Abstract
Concurrent mild traumatic brain injury (mTBI) and posttraumatic stress disorder (PTSD) are common in U.S. military service members and veterans. Tau and amyloid-beta-42 (Aβ42) are proteins that have been linked to cognitive impairment, neurological hallmarks of Alzheimer’s disease, and may also relate to recovery from mTBI. However, the role of these proteins in the maintenance or resolution of chronic symptoms has not yet been determined. Participants in the current study were 102 service members and veterans who had sustained an mTBI (n = 84) or injured controls (IC) without TBI (n = 18). They were categorized into three groups based on the presence or absence of mTBI and PTSD: IC/PTSD-Absent (n = 18), mTBI/PTSD-Absent (n = 63), and mTBI/PTSD-Present (n = 21). Concentrations of tau and Aβ42 in peripheral blood plasma were measured using Simoa™, an ultrasensitive technology, and compared across groups. Tau concentrations were highest in the mTBI/PTSD-Present group, F(2, 99) = 4.33, p = .016, compared to the other two groups. Linear multiple regression was conducted to determine the independent effects of PTSD and mTBI on tau concentrations, controlling for gender and sleep medication. PTSD was a significant and independent predictor of tau concentrations, β = .25, p = .009, ηp2 = .26. Aβ42 concentrations did not differ between the groups. The results indicated that PTSD was associated with an elevation of tau in peripheral blood and suggest that there may be increased biological effects of PTSD in this young cohort of service members and veterans following mTBI.
Since 2001, military operations in the Middle East have resulted in high rates of mild traumatic brain injury (mTBI) in U.S. service members and veterans (Defense and Veterans Brain Injury Center, 2017). Following an mTBI, most individuals are expected to recover within the first few weeks or months. However, a small percentage of individuals report symptoms many months or years postinjury; these are often the result of a variety of non–brain injury–related factors and comorbidities that are commonly found pre and postinjury (Iverson, Silverberg, Lange, & Zasler, 2013; McCrae, 2008). For military personnel, one of the most common and potentially debilitating comorbidities that occurs with mTBI is posttraumatic stress disorder (PTSD; Gates et al., 2012), which has been shown to increase the risk of neurocognitive and neurobehavioral deficits (Stricker et al., 2017), suicidality (Lemaire & Graham, 2011), and neuronal inflammation (Devoto et al., 2017). When PTSD occurs concurrently with mTBI, there may be additional psychological and/or neurobiological complications that are not attributable to brain insult alone. As such, further investigation of potential biomarkers that elucidate these associations is needed to improve care for military personnel with comorbid mTBI and PTSD.
Mild traumatic brain injuries have been linked to acute changes in proteomic biomarkers, including increases in tau, amyloid-beta (Aβ) 40, and Aβ42 (Gill et al., 2018; Washington, Villapol, & Burns, 2016). Tau is a microtubule-associated protein that can become hyperphosphorylated, disrupting microtubule organization and creating neurofibrillary tangles (NFTs). Elevated tau concentrations have been reported in both military personnel following blunt force trauma and blast exposures (Gill et al., 2018; Olivera et al., 2015), and in athletes following concussive and subconcussive impacts (Gill, Merchant-Borna, Jeromin, Livingston, & Bazarian, 2017). Higher tau concentrations in peripheral blood has also been correlated with prolonged return to play in athletes (Gill et al., 2017). The pathology of Aβ following TBI has been less consistently studied in the literature.
Amyloid-beta is a product of amyloid precursor proteins, which are known to have both neurotoxic and neuroprotective effects. Accumulation and aggregation of both tau NFTs and Aβ plaques are hallmarks of chronic traumatic encephalopathy (CTE), Alzheimer’s disease (AD), and related dementias. Sustaining a TBI, including mTBI, has been implicated as a risk factor for dementia (Barnes et al., 2018; Nordström & Nordström, 2018; Shively, Scher, Perl, & Diaz-Arrastia, 2012). Indeed, evidence from postmortem brains in short- (6 hr–7 days postinjury) and long-term TBI survivors (1–47 years) suggests that even a single moderate-to-severe TBI is associated with an increase of Aβ deposits (Gentleman et al., 1997; Johnson, Stewart, & Smith, 2012). These deposits have also been found across all age groups, with converging evidence indicating that approximately 30% of patients test positive for Aβ deposits following a severe TBI (Smith, Johnson, & Stewart, 2013; Washington et al., 2016). To date, few studies have examined the impact of tau and Aβ on chronic somatic and mental health symptoms. One recent study indicated that military personnel who reported sustaining multiple mTBIs had elevations in total tau concentrations (Olivera et al., 2015). Furthermore, elevations in tau concentrations were associated with increased postconcussive symptoms (Olivera et al., 2015); the current study did not examine Aβ concentrations.
Posttraumatic stress disorder has been independently associated with cognitive impairments in service members and veterans and to an increased risk of AD (Qureshi et al., 2011). In a study of 174, 806 veterans who were predominantly male (96.5%) and had a mean age of 68.8 years, patients with PTSD had a two-fold increase, hazard ratio (HR) = 2.3, in the likelihood of developing incident dementia compared to those without PTSD (Yaffe et al., 2010). Even after multivariable adjustment for demographic, medical, and neuropsychiatric comorbidities, there was still a 1.77 increase in risk for patients with PTSD compared to veterans without PTSD (Yaffe et al., 2010). One potential explanation for this finding is chronic stress, which has been linked to damage in the brain, particularly in the hippocampus. Researchers have found associations between PTSD and reductions in hippocampal volume and decreased concentrations of N-acetyl-aspartate in the hippocampus (Ham et al., 2007). Furthermore, preclinical models in both transgenic and nontransgenic rodent models have indicated that chronic stress leads to increased cortical accumulation of both Aβ and abnormal hyperphosphorylation of tau (Catania et al., 2009; Dong et al., 2004). Specifically, Yang and colleagues (2014) showed that following chronic stress, phosphorylated tau was found in the hippocampus and frontal cortex, with associated alterations in behavior. Therefore, there is a possibility that tau and Aβ may contribute to the link between PTSD and dementia. In a recent interim report of a study in which amyloid positron emission topography (PET) is being used, the authors noted that among a sample of 146 Vietnam-era veterans, participants with a history of PTSD and those with TBI and PTSD had worse cognitive functioning compared to controls without TBI or PTSD (Weiner, Crane, Montine, Bennett, & Veitch, 2017). However, the authors did not report a significant association between amyloid PET and TBI or PTSD. As this study is ongoing, the authors were careful to indicate that tau PET may shed further light on the associations among TBI, PTSD, and AD. To our knowledge, no studies have specifically examined if tau and Aβ42 concentrations, measured in peripheral blood plasma, are increased in individuals with PTSD nor whether there are any additive effects of having a history of mTBI with concurrent PTSD symptomology.
