Abstract
Background
The NCCN Distress Thermometer (DT) uses a 10-point scale (0=none, 10=extreme) to measure patient-reported distress. We sought to examine the relationship between treatment and NCCN DT scores in breast cancer patients over time.
Methods
We included women≥18 diagnosed with Stage 0-IV breast cancer at a 3-hospital health system from January 2014 to July 2016. Linear mixed-effects models adjusted for covariates including stage, race/ethnicity, insurance, and treatment sequence (neoadjuvant vs adjuvant) were used to estimate adjusted mean changes in DT score (MSCs) per week for patients undergoing lumpectomy, mastectomy only, and mastectomy with reconstruction (MR).
Results
We analyzed 12,569 encounters for 1029 unique patients (median score=4; median follow-up=67 weeks). MR patients (n=118) were younger and more likely to be married, white, and privately insured than lumpectomy (n=620) and mastectomy-only (n=291) patients (all p<0.01). After adjusting for covariates, distress scores declined significantly across all 3 surgical cohorts, with MR patients’ having both the most preoperative distress and the greatest decline in distress prior to surgery (MSC/week: MR −0.073 vs Lumpectomy −0.031 vs mastectomy-only −0.033, p=0.001). Neoadjuvant therapy was associated with a longitudinal decline in distress for lumpectomy (−1.023) and mastectomy-only (−0.964) patients. Over time, DCIS (−0.503) and black race (−1.198) were associated with declining distress among lumpectomy and MR patients, respectively, while divorced mastectomy-only patients (0.948) and single lumpectomy patients (0.476) experienced increased distress (all p<0.05).
Conclusion
When examined longitudinally in consecutive patients, the NCCN DT can provide patient-reported data to inform expectations and guide targeted support for breast cancer patients.
Keywords: breast cancer, distress, health disparities, modifiable risk factors, patient-reported outcomes
Precis
The NCCN Distress Thermometer (DT) is a patient-reported outcome measure that was completed at 12,569 visits by 1029 women with breast cancer in a single health system over 2.5 years. When examined longitudinally in consecutive patients, the NCCN DT can provide patient-, disease-, and treatment-specific data about the cancer care trajectory, thereby informing expectations and guiding both targeted support and shared decision-making for breast cancer patients.
Introduction
The collection of patient-reported outcomes (PROs) is increasingly recognized as an important component of oncologic care. By providing patient perspective on the experience of illness, PROs complement the objectively defined measures of health and treatment success (e.g., survival and recurrence) typically collected by providers. Furthermore, PROs and the evidence-based interventions prompted by their collection have now been shown to facilitate improved compliance1,2 and fewer hospitalizations and emergency room (ER) visits in patients receiving chemotherapy,1 benefits that have translated into cost savings at the level of the health system3 and even into prolonged survival in cancer patients.4,5
The National Comprehensive Cancer Network (NCCN) Distress Thermometer (DT) and Problem List is an ultra-short PRO measure (PROM) first developed to rapidly screen for physical and psychosocial distress among prostate cancer patients.6,7 It has since been prospectively validated for various disease sites8–15 and in a diverse array of countries and languages16–26 around the world. “Distress” is a modifiable risk factor associated with both adverse patient outcomes2 and less shared decision-making10, defined by the NCCN as “a multifactorial unpleasant experience of a psychological (i.e., cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer.”27 At any given time, distress reflects an interplay between the intrinsic personality traits of respondents and their dynamic psychological states, but there is limited data as to what effect cancer treatments might have on patient distress levels over time.
Here, we describe our institutional experience following implementation of the NCCN DT as a tool for measuring patient distress in breast cancer patients. Specifically, we sought to determine whether treatment characteristics were associated with longitudinal differences in distress.
Methods
Data Sources
The NCCN DT is a single-item PROM consisting of a visual analog scale on a schematic thermometer; distress levels range in 1-unit increments from 0 (no distress) to 10 (extreme distress; eFigure 1).28 It is associated with a 39-item Problem List that allows patients to report potential sources of distress, grouped into 5 domains: practical, physical, familial, emotional, and spiritual/religious.
Since the introduction of an Epic-based electronic health record (EHR) at our institution in June 2013, clinics were charged with having nurses administer the NCCN DT to all patients at all visits (with the exception of encounters for administration of chemotherapy or radiation) as part of vitals collection prior to seeing physicians or advanced practice providers. Scores≥4, shown in previous studies to achieve optimal sensitivity and specificity for detecting distress,11,18,24,29 are considered clinically significant in our practice and require action, specifically, notification of the supervising provider and referral to one of several programs within Cancer Support Services depending on the specific items selected by the patient on the DT Problem List. Scores are entered into the patient’s EHR and can be abstracted in conjunction with other demographic and clinical information.
Cohort
Clinical data were collected from the EHR for all women≥18 years old with newly diagnosed Stage 0-IV breast cancer who were first seen at one of 3 hospitals – two in Durham and one in Raleigh – within the Duke Health System between January 2014 and July 2016 and had their first NCCN DT score recorded following diagnosis and prior to receiving any treatment. This cohort was cross-verified with Duke’s institutional tumor registry. The beginning of the inclusion period was selected to allow for a 6-month interval following system-wide implementation of the NCCN DT and new EHR in June 2013. Length of follow-up was at least 27 weeks (i.e., approximately 6 months) for all patients by the time of data analysis. Patients with recurrent disease, bilateral metachronous breast cancer (often indistinguishable from recurrent disease), missing data, unconfirmed surgery type, or who received all treatment outside of the Duke University Health System were excluded. Because a large proportion (>40%) of non-operative patients seen during this period had their first treatment before their first NCCN DT score was recorded, all patients who were managed non-operatively were excluded from our analysis to avoid any bias associated with the selective inclusion of non-operative patients for whom complete data were available.
