via hydrophobic and ionic interactions at an entrapment efficiency of 78.4±0.8%. In the case of L-LysoPC-BPD, BPD was covalently anchored onto (16:0)LysoPC, which serves as a lipid component in the liposome formation with entrapment efficiency of 92.7±1.5%. This corresponded to approximately 267±14 BPD molecules per liposome for L-BPD, and 273±5 (16:0)LysoPC-BPD molecules per liposome for L-LysoPC-BPD. The liposomal formulation facilitated the monomerization of the photosensitizers and maintained the fluorescence emission signal of BPD molecules in physiologically relevant environments (Figure 3C).
Figure 3.
Photophysical characterization of nanoliposomal BPD (L-BPD) and nanoliposomal (16:0)LysoPC-BPD (L-LysoPC-BPD). (A) Nanoliposomes synthesized via bilayer encapsulation BPD or (16:0)LysoPC-BPD resulted in formation of monodispersed nanoliposomes around 150 nm (PdI ≤ 0.1) and 140 nm (PdI < 0.1) respectively (n=3). (B) Representative absorbance spectra of L-BPD and L-LysoPC-BPD in DMSO. Q band of BPD (690 nm, wavelength for light activation) was not altered in either nano-formulation. (C) Fluorescence spectra of L-BPD and L-LysoPC-BPD in phosphate-buffered saline.
3.4. Porphyrin-lipid nanovesicle L-LysoPC-BPD escapes efflux by P-gp and ABCG2
As little data is available on the impact of nanoliposomal formulation on the efflux of photosensitizers, we next compared BPD efflux using L-BPD and L-LysoPC-BPD nanoformulations in the selected cell lines (Figure 4). In MCF-7 MX100 cells overexpressing ABCG2, incubation of FTC increased the intracellular fluorescence of L-BPD by 5.3±1.3 fold (Figure 4A); however, no significant change in the intracellular fluorescence of L-LysoPC-BPD was observed in the presence of FTC (Figure 4B, C). In MCF-7/VP cells overexpressing MRP1, the addition of MK571 did not alter the intracellular fluorescence of L-BPD (Figure 4D) or L-LysoPC-BPD (Figure 4E, F). In MCF-7 TX400 cells overexpressing P-gp, the addition of valspodar led to a 3.0±1.3-fold increase in intracellular fluorescence of L-BPD (Figure 4G). On the other hand, only a modest 1.6±0.1-fold increase in the intracellular fluorescence of L-LysoPC-BPD was observed in the presence of valspodar (Figure 4H, I). We observed that although L-LysoPC-BPD evades efflux by ABCG2, it only partially evades P-gp.
Figure 4.
Transport of nanoliposomal BPD (L-BPD) and nanoliposomal (16:0)LysoPC-BPD (L-LysoPC-BPD) by breast cancer cell lines overexpressing (A-C) ABCG2 (MCF-7 MX100), (D-F) MRP1 (MCF-7/VP), or (G-I) P-gp (MCF-7 TX400). Cells were incubated with 1 µM L-BPD or L-LysoPC-BPD for 1 hr at 37°C. Subsequently, cells were washed and incubated in BPD-free complete medium with or without the desired inhibitor (10 µM FTC for ABCG2, 25 µM MK571 for MRP1, or 3 µg/ml valspodar for P-gp) for 1 hr at 37°C. Intracellular fluorescence of BPD or (16:0)LysoPC-BPD were determined using a flow cytometer equipped with the appropriate filter. (A, B, D, E, G, H) Representative data are shown. The mean intracellular fluorescence signal of BPD and (16:0)LysoPC-BPD with or without ABC transporter inhibitors in (C) MCF-7 MX100, (F) MCF-7/VP, and (I) MCF-7 TX400 cell lines. (n=3, *P< 0.05, **P< 0.01, ***P< 0.001, n.s.: nonsignificant, two-tailed t-test). NT: not treated, control. (n=3, *P< 0.05, **P< 0.01, ***P< 0.001, n.s.: nonsignificant, two-tailed t-test).