To examine these relations, we compared concentrations of tau and Aβ42 in peripheral blood plasma within a cohort of U.S. service members and veterans with and without a history of mTBI and concurrent PTSD. The purpose of this study was to determine if biomarkers, specifically tau and Aβ42, implicated in chronic neurological symptoms following an mTBI relate to PTSD symptomology. We also wanted to determine if mTBI with concurrent PTSD resulted in elevations in these biomarkers in comparison to mTBI without PTSD as well as injured controls (IC) without PTSD.
Method
Participants
Participants were 107 U.S. military service members who were prospectively enrolled in a larger study designed to examine the natural history of recovery from TBI in service members and veterans (i.e., 15-Year Longitudinal TBI Study; Defense and Veterans Brain Injury Center [DVBIC], Sec721 NDAA FY2007). Patients were targeted for recruitment and consent if they presented to Walter Reed National Military Medical Center (WRNMMC; Bethesda, MD) following a TBI or if they had sustained a soft-tissue or orthopedic injury without TBI (i.e., the IC group). Men and women were enrolled in the study if they were (a) 18 years of age or older, (b) an active duty service member or other DEERS-eligible veteran, and (c) able to read and understand English. General exclusion criteria included (a) lack of proficiency in conversational English and (b) a history of significant neurological or psychiatric conditions unrelated to the injury event or deployment (e.g., meningioma, bipolar disorder).
TBI classification.
Diagnosis and classification of TBI was based on information gathered via medical record review and a comprehensive lifetime TBI history interview undertaken by clinical research personnel. The comprehensive lifetime TBI history interview was completed by masters-level clinical research personnel who were specifically trained (by RTL and SML) to evaluate the presence and severity of TBI. The TBI history interview consisted of the Ohio State University TBI identification method (Corrigan & Bogner, 2007) and an extended semistructured clinical interview designed to (a) extract more detailed information to estimate the presence and duration of loss of consciousness (LOC), posttraumatic amnesia (PTA), alteration of consciousness (AOC), and retrograde amnesia; and (b) gather military-specific information regarding injury circumstances (e.g., type of blast, protection worn, etc.). Note that a simple self-report of being “dazed and confused” was not considered sufficient evidence to establish the presence of AOC. During the interview, every effort was made to distinguish between “confirmed” AOC (i.e., confusion due to brain injury as evidenced by reports of the person acting unusually, talking unusually, unable to follow simple commands, etc.) versus “questionable” AOC (i.e., confusion due to simply being startled or surprised, or from pain or psychological factors). Final determination and classification of TBI severity was undertaken by consensus, giving consideration to all information, during case conferencing with the interviewer and a PhD-level clinician/scientist trained in neuropsychology and TBI (RTL and SML). Time since injury (TSI) was also measured as months between injury date and assessment date.
Classification of mild TBI.
Participants were included in the mTBI (n = 84) group if they had evidence of a brain injury that was the result of a combat or non-combat-related event as indicated by one or more of the following: (a) AOC, LOC, PTA that was directly attributable to head trauma; (b) trauma-related intracranial abnormalities as indicated by neuroradiological scans; and/or (c) Glasgow Coma Scale (GCS) scores (if available). The mTBI group included participants who were classified as uncomplicated mTBI or complicated mTBI. Both uncomplicated and complicated mTBI were classified as a GCS score between 13 and 15, PTA duration less than 24 hr, LOC duration less than 30 min, and/or AOC present, and differentiated based on the absence (uncomplicated) and presence (complicated) of trauma-related intracranial abnormality as seen via computerized tomography (CT) or magnetic resonance imaging (MRI).
Classification as IC.
Participants were included in the IC group (n = 23) if (a) they experienced an injury event, (b) there was no evidence of an altered state of consciousness as a result of the injury (e.g., GCS score below 15; absence of AOC, LOC, or PTA) or intracranial abnormality, (c) the presenting complaint was not due to a neurological condition or disorder (e.g., cerebrovascular accident), and (d) they had no history of TBI.
All participants in the final sample had been evaluated three or more months postinjury, completed a battery of self-reported symptom measures with no missing items, passed symptom validity tests by scoring below the recommended cutoffs on the validity scales of the Minnesota Multiphasic Personality Inventory Second Edition–Restructured Format (MMPI-2-RF; Ben-Porath & Tellegen, 2008), and had a blood sample taken.
Procedure
Participants provided a blood sample and completed a 2.5-hr battery of self-report symptom measures that included the Neurobehavioral Symptom Inventory, PTSD Checklist–Civilian version, TBI-Quality of Life scale, Combat Exposure Scale, and MMPI-2-RF, as well as a brief interview to elicit information regarding demographic information, military status, and current medications. Current medications, as reported by participants, were then classified in accordance to type (e.g., antidepressant medications). These classifications were then dichotomized to indicate if participants were taking this medication or not. This resulted in three dichotomous (yes or no) categories: antidepressant, antianxiety, and sleep medications. Although antipsychotic medication use was included in the review, no participants in the sample were taking such medications. Participants completed these measures as part of a larger test battery administered over a 2-day period that also included clinical interviews, neuroimaging, and a variety of neurocognitive and sensory/motor measures. This study was approved by the WRNMMC Institutional Review Board in Bethesda, MD and was conducted in accordance with the guidelines of the Declaration of Helsinki.
