Graphical Abstract
Preliminary SAR studies and in vivo reactivation of EBV in SNU719 gastric carcinoma and AGS-Akata gastric carcinoma xenograft mouse model.
Keywords: Epstein-Barr virus, lytic activator, small molecule activator, oncolytic therapy
Epstein-Barr virus (EBV) is a human herpesvirus that infects over 90% of the world’s population and is the major cause of infectious mononucleosis.1,2 Latent infection can drive the formation of Burkitt’s lymphoma (BL), Hodgkin lymphomas, nasopharyngeal carcinoma (NPC), and gastric carcinoma (GC).3-6 In immunosuppressed patients, latent infection with EBV can cause post-transplant lymphoproliferative disease in immunosuppressed patients7,8 and greatly enhances the risk of developing non-Hodgkin and primary CNS lymphomas in the HIV-positive population.9,10 Most EBV-associated cancers contain viral DNA that exists predominantly as a latent infection in which only a limited set of viral genes are expressed.11 These latency associated genes are implicated in host cell proliferation and survival, and latent EBV can directly promote tumor progression. The total number of EBV-associated malignancies is estimated to exceed 200,000 new cancers per year. The near universal presence of EBV in certain tumors suggests that new EBV-targeting therapies could be developed for these malignancies. Current chemotherapeutic treatments of EBV-positive cancers include broad-spectrum cytotoxic drugs that ignore the EBV-positive status of tumors and have limited safety and selectivity. An alternative strategy, referred to as oncolytic therapy, utilizes drugs that stimulate reactivation of latent EBV to enhance the selective killing of EBV-positive tumors, especially in combination with existing inhibitors of herpesvirus lytic replication, like Ganciclovir (GCV).12-14 This targeted “oncolytic therapy” requires the initiation of the EBV lytic cycle, including expression of viral kinases and DNA polymerase that are exclusively expressed in the lytic phase, but never expressed during the latent phase of infection. This strategy aims to lower side effects associated with standard chemotherapy presently used to treat EBV-positive cancers, and provides a molecular targeted therapeutic strategy by exploiting the biology of EBV as a key etiologic disease factor.
We have previously shown that lytic-inducing agents, such as histone deacetylase (HDAC) inhibitors and DNA damaging agents, can be combined with the FDA approved antiherpesvirus nucleoside analogue, Ganciclovir (GCV), to induce maximal, EBV-dependent tumor cell killing.15 In cells containing the lytic type of EBV infection, virally encoded kinases (BGLF4 and/or the viral thymidine kinase BXLF4) are expressed which phosphorylate the prodrug, GCV, into a cytotoxic suicide substrate for viral DNA polymerase (BALF5). Phosphorylated GCV inhibits not only the virally encoded DNA polymerase, but also inhibits the host cell DNA polymerase and is thus cytotoxic. Furthermore, phosphorylated GCV can be transferred into nearby cells that are unable to phosphorylate GCV, thus inducing “bystander” killing in EBV positive cells that remain in the latent form of infection.
The major limitation of viral “oncolytic therapy” for EBV cancers is the poor efficiency and nonselectivity of viral reactivation by existing compounds. Of the many known chemical activators of the EBV lytic cycle, only the histone deacetylase inhibitors, derived from butyrate analogues, have been tested in clinical trials.16,17 In one clinical trial, arginine butyrate was found to be efficacious but was not tolerated due to toxicity, while sodium butyrate (NaB) was found to have unsuitable pharmacokinetics.16,17 Notably, a pilot study using the HDAC inhibitor, romidepsin, for the treatment of relapsed/refractory extranodal natural killer (NK)/T-cell lymphoma (ENKL) patients in Korea was discontinued due to serious adverse events, due to EBV reactivation in EBV-infected tumor cells.18 More recent studies have screened clinically approved drugs for potential activators for latent EBV and identified the proteasome inhibitor, bortezomib, as an activator of latent EBV.19 However, bortezomib induces EBV reactivation only in a small subset of EBV lymphoma types, and requires relatively high doses that produce non-specific cytotoxicity with risk of severe complications.20 Consequently, the selective and non-toxic induction of EBV lytic reactivation remains an unmet pharmacological need.