Clinical Characteristics
For eligible patients, we abstracted DT scores for all visits during the study period as well as information on patient demographics (age, race/ethnicity, marital status, insurance status), treatments received (sequence and breast surgery type), and clinical TNM stage at diagnosis (based on the 7th edition of the American Joint Commission on Cancer [AJCC] Staging Manual).
Patients were divided into 3 cohorts based on the type of breast surgery they received: lumpectomy, mastectomy only (MO), and mastectomy with reconstruction (MR). The lumpectomy cohort included women who underwent lumpectomy, i.e., partial mastectomy, with or without oncoplastic closure. Using an intention-to-treat principle, we included patients who converted from lumpectomy to mastectomy (due, for example, to positive margins, discovery of a predisposing genetic mutation, or dissatisfaction with post-lumpectomy cosmesis) in the Lumpectomy cohort. The two mastectomy cohorts included patients who underwent simple or modified radical mastectomy and received (MR) or did not receive (MO) reconstruction during the inclusion period. Based on the receipt and timing of surgery, endocrine therapy (e.g., letrozole), immunologic therapy (e.g., trastuzumab), and/or chemotherapy (e.g., doxorubicin) relative to the date of diagnosis and other treatments, patients were further divided into 2 groups based on treatment sequence: (1) Adjuvant (i.e., surgery as initial line of treatment) and (2) Neoadjuvant (i.e., endocrine therapy, immunologic therapy, and/or chemotherapy prior to surgery).
Statistical Analyses
Kruskal-Wallis and chi-square tests were used to assess differences in continuous and categorical clinical characteristics, respectively, among patients grouped by type of breast surgery received (Lumpectomy, MR, and MO) and treatment sequence (Adjuvant and Neoadjuvant); we report median values with interquartile ranges (IQR) or proportions as appropriate.
Linear mixed effects models were used to estimate associations between DT scores and surgical treatment over time after adjustment for patient covariates including age at diagnosis, insurance type, marital status, race/ethnicity, and clinical stage as well as treatment sequence. For each patient, we included a random intercept to account for person-level correlation. We also analyzed changes in the DT score during the perioperative period, defined as 27 weeks before and 27 weeks after surgery. For each type of breast surgery (Lumpectomy, MO, or MR), we regressed DT score onto the time periods before and after surgery along with individual characteristics. We fitted a piecewise linear trend over time with a change point at the time of surgery via an interaction between time and the pre-/post-surgery indicator, thereby allowing for different trajectories in pre- and post-surgical DT scores as well as “jumps” in DT score at the first time point after surgery. We report adjusted mean DT scores and adjusted mean changes in DT score per week (MSC/week) with 95% CIs. We also report pre- and post-surgical trends in distress, which were compared with a likelihood ratio test, as well as changes in distress immediately following surgery.
A significance level of 0.05 was used for all analyses, which were performed in R version 5.0; the lme4 package was used for mixed effects modeling. Random effects were included in all adjusted models, as we felt their inclusion would provide a more robust approach for the handling of heterogeneous, non-randomly missing data than other methods such as imputation. Our study was approved by the Institutional Review Board (IRB) at Duke University (IRB protocol Pro00083052).
Results