Nonfasting blood samples were collected using plastic lithium heparin tubes between around 9:00 am and 12:00 pm, prior to interviews and the battery of tests. Samples were processed within 1 hr of blood sample collection, using standard protocols (Olivera et al., 2015) and then stored at −80° C. Samples were stored until all samples had been collected, at which time batch assays were conducted. Assays were randomized over plates, with laboratory scientists blinded to participant groups. Tau and Aβ42 concentrations were measured using Simoa (Quanterix; Lexington, MA) a high-definition-1 analyzer. These methods have been published previously (Rissin et al., 2011). All assays were run in duplicate. To address possible batch effects, we reran selected samples across all the plates that were run. Intraplate variations comprised less than 3% for all samples. The reported coefficients of variations (CVs) were under 15% for all analyses. The average CVs were 4.9% and 3.2% for tau and Aβ42, respectively. The limit of detection for the assay is 0.012 pg/mL for tau and 0.044 pg/mL for Aβ42.
Measures
Posttraumatic stress disorder.
Symptoms of PTSD were measured using the 17-item PTSD Checklist–Civilian Version (PCLC; Weathers, Litz, Herman, Huska, & Keane, 1993). Each symptom is rated on a 5-point scale of 1 (not at all) to 5 (extremely), which provides a total summed score range of between 17 and 85. The PCLC was found to be highly reliable in this study (Cronbach’s α = .95). Participants were categorized into PTSD-Present (n = 26) or PTSD-Absent (n = 81) in accordance with the criteria given in the fourth edition (text revision) of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychiatric Association, 1994). Participants were classified into the PTSD-Present group based on the endorsement of moderately or more (i.e., a rating of 3 or higher) symptoms for one or more Criterion B symptoms, three or more Criterion C symptoms, and two or more Criterion D symptoms.
Postconcussion symptoms (PCSx).
Postconcussion symptoms were measured using the 22-item Neurobehavioral Symptom Inventory (NSI; Cicerone & Kathleen, 1995). Each symptom is rated on a 5-point scale of 0 (none) to 4 (very severe). The total score is obtained by summing the ratings of the 22 items, providing a range of 0 to 88. The NSI was found to be highly reliable in this study (22 items; Cronbach’s α = .94).
Quality of life following TBI.
The Traumatic Brain Injury-Quality of Life (TBI-QOL; Tulsky et al., 2016) scale is designed to provide a comprehensive evaluation of health-related quality of life for individuals following TBI. The TBI-QOL consists of 20 scales that are divided into four primary domains: Physical Health, Emotional Health, Cognition, and Social Participation. For the purposes of this study, seven of the 20 TBI-QOL scales were administered and showed high reliability, including Anger (nine items; Cronbach’s α = .97), Anxiety (10 items; Cronbach’s α = .94), Depression (10 items; Cronbach’s α = .92), Fatigue (10 items; Cronbach’s α = .96), Headaches (10 items; Cronbach’s α = .98), Pain Interference (10 items; Cronbach’s α = .96), Cognitive Concerns–General (10 items; Cronbach’s α = .97). The TBI-QOL was administered using static short forms. For each subscale, the test-taker is required to respond to each item on a 5-point scale. A total raw score for each scale was calculated by summing the responses to all items within each scale and converted to t scores. For the Cognitive Concerns–General subscale, higher t scores reflect better functioning; for the remaining six scales, high t scores reflect worse functioning.
Combat exposure.
The Combat Exposure Scale (CES; Keane et al., 1989) is a self-report measure that contains seven items meant to assess war zone–related stressors experienced by military personnel. The total scores range from 0 to 41, which is calculated by using a sum of weighted scores. Higher scores indicate more exposure to combat situations. The CES showed high reliability (seven items; Cronbach’s α = .90).
Symptom validity.
The MMPI-2-RF is a 338-item measure designed to assess psychological symptomatology. For the purposes of this study, measures included only the validity subscales: Cannot Say, Variable Response Inconsistency-random responding (VRIN-r), True Response Inconsistency-fixed responding (TRIN-r), Infrequent Responses (F-r), Infrequent Psychopathology Responses (Fp-r), Infrequent Somatic Responses (Fs), Symptom Validity (FBS-r), and Response Bias Scale (RBS; Gervais, Ben-Porath, Wygant, & Sellbom, 2010). Based on recommended cutoff scores, participants were not included in the study if they were considered to have exaggerated symptoms as determined by MMPI-2-RF responses (i.e., t scores of 100 [100T] or higher on the F-r, 90T or higher on the Fp-r, 100T or higher on the Fs, 100T or higher on the FBS, or 100T or higher on the RBS) or if their scores were not considered interpretable (i.e., score higher than 14 on the Cannot Say subscale or higher than 79T on the VRIN-r or TRIN-r subscales).
Data Analysis
Participants were categorized into groups according to the presence or absence of mTBI (i.e., mTBI or IC) and PTSD status (i.e., PTSD-Present or PTSD-Absent). This resulted in three groups: IC/PTSD-Absent, mTBI/PTSD-Absent, and mTBI/PTSD-Present. It is noted that the IC/PTSD-Present group only had five participants; as such, this group was removed from all group comparisons. There was substantial overlap between participants who were taking antidepressant and antianxiety medications. Statistically, these variables were highly correlated, r = .92, p <.001. As such, these categories were collapsed into a single variable for current antidepressant and/or antianxiety medication use.
Analyses were conducted using IBM SPSS (Version 25), and the figure was produced using GraphPad Prism 7. Continuous variables were assessed for normal distribution by inspection of histograms and the Shapiro–Wilks test. Levene’s test was used to assess equality of variances. Analysis of variance (ANOVA) and chi-square analyses were conducted to examine group differences for demographic and clinical characteristics. Pairwise post hoc comparisons with Bonferroni adjustment were used to assess significant group differences, with a significance value of p < .05. Linear multiple regression was then conducted to ascertain the independent effects of mTBI and PTSD on tau concentrations, controlling for significant covariates. Purposeful selection was undertaken to select the covariates for this model; correlational analysis was conducted to identify any variables that had significant associations with tau at a p value cut-point of .25 or less (Bursac, Gauss, Williams, & Hosmer, 2008). Standardized residuals were examined for normality by inspection of histograms and using the Shapiro–Wilks test of normality.