3.5. L-LysoPC-BPD overcomes ABCG2 and P-gp-mediated PDT resistance
The flow cytometry studies above revealed that BPD is a substrate for P-gp and ABCG2, whereas (16:0)LysoPC-BPD is no longer a substrate for ABCG2 and becomes a weaker substrate for P-gp. We subsequently quantified the longitudinal accumulation of BPD and (16:0)LysoPC-BPD photosensitizers in MCF-7 MX100 cells expressing ABCG2 and MCF-7 TX400 cells expressing P-gp to study ABCG2-mediated and P-gp-mediated PDT resistance. In MCF-7 MX100 cells treated with BPD, we observed a dramatic 97% decrease in intracellular BPD concentration from 57.9±6.8 fmoles of BPD per mg of protein (fmole/mg) at 1 hour post-incubation, to 3.6±0.6 and 1.6±0.6 fmole/mg at 16 and 24 hour post-incubation, respectively (Figure 5A). In contrast, the intracellular level of (16:0)LysoPC-BPD was maintained at 13.4±6.8 fmole/mg for up to 24 hours in MCF-7 MX100 cells (Figure 5A). Similarly, up to 98% reduction of intracellular BPD concentration to 2±0.9 fmole/mg was seen in MCF-7 TX400 cells at 24 hours (Figure 5B). The intracellular (16:0)LysoPC-BPD level was maintained for up to 16 hours at 10.3±1 fmole/mg for up to 16 hours in MCF-7 TX400 cells (Figure 5B). In both MCF-7 MX100 and MCF-7 TX400 cell lines, the intracellular levels of (16:0)LysoPC-BPD were significantly higher than that of BPD at 16 and 24 hour post-incubation, respectively (Figure 5A, B).
Figure 5.
Photosensitizer efflux and resistance to photodynamic therapy were mitigated when using (16:0)LysoPC-BPD and its nanoliposomal formulation (L-LysoPC-BPD). Selected cell lines overexpressing (A) ABCG2 (MCF-7 MX100), or (B) P-gp (MCF-7 TX400) were incubated with 1 µM BPD or (16:0)LysoPC-BPD for 4 hr at 37°C. Subsequently, cells were washed and incubated in fresh medium to allow for photosensitizer efflux for 1, 16, or 24 hrs at 37°C. Intracellular BPD concentration was determined using extraction methods with appropriate standard curves. Cytotoxicity assays were performed as described in the Materials and Methods section with BPD, L-BPD, (16:0)LysoPC-BPD and L-LysoPC-BPD on (C) MCF-7 MX100 cells or (D) MCF-7 TX400 cells. (n=3, *P< 0.05, **P< 0.01, *** P< 0.001, n.s.: nonsignificant, two-tailed t-test).
To determine if the improved retention of (16:0)LysoPC-BPD in cancer cells would result in an enhanced PDT outcome, we performed cytotoxicity assays on MCF-7 MX100 and MCF-7 TX400 treated with (16:0)LysoPC-BPD, free BPD, or their liposomal formulations at 24 hours after light irradiation (Figure 5C, D). Both MCF-7 MX100 and MCF-7 TX400 cell lines showed resistance to PDT using BPD and L-BPD with only less than 20% killing at light fluences of 5, 10 and 20 J/cm2. Resistance to PDT was abrogated when using (16:0)LysoPC-BPD or L-LysoPC-BPD at the same incubation conditions. In MCF-7 MX100 and MCF-7 TX400 cell lines, a light-dose dependent reduction of cell viability by approximately 40%, 50% and 70% was observed at 5, 10 and 20 J/cm2, respectively. The half maximal inhibitory concentration (IC50) values for MCF-7 MX100 and MCF-7 TX400 cells were determined to be similar at ~2.5 µM ✕ J/cm2 for (16:0)LysoPC-BPD and ~ 5 µM ✕ J/cm2 for L-LysoPC-BPD. The higher IC50 value for L-LysoPC-BPD in comparison to the IC50 value for (16:0)LysoPC-BPD is presumably due to the stealth properties of PEG (~3 mol%) grafted on L-LysoPC-BPD that limits photosensitizer uptake.[40] We did not determine the IC50 of BPD and L-BPD in MCF-7 MX100 and MCF-7 TX400 cells, due to their strong resistance to PDT and minimal cell killing.
