Abstract
Purpose
This study aimed to identify biomarkers of resistance to endocrine therapy in ER+ breast cancers (BC) treated with prolonged neoadjuvant letrozole.
Experimental Design
We performed targeted DNA and RNA-sequencing in 68 ER+ BC from patients treated with preoperative letrozole (median 7 months).
Results
Twenty-four tumors (35%) exhibited a PEPI score ≥4 and/or recurred after a median of 58 months and were considered endocrine resistant. Integration of the 47 most upregulated genes (log FC>1, FDR<0.03) in letrozole-resistant tumors with transcription binding data showed significant overlap with 20 E2F4-regulated genes (p=2.56E-15). In patients treated with the CDK4/6 inhibitor palbociclib before surgery, treatment significantly decreased expression of 24 of the 47 most upregulated genes in letrozole-resistant tumors, including 18 of the 20 E2F4 target genes. In long term estrogen-deprived ER+ BC cells, palbociclib also downregulated all 20 E2F4-target genes and P-RB levels, whereas the ER downregulator fulvestrant or paclitaxel only partially suppressed expression of this set of genes and had no effect on P-RB. Finally, an E2F4 activation signature was strongly associated with resistance to aromatase inhibitors in the ACOSOG Z1031B neoadjuvant trial and with an increased risk of relapse in adjuvant treated ER+ tumors in METABRIC.
Conclusions
In tumors resistant to prolonged neoadjuvant letrozole, we identified a gene expression signature of E2F4 target activation. CDK4/6 inhibition suppressed E2F4 target gene expression in estrogen-deprived ER+ BC cells and in patients’ ER+ tumors, suggesting a potential benefit of adjuvant CDK4/6 inhibitors in patients with ER+ breast cancer who fail to respond to preoperative estrogen deprivation.
Keywords: Neoadjuvant endocrine therapy, E2F4, CDK4/6 inhibitors
INTRODUCTION
Most breast cancers express estrogen receptors (ER+) and are diagnosed in post-menopausal women (1). Therapeutic estrogen deprivation by use of aromatase inhibitors (AIs) has been shown to reduce the risk of relapse and death after curative surgery (2). However, ~20% of patients with ER+ breast cancer eventually relapse suggesting mechanisms of de novo or acquired antiestrogen resistance to explain these recurrences (3).
Neoadjuvant trials with antiestrogens offer an opportunity to interrogate mechanisms of drug resistance that, in turn, could inform the choice of adjuvant therapy. Most of these neoadjuvant endocrine therapy studies have profiled pre-treatment tumor biopsies to investigate genomic alterations associated with response. For example, Ellis et al. performed whole genome sequencing of 77 early breast cancers before treatment with neoadjuvant letrozole. This study revealed that TP53 mutations are associated with drug resistance and that poorly responding tumors harbor more structural variations and mutations than sensitive tumors (4). However, profiling tumors after estrogen deprivation therapy instead of before treatment might be more clinically relevant since it would integrate both the intrinsic biology of the tumor and the response to endocrine therapy. For example, three neoadjuvant trials (5,6,7) have shown that maintenance of a high tumor cell proliferation, as measured by Ki67 immunohistochemistry (IHC) in a biopsy obtained 2 weeks after treatment with an AI, is associated with an increased risk of cancer recurrence. Although some short term presurgical studies have identified druggable alterations associated with drug resistance, defined as a high on treatment (~2-week) Ki67 score, only minor changes in variant allele frequency were detected between the baseline biopsy and drug-treated surgical specimen (8). This suggests the possibility that a short course of neoadjuvant endocrine therapy may only capture de novo mechanisms of antiestrogen resistance but fail to identify mechanisms of acquired resistance, such as the clonal expansion of drug-tolerant cells driven by resistant mutations and/or gene expression changes. Miller et al. (9) used whole genome sequencing and RNA-seq in 22 ER+ breast cancers before and after 4 months of estrogen deprivation with an AI showing significant temporal and spatial heterogeneity and a subclonal tumor composition that markedly changed upon treatment. Another study of gene expression of 18 matched pairs before and after three months of letrozole showed treatment-induced enrichment of cells with tumor-initiating and mesenchymal signatures (10). Taken together, these studies suggest that longer durations of therapy may be required to assess the full impact of AI-induced estrogen deprivation on the selection of drug-resistant populations.
We report herein a study where we performed targeted DNA sequencing and whole transcriptome analysis on whole tumor sections from a cohort of 68 operable ER+ breast cancers treated with the aromatase inhibitor letrozole for a median of 7.2 months before surgery and a with a median follow up of 5 years. To define endocrine-resistant tumors, we used breast cancer relapse and the preoperative endocrine prognostic index (PEPI). This is a well validated independent prognostic factor in the setting of neoadjuvant endocrine therapy that evaluates post-treatment ER levels, Ki67 score, tumor size and axillary lymph nodal status (11). By incorporating data from treated surgical specimens rather than core biopsies, the PEPI score is less impacted by spatial intra-tumor heterogeneity (8) and may represent a strong surrogate of multiple drug-tolerant clonal populations.
MATERIALS AND METHODS
Patients and tumor specimens
Tumors were from a cohort of newly diagnosed elderly patients with newly diagnosed, operable ER+/HER breast cancer treated with neoadjuvant letrozole at the Instituto Valenciano de Oncología in Valencia, Spain (12). Patients gave informed consent according to a protocol approved by the Instituto Valenciano de Oncología Institutional Review Board. They all underwent definitive surgery and had available tumor material for study endpoints. Patients were followed with serial ultrasound every 2–3 months during their preoperative treatment. Response to neoadjuvant therapy was annotated according to RECIST response criteria (13). Tumor specimens were promptly fixed after acquisition in 10% neutral buffered formalin for 18 to 24 h and embedded in paraffin. Immunohistochemistry (IHC) was conducted in formalin-fixed, paraffin-embedded (FFPE) tumor blocks from both the diagnostic biopsy and the post-treatment whole surgical specimen. Tumor sections were subjected to IHC using Ki67 (Dako #M7240), ER (Santa Cruz #sc542), PR (Dako #M3569), and HER2 (Cell Signaling #2242) antibodies according to methods reported elsewhere (14). FFPE tumor sections were scanned at 100x magnification, and the area containing the highest number of Ki67 positive cells was selected. Positive and negative tumor cells were manually counted at 400x; the percentage of positive cells was calculated with at least 1,000 viable cells. Ki67 IHC was scored by two independent expert breast pathologists (MVE and JMG). Patients were assigned a score according to the Preoperative Endocrine Prognostic Index (PEPI score: composite score of post-treatment ER, Ki67, T and N status) (11).
Statistical Considerations
Comparisons between groups were performed using the t-test for continuous variables and Fisher's exact test for categorical variables. Time to breast cancer recurrence (BCR) was defined as the time from surgery to first local, regional, or distant disease recurrence. Patients without documented disease recurrence were censored at the date of their last disease evaluation. Time to BCR was estimated using the Kaplan-Meier method. Cox modeling was used to assess whether time to BCR differed with respect to PEPI status or E2F4 activation. All statistical tests were two sided, and differences were considered statistically significant when p<0.05. False discovery rate (FDR), used for correcting p-values for multiple hypothesis testing, was computed using the Benjamini-Hochberg procedure. For gene expression analysis in POP trial, we used an ANCOVA model to assess the effect of the treatment arms on the change from baseline to surgery. The covariates included in the model were the gene expression at baseline and the treatment arm. RT-PCR were performed in biological duplicate and results are expressed as means±s.e.m. R version 3.3.0 and GradPad prism version 6 were used for the statistical analyses and visual representations.