We analyzed 12,569 encounters for 1029 unique patients (Table 1, Figure 1). Median age at diagnosis was 58. 65.2% of patients were white, 59.2% were married, 60.3% underwent lumpectomy, and 59.8% had early-stage (Stage 0-I) disease. Median length of follow-up after diagnosis was 67 weeks. Median DT score at first appointment was 4 (IQR 1-7), a level consistent with clinically significant distress at our institution. Unadjusted median scores were similar across the 3 surgical cohorts (p=0.13) and regardless of treatment sequence (p=0.55, eTable 1).
Table 1.
Newly Diagnosed Breast Cancer Patients with Baseline Visit at Duke, January 2014-July 2016 – Characteristics by Breast Surgery Type (n=1029)
| Lumpectomy | MO | MR | Total | p-value | |
|---|---|---|---|---|---|
| N | 620 | 291 | 118 | 1029 | |
| Age at Diagnosis (years) - median (interquartile range) | 61.0 (50.0 - 68.0) | 59.0 (48.0 - 66.0) | 46.0 (40.0 - 56.0) | 58.0 (48.0 - 67.0) | <0.001 |
| Average Score – median all timepoints (interquartile range) | 2.0 (0.9 - 3.4) | 2.0 (1.0 - 3.4) | 2.2 (1.2 - 3.6) | 2.0 (0.9 - 3.4) | 0.46 |
| Average Score Before Procedure - median (interquartile range) | 3.0 (1.0 - 5.6) | 3.0 (1.0 - 5.5) | 3.6 (2.0 - 5.9) | 3.0 (1.0 - 5.5) | 0.14 |
| Average Score After Procedure - median (interquartile range) | 1.4 (0.4 - 2.8) | 1.5 (0.4 - 3.0) | 1.7 (0.3 - 3.0) | 1.5 (0.4 - 2.8) | 0.81 |
| First Distress Score - median (interquartile range) | 4.0 (1.0 - 6.0) | 4.0 (1.0 - 7.0) | 5.0 (2.0 - 7.0) | 4.0 (1.0 - 7.0) | 0.13 |
| Number of Distress Scores - median (interquartile range) | 10.0 (7.0 - 15.0) | 14.0 (8.0 - 19.0) | 11.5 (6.0 - 17.0) | 11.0 (7.0 - 17.0) | <0.001 |
| Number of Distress Scores Before Procedure - median (interquartile range) | 2.0 (1.0 - 3.0) | 3.0 (2.0 - 8.0) | 2.0 (1.0 - 3.0) | 2.0 (1.0 - 4.0) | <0.001 |
| Number of Distress Scores After Procedure - median (interquartile range) | 7.0 (4.0 - 11.0) | 8.0 (5.0 - 13.0) | 7.5 (4.0 - 11.8) | 7.0 (4.0 - 11.0) | 0.01 |
| Weeks to First Surgery - median (interquartile range) | 7.3 (5.1 - 14.3) | 11.1 (6.9 - 24.5) | 7.9 (5.9 - 21.1) | 8.0 (5.7 - 20.1) | <0.001 |
| Marital Status | 0.001 | ||||
| Married | 353 (56.9%) | 164 (56.4%) | 92 (78.0%) | 609 (59.2%) | |
| Single | 96 (15.5%) | 57 (19.6%) | 11 (9.3%) | 164 (15.9%) | |
| Divorced | 82 (13.2%) | 31 (10.7%) | 11 (9.3%) | 124 (12.1%) | |
| Widowed | 56 (9.0%) | 26 (8.9%) | 1 (0.8%) | 83 (8.1%) | |
| Unknown | 33 (5.3%) | 13 (4.5%) | 3 (2.5%) | 49 (4.8%) | |
| Race/Ethnicity | 0.003 | ||||
| Non-Hispanic White | 388 (62.6%) | 189 (64.9%) | 94 (79.7%) | 671 (65.2%) | |
| Non-Hispanic Black | 169 (27.3%) | 74 (25.4%) | 14 (11.9%) | 257 (25.0%) | |
| Other | 53 (8.5%) | 17 (5.8%) | 8 (6.8%) | 78 (7.6%) | |
| Hispanic | 10 (1.6%) | 11 (3.8%) | 2 (1.7%) | 23 (2.2%) | |
| Insurance | |||||
| Private | 257 (41.5%) | 128 (44.0%) | 90 (76.3%) | 475 (46.2%) | <0.001 |
| Medicare | 305 (49.2%) | 127 (43.6%) | 17 (14.4%) | 449 (43.6%) | |
| Medicaid | 8 (1.3%) | 3 (1.0%) | 2 (1.7%) | 13 (1.3%) | |
| Other | 10 (1.6%) | 12 (4.1%) | 4 (3.4%) | 26 (2.5%) | |
| Unknown | 40 (6.5%) | 21 (7.2%) | 5 (4.2%) | 66 (6.4%) | |
| Treatment Group | <0.001 | ||||
| Adjuvant/Surgery First | 543 (87.6%) | 220 (75.6%) | 103 (87.3%) | 866 (84.2%) | |
| Neoadjuvant | 77 (12.4%) | 71 (24.4%) | 15 (12.7%) | 163 (15.8%) | |
| TNM Clinical Stage | <0.001 | ||||
| Clinical Stage 0 | 103 (16.6%) | 26 (8.9%) | 22 (18.6%) | 151 (14.7%) | |
| Clinical Stage I | 355 (57.3%) | 67 (23.0%) | 42 (35.6%) | 464 (45.1%) | |
| Clinical Stage II | 145 (23.4%) | 133 (45.7%) | 45 (38.1%) | 323 (31.4%) | |
| Clinical Stage III | 12 (1.9%) | 57 (19.6%) | 9 (7.6%) | 78 (7.6%) | |
| Clinical Stage IV | 5 (0.8%) | 8 (2.7%) | 0 (0.0%) | 13 (1.3%) | |
MO, mastectomy only. MR, mastectomy with reconstruction
Figure 1.
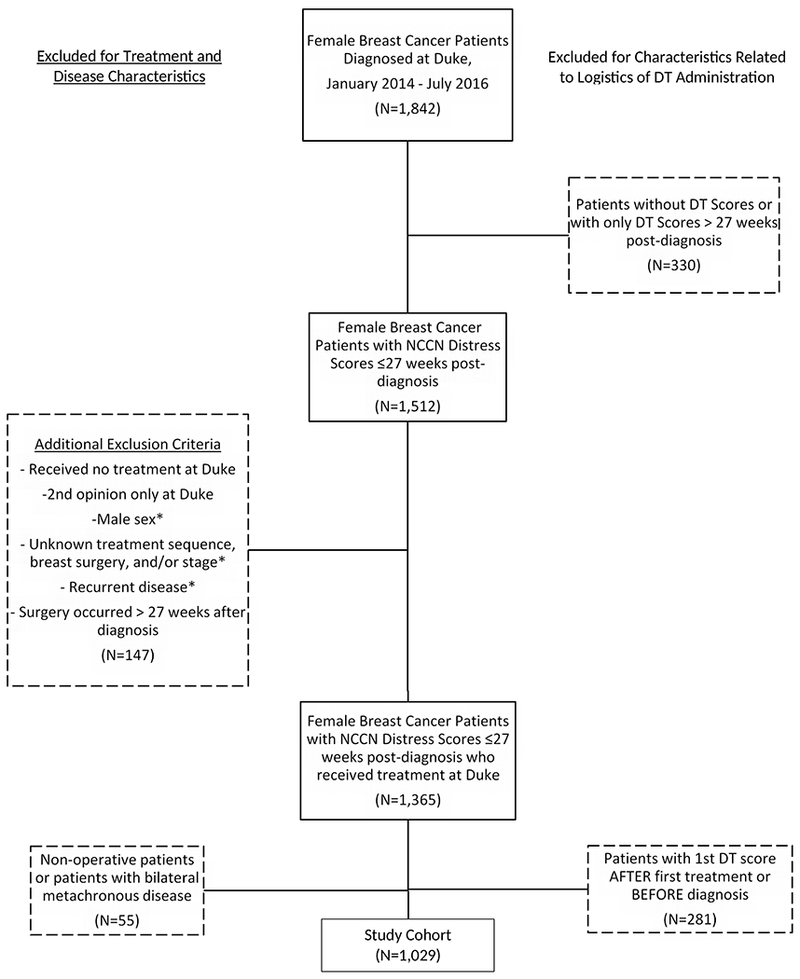
Patient Flow Diagram - Newly Diagnosed Breast Cancer Patients at Duke, January 2014-July 2016