4. Discussion
Benzoporphyrin derivative (BPD) liposome injection (Visudyne®) has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with wet (neovascular/exudative) age-related macular degeneration,[41] and it is currently being tested in a Phase II clinical trial for photodynamic therapy (PDT) of metastatic breast cancer for which curative treatments are unavailable (). Data from The Cancer Genome Atlas (TCGA) database indicate that multiple ABC transporters (i.e., ABCG2 and P-gp) are co-expressed in breast tumors and several other cancer types.[6] It has been shown that there is a positive correlation with the number of co-expressed ABC transporters and reduced relapse-free survival time in cancer patients.[8] Unfortunately, the lack of clinical success with small-molecule ABC transporter inhibitors over the past 40 years has led to a serious setback for the field.[6] This is a testimony to the desperate need for a conceptual shift to a new strategy that has the potential to overcome multidrug resistance in cancer cells. This study demonstrates that phospholipid-conjugated photosensitizing drugs can escape efflux by multiple ABC transporters and might be a way to circumvent PDT resistance.
We first examined the effect of ABCG2, P-gp, or MRP1 expression on the efflux of BPD photosensitizer using a panel of well-characterized breast cancer cell lines. The selective ABCG2 inhibitor (FTC) and P-gp inhibitor (Valspodar) increased the intracellular fluorescence of BPD in cancer cells overexpressing ABCG2 and P-gp, respectively. On the other hand, in the presence of an MRP1 inhibitor (MK571), cells expressing high levels of MRP1 did not show increased intracellular fluorescence of BPD as measured by flow cytometry. While our observation that BPD is an ABCG2 substrate agrees with previous findings,[34, 35, 38] this study provides new knowledge that BPD is indeed a P-gp substrate, but not an MRP1 substrate.
Visudyne® is a lyophilized mixture composed of BPD, unsaturated egg phosphatidylglycerol, and dimyristoyl phosphatidyl choline, which needs to be reconstituted with aqueous buffer to form polydispersed liposomal vesicles of BPD (200 nm – 1 µm in diameter) prior to administration.[42] Based on this information, we compared the effect of ABCG2, P-gp, or MRP1 expression on the efflux of ‘phospholipid-mixed’ BPD and our ‘phospholipid-conjugated’ BPD. We show that simply mixing phospholipids and BPD does not mitigate the efflux of BPD by ABCG2 and P-gp in breast cancer cells. In contrast, covalent conjugation of phospholipid was necessary to evade the ABCG2- and P-gp-mediated transport of the phospholipid-BPD conjugates, verifying our hypothesis that photosensitizers with a more complex biomolecular structure will be effluxed in a less effective manner by a variety of ABC transporters. Our findings are in agreement with the study by Liu et al. showing that attachment of galactose to HPPH also reduced the HPPH efflux by ~50% in the RIF-1 murine radiation-induced fibrosarcoma cell line.[35] Discovery and development of bioconjugation-based photosensitizers that are poor substrates for ABC efflux pumps remains an understudied area that should be further explored.