DNA and RNA extraction
DNA and RNA were extracted from 4–8 10-μm unstained whole FFPE tumor sections from surgical specimens (see supplementary methods)
DNA Targeted Cancer Gene Sequencing
DNA alterations in 303 cancer-related genes using targeted exon capture by hybridization followed by next gen sequencing (NGS) were interrogated. Our custom design panel also included probes targeting common polymorphisms tiled throughout the genome to assist in identification of copy number changes. Custom design was performed using the Nimblegen Nimble Design software (see supplementary methods).
Variant calling
To identify all variants in the samples, we used the GATK Haplotype Caller (15) for SNVs and indels. All reads with a mapping quality less than 70 were removed. Variants were annotated with ANNOVAR (16) using the genes’ canonical transcripts as defined by Ensembl. Custom scripts were written to identify variants affecting splice sites using exon coordinates provided by Ensembl. Any spurious variant call with suspicious sequencing artifacts was removed. All SNVs and indels present in ExAC (17) with a population alternate allele frequency >0.1%, that were not present in COSMIC, were considered germline and subsequently removed. We also removed variants with allele frequency between 0.45–0.55, if not present in COSMIC. As a result, we obtained 330 non-synonymous mutations in 153 genes. Mean depth of coverage across all samples was 319X (min: 25, max: 597) (Fig. Suppl. S2 & Suppl. Table S7
Driver and actionable mutations
To exclude possible passenger mutations we selected all frameshift, nonsense and splice variants, and missense mutations and indels known or predicted to be damaging by at least 2 out of 4 methods [SIFT (18), GERP++ (19), PolyPhen2 (20), OncodriveMUT (21)]. We classified variants as clinically actionable using www.cancergenomeinterpreter.org.
cDNA library preparation for RNA sequencing
Total RNA was quantified using a Qubit (Life Technologies) and quality was assessed using an Agilent Bioanalyzer. For samples that met quality requirements, 100 ng of was used for library preparation following the manufacturer’s protocol for Illumina RNA ACCESS (see supplementary methods
RNA sequencing data analysis
Detection of differentially expressed genes (DEG) between responders and non-responders cases was performed with DESeq2 package (22), using raw RNA-seq counts as input. GO term enrichment analysis for DE genes was obtained using the online functional tool GSEA/MSigDB web site v6.1. We generated rlog transformed count data using DESeq2, filtering low expressing genes (<5% tumors with 0 count and mean >4). This resulted in 16730 transcripts that served as input for the following analysis: 1) Single-sample gene set enrichment for 125 previously published breast cancer-related gene expression signatures calculated as previously described (23) and, using a FDR<0.01, for differentially expressed signatures among subgroups; 2) PAM50 molecular subtyping using R package genefu, using non scaling option (24); 3) Sample by sample correlation matrix using Pearson distance of differentially expressed transcripts with the highest variance (n=256) with the resulting matrix used to perform hierarchical cluster analysis by ward.D2; and 4) Statistical assessment of transcriptional diversity as described before (25). Gene expression data in Suppl. Table S8.
The Cancer Genome Atlas (TCGA), METABRIC and ACOSOG Z1031B data
Somatic mutations, RNA normalized gene expression, and clinical information for the breast invasive breast carcinoma TCGA cohort (26) and METABRIC (27) were downloaded using the cBIO platform. ER+ breast cancers were selected for comparison of somatic mutations (in TCGA) and PAM50 subtypes and survival (in METABRIC). Agilent gene expression arrays (GSE87411) were downloaded and used to compare E2F4 activation signatures between pre-treatment and 2–4 weeks post-treatment samples from 109 patients’ tumors in the ACOSOG Z1031B neoadjuvant trial (7).
Methods in POP trial
E2F4 activation signature
The E2F4 activation signature was generated by selecting those genes significantly upregulated (log fold change>1, FDR <0.03) in letrozole non-responder vs responder tumors that were also significant downregulated (FDR<0.01) by a 14-day treatment with palbociclib in ER+ tumors in the POP trial (NCT02008734). Eighteen of these genes are predicted to be E2F4 targets: ANLN, ARHGAP11A, BUB1, CASC5, CDCA5, CDK1, CLSPN, DIAPH3, DTL, FAM111B, HIST1H3B, HIST1H3F, HMMR, KIAA1524, KIF18A, KIF4A, KPNA2, MAD2L1, PRR11, RRM2, STMN1, TICRR, TPX2, ZNF367. An E2F4 activation z-score was developed by adding values across all genes for each tumor to generate an un-escalated E2F4 score. The un-escalated E2F4 score was then standardized to a z-score by subtracting from each patient’s score the mean score in the cohort, and then dividing it by the scores’ standard deviation.
Cell lines
MCF-7 cells and CAMA1 (from the American Type Culture Collection, ATCC, authenticated by the STR method) were maintained in Improved modified Eagle's medium (IMEM)/10% FBS (Gibco). LTED cell lines were generated by culturing cells under hormone-depleted conditions [phenol red–free IMEM/10% dextran–charcoal-treated FBS (DCC-FBS, Hyclone; contains <0.0367 pmol/L 17β-estradiol)] as described previously (28). Mycoplasma testing was conducted before use. Experiments were performed less than 3 months after thawing early passage cells.
Quantitative Reverse Transcription Polymerase Chain Reaction
Cells were harvested, and their RNA was extracted using the RNeasy Mini Kit (QIAGEN Sciences Inc., Germantown, MD). RNA (1 μg) was reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (SuperScript® III First-Strand (Invitrogen, Carlsbad, CA). Real-time PCR reactions were conducted in 96-well plates using the iCycler iQ (Bio-Rad) and primers obtained from SABiosciences (Qiagen). Threshold cycle values were normalized for the housekeeping gene GAPDH. Specific primers for the genes of interest were designed using the tool NCBI/ Primer-BLAST. The sequences of the primers set used for this analysis are listed in supplementary methods.