DT, distress thermometer. *Confirmed on manual reveiw
Patients undergoing MR (n=118) were significantly younger than those undergoing Lumpectomy (n=620) and MO (n=291) patients (p<0.001). As compared to Lumpectomy and MO patients, a greater proportion of MR patients were married (MR 78% vs Lumpectomy 56.9% and MO 56.4%, p=0.001) and white (MR 79.7% vs Lumpectomy 62.6% and MO 64.9%, p=0.003). Patients undergoing Lumpectomy had the highest rates of early-stage (Stage 0-1) disease (73.9%) followed by MR (54.2%) and MO patients (31.9%, p<0.001)
A majority of patients (84.2%, n=866) received surgery first, with higher rates of neoadjuvant treatment in patients who underwent MO (24.4%) as compared to those ultimately undergoing Lumpectomy (12.4%) and MR (12.7%, p<0.001). Patients undergoing surgery first were older than those receiving neoadjuvant treatment (median age 59 vs 55, p<0.001, eTable 1). As expected, a majority of patients (87.7%) who underwent neoadjuvant treatment had higher stage (II-IV) disease (p<0.001). A higher proportion of non-white patients received neoadjuvant treatment as compared to white patients. Notably, blacks constituted ¼ of the entire cohort but made up 44% of those receiving neoadjuvant treatment (p<0.001). Patients undergoing mastectomy waited approximately 2 weeks longer than those undergoing lumpectomy to have surgery (9.9 vs 7.3 weeks, p<0.001), but this difference was driven by longer wait times among MO patients, who had higher rates of neoadjuvant therapy and waited a median of 11.1 weeks to undergo surgery while those receiving reconstruction waited a median of 7.9 weeks (p<0.001), a length of time comparable to the median wait time of 7.3 weeks seen among lumpectomy patients. Consistent with these differences in time-to-surgery, MO patients had a greater number of DT scores recorded before and after surgery as compared to lumpectomy and MR patients (Table 1, both p<0.05).
When adjusted for stage, treatment sequence, age, marital status, type of insurance, and race/ethnicity, we observed an overall reduction in distress scores over time for all patients. However, we observed different longitudinal trends between the 3 surgical cohorts. MR patients had both the highest preoperative levels of distress and the greatest decline in distress prior to surgery (MSC/week: MR −0.073 vs Lumpectomy −0.031 vs MO −0.033, p=0.001, Table 2, Figure 2). Patients in all 3 cohorts experienced significant declines in distress immediately after surgery (Lumpectomy −1.148, MO −0.711, MR −0.897, all p<0.001) and continued to experience a decline in surgery throughout the postoperative period, but in contrast to the preoperative period, rates of change for the 3 cohorts were similar postoperatively, with scores ultimately converging at a distress level of approximately 2 (p=0.37).
Table 2.
Linear Mixed-Effects Models Estimating the Association between Changes in NCCN Distress Thermometer Scores Over Time and Type of Breast Surgery, January 2014-July 2016 (n=1029)
| Lumpectomy | Mastectomy Only | Mastectomy + Reconstruction | ||||
|---|---|---|---|---|---|---|
| MSC/week (95% CI) | p-value | MSC/week (95% CI) | p-value | MSC/week (95% CI) | p-value | |
| Trend in Distress Pre-Surgery* | −0.031 (−0.043, −0.019) | <0.001 | −0.033 (−0.046, −0.020) | <0.001 | −0.073 (−0.102,−0.045) | <0.001 |
| Change Immediately after Surgery | −1.148 (−1.309, −0.988) | <0.001 | −0.711 (−0.919, −0.504) | <0.001 | −0.897 (−1.280,−0.514) | <0.001 |
| Trend in Distress After Surgery** | −0.005 (−0.007,−0.003) | <0.001 | −0.003 (−0.006, −0.001) | 0.01 | −0.010 (−0.014,−0.005) | <0.001 |
| Lumpectomy | Mastectomy Only | Mastectomy + Reconstruction | ||||
| Difference in MSC/week* (95% CI) | p-value | Difference in MSC/week* (95% CI) | p-value | Difference in MSC/week* (95% CI) | p-value | |
