Abstract
Purpose
Diabetes leads to progressive complications such as diabetic retinopathy, which is the leading cause of blindness within the working-age population worldwide. Interleukin (IL)-17A is a cytokine that promotes and progresses diabetes. The objective of this study was to determine the role of IL-17A in retinal capillary degeneration, and to identify the mechanism that induces retinal endothelial cell death. These are clinically meaningful abnormalities that characterize early-stage non-proliferative diabetic retinopathy.
Methods
Retinal capillary degeneration was examined in vivo using the Streptozotocin (STZ) diabetes murine model. Diabetic-hyperglycemia was sustained for an 8-month period in wild type (C57BL/6) and IL-17A−/− mice to elucidate the role of IL-17A in retinal capillary degeneration. Further, ex vivo studies were performed in retinal endothelial cells to identify the IL-17A-dependent mechanism that induces cell death.
Results
It was determined that diabetes-induced retinal capillary degeneration was significantly lower in IL-17A−/− mice. Further, retinal endothelial cell death occurred through an IL-17A/IL-17R →Act1/FADD signaling cascade, which caused caspase-mediated apoptosis.
Conclusion
These are the first findings that establish a pathologic role for IL-17A in retinal capillary degeneration. Further, a novel IL-17A-dependent apoptotic mechanism was discovered, which identifies potential therapeutic targets for the early onset of diabetic retinopathy.
Keywords: Diabetic retinopathy, IL-17A, Act1, FADD, capillary degeneration
1. Introduction
Diabetic retinopathy is a diabetes-mediated retinal microvascular disease, and is the leading cause of vision loss within the working-age population worldwide1. Gradual, asymptomatic alterations in the retinal microvasculature can lead to capillary non-perfusion, which is one of the earliest detectable symptoms of non-proliferative diabetic retinopathy2,3. In response to this vasoregression, angiogenesis of retina vessels are induced, leading to proliferative diabetic retinopathy and vision loss1.
Circulating Th17 cells and IL-17A have been identified in the sera of Type I diabetic patients, and have been correlated to the progression of diabetic complications4. Further, autocrine IL-17A signaling in Muller glia has been shown to enhance early stage vascular permeability in the blood retinal barrier of diabetic mice5,6. However, the extent to which IL-17A contributes to retinal endothelial cell death and capillary degeneration, as well as the precise mechanism by which IL-17A induces capillary non-perfusion is still not known.
In the current study, hyperglycemic conditions initiated IL-17A production in STZ-diabetic mice, which was quantified in the sera and retina. Ablation of IL-17A production in IL-17A−/− diabetic mice impaired retinal capillary degeneration. These observations were extended to show that IL-17A induces retinal endothelial cell death through the Act1 adaptor molecule downstream of the IL-17A receptor. Finally, it was determined that Act1-dependent FADD (Fas-Activated Death Domain) activates caspase 8 and 3 to initiate retinal endothelial cell apoptosis. These are the first findings to establish a pathologic role for IL-17A in retinal capillary nonperfusion, as well as identify a novel apoptotic mechanism that impairs retinal endothelial cells.
2. Materials and Methods
2.1. Streptozotocin (STZ)-induced diabetic mice.
CWRU IACUC and LSVAMC ACORP approved the animal protocols employed in this study. C57BL/6J and IL-17A−/− mice were obtained from Jackson Laboratories. Diabetes was induced in 8–10 week old male mice by intraperitoneal injections of streptozotocin (60 mg/kg) on 5 consecutive days, as previously described7–9. Alternatively, heterozygous and homozygous B6.BKS(D)-LeprdblJ mice (db/db) were examined at 8–10 weeks of age, during spontaneous diabetic onset for systemic IL-17A analysis. Diabetes was defined by non-fasted blood glucose concentrations greater than 250 mg/dl, which was verified using glucose-dehydrogenase-based strips on three consecutive occasions beginning 1 week after the last STZ injection; hyperglycemia was also quantified by hemoglobin A1c levels using a Bio Rad D-10 analyzer. Insulin (0–0.2 U) was administered as needed to maintain proper body weight.