Immunoblot Analysis
Cells were washed in PBS, harvested and lysed in NP-40 buffer [10 mM Tris (pH 7.4), 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1mM EGTA, 1 mM sodium pyrophosphate, 50 mM NaF, 10 nM beta-glycerophosphate, 5 mM Na3VO4, 10% glycerol, 1 mM PMSF, and protease inhibitors] for 10 min on ice. Protein concentrations of the lysates were determined by the BCA assay (Pierce Chemical Co., Rockford, IL). Samples were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked with 3% nonfat dry milk in TBST for 1 h at room temperature and then incubated overnight at 4°C with the appropriate primary antibody. Antibodies specific for RB (#9309; 1:1000), S780 P-RB (#9307; 1:1000), and beta-actin (#4970; 1:1000) were obtained from Cell Signaling Technology (Denver, MA); an ERα (#8002) antibody was from Santa Cruz Technology (Santa Cruz, CA). Following incubation with horseradish peroxidase-conjugated secondary antibodies, proteins were visualized using an enhanced chemiluminescence detection system.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
RESULTS
PEPI score predicts long term outcome after prolonged neoadjuvant letrozole
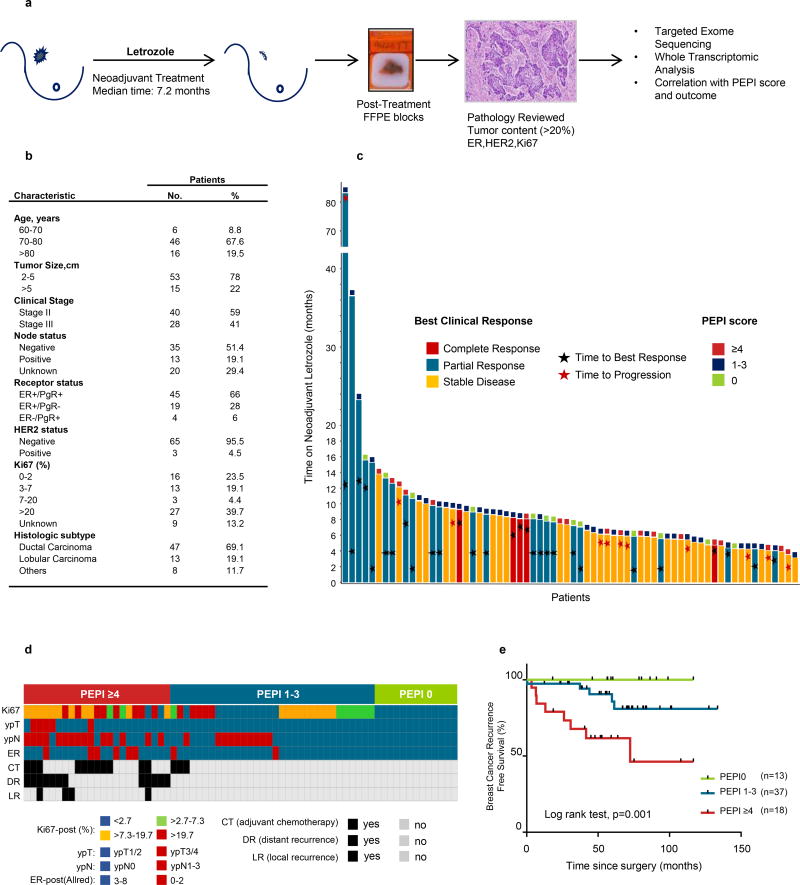
After informed consent, we treated 68 postmenopausal women with ER+ operable breast cancer with neoadjuvant letrozole followed by surgery (Fig. 1a & 1b; Suppl. Fig. S1). Patients were treated for a median 7.2 months (interquartile range, 5.4–9.2). Median age was 77 years (range, 60–86); 40 (59%) had stage II and 28 (41%) had stage III cancer. Twenty-nine patients (42.5%) achieved a complete or partial response as measured by ultrasound; 10 experienced progressive disease within a mean of 5 months and underwent surgery. The median time to achieve a best objective response (complete or partial response) was 6.3 months (range 2–16) (Fig. 1c). After surgery, patients were classified according to their PEPI score (11) (Suppl. Table S1). Thirteen (19%) patients had a PEPI score 0, 36 (52%) were PEPI 1–3, and 19 patients (28%) were PEPI ≥4. Adjuvant treatment consisted of endocrine therapy (96%), chemotherapy (CT) for 14 patients (20.5%) with high risk features (10 with PEPI≥4, 4 with PEPI 1–3), and radiotherapy (57%) for those patients who underwent breast conserving surgery or mastectomy if the primary tumor was ≥4 cm or had ≥4 axillary lymph nodes involved with cancer (Fig. 1d). With a median follow-up of 58 months (range, 50–80), 13 patients (19%: 8 with PEPI≥4, 5 with PEPI 1–3) exhibited a breast cancer recurrence (12 metastatic, 1 loco-regional). The 5-year recurrence free survival rate was 100%, 85% and 61% for PEPI 0, PEPI 1–3 and PEPI ≥4, respectively (Log rank test, p=0.001) (Fig. 1e). Patients with PEPI≥4 continued to exhibit a poor prognosis after adjusting for adjuvant CT (risk of relapse for adjuvant CT, HR: 2.84, p=0.052). The probability of achieving PEPI 0 correlated with a clinical response to neoadjuvant letrozole, with a response rate of 34% for PEPI 0 vs. 5% for PEPI>0 (p=0.002), but not to the length of neoadjuvant treatment (Suppl. Table S2).
Figure 1. PEPI score predicts long term outcome after prolonged neoadjuvant letrozole.
a) Study design. b) Patients characteristics. c) Individual patient response to prolonged neoadjuvant letrozole. Each bar represents a patient. The length of the bar shows duration of therapy; the color of the bar shows the best clinical response observed; stars mark the timing of the response; squares at the end of the bar show the PEPI score achieved. d) Each column represents a patient and its individual PEPI score assignment, adjuvant systemic treatment and breast cancer events. e) Breast cancer recurrence free survival by PEPI score.
Targeted gene sequencing identifies clinically actionable mutations in endocrine resistant tumors
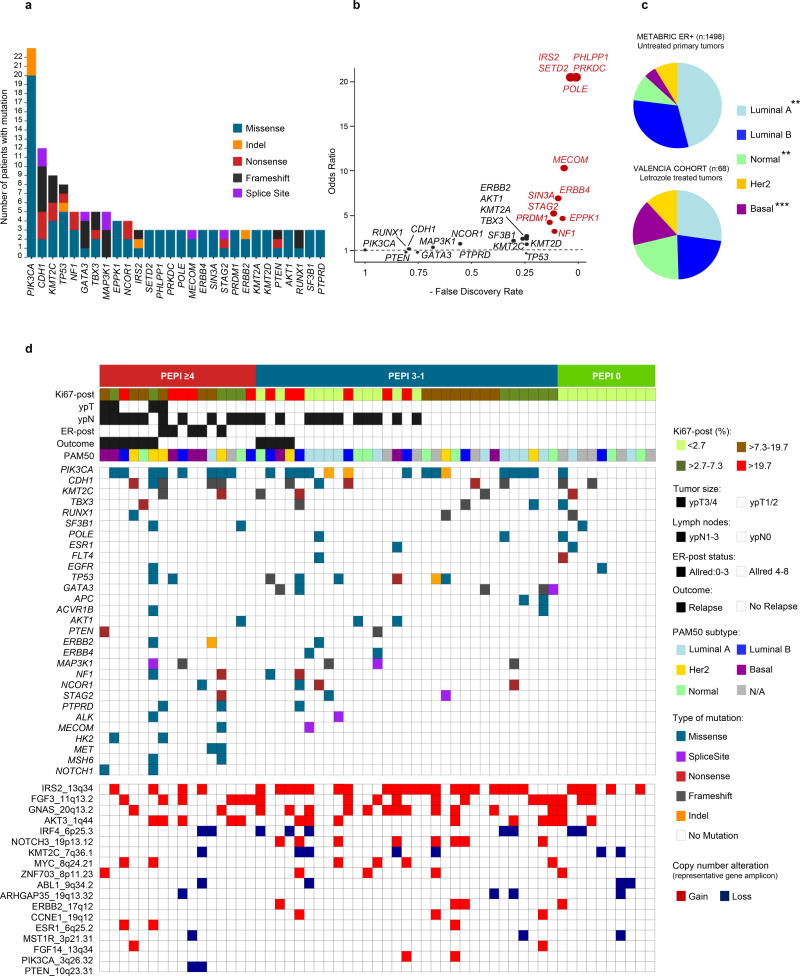
We performed targeted gene sequencing of 303 cancer related genes, with a median depth of 320X. After applying a filtering algorithm (Suppl. Fig. S2), the median number of non-synonymous somatic mutations per tumor was 4 (range 0–46); in 5 tumors (3 PEPI 0, 2 PEPI 1–3) no somatic mutations were identified. There were 8 genes mutated in at least 5 patients: PIK3CA (40%), CDH1 (21%), KMT2C (16%), TP53 (14%), NF1 (9%), GATA3 (9%), TBX3 (9%) MAP3K1 (9%) (Fig. 2a). We detected only 1 ESR1 ligand binding domain mutation, concordant with their low frequency in patients with progression on adjuvant AIs (29). Using a FDR<0.1, 12 genes were more frequently mutated in our cohort of residual tumors after long exposure to letrozole compared to untreated ER+ breast cancer in TCGA. These genes are involved in transcriptional regulation (MECOM, SETD2, SIN3A, STAG2 and PRDM1), DNA repair (POLE, PRKDC), tumor suppression (NF1, PHLPP1), growth factor signaling (ERBB4, IRS2) and cytoskeleton remodeling (EPKK1) (Fig. 2b). Using RNA-seq data we assigned PAM50 intrinsic subtypes to each tumor and investigated subtype composition after prolonged estrogen deprivation with letrozole. As presented in Figure 2c, the distribution of the intrinsic subtypes varied considerably compared to a cohort of untreated ER+ breast cancers in the METABRIC database (27). There was an increase in cancers with Basal-like and Normal gene expression and a decrease in Luminal A tumors, suggesting treatment with letrozole remodeled the transcriptional landscape of these tumors. Tumors with Basal/HER2-enriched gene expression were enriched among the letrozole resistant tumors (9 of 15, or 56%, exhibited a PEPI score ≥4 and none had a PEPI score of 0).