Results
Demographic, Injury, and Clinical Characteristics of the Sample
The majority of the sample was male (92.5%), with a mean age of 34.17 years (SD = 10.10 years; Table 1). There were no significant differences for the demographic and injury characteristics across the three groups, with the exception of TSI, CES score, and antidepressant or anxiety medication intake. The TSI for all participants ranged between 3 and 121 months (M = 45.92 months, SD = 37.92). Pairwise post hoc comparisons using Bonferroni corrections revealed that the IC/PTSD-Absent group sustained their injuries more recently than both the mTBI/PTSD-Absent and mTBI/PTSD-Present groups. However, there were no differences in TSI between the mTBI groups. Pairwise post hoc comparisons for history of combat exposure revealed that the mTBI/PTSD-Present group reported significantly higher levels of combat exposure than the mTBI/PTSD-Absent group, p = .011, but not significantly higher levels than the IC group, p = .056. The mTBI/PTSD-Absent and IC groups were statistically equivalent in scores on the CES, p = 1.00. A chi-square analysis revealed that there was a significant difference in the number of people who were taking antidepressant and/or antianxiety medications across the groups. A z-proportional test of differences indicated that both the mTBI/PTSD-Absent and mTBI/PTSD-Present groups had higher than anticipated medication intake. The mTBI/PTSD-Present group demonstrated the highest proportion of people (~60%) who indicated that they were taking antidepressant and/or antianxiety medications.
Table 1.
Descriptive Statistics and Group Comparisons for Demographic, Injury, and Clinical Characteristics, by Group
| Variable | IC/PTSD-Absent (n = 18) |
mTBI/PTSD-Absent (n = 63) |
mTBI/PTSD-Present (n = 21) |
Statistical Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | n | % | M | SD | n | % | M | SD | n | % | ||
| Demographic and Injury Variables | |||||||||||||
| Age (years) | 35.56 | 12.39 | 33.02 | 9.42 | 36.14 | 9.89 | F(2, 99) = 0.98 | ||||||
| Sex (male) | 15 | 85.3 | 59 | 93.7 | 21 | 100 | χ2(2, N = 102) = 4.28 | ||||||
| Race | χ2(2, N = 93) = 4.49 | ||||||||||||
| White | 11 | 68.8 | 47 | 82.5 | 15 | 75.0 | |||||||
| African American | 3 | 18.8 | 7 | 12.3 | 5 | 25.0 | |||||||
| Other | 2 | 12.5 | 3 | 5.3 | - | - | |||||||
| Service branch a | χ2(2, N = 61) = 15.16 | ||||||||||||
| Army | 10 | 76.9 | 21 | 55.2 | 8 | 80.0 | |||||||
| Navy | 2 | 15.4 | 4 | 10.5 | 1 | 10.0 | |||||||
| Air Force | - | - | 5 | 13.2 | - | - | |||||||
| Marines | 1 | 7.7 | 8 | 21.0 | 1 | 10.0 | |||||||
| Deployments | 3.23 | 2.98 | 1.89 | 2.17 | 1.89 | 2.17 | F(2, 57) = 1.83 | ||||||
| Time since injury (months) | 23.22 | 27.65 | 49.51 | 37.86 | 49.51 | 37.86 | F(2, 99) = 4.86* | ||||||
| CES | 17.06 | 7.83 | 17.21 | 7.27 | 17.21 | 7.27 | F(2, 99) = 4.71* | ||||||
| Number of TBIs | - | - | 1.24 | 0.56 | 1.43 | 0.68 | F(1, 82) = 1.64 | ||||||
| TBI Severity | χ2(1, N = 84) = 2.05 | ||||||||||||
| Uncomplicated mTBI | - | - | 49 | 77.8 | 13 | 61.9 | |||||||
| Complicated mTBI | - | - | 14 | 22.2 | 8 | 38.1 | |||||||
| Antidepressant/anxiety medication (Yes) | 5 | 27.8 | 15 | 23.8 | 13 | 61.9 | χ2(2, N = 102) = 10.65** | ||||||
| Sleep medication (Yes) | 2 | 11.1 | 8 | 12.7 | 2 | 9.5 | χ2(2, N = 102) = 0.16 | ||||||
| Clinical Variables | |||||||||||||
| NSI | 17.94 | 9.29 | 16.29 | 12.99 | 44.38 | 8.82e,g | F(2, 99) = 47.15*** | ||||||
| TBI-QOL scales | |||||||||||||
| Anger | 45.81 | 7.84 | 44.79 | 7.48 | 61.73 | 9.73e,g | F(2, 97) = 35.78*** | ||||||
| Anxiety | 50.31 | 10.01 | 47.14 | 8.22 | 62.51 | 4.48e,g | F(2, 96) = 27.88*** | ||||||
| Depression | 47.11 | 7.75 | 43.15 | 5.45b | 56.89 | 5.15e,g | F(2, 97) = 42.90*** | ||||||
| Cognition-General Concerns | 36.61 | 8.89 | 39.13 | 8.97 | 29.35 | 5.45c,g | F(2, 96) = 10.71*** | ||||||
| Fatigue | 53.20 | 6.84 | 50.53 | 8.29 | 62.57 | 6.08d,g | F(2, 97) = 19.40*** | ||||||
| Headaches | 50.92 | 7.64 | 47.52 | 8.56 | 55.59 | 4.93g | F(2, 99) = 8.68*** | ||||||
| Pain Interference | 55.44 | 7.62 | 51.87 | 8.11 | 61.08 | 7.35g | F(2, 97) = 10.86*** | ||||||
| Biomarkers | |||||||||||||
| Tau | 2.57 | 1.01 | 2.84 | 1.05 | 3.52 | 1.25c,f | F(2, 99) = 4.33* | ||||||
| Aβ42 | 8.11 | 1.76 | 8.22 | 1.90 | 8.33 | 2.42 | F(2, 99) = 0.06 | ||||||
Note: N = 102. IC = injured control; PTSD = posttraumatic stress disorder; TBI = traumatic brain injury; mTBI = mild traumatic brain injury; CES = Combat Exposure Scale; NSI = Neurobehavioral symptom inventory; TBI-QOL =Traumatic Brain Injury-Quality of Life Scale.
p < .05
p < .01
p < .001.