2.2. ELISA analysis.
Sera or retinal protein lysates were collected from db/db or C57BL/6 mice, and analyzed using 2-site ELISA according to the manufacturer’s directions (R&D Biosciences).
2.3. Flow cytometry.
For apoptosis analysis, Annexin V (eBioscience), Caspase 8 (active) FITC, and Caspase 3 (active) Red Staining detection kits (Abcam) were used according to manufacturer’s instructions. Cells were analyzed using a C6 Accuri flow cytometer (BD); gates were set to isotype controls, and compensated using FlowJo software.
2.4. Quantitative PCR.
RNA was extracted, cDNA generated, and qPCR was performed using SYBR green with Act1 (GeneBank ID# NM_001130518, NM_021532) and Actb (GeneBank ID# NM_001243262, NM_171846) primers. Qualitative images are PCR products run on a 2% agarose gel and visualized using ethidium bromide.
2.5. Capillary degeneration.
Acellular capillaries were quantified in the retinal vasculature as previously described2,3,7,8. Eyes were fixed with 10% formalin. Retinas were incubated in elastase for 2h followed by acidic buffer overnight. Retinal vasculature was stained with hematoxylin and periodic acid-Schiff. Acellular capillaries were quantified in 7 field areas between the optic nerve and the periphery (200X magnification).
2.6. Human retinal endothelial cells.
Human retinal endothelial cells of the microvasculature (hREC) were purchased from Cell Systems, and stimulated with 100 ng/ml of recombinant IL-17A (optimal concentration for apoptosis within the in vitro life span of the retinal endothelial cells (Supplemental Fig. S1A–B)). For Act1 and FADD knockdown experiments, cells were transfected with Act1, FADD, or scrambled control shRNA lentiviral particles according to manufacturer’s instructions (Santa Cruz). Gene silencing was confirmed by RT-PCR using manufacturer’s primers (Sc-29634-PR (Act1) and Sc-35352-PR (FADD), Santa Cruz).
2.7. Murine retinal endothelial cells.
Murine retinal endothelial cells (mREC) originally were a kind gift of Dr. Nader Sheibani and isolated from the retinas of C57BL/6 mice as previously described10. Retinas were digested in collaganase and mREC were purified using magnetic beads coated with endothelial cell adhesion molecule-1, bound VE-Cadherin positive cells were isolated, and mREC purity was confirmed to be >99% pure.
2.8. Viability assays.
3 × 105 human and murine retinal endothelial cells per well were stimulated with 100ng/ml rIL-17A (R&D), and either: 1) incubated with CellBrite cytoplasmic membrane stain and 1003bcμg/ml Propidium Iodide and imaged using a Leica DMI 600B inverted microscope, 2) stained with 0.25μg/tube 7-AAD (BD Bioscience) for quantification of cell viability11, or 3) incubated with Annexin V (eBioscience), active Caspase 8 or 3 (Abcam) antibodies for apoptosis analysis per manufacturer’s directions.
2.9. Immunoblot analysis.
Protein was isolated from cells using RIPA buffer (Thermo Scientific), denatured at 95°C in Laemmli buffer (Bio-Rad), separated on a 4–15% gel (Mini-Protean TGX, Bio-rad), and transferred to PVDF membrane (Immobilon Millipore). Blots were probed with anti-Act1 (WWW-18, Santa Cruz), anti-FADD (NBP1–4552, Novus Biologicals), and anti-β-actin (8229, Abcam) primary antibodies, followed by Li-Cor secondary antibodies for protein analysis using Li-Cor Odyssey Fc Infrared Imaging System.
2.10. Statistical analysis.
Statistical analysis was performed using a two-way ANOVA analysis and an unpaired t-test with Tukey’s post-hoc analysis (Prism, GraphPad Software). A p-value <0.05 was considered significant.