Monodispersed nanoscopic liposomes (i.e., 100–200 nm, polydispersity less than 0.1) have proven to be valuable and flexible photosensitizer delivery vehicles that allow light-triggered release of enclosed materials that home in or accumulate at desired sites preferentially to reduce the side effects of the treatment.[27, 43] Nanoliposomal ‘unconjugated, free-form’ porphyrins and phthalocyanines to tumors was first demonstrated by Jori et al. in the 80–90’s.[44–46] Pioneered by Zheng, Lovell and colleagues, porphysomes are nanoliposomes containing ‘lipid-anchored’ porphyrins can be light activated for in vivo photothermal or photodynamic tumor ablation.[47–49] Recognizing these advances, we develop monodispersed nanoliposomes (PdI ≤ 0.1) containing ‘unconjugated, free-form’ BPD, or ‘lipid-anchored’ BPD and compare their transport by ABCG2 and P-gp in cancer cells. Nanoliposomes containing ‘lipid-anchored’ BPD (i.e., porphysomes) evaded photosensitizer efflux by ABCG2 and P-gp in breast cancer cells, and maintained BPD concentration in cells up to at least 24 hours post-incubation for effective photodynamic therapy outcomes. On the other hand, ‘unconjugated, free-form’ BPD in nanoliposomes remained a substrate of ABCG2 and P-gp. Similarly, up to 98% of the intracellular BPD was removed from ABCG2- and P-gp-overexpressing cells at 24 hour postincubation, resulting in pronounced resistance to PDT.
In addition to PDT of cancer cells, the fluorescence signal generated from the relaxation of excited-state photosensitizers can be used for fluorescence imaging and image-guided resection of disseminated tumors.[26, 50] Our data indicates that, in tumors expressing ABCG2 and P-gp, intracellular photosensitizers levels could be maintained when using ‘lipid-anchored’ BPD and its liposomal formation. Thus, fluorescence imaging of tumors with lipid-anchored’ BPD and porphysomes could potentially be more effective for tumors which express ABCG2 and P-gp, compared to using free BPD and its liposomal formation.
In conclusion, we show that BPD photosensitizers are readily transported by P-gp and ABCG2, but not MRP1. ABCG2 and P-gp protect cancer cells from PDT with BPD. Lipidation camouflages BPD to mitigate P-gp and ABCG2-mediated efflux and improves intracellular BPD retention for enhanced PDT efficiency. Porphyrin-lipid nanovesicles also overcome ABC transporter-mediated resistance to PDT in cancer cells. Further studies to confirm whether or not these formulations are indeed poor substrates for efflux pumps and whether they could improve tumor photosensitivity and imaging in vivo are warranted.
Supplementary Material
Highlights.
Benzoporphyrin derivative is a substrate for both P-gp and ABCG2, but not MRP1.
Lipidation mitigates benzoporphyrin derivative efflux by P-gp and ABCG2.
Porphyrin-lipid nanovesicles bypass the P-gp and ABCG2 pumps implicated in the development of resistance to PDT.
Acknowledgements and Funding:
This work was supported by the National Institutes of Health [grant number R00 CA194269] and the UMD start-up fund (to H-C.H.). R.W.R., S.V.A., and M.M.G. acknowledge support from the National Institutes of Health (NIH) Intramural Research Program, National Cancer Institute, Center for Cancer Research. The fluorescence-activated cell sorting (FACS) flow cytometry study was conducted with support from the BioWorkshop hosted by the Fischell Department of Bioengineering at the University of Maryland, College Park.