Service branch classifications were collapsed according to service type: “Army” includes participants who identified themselves as Army National Guard and Army Reserves; and “Navy” includes participants who identified themselves as Naval Corps and Naval Reserves.
Significant difference of p < .05 for pairwise post hoc comparisons between the IC/PTSD-Absent mTBI/PTSD-Absent groups on the clinical outcomes and biomarkers.
Significant difference for pairwise post hoc comparisons between IC/PTSD-Absent and mTBI/PTSD-Present groups on the clinical outcomes and biomarkers; c p < .05, dp < .01, ep < .001; no letter indicates that there was no significant difference between these two groups.
Significant difference for pairwise post hoc comparisons between the mTBI/PTSD-Absent and mTBI/PTSD-Present groups on the clinical outcomes and biomarkers; fp < .05, gp < .001; no letter indicates that there was no significant difference between these two groups.
Note. N = 107 (whole sample). R2 = .137.
In regard to the clinical variables, the mTBI/PTSD-Present group reported significantly higher scores on the NSI and the Fatigue, Depression, Anger, and Anxiety subscales of the TBI-QOL compared to both the mTBI/PTSD-Absent and the IC/PTSD-Absent groups (Table 1). The mTBI/PTSD-Present group also had significantly lower scores on the Cognitive Concerns–General subscale, which indicates a higher level of functional impairment, than both the mTBI/PTSD-Absent and the IC/PTSD-Absent groups, who did not significantly differ. The mTBI/PTSD-Present group also reported significantly higher scores on the TBI-QOL Headaches and Pain Interference subscales compared to the mTBI/PTSD-Absent group.
Associations and Effects of PTSD and mTBI on Tau and Aβ42
Tau was significantly elevated in the mTBI/PTSD-Present group compared to both the mTBI/PTSD-Absent, p = .043, d = 0.59 (medium effect size), and the IC/PTSD-Absent, p = .022, d = 0.83 (large effect size) groups, F(2, 99) = 4.33, p = .016 (see Figure 1 and Table 1). The mTBI/PTSD-Absent and IC/PTSD-Absent groups did not significantly differ in tau concentration. Among all of the groups, there were no significant differences in Aβ42 concentrations, p = .943 (Table 1).
Figure 1.
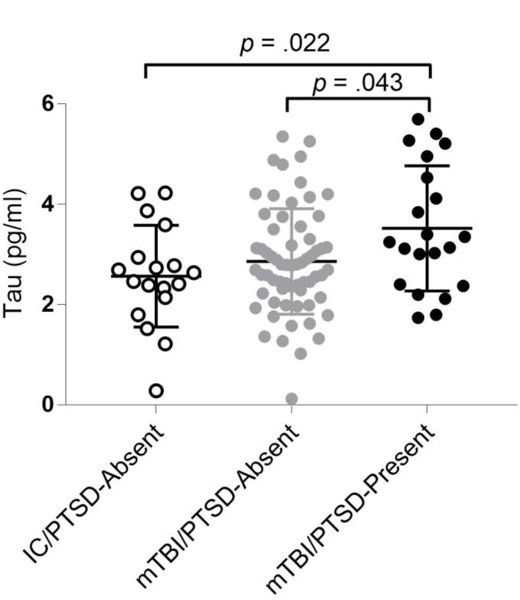
Group differences (N = 102) in tau concentrations (pg/mL). IC = injured controls; PTSD = posttraumatic stress disorder; mTBI = mild traumatic brain injury.
Correlation analyses for continuous variables and ANOVAs for categorical variables were conducted to identify any factors that were significantly associated with tau, which should be controlled for within the analysis in accordance with the purposeful selection method (Bursac et al., 2008). Gender, F(1, 105) = 1.68, p = .198; and intake of sleep medication, F(1, 105) = 6.05, p = .016, were identified for inclusion in the model. The final linear multiple regression model examined the effect of mTBI status (mTBI vs. IC) and PTSD symptoms (moderate/severe vs. absent) on tau concentrations, controlling for gender and current use of sleep medications. Normality was assessed by examining the standardized skewness and using the Shapiro–Wilks test, p = .114, indicating that the residuals were statistically normal. The overall model was significant F(4, 102) = 4.05, p = .004 (Table 2) and accounted for 13.7% of change in tau concentrations, which is classified as a medium effect size (Cohen, 1988). Current use of sleep medications and PTSD severity were significant independent predictors of tau concentrations (Table 2). Participants who were not taking sleep medications had tau concentrations that were, on average, 0.79 pg/mL higher than those participants who were taking sleep medications. Furthermore, participants classified as having moderate to severe PTSD symptoms had tau concentrations that were an average of 0.65 pg/mL higher than participants classified as PTSD-Absent, after controlling for gender and mTBI status.
Table 2.
Linear Multiple Regression Model of the Effects of Posttraumatic Stress Disorder (PTSD) Symptoms and Mild Traumatic Brain Injury (mTBI) status on Tau, Controlling for Gender and Sleep Medications
| Variable | β | B | 95% CI B | t(4) | p | ηp2 |
|---|---|---|---|---|---|---|
| Gendera | .09 | 0.37 | [−0.43, 1.17] | 0.93 | .356 | .091 |
| Sleep medicationb | −.22 | −0.79 | [−1.44, −0.13] | −2.39 | .019 | −.230 |
| mTBIc | .08 | 0.23 | [−0.29, 0.74] | 0.87 | .384 | .086 |
| PTSDd | .25 | 0.65 | [0.17, 1.13] | 2.67 | .009 | .256 |
The referent group was female participants.
The referent group was participants not taking sleep medications.
The referent group was the participants who had no history of mTBI.
The referent group was the PTSD-Absent group.