Figure 2. Targeted exome sequencing identifies clinically actionable mutations and a unique distribution of breast cancer subtypes in endocrine resistant tumors.
a) Frequency and type of non-synonymous recurrent gene mutations in 57 tumors from patients treated with neoadjuvant letrozole. b) Comparison of mutations detected in this cohort vs. primary untreated ER+ breast cancers in TCGA [Cell 2015, (45)]; in red are genes with Fisher test FDR<0.1. c) Distribution of PAM50 intrinsic subtypes in our cohort (Fig. 1) and in ER+ early breast cancers from METABRIC (p-value by Fisher-test for the comparison among cohorts **<0.001, ***<0.0001). d) Tile plot showing the distribution of recurrent driver mutations (n≥2) and copy number alterations according to PEPI score and PAM50 subtypes; each column represents a patient.
We found 180 driver mutations in 99 genes (Suppl. Table S3a). Figure 2d shows the distribution of genes with 2 or more driver mutations (see Methods) according to PEPI score, PAM50 subtype and patient outcome. Tumors were classified as non-responder (PEPI ≥4 and/or recurrence) or responder (PEPI <4 and no recurrence). We could not find any statistically significant difference in the frequency of mutations or copy number alterations between the two groups. However, the distribution of these alterations was asymmetrical with some mutations approaching overrepresentation in PEPI ≥4 vs. PEPI 0 (PIK3CA: 50% vs 10%, p=.08) and several alterations being absent in tumors with PEPI 0 (i.e., TP53, AKT1, PTEN, ERBB2). Other driver mutations, found to be more frequent in the letrozole-treated tumors in this cohort compared to those in TCGA (i.e., NF1, STAG2, ERBB4, MECOM), were only detected in tumors with PEPI >0.
For copy number alterations (CNAs), we focused on allelic imbalances (B-allele frequency>3) in previously reported recurrently altered genomic regions. We detected 159 CNAs in 28 amplicons. These amplicons contained genes such as CCND1 (16%), FGFR1 (14%), MYC (10%), ERBB2 (7%) or ESR1 (5%). Amplicons with copy number loss included genes such as KMT2C (12%) and PTEN (3%). Thirty-nine CNAs events in 9 genes were considered drivers (Suppl. Table S3b).
One hundred two mutations in 48 driver genes and all driver CNAs were classified as clinically actionable (see Methods). Actionable mutations in the phosphoinositide 3-kinase (PI3K) pathway (PIK3CA, AKT1, TSC2, and/or loss or truncation mutations of PTEN) were overrepresented in the PEPI ≥4 group compared to PEPI 0 (70% vs 10%, p=0.003). We also evaluated the association of each actionable mutation or CNA with the expression of a proliferation signature (PCNA), the intrinsic subtype and the PEPI score. This allowed us to identify a subset of druggable somatic alterations (i.e., NF1 loss, TP53, NOTCH1, FGFR4, JAK1, PTPRD) associated with multiple poor prognosis features (e.g., high PEPI, high PCNA score, and luminal B/HER2-enriched/basal subtypes), thus supporting the development of drugs targeting these alterations (Suppl. Fig. S3 & Suppl. Table S3c, S4).
Endocrine resistant tumors show enrichment in genes involved in proliferation through heterogeneous transcriptional and mutational profiles
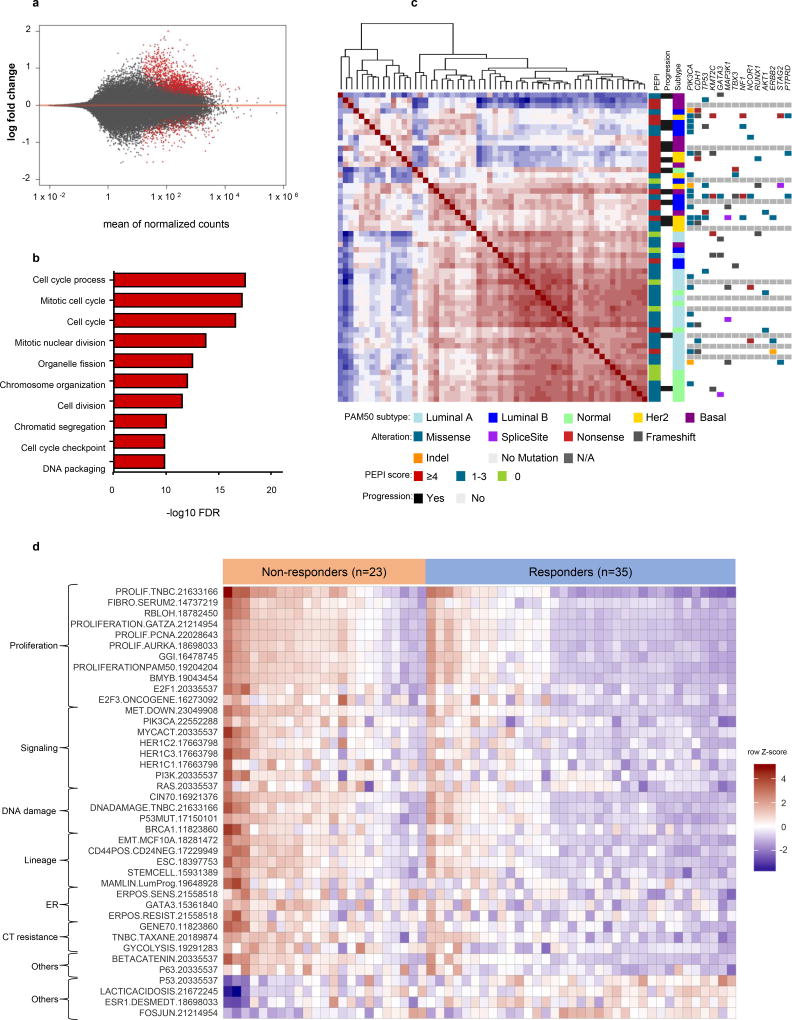
We next performed comparative transcriptional analyses on 58 tumors. Analysis between responders and non-responders showed 566 differentially expressed (DE) genes with a FDR<0.05, dominated by upregulated genes (458) in non-responder vs responder tumors (Fig. 3a). Gene ontology enrichment analysis of DE genes showed that non-responding tumors were enriched for cell cycle related genes while no-overlap was found among responders (Fig 3b).