Abbreviations:
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- (16:0)LysoPC
1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine
- HPPH
2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DMAP
4-(dimethylamino) pyridine
- ABC
ATP-binding cassette
- BPD
Benzoporphyrin derivative
- BSA
Bovine serum albumin
- BCRP; encoded by ABCG2
Breast cancer resistance protein
- DMSO
Dimethyl sulfoxide
- DOTAP
Dioleoyltrimethylammoniumpropane
- DPPC
Dipalmitoylphosphatidylcholine
- DSPE-PEG
Distearoylphosphatidylethanolamine-methoxy polyethylene glycol
- EMEM
Eagle’s minimum essential medium
- FACS
Fluorescence-activated cell sorting
- FBS
Fetal bovine serum
- FTC
Fumitremorgin C
- IC50
Half maximal inhibitory concentration
- MRP1
Multidrug resistance-associated protein 1
- DIPEA
N,N-diisopropylethylamine
- L-LysoPC-BPD
Nanoliposomal (16:0)Lyso-BPD
- L-BPD
Nanoliposomal BPD
- NIH
National Institutes of Health
- NIR
Near-infrared
- P-gp
P-glycoprotein
- PBS
Phosphate-buffered saline
- PDT
Photodynamic therapy
- PdI
Polydispersity index
- PVDF
Polyvinylidene difluoride
- PpIX
Protoporphyrin IX
- ROS
Reactive oxygen species
- TKI
Tyrosine kinase inhibitor
- FDA
U.S. Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- [1].Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: role of ATP-dependent transporters, Nat Rev Cancer, 2 (2002) 48–58. [DOI] [PubMed] [Google Scholar]
- [2].Schinkel AH, Jonker JW, Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview, Advanced drug delivery reviews, 55 (2003) 3–29. [DOI] [PubMed] [Google Scholar]
- [3].Gottesman MM, Lavi O, Hall MD, Gillet JP, Toward a Better Understanding of the Complexity of Cancer Drug Resistance, Annual review of pharmacology and toxicology, 56 (2016) 85–102. [DOI] [PubMed] [Google Scholar]
- [4].Tamaki A, Ierano C, Szakacs G, Robey RW, Bates SE, The controversial role of ABC transporters in clinical oncology, Essays in biochemistry, 50 (2011) 209–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sharom FJ, ABC multidrug transporters: structure, function and role in chemoresistance, Pharmacogenomics, 9 (2008) 105–127. [DOI] [PubMed] [Google Scholar]
- [6].Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM, Revisiting the role of ABC transporters in multidrug-resistant cancer, Nat Rev Cancer, 18 (2018) 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choi YH, Yu AM, ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development, Current pharmaceutical design, 20 (2014) 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bartholomae S, Gruhn B, Debatin KM, Zimmermann M, Creutzig U, Reinhardt D, Steinbach D, Coexpression of Multiple ABC-Transporters is Strongly Associated with Treatment Response in Childhood Acute Myeloid Leukemia, Pediatric blood & cancer, 63 (2016) 242–247. [DOI] [PubMed] [Google Scholar]
- [9].Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q, Photodynamic Therapy, JNCI: Journal of the National Cancer Institute, 90 (1998) 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dolmans DE, Fukumura D, Jain RK, Photodynamic therapy for cancer, Nat Rev Cancer, 3 (2003) 380–387. [DOI] [PubMed] [Google Scholar]
- [11].Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T, Imaging and photodynamic therapy: mechanisms, monitoring, and optimization, Chem Rev, 110 (2010) 2795–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Banerjee SM, MacRobert AJ, Mosse CA, Periera B, Bown SG, Keshtgar MRS, Photodynamic therapy: Inception to application in breast cancer, The Breast, 31 (2017) 105–113. [DOI] [PubMed] [Google Scholar]
- [13].Yano T, Hatogai K, Morimoto H, Yoda Y, Kaneko K, Photodynamic therapy for esophageal cancer, Ann Transl Med, 2 (2014) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Simone CB 2nd, Friedberg JS, Glatstein E, Stevenson JP, Sterman DH, Hahn SM, Cengel KA, Photodynamic therapy for the treatment of non-small cell lung cancer, Journal of thoracic disease, 4 (2012) 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Matei C, Tampa M, Poteca T, Panea-Paunica G, Georgescu SR, Ion RM, Popescu SM, Giurcaneanu C, Photodynamic therapy in the treatment of basal cell carcinoma, Journal of medicine and life, 6 (2013) 50–54. [PMC free article] [PubMed] [Google Scholar]
