Sea urchin embryos are excellent for in vivo functional studies because of their transparency and tractability in manipulation. They are also favorites for pharmacological approaches since they develop in an aquatic environment and addition of test substances is straightforward. Precise interpretations from such experiments though are often not feasible because of potential pleiotropic effects of the reagents, and the uncertainty of which cells are impacted by the perturbant.
The Wnt and Delta/Notch pathways are intensively studied due to their influence and prevalence in animal development and homeostasis [1–4]. These pathways are particularly well studied in sea urchins. In the canonical Wnt pathway, the Wnt ligand binds to its Frizzled receptors as well as its LRP5/6 co-receptors to stabilize the β-catenin protein and induce its nuclear translocation to regulate the transcription of target genes. The Wnt pathway in the sea urchin is involved in the specification of the endomesoderm [5], the posterior ectoderm [6] and the neuroectoderm [7]. In contrast, the Delta/Notch pathway is required for mesoderm fate, in part by transcription of the transcription factor FoxY to specify the future coelomic pouch cell progenitors [8]. The Delta ligand is enriched in the sea urchin micromeres and activates the Notch receptor in adjacent cells. This interaction induces proteolytic cleavage of the cytoplasmic domain of Notch, which then translocates into the nucleus to regulate transcription [9].
Here we use single cell mRNA sequencing (scRNA-seq) for the first time as a metric of cell types in the sea urchin embryo and to determine the selectivity of two commonly used inhibitors, one each for the Wnt and the Delta/Notch pathways, on these nascent cell types. Wnt signaling was inhibited using the small, cell permeable molecule c59. C59 inhibits Wnt signaling in vivo by inhibiting Porcupine (PORCN), and thereby blocking progression of Wnt in the secretory pathway [10]. DAPT (N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) is a γ-secretase inhibitor and blocks cleavage of the Notch intracellular domain following interaction with Delta [11].
Cells for scRNA-seq were collected from Strongylocentrotus purpuratus embryos at early and late gastrula stages and processed via DropSeq [12]. On a microfluidic chip, a single cell suspension from dissociated embryos, barcoded hydrogel beads, and oil meet to form individual droplets containing one cell and one barcoded bead. Beads are coated with primers that contain a barcode unique to each bead, to mark individual cells, and each primer also has a unique molecular identifier (UMI) to mark individual transcripts for quantitation purposes. When a cell is lysed within the droplet, polyadenylated mRNAs are captured and barcoded by the primers. A total of 15,798 single cells were profiled from eight samples on the 10× Chromium Controller. Sequencing of these mRNAs provided 67.2K to 142.2K reads per cell and on average 80% of the transcripts mapped confidently to the S. purpuratus genome. The scRNA-seq datasets from control and drug treated embryos were aligned by canonical correlation analysis (CCA) using the R package, Seurat. CCA analysis links individual cells across datasets based on similar gene expression profiles. Visualizing the scRNA-seq data by T-Distributed stochastic neighbor embedding (t-SNE) plots, we identified various cell types by distinct mRNA profiling in gastrulae, a period of diversification of cell fates. In the t-SNE plot, the distances between clusters correlate with the differences in the mRNA profiles. Both conserved and differentially expressed markers were extracted for each cluster across a control-drug treatment pair. Quality control for the scRNA-seq datasets included setting lower and upper thresholds for gene number per cell. A lower threshold of 400 genes was used to omit empty droplets, and an upper threshold of 2500 genes was set to exclude doublets, cases where two cells were encapsulated in one droplet, from further analysis.
Several predictions were borne out by this analysis. First, we identified 11 distinct cell types based on mRNA profiling. This number is compatible with results from gene regulatory analysis (GRN) and in situ hybridizations (echinobase.org) but uses a distinct metric for identification. In addition to morphology, phenotype, and functionality, we anticipate RNA-profiling to enhance the definition of cell-types. Second, the cell lineages affected by Wnt and Delta/Notch inhibition were distinct from each other. For example, several marker transcripts expressed in the future pigment cell lineage (cluster 5) were specifically downregulated selectively after DAPT treatment ([13]), concurrently we observed an increase in proportion of oral NSM cells (cluster 9). We speculate that the mRNA profiles for the pigment cell lineage have shifted to more align with the lineage of oral NSM cells. Each of these cell types comes from a recent common lineage (Veg2 tier of cells) and suggests plasticity in this lineage under control of the Delta/Notch pathway. In contrast, Foxq2, among other transcripts, was upregulated in many of the cell types after C59 treatment, consistent with prior work indicating its repression by Wnt signaling ([14] [15]). In clusters where a change in Foxq2 mRNA level was not detected, a vast majority of the cells in the cluster do not express Foxq2. Finally, cluster analysis showed nearly complete separation of pigment cells in DAPT versus vehicle control (cluster 5), and skeletogenic cells in Wnt inhibitor versus vehicle control (cluster 8). These data support the specificity and distinct effects of the Wnt and Delta/Notch pathways in differential cell lineages and illuminate how these conserved pathways selectively regulate cell fates at a single cell level.