| Age at Diagnosis (per 1 year) | −0.007 (−0.025, 0.011) | 0.42 | 0.006 (−0.029,0.018) | 0.64 | −0.007 (−0.042,0.029) | 0.71 |
| Marital status | ||||||
| Married | -REF- | - | -REF- | - | -REF- | - |
| Single | 0.476 (0.071, 0.882) | 0.02 | 0.500 (−0.075,1.075) | 0.09 | 0.665 (−0.478,1.808) | 0.26 |
| Divorced | 0.282 (−0.151, 0.715) | 0.20 | 0.948 (0.226,1.671) | 0.01 | −0.001 (−1.180,1.178) | 0.99 |
| Widowed | 0.171 (−0.352, 0.693) | 0.52 | 0.436 (−0.393,1.265) | 0.30 | −0.572 (−4.435,3.290) | 0.77 |
| Unknown | 0.329 (−0.302, 0.959) | 0.31 | −0.106 (−1.237,1.024) | 0.85 | 0.565 (−1.731,2.861) | 0.63 |
| Treatment Categories: | ||||||
| Adjuvant | -REF- | - | -REF- | - | -REF- | - |
| Neoadjuvant | −1.023 (−1.499, −0.548) | <0.001 | −0.964 (−1.489,−0.439) | <0.001 | 0.132 (−0.924,1.188) | 0.81 |
| Race/Ethnicity: | ||||||
| Non-Hispanic White | -REF- | - | -REF- | - | -REF- | - |
| Non-Hispanic Black | −0.206 (−0.538, 0.127) | 0.23 | −0.376 (−0.895,0.143) | 0.16 | −1.198 (−2.233,−0.162) | 0.03 |
| Other | −0.197 (−0.713, 0.319) | 0.45 | 0.225 (−0.728,1.178) | 0.64 | −1.544 (−3.029,−0.059) | 0.04 |
| Hispanic | 0.030 (−1.090, 1.151) | 0.96 | −0.709 (−1.970,0.551) | 0.27 | −0.617 (−3.395,2.161) | 0.66 |
| Insurance | ||||||
| Private | -REF- | - | -REF- | - | -REF- | - |
| Medicare | −0.001 (−0.412,0.411) | 0.99 | 0.491 (−0.100,1.082) | 0.10 | 0.564 (−0.533,1.661) | 0.32 |
| Medicaid | 0.975 (−0.243,2.193) | 0.12 | 5.151 (2.995,7.306) | <0.001 | 2.175 (−0.788,5.138) | 0.15 |
| Other | −0.082 (−1.205,1.040) | 0.89 | 0.094 (−1.073,1.261) | 0.87 | 0.285 (−1.552,2.123) | 0.76 |
| Unknown | −0.012 (−0.590,0.566) | 0.97 | 0.963 (0.103,1.824) | 0.03 | 0.457 (−1.134,2.048) | 0.57 |
| TNM Clinical Stages: | ||||||
| Clinical Stage 0 | −0.503 (−0.896, −0.109) | 0.01 | −0.581 (−1.469,0.308) | 0.20 | 0.328 (−0.634,1.291) | 0.51 |
| Clinical Stage I | -REF- | - | -REF- | - | -REF- | - |
| Clinical Stage II | −0.022 (−0.390, 0.347) | 0.91 | −0.181 (−0.749,0.387) | 0.53 | −0.596 (−1.390,0.198) | 0.14 |
| Clinical Stage III | −0.642 (−1.658, 0.374) | 0.22 | −0.064 (−0.763,0.635) | 0.86 | −0.454 (−1.734,0.827) | 0.49 |
| Clinical Stage IV | −0.801 (−2.375, 0.772) | 0.32 | −0.892 (−2.242,0.457) | 0.20 | - | - |
MSC/week, mean score change per week. *vs reference group. Models are adjusted for age, marital status, race/ethnicity, clinical stage, and treatment group. Highlighted cells contain statistically significant findings.
Likelihood ratio test p=0.001 among treatment groups.
Likelihood ratio test p=0.37 among treatment groups.
Figure 2.
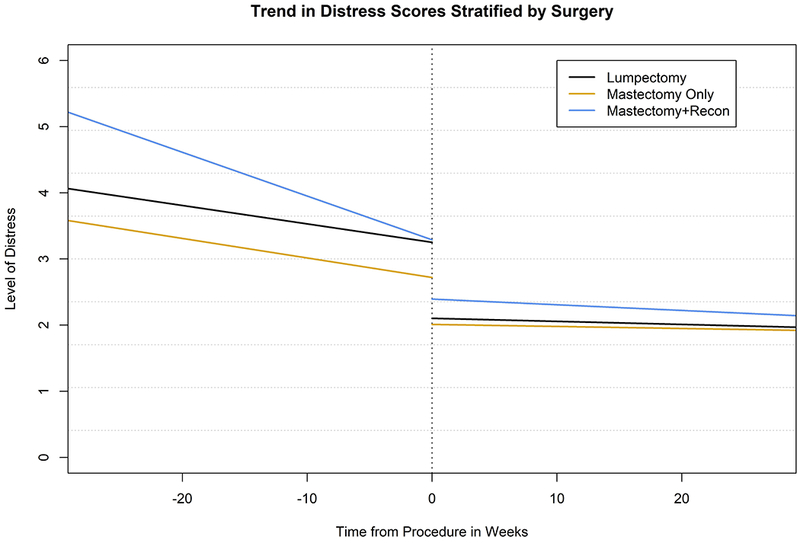
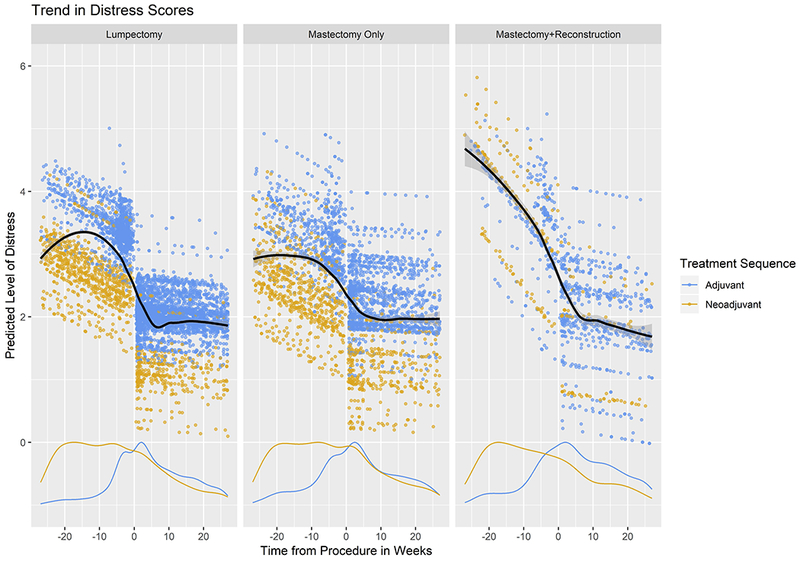
Adjusted Mean Change in NCCN Distress Thermometer Score per Week, Breast Cancer Patients at Duke, January 2014-July 2016 (n=1029): (a) Linear Trend Plot (b) Scatter Plot with Loess Smoothing and Scaled Density Curve – Treatment Sequence
Among lumpectomy patients, being single (vs married) was associated with worsening distress over time (0.476), while receipt of neoadjuvant therapy (−1.023) vs having surgery first and having noninvasive cancer, i.e., ductal carcinoma in situ (DCIS, −0.503) as opposed to Stage I disease were associated with declining distress over time (all p<0.05). Among patients undergoing mastectomy without reconstruction, being divorced (0.948) vs being married and having Medicaid (5.151) as opposed to having private insurance were associated with worsening distress over time, while receipt of neoadjuvant therapy was again associated with improvement in distress over time (−0.964, all p<0.05). Among mastectomy patients undergoing reconstruction, non-Hispanic black race (−1.198) as compared to non-Hispanic white race was the only characteristic associated with improved distress over time (p=0.03).
