Abstract
Background:
In the fifth National Wilms Tumor Study (NWTS-5), the 4-year event-free (EFS) and overall survival (OS) estimates for 29 patients with stage I focal (n=10) or diffuse (n= 19) anaplastic Wilms tumor (AWT) treated with vincristine and dactinomycin without flank radiation were 69.5% and 82.6%, respectively. The Children’s Oncology Group AREN0321 study evaluated whether adding doxorubicin and flank radiation improves survival for these patients.
Patients and Methods:
Tumor histology and stage were confirmed by real-time central pathology, surgery, and radiology review. Patients received 25 weeks of vincristine, dactinomycin and doxorubicin (cumulative dose 150 mg/m2) with flank radiation (1080 cGy). We retrospectively analyzed outcomes of all patients with stage I AWT enrolled on National Wilms Tumor Studies (NWTS) 1–5 and AREN0321 with respect to treatment regimens.
Results:
Eighteen patients with stage I AWT (8 focal and 10 diffuse) were enrolled on AREN0321. With median follow-up of 4.6 years, the 4-year EFS and OS were 100%. One patient with diffuse AWT had pulmonary relapse 4.12 years after diagnosis. In the 112 patients with stage I AWT treated on NWTS 1–5 and AREN0321, the EFS was significantly improved with doxorubicin treatment (p=0.01; 4-year EFS: 97.2% (95% CI: 91.3–100) vs. 77.5% (95% CI: 67.6–87.4)), but not by flank radiation (p=0.15).
Conclusions:
Treatment of stage I AWT with vincristine, dactinomycin, doxorubicin, and flank radiation on AREN0321 yielded excellent survival outcomes. Retrospective analysis of AREN0321 and NWTS patients suggests that doxorubicin had a greater contribution to the excellent outcomes than radiation.
Keywords: Wilms tumor, stage I, diffuse anaplasia, focal anaplasia, treatment, vincristine, dactinomycin, doxorubicin, radiation, outcome
Introduction
Approximately 5–10% of Wilms tumors demonstrate anaplastic histology, which is defined by the presence of polyploid atypical mitotic figures, large nuclear size, and hyperchromasia [1, 2]. The definition of anaplasia is further refined to specify whether the anaplasia is diffuse or focal based on the geographic distribution of anaplastic cells within the tumor [3]. The presence of anaplasia is one of the most powerful adverse prognostic factors for Wilms tumor. Wilms tumors with diffuse anaplasia are associated with inferior event-free survival (EFS) and overall survival (OS) estimates compared to tumors of favorable histology; tumors with focal anaplasia have an intermediate prognosis. The third National Wilms Tumor Study (NWTS-3) was the first study to augment therapy for anaplastic Wilms tumor, with outcomes improving with the addition of cyclophosphamide, and later etoposide, to treatment regimens [4, 5].
On NWTS-5, patients with Stage I diffuse or focal anaplastic Wilms tumor were treated with vincristine and dactinomycin without flank radiation (Regimen EE4A), the same regimen used for favorable histology Wilms tumor. The rationale for this approach was that previous studies showed relatively favorable outcomes for stage I anaplastic Wilms tumor, though some of these studies included combinations of doxorubicin, cyclophosphamide, and/or flank radiation [6, 7]. Moreover, because stage I tumors are confined to the kidney and completely resected, it was thought that all the anaplastic cells are removed and additional therapy is not required. However, among patients with Stage I focal and diffuse anaplasia enrolled on NWTS-5, the 4-year EFS and OS estimates were only 69.5% (95% CI, 46.9–84.0) and 82.6% (95% CI, 63.1–92.4), respectively. By contrast, the 4-year EFS and OS estimates for 473 patients with stage I favorable histology Wilms tumor were 92.4% (95% CI, 89.5–94.5) and 98.3% (95% CI, 96.4–99.2) [5]. The sites of relapse among the patients with stage I anaplastic Wilms tumor included the lung (1), operative bed (1), abdomen and pelvis outside the operative bed (2), liver (2), and other sites (2).
To improve upon these results, the Children’s Oncology Group (COG) AREN0321 study evaluated the treatment of patients with stage I anaplastic Wilms tumor with vincristine, dactinomycin, doxorubicin (Regimen DD4A), and flank radiation. Here we report the outcomes of patients treated with this approach, and the results of a retrospective analysis of all patients with stage I anaplastic Wilms tumor treated on NWTS 1–5 and COG AREN0321 to identify treatment factors that influenced outcomes.
Patients and Methods
The AREN0321 study (ClinicalTrials.gov identifier: ) was approved by the National Cancer Institute Pediatric Central Institutional Review Board (IRB) and local IRBs according to institutional policy. The NWTS 1–5 were approved by the local IRBs. All participants or their legally authorized guardians provided consent.