Development of new small molecule inducers of EBV lytic reactivation has been limited by incomplete knowledge of the biochemical pathways controlling EBV latency. Histone deacetylase inhibitors, including butyrate-derivatives and trichostatins, have the generic ability to reduce chromatin repression which is known to maintain EBV latency.21 Other lytic inducers include phorbol esters, calcium ionophores, hypoxia, TNF agonists, and B-cell receptor ligands.22 Several of these lytic inducers have common and convergent signaling pathways that have been tracked to the transcriptional regulatory elements of the viral immediate early genes, and include transcription factors in the AP1 and MEF2D pathways.23,24 Several inhibitors of EBV lytic cycle are known, including EBV-encoded latency proteins, LMP1, LMP2, and EBNA1. However, the mechanisms through which these viral proteins restrict lytic cycle gene expression are not completely understood. It is also widely believed that some natural products (e.g. phorbol esters) and environmental co-factors (e.g. malaria surface antigens) stimulate EBV reactivation, but it is not clear how these could be suitable for oncolytic therapy.25 Consequently, a better understanding of the chemical biology of EBV reactivation is of great biomedical significance for both the development of therapeutic agents for potential oncolytic therapy, and as a probe for biochemical pathways regulating the EBV latent-lytic switch.
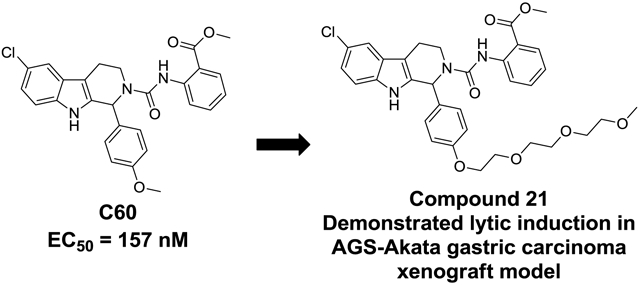
Herein we report the preliminary Hit-to-Lead optimization of a new class of small molecules that reactivate latent EBV. A small molecule reactivator of latent EBV in the nanomolar potency range with good preliminary in vitro ADME properties has the potential to greatly expand the field of study by providing a useful probe for biological evaluation and target identification, as well as a starting point for a new therapeutic agent for clinical evaluation in synergy with the anti-viral agent GCV. We have previously reported on the C60 series that was discovered through a high throughput screening campaign.26 From the 66,840 compounds screened we confirmed the activity of the top five hit compounds through testing in the primary and secondary confirmatory assays as well as re-testing freshly purchased powder samples that were analytically characterized. This provided a small set of closely related urea analogs showing initial potency structure activity relationships (SAR) for the series (Table 1). Compound C60 was selected for further follow studies because it showed the greatest potency in MutuI cell based assays (EC50 = 157 nM) and consistently stimulated EBV lytic reactivation in multiple cell types.26 Compound C60 showed greatly improved potency compared to NaB and TPA which typically require millimolar concentrations to trigger the latent to lytic switch27,28,29. It was shown to be an EBV activator that functions synergistically with Ganciclovir (GCV) to selectively kill EBV-positive cells through a distinct mechanism of action from that of the histone deacetylase inhibitor, sodium butyrate, or 12-O-tetradecanoylphorbol-13-acetate (TPA).25 Importantly, neither C60 nor GCV alone showed any cytotoxicity up to 30 μM in contrast to NaB, romidepsin, or TPA.
TABLE 1.
 | ||||
|---|---|---|---|---|
| ID | R1 | R2 | R3 | EC50 (nM)a |
| C09 | −OMe | −CH3 | 576 | |
| C53 | −OMe | −CH3 |  |
1079 |
| C50 | −Cl | −OMe | 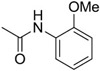 |
169 |
| C60 | −Cl | −OMe | 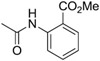 |
157 |
| C67 | −Cl | −OMe | 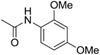 |
1128 |
EC50 values represent the average of at least 3 separate experiments.