To address potential selection bias, we compared the 813 patients who were excluded due to missing/incomplete treatment information and/or NCCN DT scores to the 1029 patients included in our analysis and found that excluded patients included a significantly higher proportion of patients with Stage IV disease (excluded 13.8% vs included 1.3%), fewer patients with private insurance (excluded 22.2% vs included 46.2%), and fewer patients who received surgery at the main hospital within our system (excluded 33.2% vs included 95.8%, all p<0.001).
Discussion
Our study is the first to demonstrate an association between NCCN DT scores and type of surgical procedure among patients with breast cancer or any other malignancy and the largest outside of a clinical trial context to examine longitudinal patient-reported distress using the NCCN DT. We found that many newly diagnosed breast cancer patients had clinically significant distress levels at presentation, but that over time, distress levels eventually declined over time to low levels for a majority of women. However, we also found that there were patient-, disease-, and treatment-specific differences in how distress changed over time, revealing potentially vulnerable groups of women – those who are unmarried and those with Medicaid – for whom additional targeted support may be needed. Finally, our study illustrates the real-world benefits and challenges of incorporating PROMs into routine oncologic practice, even with a simple instrument such as the NCCN DT. While there is limited data in smaller studies that the NCCN DT is a valid tool for assessing distress throughout treatment and survivorship,14,18 our goal was to examine the reliability of the NCCN DT as a routine and stable indicator of the patient experience over time and across modalities of treatment. We are reassured by the relatively high intra-class correlation coefficients for the overall study cohort before (~0.6) and after (~0.4) surgery, thereby demonstrating point-to-point reliability of DT score measures over time.
After adjusting for known covariates, patients at our institution who ultimately chose to pursue mastectomy with reconstruction had higher baseline levels of distress than patients undergoing lumpectomy and mastectomy without reconstruction, a finding that may reflect the complex interplay between trait and state psychological factors in patients making treatment decisions. Elevated distress has been shown to be associated with pre-existing depression and pre-diagnosis utilization of psychosocial support services in breast cancer patients,13,30 and notably, many women cite “peace of mind” as a reason for choosing both unilateral therapeutic and contralateral risk-reducing mastectomy.31,32 But given that both pre-existing anxiety and body-image concerns33,34 have been more commonly observed among women opting for mastectomy with reconstruction, it may be that peace of mind is, at baseline, already disproportionately prioritized by women who choose this surgical option. Nevertheless, we were reassured by the significant decline in distress experienced by MR women over time, and, notably, that this decline was particularly pronounced among black women. Accordingly, our study demonstrates that women considering mastectomy with reconstruction should be counseled that it may take some time for them to experience the anticipated reduction in psychosocial distress that may be driving their surgical decision-making but that such peace of mind is potentially achievable with sufficient support and resources.
Of note, receipt of neoadjuvant treatment was associated with a greater decline in perioperative distress among patients who underwent lumpectomy and mastectomy without reconstruction as compared to those who received surgery first (Figure 2), a finding that may reflect that these patients had more time to come to terms with their diagnosis prior to surgery. While there is some evidence that receipt of neoadjuvant therapy may influence surgical decision-making among breast cancer patients,35 an association between neoadjuvant chemotherapy and distress levels was not observed among patients receiving mastectomy with reconstruction in the current study, likely to due to the relatively small number (15) of neoadjuvant patients undergoing reconstruction within 6 months of surgery.
Across all 3 surgical cohorts in our study, the adjusted mean distress score of patients was >4, which is the threshold for clinically significant distress in our health system and throughout the literature,11,18,24,29 though a few recent studies have suggested that a cutoff of 7 is potentially more accurate in identifying patients at high risk for chronic distress and need for clinical intervention.13,30 At our institution, the NCCN DT is administered routinely as part of the patient intake process and is used to assist in making referrals to various cancer support service programs including Child Life, Financial Care Counseling, Patient Navigation, and on-site counseling with licensed therapists for patients with distress scores ≥4; from our own internal tracking system, we estimate that approximately 85% of patients are appropriately triaged to one or more support services based on the DT score they provide at clinic intake. Being unmarried and having Medicaid were both associated with potential worsening of distress over time, and these findings represent an opportunity for us to provide targeted support to these women, who may have less support and more logistical barriers to care. The impact of such targeted interventions on DT scores is the focus of a separate ongoing analysis.