Patients
Eligible patients had stage I focal or diffuse anaplastic Wilms tumor. All patients underwent upfront nephrectomy and began protocol therapy no later than Day 14 after nephrectomy. The standard operation was a unilateral radical nephroureterectomy with lymph node sampling. Expert central review of a complete set of pathology slides, the pathology reports, surgical summaries, and protocol required imaging studies was performed before enrollment on AREN0321 through mandatory enrollment on the COG AREN03B2 biology and classification protocol. Stage I disease was defined according to the following criteria: 1) tumor is limited to kidney and is completely resected, 2) the renal capsule is intact, 3) the tumor was not ruptured or biopsied prior to removal, 4) the vessels of the renal sinus are not involved, 5) there is no evidence of tumor at or beyond the margins of resection, and 6) regional lymph nodes that were examined microscopically were free of tumor.
Patients had to be younger than 30 years old, have a Karnofsky or Lansky performance status ≥ 50, have not received systemic chemotherapy or radiation, have adequate cardiac function defined as shortening fraction ≥ 27% by echocardiogram or ejection fraction ≥ 50% by radionuclide angiogram, have adequate liver function defined as total serum bilirubin ≤ 1.5 x normal for age and liver transaminases < 2.5 x normal for age. Female patients of childbearing age had to have a negative pregnancy test, and if lactating, had to stop breast‐feeding. Sexually active patients of childbearing potential had to use effective contraception.
Protocol therapy
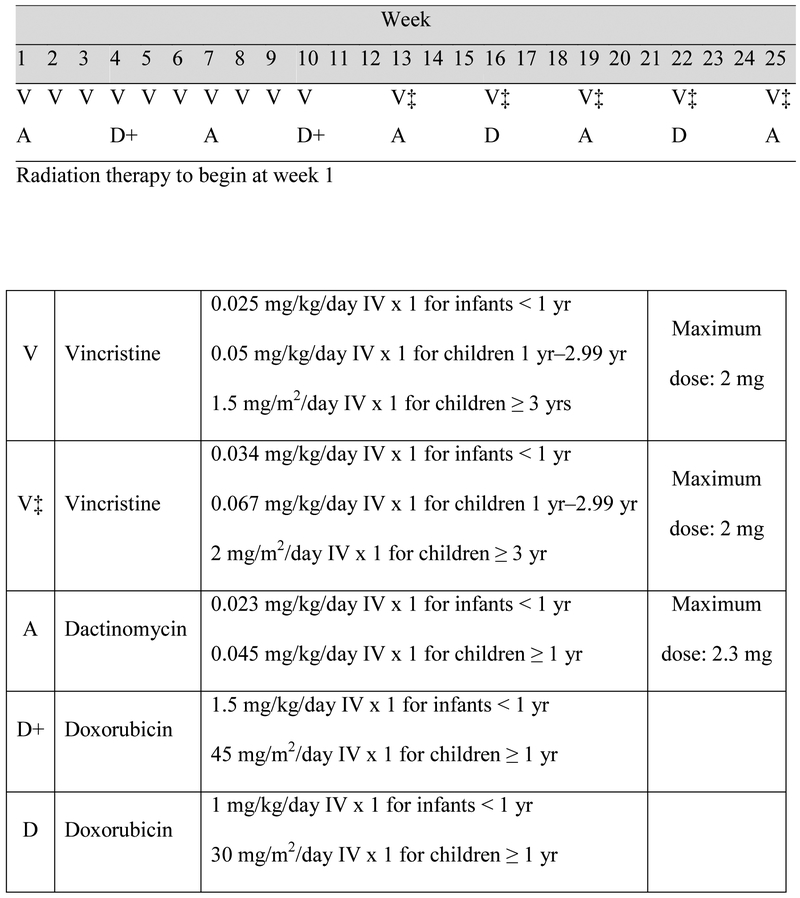
Patients received Regimen DD4A with vincristine, doxorubicin, dactinomycin, and radiation to the flank (Figure 1). Absolute neutrophil count ≥ 750/μL and platelet count ≥ 75,000/μL were required to start myelosuppressive chemotherapy. Vincristine was held until peristalsis was established after nephrectomy, and was continued during the weekly schedule regardless of blood counts. Radiation therapy (10.8 Gy) to the flank was delivered as previously described concurrently with initiation of chemotherapy by Day 14 after nephrectomy [8, 9]. Radiation therapy was delivered at COG approved centers. The detailed treatment plan and dosimetry were required to be submitted to the Quality Assurance Review Center for review.
Figure 1.
Treatment schema for regimen DD4A and chemotherapy dosing.
Patient evaluation
Laboratory testing, computed tomography (CT) of the chest, abdomen and pelvis, electrocardiography, and echocardiography were performed at baseline and end of therapy. The laboratory tests included complete blood counts, and serum creatinine, alanine aminotransferase, bilirubin, electrolytes, calcium, phosphorus, magnesium, total protein/albumin, and urinalysis. During therapy, an echocardiogram was repeated before week 16. After completion of therapy, patients were followed for disease surveillance every 3 months during the first 2 years, every 6 months during the third and fourth years, and every 12 months during the fifth year. CT of the chest, abdomen, and pelvis was obtained at every visit during the first 2 years and switched to chest radiography and abdominal ultrasonography during the subsequent years.