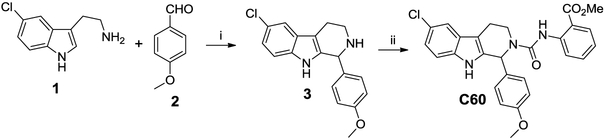
Compound C60 is a tetrahydro-beta-carboline with structural similarity to tryptoline indole alkaloid natural products which are wide spread in plants and animals. This class of compounds is known for their broad biological activity which includes anti-viral activity, inhibition of VEGF production, and anti-cancer indications.30 A robust synthesis route was established for the re-synthesis of C60 as shown in Scheme 1. A Pictet-Spengler reaction using 5-chloro tryptamine (1) with p-anisaldehyde (2) under acid catalyzed conditions provided the corresponding tetrahydro beta-carboline core structure (3), following a slightly modified literature protocol.31 Reaction with the isocyanate, methyl 2-isocyanato-benzoate, resulted in C60 as a white solid after chromatographic purification. Testing the re-synthesized material confirmed the original activity. We then evaluated Compound C60 for its in vitro ADME properties. Our initial optimization focused on improving the liabilities in this series which are poor water solubility and a high lipophilicity. Both properties may limit the distribution and in vivo pharmacokinetics of the series. The lead showed good stability in liver microsomes, and good plasma stability in addition to potency in the 150-200 nM range in cells.
Scheme 1.
Reagents: (i) 0.5 M HCl, reflux 14h. (ii) methyl-2-isocyanato benzoate, DCM, DIEA, rt, 3h.
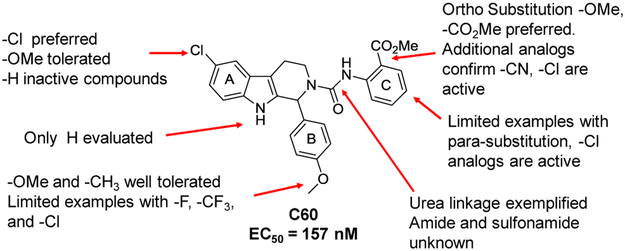
We then set out to develop additional SAR for the C60 series to help guide the improvement of the molecular properties while maintaining potency in the cellular assay. Re-examining the results from the high-throughput screening effort identified 199 analogs containing the tetrahydro-beta-carboline core scaffold out of the 66,840 compounds screened. These analogs were cherry picked and then evaluated in a follow-up screen. Using a cell-based luciferase reporter assay for EBV reactivation we tested activity at 2 concentrations (2 and 10 μM). Representative examples are shown in Table 2 for SAR comparison where we looked at percentage of activity compared to Compound C60 to provide a relative potency for the average values at the 2 μM concentrations. The 199 tetrahydro-β-carboline analogs evaluated in the follow-up HTS campaign had either chlorine (−C1), methoxy (−OMe), or hydrogen (−H) in the 6-position of the A ring. There was a clear preference for the Cl in the 6-position (i.e. C60 vs 4; C50 vs 6). Figure 1 shows a summary of the SAR of the 199 available tetrahydro-β-carboline analogs evaluated in this follow-up screen. Para-methoxy substitution on the aromatic ring, B, provided potent analogs, other substituents such as a methyl and fluorine were tolerated, however additional analogs are required in order to fully explore the SAR for the B ring. Comparison of the substitution pattern for the aromatic urea ring, C, suggested that the ortho substitution was preferred (i.e. C60 vs. 5; C50 vs. 7 or 8). Additional analogs with electron withdrawing and electron donating substitution, and di-substitution on the urea aromatic C ring would be helpful to confirm and expand this SAR. We synthesized additional urea analogs and found that −CN and −C1 are well tolerated in the ortho position of the C ring. Also −C1 is well tolerated in the para position, and only shows a slight loss in activity in the meta position of the C ring.
TABLE 2.
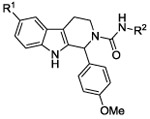 | |||
|---|---|---|---|
| ID | R1 | R2 | Rel potencya |
| C60 | −Cl | 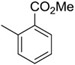 |
100 |
| 4 | −H | 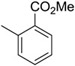 |
14 |
| 5 | −Cl | 17 | |
| C50 | −Cl | 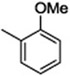 |
79 |
| 6 | −H | 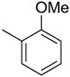 |
15 |
| 7 | −Cl | 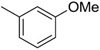 |
43 |
| 8 | −Cl | 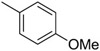 |
31 |
The relative potency is based on the average of two independent experiments. Rel potency of Cpd= [Cpd % inhib / C60 % inhib] * 100.