Figure 3. Resistant tumors to letrozole show enrichment in cell cycle related genes through a heterogeneous transcriptional and mutation profile.
a) MA plot showing the log2 fold changes from non-responders (PEPI ≥4 and/or breast cancer recurrence) over responder tumors (PEPI <4 and no breast cancer recurrence) of normalized counts (i.e., the average of counts normalized by size factor). Points in red represent normalized counts with an adjusted p-value <0.05. b) GO enrichment of genes overexpressed in non-responder tumors. c) Dendrogram and unsupervised clustered correlation matrix (red positive and blue negative correlation) of 58 breast cancers using Pearson distance. Differentially expressed genes between responders and non-responder tumors were used to compute Pearson distance among the tumors and subsequent hierarchical clustering. Recurrent mutations, PEPI score, patient outcome, and PAM50 subtype are represented in columns in the right panel for each case. d) Single sample gene set enrichment analysis using a set of 125 breast cancer related signatures shows differentially enrichment (FDR<0.05) of 40 signatures between responder and non-responder tumors.
To analyze the degree of variability among the tumors in their transcriptional response to estrogen deprivation with letrozole, DE genes between responders and non-responders were used to perform a correlation matrix followed by unsupervised hierarchical clustering. This analysis revealed two main clusters: one relatively homogenous cluster integrating most of the responders and a second heterogeneous cluster enriched with non-responders. Measurement of the transcriptional diversity showed a greater average distance to the median for the non-responder compared to responding tumors (permutation test for homogeneity of multivariate dispersions <0.01). We also observed significant heterogeneity in somatic mutations between the two groups, with a greater mean number of mutations in non-responders vs. responders (12 vs 3, p<0.0001) (Fig 3c).
To investigate processes that are enriched in responders vs non-responders, we analyzed differential signature enrichment using a set of 125 previously published breast cancer-related gene expression signatures (23). Thirty-six signatures were enriched in non-responders and 4 in the responding tumors (FDR<0.05). Endocrine resistant tumors showed an enrichment of a diverse set of signatures involved cell cycle/proliferation, signaling pathway (EGFR1, PI3K, RAS), DNA repair, breast cancer stemness, ER signaling, and resistance to chemotherapy. Endocrine sensitive tumors were enriched for signatures involved in p53 signaling, genes associated with ER expression, lactic acidosis response and Fos-Jun kinase signaling (Fig 3d). These results suggest some ER+ breast cancers adapt to evade estradiol deprivation through different transcriptional programs that ultimately confer the ability of sustain cell cycle progression.
A CDK4/6 inhibitor-sensitive E2F4 transcriptional program is associated with estrogen-independent proliferation in letrozole-resistant tumors
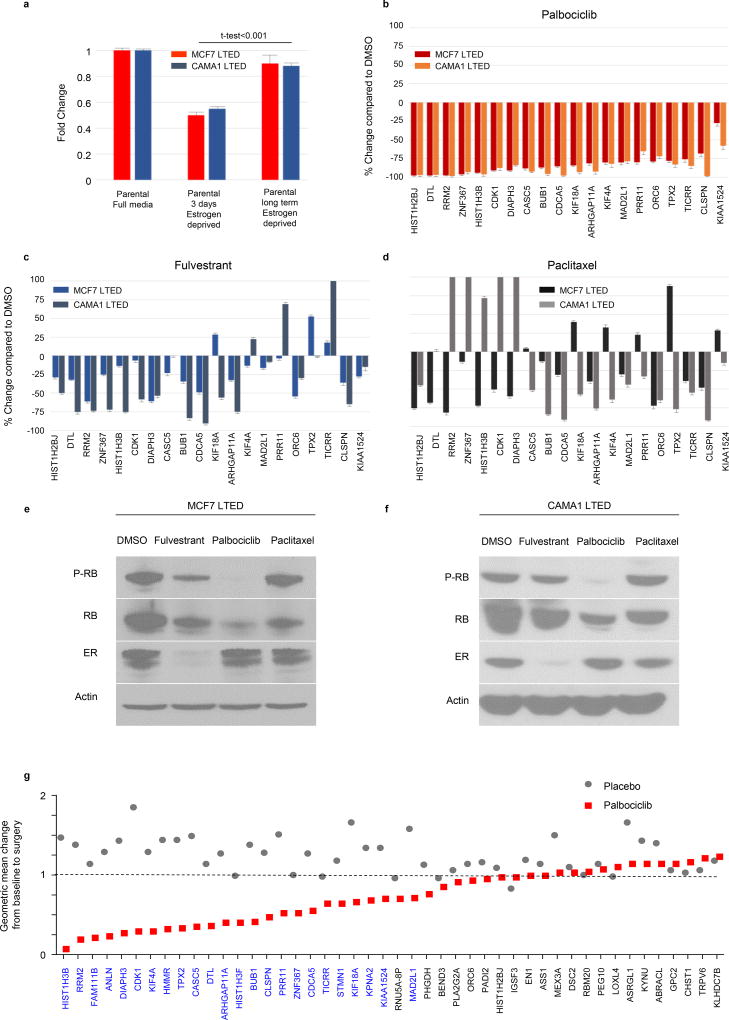
To identify transcriptional programs with differential activity between sensitive and resistant tumors, we integrated transcription factor binding data from ChIP-seq studies (CheA 2016 and ENCODE-TF ChIP-seq 2015) with expression of the 47 most upregulated genes in non-responder tumors (log FC>1, FDR<0.03), using the platform Enrichr (30). E2F4 was the transcription factor whose targets demonstrated the most significant overlap with upregulated genes in the resistant list (overlap 20/710, adjusted p=2.56E-15) (Suppl. Table S5). E2F4 is repressed by binding to unphosphorylated Rb. Upon phosphorylation by a cyclinD/CDK4/6 complex, Rb is inactivated and uncoupled from E2F4 which, in turn, can induce transcription of genes associated with progression into S phase of the cell cycle and cell survival (31). Thus, we next tested if the 20 E2F4-regulated genes were overexpressed in ER+ breast cancer cells adapted to long term estrogen deprivation (LTED), and if they could be modulated by treatment with the CDK4/6 inhibitor palbociclib. We found upregulation of these genes in MCF7/LTED and CAMA1/LTED cells compared to parental MCF7 and CAMA1 cells, respectively (Fig 4a). Treatment with palbociclib significantly downregulated the expression of all 20 E2F4-regulated genes (median decrease 78%) with a simultaneous decrease in P-RB levels. Treatment with the ER downregulator fulvestrant or with paclitaxel only partially suppressed the expression of this set of genes and had no effect on P-RB levels (Fig. 4b-4f).
Figure 4. An E2F4 transcriptional program is associated with estrogen-independent proliferation in letrozole-resistant tumors and is modulated by CDK4/6 inhibitors.