Retrospective Analysis of NWTS 1–5 Data
The results of NWTS 1–5 were previously reported [1, 5–7, 10]. Patients with stage I diffuse or focal anaplastic Wilms tumor were identified using the NWTS database. Patients who were confirmed by retrospective central pathology and study chair review to have stage I disease and anaplastic histology were included in the analysis. The treatment regimens used on NWTS 1–5 are summarized in Table 1.
Table 1.
Treatment regimens used for patients with stage I anaplastic histology Wilms tumor enrolled on NWTS 1–5
| Study | Regimen Name | Chemotherapy Regimen | XRT | # patients (FA/DA) |
|---|---|---|---|---|
| NWTS-1 | A | AMD × 15 mo | Yes | 0/2 |
| B | AMD × 15 mo | No | 0/2 | |
| NWTS-2 | C | AMD/VCR × 15 mo | Yes | 1/0 |
| D | VCR/AMD/DOX × 15 mo | Yes | 0/1 | |
| E | VCR/AMD × 6 mo | No | 1/1 | |
| F | VCR/AMD × 15 mo | No | 0/4 | |
| NWTS-3 | DD | VCR/AMD/DOX × 15 mo | No | 1/2 |
| DD-RT/DD2000 | VCR/AMD/DOX × 15 mo | Yes | 2/8 | |
| EE | VCR/AMD × 25 wk | No | 1/4 | |
| J | VCR/AMD/DOX/CTX × 15 mo | Yes | 0/4 | |
| L | VCR/AMD × 10 wk | No | 2/0 | |
| NWTS-4 | EE | VCR/AMD-std. × 25 wk | No | 8/9 |
| EE4A | VCR/AMD-PI × 18 wk | No | 7/6 | |
| K | VCR/AMD-std × 65 wk | No | 0/1 | |
| NWTS-5 | EE4A | VCR/AMD-PI × 18 wk | No | 8/19 |
FA, focal anaplasia; DA, diffuse anaplasia; VCR, vincristine; wk, weeks; mo, months; AMD, dactinomycin; std, standard dosing; PI, pulse intensive dosing; DOX, doxorubicin; CTX, cyclophosphamide; XRT, radiation therapy
Definitions of Focal and Diffuse Anaplasia
Focal anaplasia was defined as the presence of one or a few sharply localized regions of anaplasia within a primary tumor, the majority of which do not contain nuclear atypia. The topographic definition of focal anaplasia required careful documentation of the exact site from which every section was obtained (e.g., on a diagram, specimen photocopy, and/or polaroid photograph of the gross specimen). Diffuse anaplasia was defined as: a) anaplasia in any extrarenal site, including vessels of the renal sinus, extracapsular infiltrates, nodal or distant metastases; b) anaplasia in a random biopsy specimen; c) anaplasia unequivocally expressed in one region of the tumor, but with extreme nuclear pleomorphism approaching the level of anaplasia elsewhere in the tumor; d) anaplasia in more than one tumor slide unless: 1) every slide showing anaplasia came from the same region of the tumor; or 2) anaplastic foci on the various slides are small and surrounded on all sides by non-anaplastic tumor.
Statistical Methods
Data from AREN0321 patients who were confirmed to have stage I anaplastic Wilms tumor upon central pathology review were analyzed. The clinical characteristics of patients treated on AREN0321 and NWTS-5 were compared using the Wilcoxon rank-sum test for age, Chi-square test for gender and histologic subtype, and Fisher’s exact test for race/ethnicity. Further analyses were conducted including patients treated on NWTS 1–5 to evaluate the outcomes of patients with stage I anaplastic Wilms tumors according to treatment with doxorubicin, flank radiation, or both, and according to histologic subtype (focal or diffuse). The T-test was used to examine the relationship between tumor weight and tumor relapse. Both the EFS and OS were calculated from the time of diagnosis. The definition of an event included relapse, second malignant neoplasm, and death of any cause, whichever occurred first. Local relapse was defined as relapse in the operative bed, abdomen outside the operative bed, or pelvis; tumor spread to the liver was considered distant metastasis. The 4-year EFS and OS rates were estimated using the method of Kaplan and Meier [11] with confidence intervals estimated by the Peto-Peto method [12]. The EFS and OS were compared between groups using the log-rank test [13]. The median time of follow-up was calculated based on the Kaplan-Meier estimates [14]. Patient follow-up was current through December 31, 2015.