Figure 1.
Summary of the SAR based on 199 analogs available from our screening collection.
Although amide linked compounds were contained in the 199 analog follow-up set, all of these compounds contained the non-optimal −H in the 6-position of the indole A ring, and were thus weakly active or inactive. Also sulfonamide analogs were not available in this small set. Thus, we synthesized additional C60 analogs to fill in some of the initial gaps in the SAR focusing on understanding the potency determinates for the series. Representative analogs are shown in Table 3. The synthesis of amide or sulfonamide analogs utilized the 6-chloro-1-(4-methoxyphenyl)-2,3,4,9-tetrahydro-β-carboline intermediate, 3, (Scheme 1) followed by reaction with either an acid chloride or sulfonyl chloride in the presence of Hunigs base. The scaffold, 3, was active in the same potency range as C60 and C50. Ortho substitution of the amides is least preferred (i.e. 10, 13, 15), in contrast to what was observed for the urea C ring. Para substitution provided amide analogs with comparable potency to C60. Meta and para substitution appear to be equally potent (i.e. 11 and 12). Para methoxy seems best preferred (11), with para-Cl substituted analog 9 only slightly less potent, while the electron withdrawing para-trifluormethyl is least preferred (14). The two sulfonamide analogs, 16 and 17, are significantly less potent.
TABLE 3.
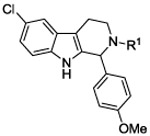 | ||
|---|---|---|
| ID | R1 | EC50 (nM)a |
| C60 | 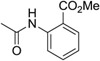 |
157 |
| 3 | −H | 215 |
| 9 | 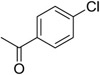 |
228 |
| 10 | 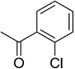 |
2432 |
| 11 | 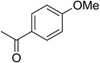 |
154 |
| 12 | 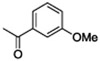 |
168 |
| 13 | 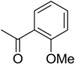 |
4267 |
| 14 | 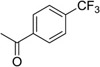 |
434 |
| 15 | 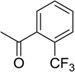 |
1576 |
| 16 | 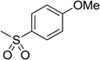 |
8030 |
| 17 | 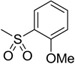 |
4008 |
EC50 values represent the average of at least 3 separate experiments.
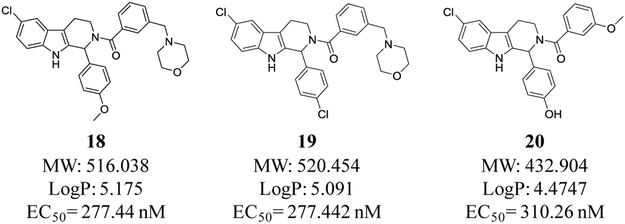
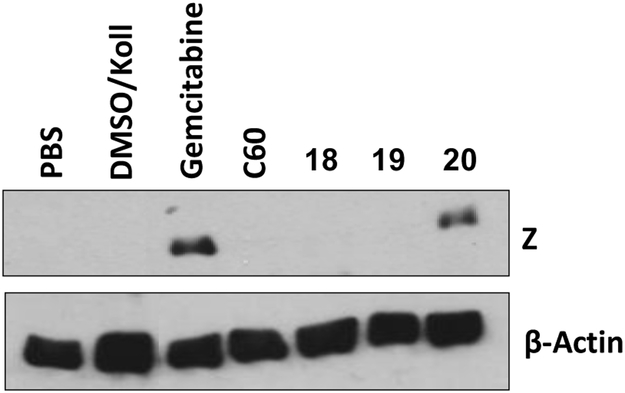
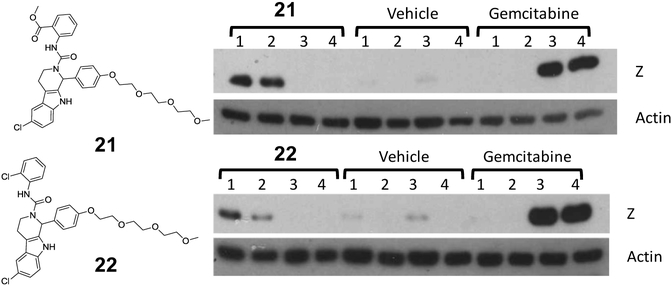
Compound 12 was chosen for ADME profiling to compare against C60 (Table 4). Water solubility improved and lipophilicity, measured by cLogP, was reduced. Interestingly liver microsomal stability is comparable to C60. In an attempt to further improve water solubility for a preliminary in vivo experiment we synthesized three additional analogs, two containing a morpholine group in the meta-position (18 and 19) and a phenol analog of 12 (20) (Figure 2). These analogs maintained potency, and were more soluble as demonstrated by their improved solubility characteristics when formulated in the 10% DMSO/ 10% solutol/ 80% PBS formulation mixture. We then tested these analogs in an in vivo xenograft model of EBV-associated gastric carcinoma. SNU719 gastric carcinoma cells (1×107) were injected subcutaneously into the left and right flanks of NSG mice. After 28 days post-injection, the animals were injected once per day with control vehicle (5% DMSO/ 5% Solutol), PBS, C60 or C60 analogues by i.p. administration (30 mg/kg). Animals were euthanized and tumor masses were collected after 4 days of treatment. Tumor masses were homogenized and immunoblot analysis was performed to detect the lytic EBV protein, BZLF1 (Z) and β-actin (loading control). Figure 3 shows that 20 modestly reactivates EBV as demonstrated by the presence of the lytic EBV protein BZLF1 (Z in Fig. 3).