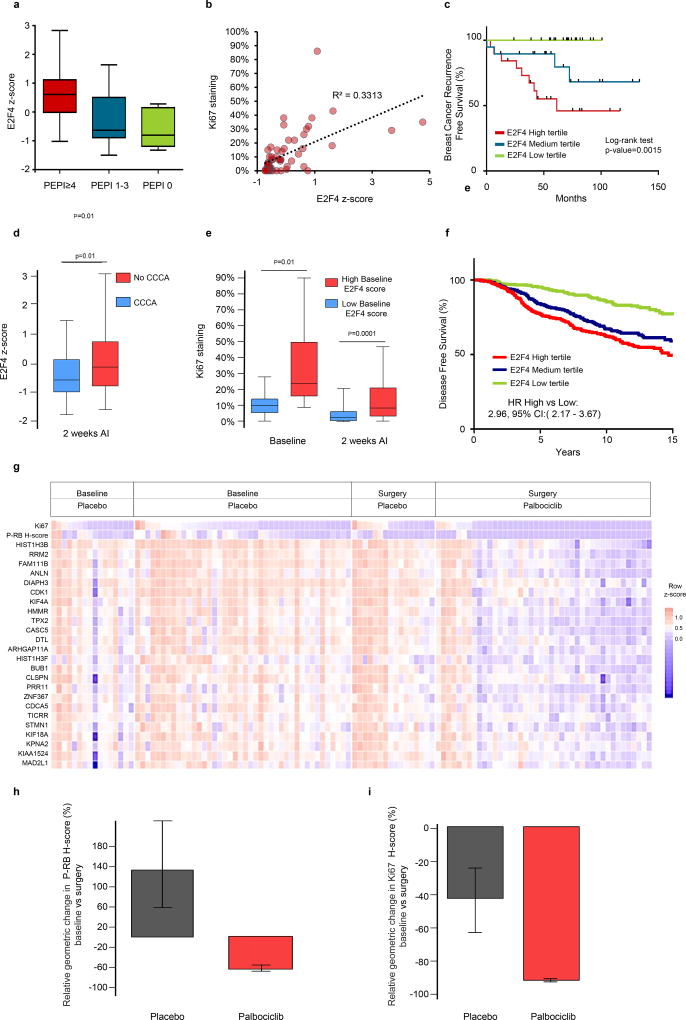
a) The expression levels of 20 E2F4-regulated genes overexpressed in non-responder tumors were assessed by RT-PCR in MCF7 and CAMA1 long term estrogen deprived (LTED) cells and normalized to their expression in MCF7 and CAMA1 parental cells, respectively. Data are presented as the 20 genes mean fold change +/− SEM. b) Expression levels of the same 20 genes were assessed by RT-PCR in MCF7/LTED and CAMA1/LTED cells after treatment with palbociclib 1 μM for 24 h. c) fulvestrant 1 µM for 24 h or d) paclitaxel 20 nM for 24 h. Data are presented as mean % change +/− SEM relative to treatment with DMSO of two independent replicates. e,f) Immunoblots of lysates from MCF7/LTED or f) CAMA1/LTED cells treated with DMSO, fulvestrant 1 μM, palbociclib 1 μM or paclitaxel 20 nM for 24 h. g) The geometric mean change, between baseline and surgery, for the top 47 genes associated with letrozole resistance in the study cohort, were assessed in tumor samples from 60 ER-positive/HER2-negative primary tumors treated with placebo or palbociclib for 15 days in the POP trial (NCT02008734); in blue text are genes predicted to be E2F4 targets.
Next, we investigated if these genes could be modulated in primary breast cancers in patients enrolled in the Pre-Operative Palbociclib (POP) trial (NCT02008734) (32). In this study, patients with newly diagnosed, operable ER+/HER2− breast cancer received palbociclib daily or placebo x14 days leading up to breast cancer surgery. Tumor cell proliferation and CDK4/6 inhibition were assessed by Ki67 IHC and P-RB IHC, respectively, in a pre-treatment biopsy and in the (post-treatment) surgical specimen. Consistent with the inhibition of CDK4/6, treatment with palbociclib induced a significant reduction of P-RB levels and Ki67(32). Next, we used gene expression array data from pre- and post-palbociclib tumors in this trial to assess expression of the 47 most upregulated genes in the tumors resistant to prolonged neoadjuvant letrozole (Fig. 1). Treatment with palbociclib, but not with placebo, significantly decreased expression of 24 of 47 of these resistance-associated genes (FDR<0.01); among these were 18 of the 20 E2F4 target genes (Fig. 4g & Suppl. Table S6).
An E2F4 target gene signature is associated with resistance to neoadjuvant and adjuvant endocrine therapy
We generated a signature of E2F4 transcriptional activation, using the 24 genes associated with resistance to neoadjuvant letrozole and relapse in our cohort that were also significantly downregulated by palbociclib treatment in tumors in the POP trial, as compared to the placebo control group. We next assessed the ability of this set of genes to predict breast cancer recurrence in ER+ treated with endocrine therapy. First, we tested the signature in the cohort of patients treated with prolonged neoadjuvant letrozole and showed that tumors within PEPI ≥4 had significantly higher E2F4 activation signature than tumors with PEPI 1–3 or PEPI 0 (Fig. 5a), and that E2F4 score was moderately correlated with post-treatment Ki67 levels (Fig 5b). Further, the 5-year relapse-free survival was 100%, 79% and 45%, for patients in the low, medium or high tertile of the E2F4 gene expression signature, respectively (log-rank test, p=0.0015) (Fig. 5c).
Figure 5. An E2F4 target gene signature is associated with resistance to neoadjuvant and adjuvant endocrine therapy.
a) An E2F4 activation signature was enriched in tumors with PEPI≥4, b) correlated with post-treatment Ki67 levels and c) was associated with increased risk of breast cancer recurrence in the cohort of patients treated with prolonged neoadjuvant letrozole (Fig. 1). d) Box plots comparing the E2F4 signature score in ER+/HER2− tumors from patients in the ACOSOG Z1031B study (n=110, NCT01953588) after treatment with an aromatase inhibitor (AI). According to the 2-week Ki67 score, tumors were classified as achieving complete cell arrest (CCCA, Ki67 ≤2.7%) or no-CCCA (Ki67 >2.7%), p-value for t-test. e) Box plot correlating high vs. low E2F4 signature score with the Ki67 score at baseline and after 2-weeks of treatment with an AI in tumors from the ACOSOG Z1031B study. Tumors with a high E2F4 score at baseline exhibited a lower rate of CCCA upon treatment compared to tumors with a low baseline E2F4 score (18% vs. 50%), p-value for t-test. f) Disease free survival in patients with ER+ breast cancer treated with adjuvant endocrine therapy in the METABRIC database (n=1498) according to E2F4 signature score tertiles. g) Tile plot showing baseline and surgery gene expression values for each of the components of the E2F4 gene signature, Ki67 and P-RB score from 60 ER+/HER2- tumors treated in the POP trial with either placebo or two weeks of palbociclib. h, i) Geometric mean change (± SD) in P-RB H-score (h) and Ki67 score (i) in 30 ER+/HER2− tumor pairs before and after a two-week treatment with placebo or palbociclib. Tumors are those with a high baseline E2F4 score.
To externally validate the performance of the signature, we used gene expression data from patients treated with neoadjuvant AIs in the ACOSOG Z1031B study (n=110) (7). In this trial, tumors that failed to achieve a complete cell cycle arrest (CCCA), defined as an on-treatment, 2-week Ki67 ≤2.7%, exhibited higher E2F4 signature score (Fig. 5d). Also, tumors with a high E2F4 score at baseline had a higher baseline Ki67 score and a worse response to AIs compared to those tumors with a low E2F4 score. CCCA rate was 18% vs 50% for high and low baseline E2F4 scores, respectively (p<0.001) (Fig 5e). Of note, up to 40% of tumors with a high baseline E2F4 score switched to a low E2F4 score after 2-week treatment with an AI (Suppl Fig. S4a). To assess the predictive value of the E2F4 activation signature in the adjuvant setting, we selected patients with ER+ breast cancer treated with adjuvant endocrine therapy in the METABRIC cohort (n=1408). Patients with E2F4 scores in the higher tertile showed an increased risk of relapse (HR: 2.96, 95% CI: 2.176 – 3.670) and death (HR: 1.59, 95% CI: 1.32–1.94) compared to those in the lower tertile (Fig. 5f & Suppl Fig. S4b). In addition, we evaluated luminal PAM50 subtypes and noted a significant association the E2F4 signature score with survival in both Luminal A and Luminal B breast cancer subtypes (Suppl. Fig. S4c & S4d).