3. Results
3.1. Diabetes induces IL-17A production.
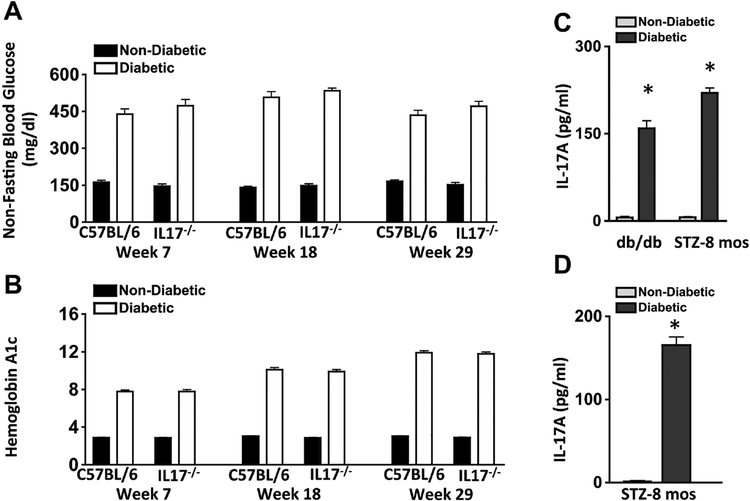
Diabetes-mediated hyperglycemia was sustained throughout an 8-month period in streptozotocin (STZ) diabetic mice (n=10 mice/group). The severity of hyperglycemia was similar among diabetic wild type (C57BL/6) and IL17A−/− mice (Fig. 1A–B).
Figure 1. Analysis of diabetes and IL-17A production in mice.
Assessment of Non-Fasting Blood Glucose (A) and Glycated Hemoglobin A (B) in non-diabetic (black) and STZ-injected diabetic (white) mice throughout the 8-month duration to confirm diabetes in C57BL/6 and IL-17−/− mice (n=10 mice/group). C) Protein levels of IL-17A in serum of non-diabetic (grey) and spontaneous diabetic (black) db/db mice, and non-diabetic (grey) and STZ-induced diabetic (black) mice. Sera samples (n=5/group) were collected 2-months or 8-months after diabetic conditions were confirmed in db/db and STZ-diabetic mice respectively. D) Quantification of IL-17A in retinas (n=6) of non-diabetic (grey) and diabetic (black) mice, 8-months after diabetic conditions was confirmed. * = p<0.01 per unpaired student’s t-test. Data are representative of three separate experiments.
Sera were evaluated in non-diabetic and STZ-diabetic C57BL/6, and non-diabetic (heterozygous B6.BKS(D)-LeprdblJ) and spontaneous diabetic (homozygous B6.BKS(D)-LeprdblJ) db/db mice (Fig. 1C). IL-17A was detected in all (n=5) of the diabetic mice but not in any of the non-diabetic mice, substantiating that IL-17A is induced by diabetes. Further, protein lysates were collected from the retinas of non-diabetic and STZ-diabetic mice (n=6) to quantify IL-17A. Approximately 200 pg/ml of IL-17A was detected in the retinas of diabetic, but not non-diabetic mice 8- months post-diabetes (Fig. 1D). Similar results were detected in two separate experiments (data not shown). These results confirm that diabetes induces IL-17A production, which is detectable in diabetic retinas.