TABLE 4.
In vitro drug-like properties comparison of C60 and 12
| ID | EC50(nM) | Mol Wt | cLogP | % Plasma Protein Binding |
Plasma Stability | Liver Microsomal Stability | water Solubility | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | Human | Mouse | Human | Mouse CLint/ t1/2 min |
Human CLint/ t1/2 min |
PBS buffer pH 7.4 μg/mL |
||||
| IDEALPROBE | <100 | <500 | 2.5-5.0 | <95% | <95% | >2h | >2h | <2/ >60 | <2/ >60 | >500 |
| C60 | 157 | 489.95 | 5.3 | NA | NA | >6h | >6h | <0.5/ >90 | 0.93/ 77.8 | <10 (poor) |
| 12 | 168 | 446.93 | 4.7 | NA | NA | >6h | >6h | 2.3/31 | 0.67/>90 | 166 |
Figure 2.
Analogs of compound 12 with water solubilizing morpholino groups in 18 and 19, and a phenolic moiety in 20.
Figure 3.
SNU719 gastric carcinoma tumors were collected after 4 days of treatment with C60, 18, 19, and 20 (30 mg/kg; i.p.). Tumor masses were homogenized and immunoblot analysis was performed to detect the lytic EBV protein, BZLF1 (Z) and β-actin (loading control).
We then synthesized C60 analogs, 21 and 22, which have a tri-polyethylene glycol (PEG) moiety attached to the phenolic oxygen (Fig. 4) to further improve water solubility32 and biodistribution. These are very similar urea analogs (see Fig. 1) to C60 and the related ortho chloro C-ring analog (not shown) which were consistently showing good activity in the cell based reactivation assay. The main difference was the incorporation of the PEG moiety instead of the methoxy in the B ring (Fig. 1) which significantly improved water solubility, demonstrated by their ease of formulation. We had also shown that the PEG moiety was tolerated and the two analogs had comparable activity to their methoxy substituted counterparts based on Western blot analysis (not shown). We wanted to confirm that compounds, very similar to C60, i.e Compounds 21 and 22 both in the urea series but with improved water solubility, could also reactivate EBV. Therefore these analogs were evaluated in the AGS-Akata gastric carcinoma cell xenograft model. This is a closely related gastric carcinoma model compared to the SNU719 model. The AGS-Akata is a super infected gastric carcinoma cell line routinely used in the Kenney lab.33 This model would provide analogous preliminary data on the ability of these C60 analogs to reactivate EBV in another cellular background, thus we choose to use this model to test these more water soluble analogs of C60. AGS-Akata cells (1×107) were injected into each flank of NSG mice. There are 4 mice per group, i.e. a Compound treated group (21 or 22), a Vehicle control group, and a Gemcitabine treated group. After 35 days, mice were injected with 60 mg/kg of Gemcitabine (once), 25 mg/kg of 21 or 22 (once a day for 3 days), and vehicle (5% DMSO and 5% Solutol) (once a day for 3 days). Tumor masses were homogenized and immunoblots were performed to detect the lytic EBV, BZLF1(Z; immediate early protein) and the loading control β-actin. These preliminary in vivo experiment suggest that the C60 analogs are reactivating latent EBV as demonstrated by the presence of the EBV lytic protein, BZLF1 shown in both animal models. The fact that BZLF1 is not observed from all the animals in the study is mostly due to picking the incorrect section of tumor for Western Blot analysis, or picking a part of the tumor which is not getting the drug delivered adequately to the tumor (perhaps due to poor blood supply to the tumor).