Discussion
Herein, we report for the first time that tau in plasma is significantly elevated in military personnel who have sustained an mTBI and display concurrent PTSD symptomology. Participants in the mTBI/PTSD-Present group also reported a significantly higher level of PCSx and endorsed more clinical symptoms on the TBI-QOL than the other groups. Furthermore, in a model that accounted for gender and mTBI, PTSD had a significant and independent effect on tau concentrations. These results indicate that within this cohort, tau-related changes were in part associated with the presence of PTSD. This finding supports evidence from studies of civilians and athletes that has demonstrated acute elevations in tau related to poor recovery, as shown by higher levels of neurological symptoms and deficits (Bogoslovsky et al., 2017; Gill et al., 2017). Participants with an mTBI and concurrent PTSD were, on average, 58.5 months postinjury, indicating that their reported PTSD symptoms were chronic in nature. Previous research in cohorts of older veterans has shown links between TBI and AD as well as PTSD and AD later in life (Flatt, Gilsanz, Quesenberry, Albers, & Whitmer, 2018). Thus, our observed increase in tau in peripheral blood plasma may provide some insights into the nature of the risk for dementia following TBI. These findings suggest the need for additional longitudinal studies that include biomarkers, such as tau, to determine if mTBI and comorbid PTSD may increase the risk of neuronal pathology later in life.
Higher concentrations of tau following a mTBI in service members and veterans with PTSD may also relate to the observed reductions in overall neuronal volume (Averill et al., 2017), specifically in the volume and function of the hypothalamus, prefrontal cortex, and amygdala (Fitzgerald, DiGangi, & Phan, 2018). Therefore, it may be that the increased tau concentrations observed following TBIs increases vulnerability of neurons and may relate to a higher risk for PTSD and other chronic symptoms. Conversely, PTSD may also trigger neuronal degradation independently or in conjunction with mTBI. Karabatsiakis et al. (2015) was the first to profile serum biomarkers of PTSD and showed that participants with PTSD had significantly higher levels of phospholipids and other compounds compared to those without PTSD. This indicates that PTSD is associated with processes of neuroinflammation and neuronal membrane degradation (Harper et al., 2014). These biomarker changes were also observed in patients with AD (Wood, 2012), thus strengthening the hypothesized crosslink between PTSD and AD. Additional studies that include blood-based biomarkers in combination with neuronal imaging will allow for additional insights into the links between tau, neuronal loss, and possible neurodegenerative risks.
Another possible explanation for the association between mTBI, PTSD, and tau is sleep. Sleep disturbances have long been recognized as a prominent symptom of PTSD; however, disturbed sleep has also been posited as a potential risk factor for PTSD (Gehrman et al., 2013; Mellman et al., 2014). These reciprocal associations are also mirrored in mTBI in that sleep disturbances have been correlated with an increased risk of sustaining injuries such as TBI and, conversely, sleep disturbances are a known consequence following TBI of all severities (Sandsmark, Elliott, & Lim, 2017). Sleep disturbances have also been linked to increased symptom reporting and have been shown to hinder recovery following TBI (White et al., 2016). Finally, there is converging evidence that sleep affects protein concentrations in the central nervous system, including in terms of alterations in levels of both tau and Aβ (Ju et al., 2017; Motamedi et al., 2018). Sleep is the clearance mechanism for the glymphatic system, ridding the brain of excess proteins, including tau (Iliff et al., 2014; Louveau et al., 2015). Therefore, disruptions to normal sleep may impede this clearance, leading to an accumulation of proteins. In this study, taking sleep medication was a significant and independent predictor of decreased tau. It may be that the sleep medication increases sleep quality and therefore assists with glymphatic regulation of proteins. Although we were unable to assess if participants who were not taking sleep medications actually had poorer sleep than those who were taking sleep medications, this finding does lend support to the idea that sleep is an important consideration. Sleep problems are pervasive in service members and veterans (Mysliwiec et al., 2013), with reports indicating that sleep disorders such as obstructive sleep apnea (OSA) are actually increasing in military personnel (Rogers, Stahlman, Hunt, Oh, & Clark, 2016). Moreover, recent research (Motamedi et al., 2018) has indicated that adults with OSA have elevated tau concentrations compared to healthy adults. As such, it is important that future research includes measures of sleep quality and quantity when considering the associations among PTSD, mTBI, and proteins such as tau and Aβ. Although our findings provide some initial insights into possible links, further research is required.
This study had a number of strengths, including its rigorous assessment and categorization of TBIs and validated, high-sensitivity measurement of tau and Aβ42. There are also several limitations of note. First, due to the limited sample size of participants classified as IC with concurrent PTSD symptoms (n = 5), we were unable to conduct comparisons between the four groups. This limits the generalizability of the findings in this study, and future research should attempt to include this group to further elucidate the associations between PTSD and tau reported herein. Furthermore, we were unable to determine if the tau measured was centrally derived, resultant from neuronal injury of the TBI, or due to secondary peripheral factors. Future research should endeavor to examine neuronally derived tau, for example, from exosomes (Gill et al., 2018). This study was of a relatively small cohort of military personnel, and as per much research in the field, we had an overrepresentation of white male participants in this sample. Therefore, to improve generalizability, we recommend that future studies should aim to recruit more participants and seek increased gender and racial diversity to ensure accurate representation and increase generalizability to the wider military and civilian communities. Overall, the observation of higher levels of tau in the peripheral blood plasma of service members and veterans with concurrent mTBI and PTSD provides insights into the possible links between mTBI and the increased neurobiological effects of PTSD.