- [16].Oinuma T, Nakamura T, Nishiwaki Y, Report on the National Survey of Photodynamic Therapy (PDT) for Gastric Cancer in Japan (a secondary publication), Laser therapy, 25 (2016) 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang W, Zhang A, Sun W, Yue Y, Li H, Efficacy and safety of photodynamic therapy for cervical intraepithelial neoplasia and human papilloma virus infection: A systematic review and meta-analysis of randomized clinical trials, Medicine, 97 (2018) e10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Betrouni N, Boukris S, Benzaghou F, Vascular targeted photodynamic therapy with TOOKAD(R) Soluble (WST11) in localized prostate cancer: efficiency of automatic pre-treatment planning, Lasers Med Sci, 32 (2017) 1301–1307. [DOI] [PubMed] [Google Scholar]
- [19].Biel MA, Photodynamic therapy of head and neck cancers, Methods in molecular biology (Clifton, N.J.), 635 (2010) 281–293. [DOI] [PubMed] [Google Scholar]
- [20].Akimoto J, Photodynamic Therapy for Malignant Brain Tumors, Neurol Med Chir (Tokyo), 56 (2016) 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AWR, Photodynamic therapy for cancer of the pancreas, Gut, 50 (2002) 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, Kent E, Bown SG, Hasan T, Pogue BW, Pereira SP, Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer, Br J Cancer, 110 (2014) 1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tatter SB, Shaw EG, Rosenblum ML, Karvelis KC, Kleinberg L, Weingart J, Olson JJ, Crocker IR, Brem S, Pearlman JL, Fisher JD, Carson K, Grossman SA, An inflatable balloon catheter and liquid 125I radiation source (GliaSite Radiation Therapy System) for treatment of recurrent malignant glioma: multicenter safety and feasibility trial, Journal of neurosurgery, 99 (2003) 297–303. [DOI] [PubMed] [Google Scholar]
- [24].Friedberg JS, Radical pleurectomy and photodynamic therapy for malignant pleural mesothelioma, Annals of Cardiothoracic Surgery, 1 (2012) 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Simone CB, Cengel KA, Photodynamic Therapy for Lung Cancer and Malignant Pleural Mesothelioma, Seminars in oncology, 41 (2014) 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang HC, Pigula M, Fang Y, Hasan T, Immobilization of Photo-Immunoconjugates on Nanoparticles Leads to Enhanced Light-Activated Biological Effects, Small, (2018) e1800236. [DOI] [PMC free article] [PubMed]
- [27].H. H.C., H. T., The “Nano” World in Photodynamic Therapy Austin J Nanomed Nanotechnol, 2 (2014) 1020. [PMC free article] [PubMed] [Google Scholar]
- [28].Robey RW, Steadman K, Polgar O, Bates SE, ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy, Cancer biology & therapy, 4 (2005) 187–194. [PubMed] [Google Scholar]
- [29].Juliano RL, Ling V, A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants, Biochim Biophys Acta, 455 (1976) 152–162. [DOI] [PubMed] [Google Scholar]
- [30].Gros P, Croop J, Roninson I, Varshavsky A, Housman DE, Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells, Proceedings of the National Academy of Sciences of the United States of America, 83 (1986) 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Roninson IB, Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells, Cell, 47 (1986) 381–389. [DOI] [PubMed] [Google Scholar]
- [32].Ishikawa T, Kajimoto Y, Inoue Y, Ikegami Y, Kuroiwa T, Critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor, Advances in cancer research, 125 (2015) 197–216. [DOI] [PubMed] [Google Scholar]
- [33].Sun W, Kajimoto Y, Inoue H, Miyatake S, Ishikawa T, Kuroiwa T, Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells, Photodiagnosis Photodyn Ther, 10 (2013) 42–50. [DOI] [PubMed] [Google Scholar]