This study provides important insights into the real-world challenges and opportunities afforded by the implementation of routine PROs into clinic flow. Notably, although completion of the NCCN DT at every non-treatment encounter is the goal, nearly 20% of potentially eligible patients (330/1842, Figure 1) had no NCCN scores recorded within the first 6 months following diagnosis. A significant proportion also did not have their first score recorded until after receiving their first cancer treatment, even as most of these treatments were administered at our institutions. These statistics reflect not only the realities of variation within clinical care but also the need for standardized processes and ample resources to allow for longitudinal data collection in the context of a system-wide EHR.
We also noted important differences among different patient populations. Patients with metastatic breast cancer were overrepresented among patients excluded due to missing treatment information but underrepresented in our study cohort relative to other population-based analyses;36 these patients with Stage IV disease represent a highly heterogeneous population, from patients with bony metastases for many years to those succumbing to visceral involvement shortly after presentation. A majority of these patients were also managed non-operatively, and we chose to exclude non-operative patients from our analysis due to high rates of missing data. Our review highlights the need for our clinical teams to provide more consistent PRO documentation for this physically and psychologically vulnerable group in order to provide appropriate interventions as needed. At the other end of the clinical spectrum, in patients undergoing lumpectomy, having DCIS was associated with a greater decline in distress over time as compared to having Stage I disease, a finding that may provide further support for the idea that DCIS – as a disease and as a lived experience – is fundamentally different from invasive disease and may not necessitate all of the treatment most patients receive. We expect that PROMs completed as part of clinical trials focused on the feasibility of de-escalating treatment for DCIS will provide prospective validation for our findings.37
Limitations
Despite the prospective collection of DT scores, limitations of our study include those inherent to a retrospective analysis including selection bias. In addition, because this analysis was performed using data from an EHR that was not designed for research purposes, several patients were excluded from our analysis due to missing data elements. As described above, we compared these excluded patients to our cohort, and we recognize that our conclusions need to be considered and applied with these differences in mind. Indeed, we hope to use our findings of nonrandom missingness to improve our clinical practice, with plans for special attention to enhancing the continuity of care and consistency of PRO documentation among patients with metastatic and non-operative disease. We also acknowledge that comparison of DT scores between groups was performed using linear regression to determine statistical significance but that much remains understudied regarding the magnitude of difference that determines clinical significance. Better characterizing these differences will be the subject of future work evaluating PROMs in our health system. Furthermore, follow-up in our study was only limited to 6 months; additional important work is planned to confirm the applicability of the NCCN DT to long-term survivors, according to work that has been reported in smaller populations by others.14 Finally, we describe our experience at an academic, comprehensive cancer center that includes 3 different facilities and acknowledge that our conclusions might not be applicable to the clinical practice of breast oncology outside of university-based settings such as our own.
Conclusions
In summary, our study demonstrates the utility of the NCCN DT in providing longitudinal assessment of distress in breast cancer patients. Our experience demonstrates the potential of the NCCN DT as a simple but powerful tool that can be readily applied to routine oncologic care for malignancies of the breast and potentially other disease sites even in resource-limited, time-constrained contexts. At Duke, it has been successfully used as an initial screen for distress in the ambulatory cancer setting. We now hope to use it as a means to proactively identify those who may need additional resources at various points throughout treatment, thereby providing a source of cohort-level data that can assist providers and patients in the pursuit of shared treatment decision-making and appropriate resource allocation.
Supplementary Material
Acknowledgements
The authors would like to thank Heather Sperling, RN, MSN, former nurse manager for the Duke Breast Clinic and Kristy L. Everette, LRT/CTRS, Supervisor of Clinical Operations for the Duke Cancer Patient Support Program for their contributions to delineating the flow of patient care. Portions of this study were presented in a podium presentation at the 2018 Annual Meeting of the Society of Surgical Oncology on March 23, 2018.
Funding
Dr. Fayanju is supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Number 1KL2TR002554 (PI: Svetkey). Dr. Goldstein is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K25DK097279. This publication was made possible (in part) by Grant Number UL1TR001117 from NCATS, the NIH Roadmap for Medical Research, the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan), and philanthropic funds through the generosity of Sara and Bruce Brandaleone. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of NIH or any of its components.