Results
From June 2006 to November 2013, a total of 18 eligible patients with stage I anaplastic (8 focal and 10 diffuse) Wilms tumor were treated with Regimen DD4A on AREN0321. This number does not reflect the total number of patients with stage I anaplastic Wilms tumor enrolled on the AREN03B2 Renal Tumors Classification and Biology Study during this time period because accrual to the AREN0321 protocol was intermittently suspended for data review. The demographics of these 18 patients and the 27 patients with stage I anaplastic Wilms tumor treated with regimen EE4A on NWTS-5 are summarized in Table 2.
Table 2.
Clinical characteristics of 45 patients with stage I anaplastic Wilms tumors according to study
| Characteristic | AREN0321 | NWTS-5 | P-value1 |
|---|---|---|---|
| N (%) | N (%) | ||
| Number of patients | 18 | 27 | |
| Median age at diagnosis years (range) | 4.5 (1.4–13.9) | 3.8 (1.4–15) | 0.60 |
| Gender | 0.11 | ||
| Male | 11 (61) | 10 (37) | |
| Female | 7 (39) | 17 (63) | |
| Race/ethnicity | 0.18 | ||
| White | 15 (83) | 19 (70) | |
| Black | 2 (11) | 3 (11) | |
| Hispanic | - | 5 (19) | |
| Unknown | 1 (6) | - | |
| Histologic subtype | 0.31 | ||
| Focal anaplasia | 8 (44) | 8 (30) | |
| Diffuse anaplasia | 10 (56) | 19 (70) | |
| Lymph nodes sampled | 1.00 | ||
| Yes | 16 (89) | 24 (89) | |
| No | 2 (11) | 3 (11) |
Wilcoxon rank-sum test for age, Chi-square test for gender and histologic subtype, and Fisher’s exact test for race/ethnicity
Patient Outcomes on AREN0321 and NWTS-5
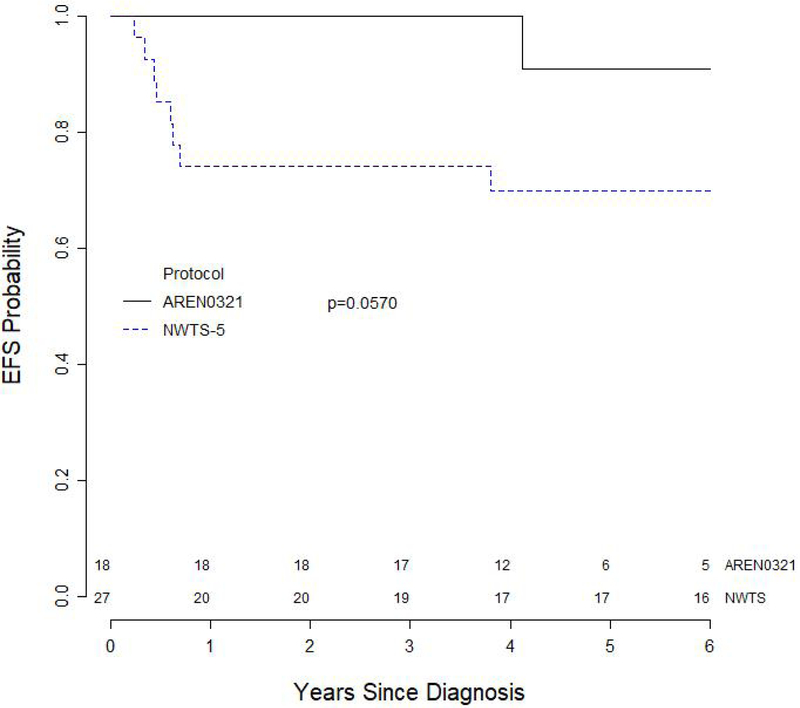
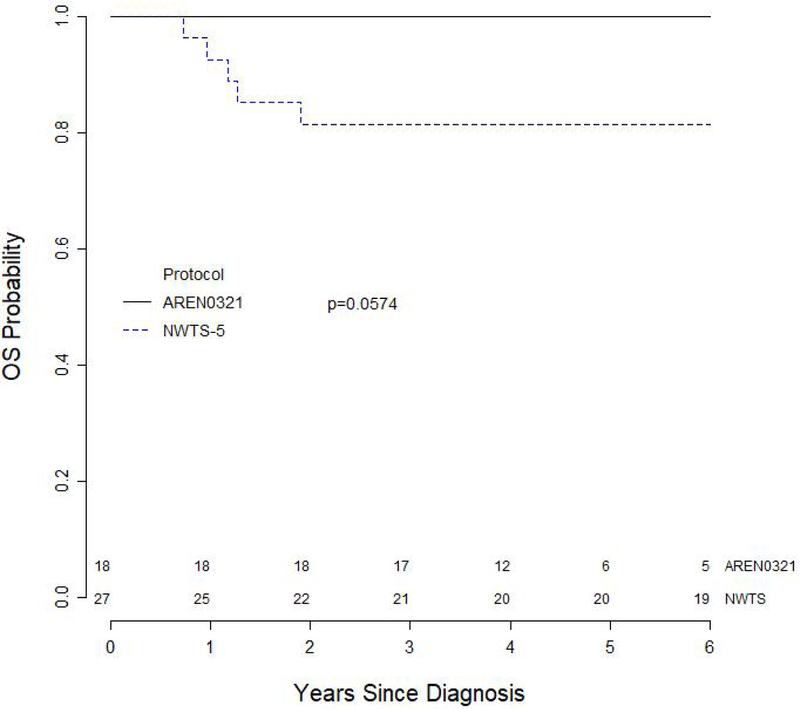
The median follow up for survivors on AREN0321 and NWTS-5 were 4.6 years and 13.3 years, respectively. Both the 4-year EFS and OS for the 18 patients treated on AREN0321 were 100%. In comparison, an updated analysis of 4-year EFS and OS for 27 patients treated on NWTS-5 were 70.0% (95% CI, 51.7%–88.2%; p=0.057) and 81.5% (95% CI, 66.1%–96.9%; p=0.057), respectively (Figures 2 and 3). One patient with diffuse anaplastic Wilms tumor treated on AREN0321 had a pulmonary relapse 4.12 years after study entry and is alive without evidence of disease 3.92 years after relapse. None of the patients treated on AREN0321 died.