Figure 4.
AGS-Akata cells (1×107) were injected into each flank of NSG mice. There are 4 mice per group, i.e. a Compound treated group, a Vehicle control group, and a Gemcitabine treated group. After 35 days, mice were injected with 60 mg/kg of Gemcitabine (once), 25 mg/kg of 21 or 22 (once a day for 3 days), and vehicle (5% DMSO and 5% Solutol) (once a day for 3 days). Tumor masses were homogenized and immunoblots were performed to detect the lytic EBV, BZLF1(Z; immediate early protein) and the loading control β-actin.
In summary, this new series of reactivators of latent EBV show promise for further hit to lead optimization to provide additional tool compounds for pharmacological evaluation in animal models of lytic therapy in combination with GCV. This series will also provide the basis for chemical biology tool compounds to further uncover the molecular target responsible for the reactivation activity and to further study the mechanism involved in the latent-lytic switch in EBV. Additional analogs and tool compounds will be reported on in due course.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from NIH RO1 CA193624 and P30 CA010815.
ABBREVIATIONS
- (EBV)
Epstein-Barr virus
- (BL)
Burkitt’s lymphoma
- (NPC)
nasopharyngeal carcinoma
- (GC)
gastric carcinoma
- (GCV)
Ganciclovir
- (DCM)
dichloromethane
Footnotes
Declaration of interest
Dr. Salvino reports grants from The Wistar Institute, grants from Drexel University, grants from University of Wisconsin-Madison, during the conduct of the study; In addition, Dr. Salvino has a patent WO 2015031759 A1 20150305 issued to The Wistar institute and Drexel University.
Supporting Information
Supplemental Tables, experimental procedures for the synthesis and characterization of the new compounds, and the in vitro activity assay is available free of charge via the Internet.
ANIMAL EXPERIMENTS
The study is compliant with all relevant ethical regulations regarding animal research. For NSG mouse experiments, mice of both sexes were used. Recipient mice were 8-10 weeks old. The University of Wisconsin-Madison is an Accreditation of Laboratory Animal Care (AAALAC) certificated facility. All the procedures and protocols related to animal handling, care, and the treatment in the study were approved by the Institutional Animal Care and Use Committee (IACUC).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Rickinson AB, and Kieff E (2007) Epstein-Barr Virus., 5th ed., Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- (2).Kieff E (2007) Epstein-Barr Virus and its replication., 5th ed., Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- (3).Thorley-Lawson DA EBV the prototypical human tumor virus--just how bad is it?, J Allergy Clin Immunol. 2005, 116 (2), 251–261; quiz 262. PMID: 16083776. DOI: 10.1016/j.jaci.2005.05.038. [DOI] [PubMed] [Google Scholar]
- (4).Klein E, Kis LL, and Klein G Epstein-Barr virus infection in humans: from harmless to life endangering virus-lymphocyte interactions, Oncogene 2007, 26, 1297–1305. PMID: 17322915. DOI: 10.1038/sj.onc.1210240. [DOI] [PubMed] [Google Scholar]
- (5).Raab-Traub N Epstein-Barr virus in the pathogenesis of NPC, Seminars in cancer biology 2002, 12, 431–441. PMID: 12450729. [DOI] [PubMed] [Google Scholar]
- (6).Fukayama M, and Ushiku T Epstein-Barr virus-associated gastric carcinoma, Pathology, research and practice 2011, 207 (9), 529–537. PMID:21944426. DOI: 10.1016/j.prp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- (7).Loren AW, Porter DL, Stadtmauer EA, and Tsai DE Post-transplant lymphoproliferative disorder: a review, Bone marrow transplantation 2003, 31(3), 145–155. PMID: 12621474 [DOI] [PubMed] [Google Scholar]