Overall, we conclude that single cell RNA-seq analysis in this embryo is revealing of the cell types present during development, of the changes in the gene regulatory network resulting from inhibition of various signaling pathways, and of the selectivity of these pathways in influencing developmental trajectories. Sea urchin embryos appear well suited to this technology.
Supplementary Material
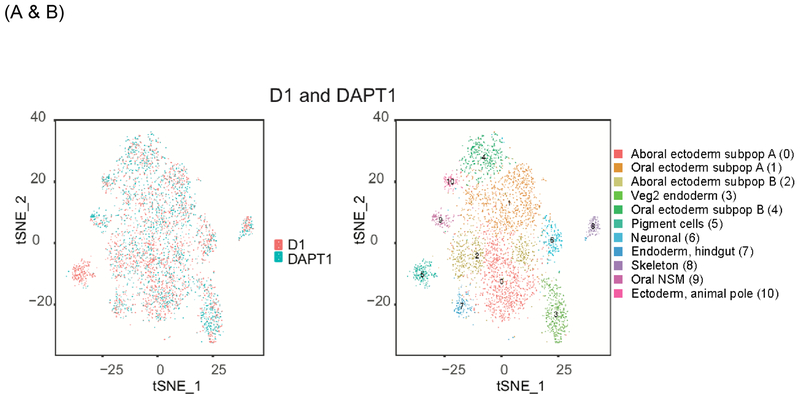
Figure1:
Single cell analysis of late gastrula treated with DMSO only (D1) or with the Delta/Notch inhibitor (DAPT1). Results are presented as t-SNE plots, in which each dot represents a transcriptome from a single cell. (A) Comparison of the transcriptomes obtained between the control (red) and the DAPT treated embryos (blue). (B) Representation of the total number of clusters obtained for D1 (DMSO control) and DAPT1 grouped together. Note the distinct change in composition of cells in cluster 5 (blue) following DAPT treatment.
Acknowledgements
The authors declare no conflicts of interest in this work, and are grateful for support from the National Institutions of Health 9RO1GM125071, R01 AG050582, P20 GM109035, P20 GM119943
References
- 1.Duchartre Y, Kim YM, and Kahn M, The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol, 2016. 99: p. 141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nusse R and Clevers H, Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell, 2017. 169(6): p. 985–999. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Engler A, and Taylor V, Notch: an interactive player in neurogenesis and disease. Cell Tissue Res, 2018. 371(1): p. 73–89. [DOI] [PubMed] [Google Scholar]
- 4.Li L, et al. , Notch signaling pathway networks in cancer metastasis: a new target for cancer therapy. Med Oncol, 2017. 34(10): p. 180. [DOI] [PubMed] [Google Scholar]
- 5.Croce J, et al. , Wnt6 activates endoderm in the sea urchin gene regulatory network. Development, 2011. 138(15): p. 3297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntyre DC, et al. , Short-range Wnt5 signaling initiates specification of sea urchin posterior ectoderm. Development, 2013. 140(24): p. 4881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Range RC, Angerer RC, and Angerer LM, Integration of canonical and noncanonical Wnt signaling pathways patterns the neuroectoderm along the anterior-posterior axis of sea urchin embryos. PLoS Biol, 2013. 11(1): p. e1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Materna SC, Swartz SZ, and Smith J, Notch and Nodal control forkhead factor expression in the specification of multipotent progenitors in sea urchin. Development, 2013. 140(8): p. 1796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopan R and Ilagan MX, The canonical Notch signaling pathway: unfolding the activation mechanism. Cell, 2009. 137(2): p. 216–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proffitt KD, et al. , Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res, 2013. 73(2): p. 502–7. [DOI] [PubMed] [Google Scholar]
- 11.Geling A, et al. , A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep, 2002. 3(7): p. 688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macosko EZ, et al. , Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell, 2015. 161(5): p. 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohguro Y, Takata H, and Kominami T, Involvement of Delta and Nodal signals in the specification process of five types of secondary mesenchyme cells in embryo of the sea urchin, Hemicentrotus pulcherrimus. Dev Growth Differ, 2011. 53(1): p. 110–23. [DOI] [PubMed] [Google Scholar]
- 14.Yaguchi S, et al. , A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev Cell, 2008. 14(1): p. 97–107. [DOI] [PubMed] [Google Scholar]
- 15.Cui M, et al. , Specific functions of the Wnt signaling system in gene regulatory networks throughout the early sea urchin embryo. Proc Natl Acad Sci U S A, 2014. 111(47): p. E5029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Materna SC and Davidson EH, A comprehensive analysis of Delta signaling in pre-gastrular sea urchin embryos. Dev Biol, 2012. 364(1): p. 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClay DR, Methods for embryo dissociation and analysis of cell adhesion. Methods Cell Biol, 2004. 74: p. 311–29. [DOI] [PubMed] [Google Scholar]
- 18.Butler A, et al. , Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol, 2018. 36(5): p. 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.