Figure 2.
Event-free survival curves for 45 patients with stage I anaplastic Wilms tumor according to study.
Figure 3.
Overall survival curves for 45 patients with stage I anaplastic Wilms tumor according to study.
Patient Outcomes on AREN0321 and NWTS 1–5
In view of the excellent survival observed in patients with stage I anaplastic Wilms tumor with the addition of doxorubicin and flank radiation to vincristine and dactinomycin on AREN0321, we conducted further analyses to assess whether the addition of doxorubicin, radiation, or both contributed to the improved outcomes. These analyses included all 112 patients with stage I anaplastic Wilms tumor confirmed by central pathology review, treated on NWTS-1 (n=4), NWTS-2 (n=8), NWTS-3 (n=24), NWTS-4 (n=31), NWTS-5 (n=27), and AREN0321 (n=18). The 4-year EFS and OS for the 112 patients were 83.8% (95% CI, 76.5–91.1) and 89.3% (95% CI, 83.1–95.4). The 4-year EFS for patients with stage I diffuse anaplasia was 79.5% (95% CI, 69.7–89.2), compared to 92% (95% CI, 82.7–100) for patients with stage I focal anaplasia (p=0.21) (Table 3). Sites of relapse among patients with stage I diffuse anaplasia were lung only (n=8), lung and other sites (n=1), liver (n=2), operative bed (n=2), abdomen outside the operative bed (n=1), pelvis (n=1), and other distant sites (n=2). Sites of relapse among patients with stage I focal anaplasia were lung only (n=3).
Table 3.
Analysis of EFS and OS for 112 patients with stage 1 focal or diffuse anaplastic Wilms tumor treated on AREN0321 and NTWS 1–5 according to treatment and histologic subtype
| Factor | Category | N | 4-year EFS (95% CI) | P-value2 | 4-year OS (95% CI) | P-value2 |
|---|---|---|---|---|---|---|
| Overall | - | 112 | 83.8% (76.5–91.1) | - | 89.3% (83.1–95.4) | - |
| Doxorubicin | Yes | 36 | 97.2% (91.3–100) | 0.01 | 97.2% (91.3–100) | 0.10 |
| No | 76 | 77.5% (67.6–87.4) | 85.5% (77.2–93.8) | |||
| Radiation | Yes | 36 | 91.7% (81.7–100) | 0.15 | 94.4% (86.2–100) | 0.26 |
| No | 76 | 80.2% (70.7–89.6) | 86.8% (78.8–94.8) | |||
| Doxorubicin + radiation1 | Yes | 33 | 97.0% (90.5–100) | 0.03 | 97.0% (90.5–100) | 0.14 |
| No | 73 | 79.4% (69.6–89.2) | 86.2% (77.9–94.5) | |||
| Histology | DA | 73 | 79.5% (69.7–89.2) | 0.21 | 84.9% (76.2–93.5) | 0.07 |
| FA | 39 | 92% (82.7–100) | 97.4% (92–100) |
EFS, Event -free survival; OS, overall survival; DA, diffuse anaplasia, FA, focal anaplasia
Excluding 6 patients who received either doxorubicin with no radiation or radiation with no doxorubicin
Log Rank test
Data on tumor weight was available for 102 patients, of whom 20 had a relapse and one died after a second malignant neoplasm. The mean tumor weight was 652 grams (212–1650 grams) for the 21 patients who had a relapse or died vs. 599.9 grams (114–2015 grams) for the 81 patients without a relapse (p=0.55).