References
- American Psychiatric Association. (1994). DSM-IV: Diagnostic and Statistical Manual of Mental Disorders (Widiger T, Frances A, Pincus H, First M, Ross R, & Davis W, Eds.). Washington DC: American Psychiatric Press Inc. [Google Scholar]
- Averill CL, Satodiya RM, Scott JC, Wrocklage KM, Schweinsburg B, Averill LA, … Abdallah CG (2017). Posttraumatic Stress Disorder and Depression Symptom Severities Are Differentially Associated With Hippocampal Subfield Volume Loss in Combat Veterans. Chronic Stress (Thousand Oaks, Calif.), 1, 10.1177/2470547017744538 . Retrieved from 10.1177/2470547017744538http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5839647/. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5839647/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, & Yaffe K (2018). Association of Mild Traumatic Brain Injury With and Without Loss of Consciousness With Dementia in US Military Veterans. JAMA Neurology, 94121 10.1001/jamaneurol.2018.0815 [DOI] [PMC free article] [PubMed]
- Ben-Porath Y, & Tellegen A (2008). MMPI-2-RF (Minnesota Multiphasic Personality Inventory-2-Restructured Form) Manual for Administration, Scoring, and Interpretation Minneapolis, MN: University of Minnesota Press. [Google Scholar]
- Bogoslovsky T, Wilson D, Chen Y, Hanlon D, Gill J, Jeromin A, … Diaz-Arrastia R (2017). Increases of Plasma Levels of Glial Fibrillary Acidic Protein, Tau, and Amyloid β up to 90 Days after Traumatic Brain Injury. Journal of Neurotrauma, 34(1), 66–73. 10.1089/neu.2015.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursac Z, Gauss CH, Williams DK, & Hosmer DW (2008). Purposeful selection of variables in logistic regression. Source Code for Biology and Medicine, 3(17), 1–8. 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, & Almeida OFX (2009). The amyloidogenic potential and behavioral correlates of stress. Molecular Psychiatry, 14(1), 95–105. 10.1038/sj.mp.4002101 [DOI] [PubMed] [Google Scholar]
- Cicerone KD, & Kathleen K (1995). Persistent postconcussion syndrome: the structure of subjective complaints after mTBI. The Journal of Head Trauma Rehabilitation, 10, 1–17. [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences (Second Edi). Hillsdale, New Jersey: Lawrence Erlbaun Associates, Publishers; Retrieved from http://www.utstat.toronto.edu/~brunner/oldclass/378f16/readings/CohenPower.pdf [Google Scholar]
- Corrigan JD, & Bogner J (2007). Initial reliability and validity of the Ohio State University TBI Identification Method. The Journal of Head Trauma Rehabilitation 10.1097/01.HTR.0000300227.67748.77 [DOI] [PubMed]
- Defense and Veterans Brain Injury Center. (2017). DoD Numbers for Traumatic Brain Injury Retrieved from http://dvbic.dcoe.mil/files/tbi-numbers/worldwide-totals-2017-Q1-Q4_feb-14-2018_v1.0_2018-03-08_0.pdf
- Devoto C, Arcurio L, Fetta J, Ley M, Rodney T, Kanefsky R, & Gill J (2017). Inflammation Relates to Chronic Behavioral and Neurological Symptoms in Military Personnel with Traumatic Brain Injuries. Cell Transplantation, 26(7), 1169–1177. 10.1177/0963689717714098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, & Csernansky JG (2004). Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience, 127(3), 601–609. 10.1016/j.neuroscience.2004.05.040 [DOI] [PubMed] [Google Scholar]
- Fitzgerald JM, DiGangi JA, & Phan KL (2018). Functional Neuroanatomy of Emotion and Its Regulation in PTSD. Harvard Review of Psychiatry, 26(3), 116–128. 10.1097/HRP.0000000000000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt JD, Gilsanz P, Quesenberry CP, Albers KB, & Whitmer RA (2018). Post-traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimer’s and Dementia, 14(1), 28–34. 10.1016/j.jalz.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, & Rosen RC (2012). Posttraumatic stress disorder in veterans and military personnel: Epidemiology, screening, and case recognition. Psychological Services, 9(4), 361–382. 10.1037/a0027649 [DOI] [PubMed] [Google Scholar]
- Gehrman P, Seelig AD, Jacobson IG, Boyko EJ, Hooper TI, Gackstetter GD, … Smith TC (2013). Predeployment Sleep Duration and Insomnia Symptoms as Risk Factors for New-Onset Mental Health Disorders Following Military Deployment. Sleep, 36(7), 1009–1018. 10.5665/sleep.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman SM, Greenberg BD, Savage MJ, Noori M, Newman SJ, Roberts GW, … Graham DI (1997). A β42 is the predominant form of amyloid b -protein in the brains of short-term survivors of head injury. NeuroReport, 8(6). Retrieved from https://journals.lww.com/neuroreport/Fulltext/1997/04140/A__42_is_the_predominant_form_of_amyloid_b.39.aspx [DOI] [PubMed] [Google Scholar]
- Gervais RO, Ben-Porath YS, Wygant DB, & Sellbom M (2010). Incremental Validity of the MMPI-2-RF Over-reporting Scales and RBS in Assessing the Veracity of Memory Complaints†. Archives of Clinical Neuropsychology, 25(4), 274–284. Retrieved from 10.1093/arclin/acq018 [DOI] [PubMed] [Google Scholar]
- Gill JM, Merchant-Borna K, Jeromin A, Livingston W, & Bazarian J (2017). Acute plasma tau relates to prolonged return to play after concussion. Neurology, 88(6), 595–602. 10.1212/WNL.0000000000003587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T, … Kapogiannis D (2018). Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Injury, 32(10), 1277–1284. 10.1080/02699052.2018.1471738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham BJ, Chey J, Yoon SJ, Sung Y, Jeong DU, Ju Kim S, … Lyoo IK (2007). Decreased N-acetyl-aspartate levels in anterior cingulate and hippocampus in subjects with post-traumatic stress disorder: A proton magnetic resonance spectroscopy study. European Journal of Neuroscience, 25(1), 324–329. 10.1111/j.1460-9568.2006.05253.x [DOI] [PubMed] [Google Scholar]
- Harper DG, Jensen JE, Ravichandran C, Sivrioglu Y, Silveri M, Iosifescu DV, … Forester BP (2014). Tissue-specific differences in brain phosphodiesters in late-life major depression. American Journal of Geriatric Psychiatry, 22(5), 499–509. 10.1016/j.jagp.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, … Nedergaard M (2014). Impairment of Glymphatic Pathway Function Promotes Tau Pathology after Traumatic Brain Injury. The Journal of Neuroscience, 34(49), 16180–16193. 10.1523/JNEUROSCI.3020-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Silverberg N, Lange R, & Zasler N (2013). Conceptualizing Outcome from Mild Traumatic Brain Injury. In Zasler N, Katz D, Zafonte R, Arciniegas D, Bullock M, & Kreutzer J (Eds.), Brain Injury Medicine: Principles and Practice (2nd Editio, pp. 470–497). New York: Demos Medical Publishing Inc. [Google Scholar]