Finally, we assessed the efficacy of palbociclib in tumors from patients in the POP trial. Treatment for 2 weeks with palbociclib suppressed Ki67 and P-RB levels expression and downregulated all genes composing the signature (Fig. 5g). In the group of tumors expressing high levels of E2F4 signature activity (n=30), treatment with the CDK4/6 inhibitor was able to suppress P-RB by 90% but Ki67 by only 67% (Fig. 5h & 5i). We speculate that the partial suppression of Ki67 despite almost complete inhibition of P-RB could be accounted for by the lack of simultaneous antiestrogen therapy. In sum, we have identified a CDK4/6 inhibitor-sensitive E2F4 activation signature that defines ER+ breast cancers with poor prognostic features. This signature is of potential use for the identification of patients with ER+ breast cancer candidates for adjuvant therapy with CDK4/6 inhibitors in combination with antiestrogens.
DISCUSSION
Neoadjuvant endocrine therapy trials offer an opportunity to discover functional genomic alterations associated with drug resistance that may inform post-operative adjuvant treatment. In this study, we performed targeted DNA and whole transcriptome sequencing in residual ER+ breast cancers treated with letrozole for a median of 7.2 months. A higher number of mutations were found in patients with a poor response to estrogen deprivation with letrozole, confirming other studies (4,8), and also suggesting a source of genetic diversity that may identify cancers that recur after adjuvant endocrine therapy. In agreement with other studies (33), we detected a different composition of intrinsic molecular subtypes to what would be expected in a cohort of untreated ER+ postmenopausal breast cancers. The increase in tumors with a Normal subtype and reduction in Luminal A tumors suggest a change induced by treatment, while the increase in tumors of the Basal-like subtype suggests a loss of luminal expression and the outgrowth of endocrine resistant cancer cell sub-populations.
We did not detect recurrent mutations or copy number alterations significantly enriched in tumors resistant to letrozole. However, there was a numerical increase in few clinically actionable mutations, such as NF1 loss and in genes like JAK1, NOTCH1, FGFR4 and PTPRD, whose role in endocrine resistance has not yet been elucidated. We found a greater number of mutations in the PI3K pathway (PIK3CA, AKT1, TSC2, and/or loss or truncation mutations of PTEN) associated with poor response to letrozole, but only those PI3K pathway mutations in Luminal B/HER2-enriched/Basal tumors were associated with poor features (high PEPI score, high proliferation, and breast cancer relapse). By applying a comprehensive set of breast cancer related gene signatures, we showed that multiple pathways are involved in evading estrogen deprivation, including gene signatures related to growth factor receptor, RAS and PI3K signaling, and cancer cell stemness. Different to some prior reports (34), we did not observe an enrichment in immune related gene expression signatures.
Integration of the 47 most upregulated genes in letrozole-resistant tumors with transcription binding data identified a set of genes controlled by the E2F4 transcription factor. Consistent with activation of E2F4 by cyclin D/CDK4/6 complexes, treatment with the CDK4/6 inhibitor palbociclib downregulated this set of genes in primary ER+ breast cancers simultaneous with a reduction in P-RB levels and tumor cell proliferation measured by Ki67 IHC. In the initial cohort of tumors treated with prolonged neoadjuvant letrozole (Fig. 1), the prognostic ability of this set of genes was independent of adjuvant chemotherapy, suggesting they may also be causal to chemotherapy resistance. In line with this hypothesis, paclitaxel was not able to suppress this set of genes in vivo. We found a marked upregulation of the E2F4 gene expression signature in AI-resistant tumors from patients in the ACOSOG Z1031B study. Of note, the endocrine resistant tumors in ACOSOG Z1031B were also resistant to neoadjuvant chemotherapy and several of the genes that compose the signature (i.e., KIF4A, KIF18A, DIAPH3, TPX2) have been causally associated with resistance to chemotherapy (20,21,(37). Taken together, these data suggest CDK4/6 inhibitors would be an excellent therapeutic strategy against ER+ breast cancers where antiestrogens do not inhibit tumor cell proliferation and/or other pharmacodynamic surrogates like the E2F4 score described herein. Two recent studies support this notion. First, the NEO-MONARCH trial showed an overall striking reduction in tumor cell proliferation (Ki67 score) in primary ER+ breast cancers treated with the CDK4/6 inhibitor abemaciclib alone or in combination with letrozole (38). In a second example, Ma and collegues (39) treated patients with ER+ breast cancer with the AI anastrozole for 28 days, at which time the Ki67 score was measured in a research biopsy and palbociclib was added. Complete cell cycle arrest rate was significantly higher after adding palbociclib to anastrozole suggesting that the addition of a CDK4/6 inhibitor can induce a more complete antiproliferative effect in tumors that exhibit partial growth suppression upon estrogen deprivation.
The role of E2F in endocrine resistance has been previously documented by our group (40) and others (41). Our results agree with studies that have shown the prognostic value of an E2F4 signature in ER+ breast cancer (42). This signature, based on 199 E2F4 target genes, identified by in vitro ChiP-seq experiments, remains a significant prognostic factor in the adjuvant setting even after adjusting for clinic-pathological variables and adjuvant therapy (endocrine and/or chemotherapy). The finding that current adjuvant treatments cannot improve the prognosis of patients exhibiting high expression of this E2F4 signature also agrees with our results that only CDK4/6 inhibition, and not chemotherapy nor fulvestrant, is able to suppress completely E2F4 target gene expression. However, the same authors reported that high levels of the 199-gene E2F4 signature are predictive of pathological complete response to neoadjuvant chemotherapy in ER+ breast cancer (43). Although this might seem contradictory, transcriptional activity of E2F4 is a marker of highly proliferative tumors, which is a recognized predictive factor of an initial response to chemotherapy. However, if a tumor does not respond to preoperative endocrine therapy or chemotherapy, it is also known that high proliferation (measured by Ki67) is a marker of poor prognosis (44). Thus, we believe that a pharmacodynamic assessment of proliferation, particularly in ER+ tumors, can clearly unmask highly proliferative tumors with a poor prognosis. Our E2F4 signature differs from the E2F4 signature mentioned above mainly on the biological and clinical contexts from which it is derived. Instead of in vitro data, we used on-treatment primary tumor data from a cohort of patients with ER+ breast cancer treated preoperatively with standard of care letrozole. Therefore, we believe that the established biology of our signature and the context of its discovery may facilitate its implementation in neoadjuvant endocrine studies testing the performance of new drugs.
Some limitations of the current study are the lack of baseline DNA/RNA sequencing data that precluded direct comparisons between untreated and on treatment samples. Thus, we used TCGA data as baseline. Indirect comparisons of genetic alterations among studies can be subjected to bias regarding software pipeline, sequencing depth and tumor content. Also, the disproportion in sample size and some particularities between the two datasets, such as age, might account for some of the differences we found.
In conclusion, we have identified genomic alterations and transcriptional phenotypes in a cohort of ER+/HER2− breast cancers resistant to prolonged estrogen deprivation. Results suggest presence of an ER-independent E2F4 gene expression program that can be blocked by inhibition of CDK4/6. We posit these tumors may require combined inhibition of ER and CDK4/6 for a maximal anti-cancer effect.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Approximately 20% of patients with early ER+ breast cancer treated with adjuvant antiestrogen therapy eventually relapse with metastatic disease. Herein we show that those tumors enriched in E2F4 target genes following prolonged neoadjuvant estrogen deprivation with letrozole may benefit from adjuvant therapy with CDK4/6 inhibitors.