For the 112 patients with stage I anaplastic histology, treatment with doxorubicin positively impacted EFS. The 4-year EFS for patients who received doxorubicin was 97.2% (95% CI, 91.3–100) vs. 77.5% (95% CI, 67.6–87.4) for those who did not (p=0.01). The 4-year EFS for patients who received doxorubicin plus radiation was 97.0% (95% CI, 90.5–100) and 79.4% (95% CI, 69.6–89.2) for those who did not (p=0.030). However, the 4-year EFS did not differ in patients who received flank radiation (91.7%, 95% CI, 81.7–100) compared to those who did not (80.2% (95% CI, 70.7–89.6), p=0.15) (Table 3). Similarly in the subgroup of patients with diffuse anaplastic Wilms tumor, the 4-year EFS was significantly associated with doxorubicin treatment (p=0.046) but not with radiation (Table 4). In the subgroup of patients with focal anaplastic Wilms tumor, there was a trend for better EFS with treatment with radiation (p=0.06) and with treatment with both doxorubicin and radiation (p=0.09; Table 5). However, there was near complete overlap between doxorubicin and radiation treatment, making it difficult to separate the effects.
Table 4.
Analysis of EFS and OS for 73 patients with stage I diffuse anaplastic Wilms tumor according to treatment
| Factor | Category | N | 4-year EFS (95% CI) | P-value2 | 4-year OS (95% CI) | P-value2 |
|---|---|---|---|---|---|---|
| Doxorubicin | Yes | 25 | 96 (88.0–100) | 0.046 | 96% (88.0–100) | 0.10 |
| No | 48 | 70.8 (57.1–84.5) | 79% (66.8–91.2) | |||
| Radiation | Yes | 25 | 88.0% (74.6–100) | 0.47 | 92% (80.9–100) | 0.33 |
| No | 48 | 75.0% (62.0–88.0) | 81.1% (69.4–92.8) | |||
| Doxorubicin + radiation1 | Yes | 23 | 95.7% (86.9–100) | 0.11 | 95.7% (86.9–100) | 0.16 |
| No | 46 | 73.9% (60.4–87.4) | 80.3% (68.1–92.4) |
EFS, Event -free survival; OS, overall survival
Excluding 4 patients who received either doxorubicin with no radiation or radiation with no doxorubicin
Log Rank test
Table 5.
Analysis of EFS and OS for 39 patients with stage I focal anaplastic Wilms tumor according to treatment
| Factor | Category | N | 4-year EFS (95% CI) | P-value2 | 4-year OS (95% CI) | P-value2 |
|---|---|---|---|---|---|---|
| Doxorubicin | Yes | 11 | 100% | 0.13 | 100% | 0.47 |
| No | 28 | 89.1% (77.1–100) | 96.4% (89.3–100) | |||
| Radiation | Yes | 11 | 100% | 0.06 | 100% | 0.43 |
| No | 28 | 89.1% (77.1–100) | 96.4% (89.3–100) | |||
| Doxorubicin + radiation1 | Yes | 10 | 100% | 0.09 | 100% | 0.47 |
| No | 27 | 88.7% (76.3–100) | 96.3% (88.9–100) |
EFS, Event -free survival; OS, overall survival
Excluding 2 patients who received either doxorubicin with no radiation or radiation with no doxorubicin
Log Rank test
Only four patients (3.6%) had local relapse, all of whom had diffuse anaplasia and lymph node sampling as part of their tumor resection. Among the 73 patients with diffuse anaplasia, one of 25 (4.0%) patients who received flank radiation and three of 48 (6.3%) patients who did not receive flank radiation had a local relapse. The one patient with local relapse who received flank radiation and two of the three patients who did not receive flank radiation died. Hence, death from local relapse occurred in 4.0% of patients who received flank radiation and 4.2% of patients who did not.
In terms of OS, there was a trend for improved 4-year OS for patients with focal anaplastic Wilms tumor (97.4%, 95% CI, 92–100) compared to diffuse anaplastic Wilms tumors (84.9%, 95% CI, 76.2–93.5) (p=0.07). No statistically significant associations were detected between OS and treatment with doxorubicin, radiation, or both (Tables 3, 4, and 5).
Discussion
The AREN0321 study demonstrated that outcomes for patients with stage I anaplastic Wilms tumor were improved with the addition of doxorubicin and flank radiation to vincristine/dactinomycin therapy. Both 4-year EFS and OS estimates were 100% on AREN0321, compared to 70.0% and 81.5%, respectively, in an updated analysis of NWTS-5. Both doxorubicin and flank radiation were incorporated into the treatment regimen on AREN0321 because the pattern of relapse for stage I anaplastic Wilms tumor on NWTS-5 included both the abdomen and distant sites. Although these agents increase the risk of long-term side effects, notably cardiotoxicity, second malignancies, and pregnancy-associated complications, the doses used were relatively low (150 mg/m2 cumulative doxorubicin dose and 1080 cGy flank radiation). The incremental risks of this therapy must be balanced against the risks of salvage therapy required for recurrence, the psychosocial effects of relapse, and the increased likelihood of tumor-related death.