Footnotes
Conflicts of Interest to Disclose: None
References
- 1.Basch E, Deal AM, Kris MG, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yee MK, Sereika SM, Bender CM, Brufsky AM, Connolly MC, Rosenzweig MQ. Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer. 2017;123(11):2061–2069. [DOI] [PubMed] [Google Scholar]
- 3.Simpson JS, Carlson LE, Trew ME. Effect of group therapy for breast cancer on healthcare utilization. Cancer practice. 2001;9(1):19–26. [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. Jama. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(8):1355–1363. [DOI] [PubMed] [Google Scholar]
- 6.Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA: a cancer journal for clinicians. 2007;57(5):278–300. [DOI] [PubMed] [Google Scholar]
- 7.Reeve BB, Mitchell SA, Dueck AC, et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. Journal of the National Cancer Institute. 2014;106(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: Prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegel MT, Collins ED, Kearing S, Gillock KL, Moore CP, Ahles TA. Sensitivity and specificity of the Distress Thermometer for depression in newly diagnosed breast cancer patients. Psycho-Oncology. 2008;17(6):556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegel MT, Moore CP, Collins ED, et al. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. 2006;107(12):2924–2931. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RL, Gold MA, Wyche KF. Distress in women with gynecologic cancer. Psycho-Oncology. 2010;19(6):665–668. [DOI] [PubMed] [Google Scholar]
- 12.Keir ST, Calhoun-Eagan RD, Swartz JJ, Saleh OA, Friedman HS. Screening for distress in patients with brain cancer using the NCCN’s rapid screening measure. Psycho-Oncology. 2008;17(6):621–625. [DOI] [PubMed] [Google Scholar]
- 13.Ploos van Amstel FK, Tol J, Sessink KH, van der Graaf WTA, Prins JB, Ottevanger PB. A Specific Distress Cutoff Score Shortly After Breast Cancer Diagnosis. Cancer Nursing. 2017;40(3):E35–E40. [DOI] [PubMed] [Google Scholar]
- 14.Ploos van Amstel FK, van den Berg SW, van Laarhoven HWM, Gielissen MFM, Prins JB, Ottevanger PB. Distress screening remains important during follow-up after primary breast cancer treatment. Supportive Care in Cancer. 2013;21(8):2107–2115. [DOI] [PubMed] [Google Scholar]
- 15.Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82(10):1904–1908. [DOI] [PubMed] [Google Scholar]
- 16.Beck KR, Tan SM, Lum SS, Lim LEC, Krishna LKR. Validation of the emotion thermometers and hospital anxiety and depression scales in Singapore: Screening cancer patients for distress, anxiety and depression. Asia-Pacific Journal of Clinical Oncology. 2016;12(2):e241–e249. [DOI] [PubMed] [Google Scholar]
- 17.Bultz BD, Carlson LE. Emotional distress: the sixth vital sign in cancer care. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(26):6440–6441. [DOI] [PubMed] [Google Scholar]
- 18.Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psycho-Oncology. 2008;17(6):538–547. [DOI] [PubMed] [Google Scholar]
- 19.Gil F, Grassi L, Travado L, Tomamichel M, Gonzalez JR. Use of distress and depression thermometers to measure psychosocial morbidity among southern European cancer patients. Supportive Care in Cancer. 2005;13(8):600–606. [DOI] [PubMed] [Google Scholar]
- 20.Groff S, Holroyd-Leduc J, White D, Bultz BD. Examining the sustainability of Screening for Distress, the sixth vital sign, in two outpatient oncology clinics: A mixed-methods study. Psychooncology. 2018;27(1):141–147. [DOI] [PubMed] [Google Scholar]
- 21.Mertz BG, Bistrup PE, Johansen C, et al. Psychological distress among women with newly diagnosed breast cancer. European Journal of Oncology Nursing. 2012;16(4):439–443. [DOI] [PubMed] [Google Scholar]
- 22.Omran S, Saeed AM, Simpson J. Symptom distress of Jordanian patients with cancer receiving chemotherapy. International journal of nursing practice. 2012;18(2):125–132. [DOI] [PubMed] [Google Scholar]
- 23.Özalp E, Cankurtaran ES, Soygür H, Özdemir Geyik P, Jacobsen PB. Screening for psychological distress in Turkish cancer patients. Psycho-Oncology. 2007;16(4):304–311. [DOI] [PubMed] [Google Scholar]
- 24.Shim E-J, Shin Y-W, Jeon HJ, Hahm B-J. Distress and its correlates in Korean cancer patients: pilot use of the distress thermometer and the problem list. Psycho-Oncology. 2008;17(6):548–555. [DOI] [PubMed] [Google Scholar]
- 25.Tuinman MA, Gazendam-Donofrio SM, Hoekstra-Weebers JE. Screening and referral for psychosocial distress in oncologic practice: use of the Distress Thermometer. Cancer. 2008;113(4):870–878. [DOI] [PubMed] [Google Scholar]
- 26.Zainal N, Hui K, Hang T, Bustam A. Prevalence of distress in cancer patients undergoing chemotherapy. Asia-Pacific Journal of Clinical Oncology. 2007;3(4):219–223. [Google Scholar]
- 27.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Distress Management, Version 2.2018 2018; http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Accessed 20 March 2018.
- 28.Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Annals of surgical oncology. 2007;14(12):3328–3334. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494–1502. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal J, Powers K, Pappas L, et al. Correlates of elevated distress thermometer scores in breast cancer patients. Supportive Care in Cancer. 2013;21(8):2125–2136. [DOI] [PubMed] [Google Scholar]
- 31.Collins ED, Moore CP, Clay KF, et al. Can Women With Early-Stage Breast Cancer Make an Informed Decision for Mastectomy? Journal of Clinical Oncology. 2009;27(4):519–525. [DOI] [PubMed] [Google Scholar]
- 32.Katz SJ, Morrow M. Contralateral prophylactic mastectomy for breast cancer: Addressing peace of mind. Jama. 2013;310(8):793–794. [DOI] [PubMed] [Google Scholar]
- 33.Montebarocci O, Dato FL, Baldaro B, Morselli P, Rossi NCF. Anxiety and Body Satisfaction before and Six Months after Mastectomy and Breast Reconstruction Surgery. Psychological Reports. 2007;101(1):100–106. [DOI] [PubMed] [Google Scholar]
- 34.Kwait RM, Pesek S, Onstad M, et al. Influential Forces in Breast Cancer Surgical Decision Making and the Impact on Body Image and Sexual Function. Annals of Surgical Oncology. 2016;23(10):3403–3411. [DOI] [PubMed] [Google Scholar]
- 35.Kantor O, Ajmani G, Wang C-H, Datta A, Yao K. The Shifting Paradigm for Breast Cancer Surgery in Patients Undergoing Neoadjuvant Chemotherapy. Annals of Surgical Oncology. 2018;25(1):164–172. [DOI] [PubMed] [Google Scholar]
- 36.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the united states. Jama. 2015;313(2):165–173. [DOI] [PubMed] [Google Scholar]
- 37.Youngwirth LM, Boughey JC, Hwang ES. Surgery versus monitoring and endocrine therapy for low-risk DCIS: The COMET Trial. Bulletin of the American College of Surgeons. 2017;102(1):62–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