- Johnson VE, Stewart W, & Smith DH (2012). Widespread Tau and Amyloid-Beta Pathology Many Years After a Single Traumatic Brain Injury in Humans. Brain Pathology (Zurich, Switzerland), 22(2), 142–149. 10.1111/j.1750-3639.2011.00513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YES, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, … Holtzman DM (2017). Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain, 140(8), 2104–2111. 10.1093/brain/awx148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsiakis A, Hamuni G, Wilker S, Kolassa S, Renu D, Kadereit S, … Kolassa I-T (2015). Metabolite profiling in posttraumatic stress disorder. Journal of Molecular Psychiatry, 3(1), 2 10.1186/s40303-015-0007-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, & Mora CA (1989). Clinical Evaluation of a Measure to Assess Combat Exposure. Psychological Assessment, 1(1), 53–55. 10.1037/1040-3590.1.1.53 [DOI] [Google Scholar]
- Lemaire CM, & Graham DP (2011). Factors associated with suicidal ideation in OEF/OIF veterans. Journal of Affective Disorders, 130(1–2), 231–238. 10.1016/j.jad.2010.10.021 [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, … Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341. 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M (2008). Mild traumatic brain injury and postconcussion syndrome: The new evidence base for diagnosis and treatment New York, NY: Oxford University Press. [Google Scholar]
- Mellman T. a, Kobayashi I, Lavela J, Wilson B, Hall Brown TS, Brown TSH, & Hall Brown TS (2014). A Relationship between REM Sleep Measures and the Duration of Posttraumatic Stress Disorder in a Young Adult Urban Minority Population. Sleep, 37(8), 1321–1326. 10.5665/sleep.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi V, Kanefsky R, Matsangas P, Mithani S, Jeromin A, Brock MS, … Gill J (2018). Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Medicine, 43, 71–76. 10.1016/j.sleep.2017.11.1121 [DOI] [PubMed] [Google Scholar]
- Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, & Roth BJ (2013). Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep, 36(2), 167–174. 10.5665/sleep.2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström A, & Nordström P (2018). Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS Medicine, 15(1), e1002496 10.1371/journal.pmed.1002496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Lejbman N, Jeromin A, French LM, Kim HS, Cashion A, … Gill J (2015). Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurology, 72(10), 1109–1116. 10.1001/jamaneurol.2015.1383 [DOI] [PubMed] [Google Scholar]
- Qureshi SU, Long ME, Bradshaw MR, Pyne JM, Magruder KM, Kimbrell T, … Kunik ME (2011). Does PTSD Impair Cognition Beyond the Effect of Trauma? Journal of Neuropsychiatry, 23(1), 16–28. 10.1176/appi.neuropsych.23.1.16 [DOI] [PubMed] [Google Scholar]
- Rissin DM, Fournier DR, Piech T, Kan CW, Campbell TG, Song L, … Duffy DC (2011). Simultaneous Detection of Single Molecules and Singulated Ensembles of Molecules Enables Immunoassays with Broad Dynamic Range. Analytical Chemistry, 83(6), 2279–2285. 10.1021/ac103161b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AE, Stahlman S, Hunt DJ, Oh G-T, & Clark LL (2016). Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR, 23(10), 2–11. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27792352 [PubMed] [Google Scholar]
- Sandsmark DK, Elliott JE, & Lim MM (2017). Sleep-wake disturbances after traumatic brain injury: Synthesis of human and animal studies. Sleep, 40(5). 10.1093/sleep/zsx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively S, Scher A, Perl D, & Diaz-Arrastia R (2012). Dementia resulting from traumatic brain injury: What is the pathology? Archives of Neurology, 69(10), 1245–1251. Retrieved from 10.1001/archneurol.2011.3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, & Stewart W (2013). Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nature Reviews. Neurology, 9(4), 211–221. 10.1038/nrneurol.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NH, Lippa SM, Green DL, McGlynn SM, Grande LJ, Milberg WP, & McGlinchey RE (2017). Elevated rates of memory impairment in military service-members and veterans with posttraumatic stress disorder. Journal of Clinical and Experimental Neuropsychology, 39(8), 768–785. 10.1080/13803395.2016.1264575 [DOI] [PubMed] [Google Scholar]
- Tulsky DS, Kisala PA, Victorson D, Carlozzi N, Bushnik T, Sherer M, … Cella D (2016). TBI-QOL: Development and Calibration of Item Banks to Measure Patient Reported Outcomes Following Traumatic Brain Injury. The Journal of Head Trauma Rehabilitation, 31(1), 40–51. 10.1097/HTR.0000000000000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington PM, Villapol S, & Burns MP (2016, January 1). Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Experimental Neurology Academic Press; 10.1016/j.expneurol.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska J. a., & Keane TM (1993). The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. Paper Presented at the Annual Meeting of International Society for Traumatic Stress Studies, San Antonio, TX, October, 1993 10.1101/gr.095406.109 [DOI] [Google Scholar]
- Weiner MW, Crane PK, Montine TJ, Bennett DA, & Veitch DP (2017, October 31). Traumatic brain injury may not increase the risk of Alzheimer disease. Neurology Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology; 10.1212/WNL.0000000000004608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, … Grashow R (2016). Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex, 74(January), 449–475. 10.1016/j.cortex.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PL (2012). Lipidomics of Alzheimer’s disease: current status. Alzheimer’s Research & Therapy, 4(1), 5 10.1186/alzrt103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, … Marmar C (2010). Posttraumatic stress disorder and risk of dementia among US veterans. Archives of General Psychiatry, 67(6), 608–613. 10.1001/archgenpsychiatry.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Guo X, Wang GH, Wang HL, Liu ZC, Liu H, … Li Y (2014). Changes in tau phosphorylation levels in the hippocampus and frontal cortex following chronic stress. Brazilian Journal of Medical and Biological Research, 47(3), 237–244. 10.1590/1414-431X20133275 [DOI] [PMC free article] [PubMed] [Google Scholar]