To assess the relative contributions of doxorubicin and flank radiation to the outstanding outcomes seen on AREN0321, we conducted a retrospective analysis of all patients with stage I anaplastic Wilms tumor treated on the present study and on NWTS 1–5. This review showed a significant improvement in EFS for patients treated with doxorubicin, but no difference in EFS according to whether flank radiation was given. The rate of local recurrence was low (3.6%) and appeared to be similar for patients who received flank radiation (4%) and patients who did not receive flank radiation (6.2%). In addition, local relapse occurred only in patients with diffuse anaplasia. These findings suggest that flank radiation may not be necessary for treatment of patients with stage I focal anaplastic Wilms tumor and raise the question of whether the possible benefit of avoiding a few local relapses is worth the risk of administering relatively low-dose flank radiation to all patients with stage I diffuse anaplastic Wilms tumor. An international collaborative effort with a larger number of patients of this rare subtype of Wilms tumor is needed to answer this question definitively.
Other groups have reported outcomes for patients with stage I anaplastic WT, though stage I in the setting of preoperative chemotherapy may have different prognostic implications from stage I in the setting of immediate nephrectomy. Among patients with stage I anaplastic Wilms tumor treated on the International Society of Pediatric Oncology (SIOP) −6 and −9 trials, one of 7 patients with focal anaplasia had tumor recurrence and none died, whereas five of 16 with diffuse anaplasia had tumor recurrence and four died [15]. In the subsequent trial SIOP 93–01, the 5-year EFS and OS for patients with stage I intermediate-risk and anaplastic Wilms tumor treated with only vincristine and dactinomycin were 87% and 95% in patients who received only four weeks of post-operative chemotherapy, but the difference in outcome between non-anaplastic and anaplastic tumors was not specified [16].
It would ideal to identify biological prognostic factors that could more precisely select patients who require additional therapy. A strong correlation between TP53 mutations and anaplasia has been established and several lines of evidence indicate that such mutations occur as a secondary event in the progression of Wilms tumor from favorable to anaplastic histology [17–19]. Recent studies have assessed the prognostic significance of TP53 mutation or loss of its chromosomal locus at 17p13 in patients with anaplastic Wilms tumor. Although tumor-specific TP53 alterations were associated with increased risk of recurrence and death [20], the marker was predictive of adverse outcome only for advanced stage disease (stage III and IV) [21]. In the latter analysis, comprehensive assessment of TP53 alterations including sequencing, copy number analysis, and immunohistochemistry, demonstrated that the vast majority of anaplastic Wilms tumor had evidence of TP53 alterations. Hence, the ability to detect a TP53 mutation in a random frozen tumor was likely a surrogate for the overall extent of TP53 mutations in the tumor, in turn reflecting the burden of anaplasia. Presently, assessment of TP53 mutational status is not helpful in stratifying therapy for patients with stage I anaplastic Wilms tumor.
Strengths of the AREN0321 study are that it was a prospective trial to evaluate the effect of augmenting therapy for stage I anaplastic Wilms tumor and that all patients underwent central review of pathology slides, surgical reports, and chest CT scans. The study population was an accurately defined and well-annotated group. A caveat to the study is the small number of patients. The retrospective analysis of the 112 patients treated on NWTS 1–5 and COG AREN0321 has helped to elucidate the contribution of doxorubicin versus flank radiation to the excellent patient outcome. However, the retrospective analysis is limited by the inclusion of patients treated over a broad time frame during which the quality of diagnostic imaging improved and the criteria for stage I changed, as did dosing schemas and duration of chemotherapy regimens.
In summary, EFS for patients with stage I anaplastic Wilms tumor appears to be improved on AREN0321 compared to NWTS-5 with the addition of doxorubicin and flank radiation to vincristine and dactinomycin. The retrospective analysis of the larger group of patients with stage I anaplastic Wilms tumor enrolled on the NWTS 1–5 and COG AREN0321 studies indicates that doxorubicin had a greater contribution to the outstanding outcomes than flank radiation. Our study provides important information that will help guide physicians in the treatment of stage I anaplastic Wilms tumor.
Highlights.
Vincristine, dactinomycin, doxorubicin and radiation in stage I anaplastic Wilms
Three-drug chemotherapy and radiation yielded 100% 4-year event-free survival
Doxorubicin had a greater contribution to the excellent outcome than radiation
Acknowledgments
The authors wish to thank the parents and children who enrolled on the Wilms tumor studies and the many investigators, pediatric oncologists, pathologists, surgeons, radiation oncologists, diagnostic imagers, and other health professionals who manage the children entered on these studies.
Research support: The research was supported by St. Baldrick’s Foundation and grants from the National Institutes of Health to the Children’s Oncology Group (U10CA180886, U10CA180899, U10CA098543, U10CA098413, and U24CA114766) and the National Wilms Tumor Study Group (). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 48th Congress of the International Society of Paediatric Oncology, Dublin, Ireland in October 2016.