- (8).Gottschalk S, Rooney CM, and Heslop HE Post-Transplant Lymphoproliferative Disorders, Annu Rev Med. 2004, 104 (8), 2272–2280. PMID: 15660500. DOI: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- (9).Hamilton-Dutoit SJ, Rea D, Raphael M, Sandvej K, Delecluse HJ, Gisselbrecht C, Marelle L, van Krieken HJ, and Pallesen G Epstein-Barr virus-latent gene expression and tumor cell phenotype in acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Correlation of lymphoma phenotype with three distinct patterns of viral latency, Am. J. Path 1993, 143 (4), 1072–1085. PMID: 8214003 PMCID: PMC1887058. [PMC free article] [PubMed] [Google Scholar]
- (10).MacMahon EM, Glass JD, Hayward SD, Mann RB, Becker PS, Charache P, McArthur JC, and Ambinder RF Epstein-Barr virus in AIDS-related primary central nervous system lymphoma, Lancet 1991, 338 (8773), 969–973. PMID: 1681341. [DOI] [PubMed] [Google Scholar]
- (11).Young LS, and Rickinson AB Epstein-Barr virus: 40 years on, Nat. Rev. Cancer 2004, 4(10), 757–768. PMID: 15510157. DOI: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- (12).Gutierrez MI, Judde JG, Magrath IT, and Bhatia KG Switching viral latency to viral lysis: a novel therapeutic approach for Epstein-Barr virus-associated neoplasia, Cancer Res, 1996, 56 (5), 969–972. PMID: 8640787. [PubMed] [Google Scholar]
- (13).Kenney S, Ge JQ, Westphal EM, and Olsen J Gene therapy strategies for treating Epstein-Barr virus-associated lymphomas: comparison of two different Epstein-Barr virus-based vectors, Human gene therapy 1998, 9 (8), 1131–1141. PMID: 9625252. DOI: 10.1089/hum.1998.9.8-1131. [DOI] [PubMed] [Google Scholar]
- (14).Moore SM, Cannon JS, Tanhehco YC, Hamzeh FM, and Ambinder RF Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues, Antimicrob. Agents Chemother, 2001, 45 (7), 2082–2091. PMID: 11408227. PMCID: PMC90604. DOI: 10.1128/AAC.45.7.2082-2091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Feng WH, Kenney SC, Valproic acid enhances the efficacy of chemotherapy in EBV-positive tumors by increasing lytic viral gene expression. Cancer Res 2006, 66, 8762–9. PMID: 16951192. DOI: 10.1158/0008-5472.CAN-06-1006. [DOI] [PubMed] [Google Scholar]
- (16).Perrine SP, Hermine O, Small T, Suarez F, O'Reilly R, Boulad F, Fingeroth J, Askin M, Levy A, Mentzer SJ, Di Nicola M, Gianni AM, Klein C, Horwitz S, and Faller DV A Phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies, Blood 2007, 109 (6), 2571–2578. PMID: 17119113. PMCID: PMC1852196. DOI: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ghosh SK, Perrine SP, Williams RM, and Faller DV Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents, Blood 2012, 119 (4), 1008–1017. PMID: 22160379 PMCID: PMC3271713. DOI: 10.1182/blood-2011-06-362434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kim SJ, Kim JH, Ki CS, Ko YH, Kim JS, Kim WS Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin. Ann Oncol. 2016, 27, 508–13. PMID: 26658891. DOI: 10.1093/annonc/mdv596. [DOI] [PubMed] [Google Scholar]