- [34].Gallagher-Colombo SM, Miller J, Cengel KA, Putt ME, Vinogradov SA, Busch TM, Erlotinib Pretreatment Improves Photodynamic Therapy of Non-Small Cell Lung Carcinoma Xenografts via Multiple Mechanisms, Cancer Res, 75 (2015) 3118–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, Pandey RA, Oseroff AR, The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2, Clin Cancer Res, 13 (2007) 2463–2470. [DOI] [PubMed] [Google Scholar]
- [36].Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE, Pheophorbide a is a specific probe for ABCG2 function and inhibition, Cancer Res, 64 (2004) 1242–1246. [DOI] [PubMed] [Google Scholar]
- [37].Huang HC, Rizvi I, Liu J, Anbil S, Kalra A, Lee H, Baglo Y, Paz N, Hayden D, Pereira S, Pogue BW, Fitzgerald J, Hasan T, Photodynamic Priming Mitigates Chemotherapeutic Selection Pressures and Improves Drug Delivery, Cancer Res, 78 (2018) 558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huang HC, Mallidi S, Liu J, Chiang CT, Mai Z, Goldschmidt R, Ebrahim-Zadeh N, Rizvi I, Hasan T, Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer, Cancer Res, 76 (2016) 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Abrahamse H, Hamblin MR, New photosensitizers for photodynamic therapy, The Biochemical journal, 473 (2016) 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Immordino ML, Dosio F, Cattel L, Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential, International journal of nanomedicine, 1 (2006) 297–315. [PMC free article] [PubMed] [Google Scholar]
- [41].Guidelines for using verteporfin (Visudyne) in photodynamic therapy for choroidal neovascularization due to age-related macular degeneration and other causes: update, Retina (Philadelphia, Pa.), 25 (2005) 119–134. [DOI] [PubMed] [Google Scholar]
- [42].Obaid G, Jin W, Bano S, Kessel D, Hasan T, Nanolipid Formulations of Benzoporphyrin Derivative: Exploring the Dependence of Nanoconstruct Photophysics and Photochemistry on Their Therapeutic Index in Ovarian Cancer Cells, Photochem Photobiol, (2018). [DOI] [PMC free article] [PubMed]
- [43].Spring BQ, Bryan Sears R, Zheng LZ, Mai Z, Watanabe R, Sherwood ME, Schoenfeld DA, Pogue BW, Pereira SP, Villa E, Hasan T, A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways, Nature nanotechnology, 11 (2016) 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reddi E, Zhou C, Biolo R, Menegaldo E, Jori G, Liposome- or LDL-administered Zn (II)-phthalocyanine as a photodynamic agent for tumours. I. Pharmacokinetic properties and phototherapeutic efficiency, British journal of cancer, 61 (1990) 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ricchelli F, Biolo R, Reddi E, Tognon G, Jori G, Liposomes As Carriers Of Hydrophobic Photosensitizers In Vivo: Increased Selectivity Of Tumor Targeting, Proc. of SPIE, 0847 (1987). [Google Scholar]
- [46].Jori G, Tomio L, Reddi E, Rossi E, Corti L, Zorat PL, Calzavara F, Preferential delivery of liposome-incorporated porphyrins to neoplastic cells in tumour-bearing rats, British journal of cancer, 48 (1983) 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WC, Cao W, Wang LV, Zheng G, Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents, Nature materials, 10 (2011) 324–332. [DOI] [PubMed] [Google Scholar]
- [48].Miranda D, Lovell JF, Mechanisms of light induced liposome permeabilization, Bioengineering & Translational Medicine, 1 (2016) 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shao S, Geng J, Ah Yi H, Gogia S, Neelamegham S, Jacobs A, Lovell JF, Functionalization of cobalt porphyrin-phospholipid bilayers with his-tagged ligands and antigens, Nature chemistry, 7 (2015) 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stummer W, Suero Molina E, Fluorescence Imaging/Agents in Tumor Resection, Neurosurgery clinics of North America, 28 (2017) 569–583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.