Conflict of interest statement: None declared
References
- [1].Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer. 1978;41:1937–48. [DOI] [PubMed] [Google Scholar]
- [2].Zuppan CW, Beckwith JB, Luckey DW. Anaplasia in unilateral Wilms’ tumor: a report from the National Wilms’ Tumor Study Pathology Center. Human Pathology. 1988;19:1199–209. [DOI] [PubMed] [Google Scholar]
- [3].Faria P, Beckwith JB, Mishra K, Zuppan C, Weeks DA, Breslow N, et al. Focal versus diffuse anaplasia in Wilms tumor--new definitions with prognostic significance: a report from the National Wilms Tumor Study Group. Am J Surg Pathol. 1996;20:909–20. [DOI] [PubMed] [Google Scholar]
- [4].Green DM, Beckwith JB, Breslow NE, Faria P, Moksness J, Finklestein JZ, et al. Treatment of children with stages II to IV anaplastic Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. Journal of Clinical Oncology. 1994;12:2126–31. [DOI] [PubMed] [Google Scholar]
- [5].Dome JS, Cotton CA, Perlman EJ, Breslow NE, Kalapurakal JA, Ritchey ML, et al. Treatment of anaplastic histology Wilms’ tumor: results from the fifth National Wilms’ Tumor Study. J Clin Oncol. 2006;24:2352–8. [DOI] [PubMed] [Google Scholar]
- [6].D’Angio GJ BN, Beckwith JB. The treatment of Wilms’ tumor: results of the Third National Wilms’ Tumor Study. Cancer 1989;64:349–60. [DOI] [PubMed] [Google Scholar]
- [7].Green DM, Breslow NE, Beckwith JB, Finklestein JZ, Grundy PE, Thomas PR, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1998;16:237–45. [DOI] [PubMed] [Google Scholar]
- [8].Kalapurakal JA, Li SM, Breslow NE, Beckwith JB, Macklis R, Thomas PR, et al. Influence of radiation therapy delay on abdominal tumor recurrence in patients with favorable histology Wilms’ tumor treated on NWTS-3 and NWTS-4: a report from the National Wilms’ Tumor Study Group. International Journal of Radiation Oncology, Biology, Physics. 2003;57:495–9. [DOI] [PubMed] [Google Scholar]
- [9].Kalapurakal JA, Dome JS, Perlman EJ, Malogolowkin M, Haase GM, Grundy P, et al. Management of Wilms’ tumour: current practice and future goals. Lancet Oncol. 2004;5:37–46. [DOI] [PubMed] [Google Scholar]
- [10].D’Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, et al. The treatment of wilms’ tumor:Results of the second national wilms’ tumor study. Cancer. 1981;47:2302–11. [DOI] [PubMed] [Google Scholar]
- [11].Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457. [Google Scholar]
- [12].Peto RP J Asymptotically efficient rank invariant test procedures. Journal of the Royal Statistical Society: Series A. 1972;135:184–206. [Google Scholar]
- [13].Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Controlled clinical trials. 1996;17:343–6. [DOI] [PubMed] [Google Scholar]
- [15].Vujanic GM, Harms D, Sandstedt B, Weirich A, de KJ, Delemarre JF. New definitions of focal and diffuse anaplasia in Wilms tumor: the International Society of Paediatric Oncology (SIOP) experience. Medical and Pediatric Oncology. 1999;32:317–23. [DOI] [PubMed] [Google Scholar]
- [16].de Kraker J, Graf N, van TH, Pein F, Sandstedt B, Godzinski J, et al. Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms’ tumour (SIOP 93–01 trial): a randomised controlled trial. Lancet. 2004;364:1229–35. [DOI] [PubMed] [Google Scholar]
- [17].Bardeesy N, Beckwith JB, Pelletier J. Clonal expansion and attenuated apoptosis in Wilms’ tumors are associated with p53 gene mutations. Cancer Res. 1995;55:215–9. [PubMed] [Google Scholar]
- [18].Bardeesy N, Falkoff D, Petruzzi MJ, Nowak N, Zabel B, Adam M, et al. Anaplastic Wilms’ tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat Genet. 1994;7:91–7. [DOI] [PubMed] [Google Scholar]
- [19].Hill DA, Shear TD, Liu T, Billups CA, Singh PK, Dome JS. Clinical and biologic significance of nuclear unrest in Wilms tumor. Cancer. 2003;97:2318–26. [DOI] [PubMed] [Google Scholar]
- [20].Maschietto M, Williams RD, Chagtai T, Popov SD, Sebire NJ, Vujanic G, et al. TP53 mutational status is a potential marker for risk stratification in Wilms tumour with diffuse anaplasia. PloS one. 2014;9:e109924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ooms AH, Gadd S, Gerhard DS, Smith MA, Guidry Auvil JM, Meerzaman D, et al. Significance of TP53 Mutation in Wilms Tumors with Diffuse Anaplasia: A Report from the Children’s Oncology Group. Clin Cancer Res. 2016;22:5582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]