- (19).Shirley CM, Chen J, Shamay M, Li H, Zahnow CA, Hayward SD, and Ambinder RF Bortezomib induction of C/EBPbeta mediates Epstein-Barr virus lytic activation in Burkitt lymphoma, Blood 2011, 117 (23), 6297–6303. PMCID: PMC3122948 PMID: 21447826. DOI: 10.1182/blood-2011-01-332379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zou P, Kawada J, Pesnicak L, Cohen JI, Bortezomib Induces Apoptosis of Epstein-Barr Virus (EBV)-Transformed B Cells and Prolongs Survival of Mice Inoculated with EBV-Transformed B Cells, J. Virol 2007, 81(18), 10029–10036. PMID: 17626072 PMCID: PMC2045383. DOI: 10.1128/JVI.02241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Gorres KL, Daigle D, Mohanram S, Miller G, Activation and repression of Epstein-Barr virus and Kaposi sarcoma-associated herpesvirus lytic cycles by short- and medium-chain fatty acids J. Virol 2014, 88 (14), 8024–8044. doi: 10.1128/JVI.00722-14 PMID: 24807711 PMCID: PMC4097796. DOI: 10.1128/JVI.00722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Murata T, Tsurumi T, Switching of EBV cycles between latent and lytic states. Rev. Med. Virol 2014, 24 (3), 142–153. doi: 10.1002/rmv.1780. PMID: 24339346. DOI: 10.1002/rmv.1780 [DOI] [PubMed] [Google Scholar]
- (23).Speck SH, Chatila T, Flemington E, Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene Trends Microbiol. 1997, 5 (10), 399–405. PMID: 9351176. DOI: 10.1016/S0966-842X(97)01129-3. [DOI] [PubMed] [Google Scholar]
- (24).Amon W, Farrell PJ, Reactivation of Epstein-Barr virus from latency Rev Med Virol. 2005, 15(3), 149–156. PMID: 15546128. DOI: 10.1002/rmv.456. [DOI] [PubMed] [Google Scholar]
- (25).Rickinson AB, Co-infections, inflammation and oncogenesis: Future directions for EBV research Semin. Cancer Biol 2014, 26, 99–115. 10.1016/j.semcancer.2014.04.004. PMID: 24751797. [DOI] [PubMed] [Google Scholar]
- (26).Tikhmyanova N, Schultz DC, Lee T, Salvino JM, Lieberman PM Identification of a New Class of Small Molecules That Efficiently Reactivate Latent Epstein–Barr Virus ACS Chem Biol 2014, 9(3), 785–795. PMCID: PMC4159771 NIHMSID: NIHMS568917 PMID: 24028149. DOI: 10.1021/cb4006326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ghosh SK, Perrine SP, Williams RM, and Faller DV Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood 2012, 119 (4), 1008–1017. PMCID: PMC3271713 PMID: 22160379. DOI: 10.1182/blood-2011-06-362434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Countryman J, Gradoville L, Bhaduri-Mclntosh S, Ye J, Heston L, Himmelfarb S, Shedd D, and Miller G Stimulus duration and response time independently influence the kinetics of lytic cycle reactivation of Epstein-Barr virus. J. Virol 2009, 83(20), 10694–10709. PMID: 19656890 PMCID: PMC2753116. DOI: 10.1128/JVI.01172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Miller G, El-Guindy A, Countryman J, Ye J, and Gradoville L Lytic cycle switches of oncogenic human gamma herpes viruses. Adv. Cancer Res 2007, 97, 81–109. PMID: 17419942. DOI: 10.1016/S0065-230X(06)97004-3 [DOI] [PubMed] [Google Scholar]
- (30).PTC Therapeutics, Inc.; Seongwoo Hwang, Peter; Young-choon Moon; Tamil Arasu; Hongyan QI; Neil Almstead Patent: 2010. WO2010/138644 A1.
- (31).X-RT, Inc. Keefe AD, Wagner RW, Clark M, Zhang Y, Gikunju D, Cuozzo J, Thomson H 2013. WO2013106414 (A1) Tryptoline derivatives having kinase inhibitory activity and uses thereof.
- (32).Binderup E, Björkling F, Hjamaa PV, Latini S, Baltzer B, Carlsen M, Binder L EB1627: a soluble prodrug of the potent anticancer cyanoguanidine CHS828. Bioorg. Med. Chem. Lett 2005, 15, 2491–2494. PMID: 15863303. DOI: 10.1016/j.bmcl.2005.03.064. [DOI] [PubMed] [Google Scholar]
- (33).Nawandar DM, Wang A, Makielski K, Lee D, Ma S, Barlow E, Reusch J, Jiang R, Wille CK, Greenspan D, Greenspan JS, Mertz JE, Hutt-Fletcher L, Johannsen EC, Lambert PF, Kenney SC Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells. PLoS Pathog. 2015, 11, e1005195 PMID: 26431332. PMCID: PMC4592227. DOI: 10.1371/journal.ppat.1005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.