Acknowledgments
This study was supported by NIH Breast SPORE grant P50 CA098131, Vanderbilt-Ingram Cancer Center Support grant P30 CA68485, Susan G. Komen for the Cure Breast Cancer Foundation grant SAC100013, a grant from the Breast Cancer Research Foundation, a grant from the Sociedad Española de Oncología Médica (SEOM), a grant from the Asociación Española Contra el Cáncer (AECC), and a grant from Grupo Español de Investigación en Cáncer de Mama (GEICAM).
References
- 1.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv048. djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 3.(EBCTCG) EBCTCG. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. The Lancet. 2015;386:1341–52. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 4.Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353–60. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Short-Term Changes in Ki-67 during Neoadjuvant Treatment of Primary Breast Cancer with Anastrozole or Tamoxifen Alone or Combined Correlate with Recurrence-Free Survival. Clin Cancer Res. 2005;11:951s–958s. [PubMed] [Google Scholar]
- 6.Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Jänicke F, et al. Letrozole Inhibits Tumor Proliferation More Effectively than Tamoxifen Independent of HER1/2 Expression Status. Cancer Res. 2003;63:6523–31. [PubMed] [Google Scholar]
- 7.Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, et al. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance) J Clin Oncol. 2017;35:1061–1069. doi: 10.1200/JCO.2016.69.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gellert P, Segal CV, Gao Q, López-Knowles E, Martin L-A, Dodson A, et al. Impact of mutational profiles on response of primary oestrogen receptor-positive breast cancers to oestrogen deprivation. Nat Commun. 2016;7:13294. doi: 10.1038/ncomms13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller CA, Gindin Y, Lu C, Griffith OL, Griffith M, Shen D, et al. Aromatase inhibition remodels the clonal architecture of estrogen-receptor-positive breast cancers. Nat Commun. 2016;7:12498. doi: 10.1038/ncomms12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–8. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llombart-Cussac A, Guerrero Á, Galán A, Carañana V, Buch E, Rodríguez-Lescure Á, et al. Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol. 2012;14:125–31. doi: 10.1007/s12094-012-0771-9. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- 15.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–4. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a High Fraction of the Human Genome to be under Selective Constraint Using GERP++ PLOS Computational Biology. 2010;6:e1001025. doi: 10.1371/journal.pcbi.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamborero D, Rubio-Perez C, Deu-Pons J, Schroeder MP, Vivancos A, Rovira A, et al. Cancer Genome Interpreter Annotates The Biological And Clinical Relevance Of Tumor Alterations. bioRxiv. 2017;140475 doi: 10.1186/s13073-018-0531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNAseq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stover DG, Coloff JL, Barry WT, Brugge JS, Winer EP, Selfors LM. The Role of Proliferation in Determining Response to Neoadjuvant Chemotherapy in Breast Cancer: A Gene Expression-Based Meta-Analysis. Clin Cancer Res. 2016;22:6039–6050. doi: 10.1158/1078-0432.CCR-16-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker JS, Mullins M, Cheang MCU, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang T, Shi W, Natowicz R, Ononye SN, Wali VB, Kluger Y, et al. Statistical measures of transcriptional diversity capture genomic heterogeneity of cancer. BMC Genomics. 2014;15:876. doi: 10.1186/1471-2164-15-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell. 2015;163:506–19. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis C, Shah SP, Chin S-F, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller TW, Hennessy BT, González-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor–positive human breast cancer. J Clin Invest. 2010;120:2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiavon G, Hrebien S, Garcia-Murillas I, Cutts RJ, Pearson A, Tarazona N, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aac7551. 313ra182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindeman GJ, Gaubatz S, Livingston DM, Ginsberg D. The subcellular localization of E2F-4 is cell-cycle dependent. PNAS. 1997;94:5095–100. doi: 10.1073/pnas.94.10.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnedos M, Cheaib B, Bayar MA, Michiels S, Scott V, Adam J, et al. Abstract CT041: Antiproliferative response and predictive biomarkers to palbociclib in early breast cancer: The Preoperative Palbociclib (POP) randomized trial. Cancer Res. 2016;76:CT041–CT041. [Google Scholar]
- 33.Dunbier AK, Anderson H, Ghazoui Z, Salter J, Parker JS, Perou CM, et al. Association between breast cancer subtypes and response to neoadjuvant anastrozole. Steroids. 2011;76:736–40. doi: 10.1016/j.steroids.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Turnbull AK, Arthur LM, Renshaw L, Larionov AA, Kay C, Dunbier AK, et al. Accurate Prediction and Validation of Response to Endocrine Therapy in Breast Cancer. J Clin Oncol. 2015;33:2270–8. doi: 10.1200/JCO.2014.57.8963. [DOI] [PubMed] [Google Scholar]
- 35.Tilghman J, Wu H, Sang Y, Shi X, Guerrero-Cazares H, Quinones-Hinojosa A, et al. HMMR Maintains the Stemness and Tumorigenicity of Glioblastoma Stem-like Cells. Cancer Res. 2014;74:3168–79. doi: 10.1158/0008-5472.CAN-13-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warner SL, Stephens BJ, Nwokenkwo S, Hostetter G, Sugeng A, Hidalgo M, et al. Validation of TPX2 as a Potential Therapeutic Target in Pancreatic Cancer Cells. Clin Cancer Res. 2009;15:6519–28. doi: 10.1158/1078-0432.CCR-09-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan MH, De S, Bebek G, Orloff MS, Wesolowski R, Downs-Kelly E, et al. Specific kinesin expression profiles associated with taxane resistance in basal-like breast cancer. Breast Cancer Res Treat. 2012;131:849–58. doi: 10.1007/s10549-011-1500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurvitz S, Abad MF, Rostorfer R, Chan D, Egle D, Huang C-S, et al. breast cancer, early stageInterim results from neoMONARCH: A neoadjuvant phase II study of abemaciclib in postmenopausal women with HR + /HER2- breast cancer (BC) Ann Oncol. 2016;27:LBA13. [Google Scholar]
- 39.Ma CX, Gao F, Luo J, Northfelt DW, Goetz MP, Forero A, et al. NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor positive breast cancer. Clin Cancer Res. 2017;23:4055–65. doi: 10.1158/1078-0432.CCR-16-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller TW, Balko JM, Fox EM, Ghazoui Z, Dunbier A, Anderson H, et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338–51. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu J, Zhu Y, Liu Y, Sun L, Lv X, Wu Y, et al. E2F7 overexpression leads to tamoxifen resistance in breast cancer cells by competing with E2F1 at miR-15a/16 promoter. Oncotarget. 2015;6:31944–57. doi: 10.18632/oncotarget.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khaleel SS, Andrews EH, Ung M, DiRenzo J, Cheng C. E2F4 regulatory program predicts patient survival prognosis in breast cancer. Breast Cancer Research. 2014;16:486. doi: 10.1186/s13058-014-0486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mark KMK, Varn FS, Ung MH, Qian F, Cheng C. The E2F4 prognostic signature predicts pathological response to neoadjuvant chemotherapy in breast cancer patients. BMC Cancer. 2017;17:306. doi: 10.1186/s12885-017-3297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast. 2015;(24 Suppl 2):S67–72. doi: 10.1016/j.breast.2015.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.