3.2. Systemic neutralization of IL-17A decreases capillary degeneration and retinal endothelial cell death.
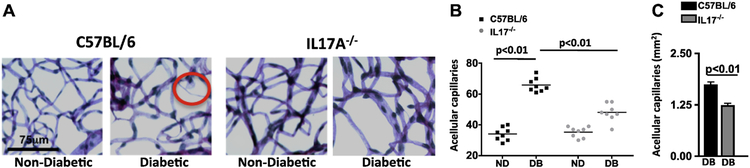
In early stages of diabetic retinopathy and in this 8-month murine model, endothelial cells can die in the retinal capillaries, resulting in acellular and degenerative capillaries that leads to capillary non-perfusion1–3,12. To ascertain if there is a role for IL-17A in retinal capillary degeneration, we isolated retinas (n=8/group) from non-diabetic and diabetic C57BL/6 and IL17A−/− mice 8-months after diabetes and quantified the number of acellular capillaries (representative example is circled in Fig. 2A). The number of acellular capillaries in the retinas of diabetic mice was significantly higher than in non-diabetic mice (Fig. 2B), but was significantly lower in the retinas of diabetic IL17A−/− than C57BL/6 mice (Fig. 2B–C). These results are representative of two separate experiments with similar results.
Figure 2. IL-17A elicits capillary cell death and degeneration in the retina.
A) Representative images of acellular capillaries and degeneration in retinal capillary beds of C57BL/6 and IL17A−/− non-diabetic and diabetic mice (scale bars of all images = 75 μm). Circled area highlights an acellular capillary. B) Quantification of acellular capillaries within a 1.10 mm2 area of each retina of non-diabetic (ND) and diabetic (DB) C57BL/6 (square black data point) and IL17A−/−(grey circle data point) mice. Each data point represents an individual retina from 8 different mice. p-value was first equated by two-way ANOVA analysis and then an unpaired t-test with Tukey’s post-hoc analysis. C) Mean quantifications of acellular capillary degeneration in retinas (n=8) of diabetic C57BL/6 (black) and IL17A−/− (grey) mice normalized to non-diabetic controls. All samples were collected 8 months after diabetic conditions were confirmed. All data is representative of two separate experiments with similar results.
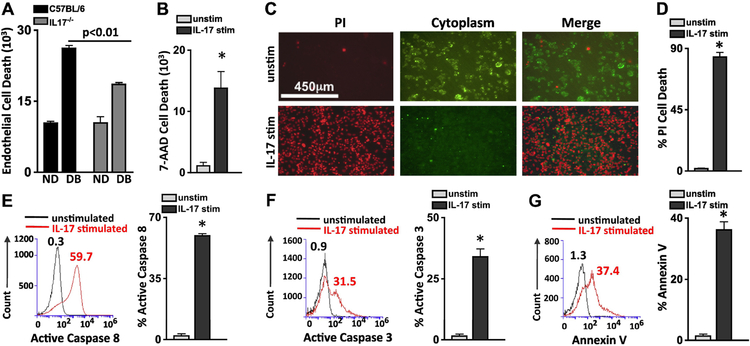
To determine if IL-17A regulates cell death in retinal endothelial cells, we co-cultured murine retinal endothelial cells (mREC) with bone marrow cells from non-diabetic and diabetic C57BL/6 and IL-17A−/− mice (Fig. 3A). After 18h of stimulation, there was a significant increase in retinal endothelial cell death (CD144+/7-AAD+) in mREC co-cultured with diabetic than non-diabetic bone marrow cells; as previously reported11. However, there was a significant decrease in retinal endothelial cell death in mREC co-cultured with bone marrow cells of diabetic-IL17A−/− mice than diabetic-C57BL/6 mice (Fig. 3A). Next, we stimulated human retinal endothelial cells with 100 ng/ml of recombinant (r)IL-17A for 18h, and then quantified 7-AAD+ (dead) hREC by flow cytometry. As shown in Fig. 3B, there were ~300 dead cells in the unstimulated samples but >18,000 dead hREC in all samples stimulated with rIL-17A. Further, we stimulated retinal endothelial cells with rIL-17A, then stained the cells with cytoplasmic membrane dye (green viable cells) and Propidium iodide ((PI) which is a red indicator for membrane permeability seen in dead cells), and then acquired live cell images. After 48h, most of the IL-17A stimulated hREC (Fig. 3C) and mREC (Supplemental Fig. S1C) were dead, whereas the unstimulated hREC and mREC remained viable. Flow cytometry showed 81.6% of the IL-17A stimulated hREC were PI positive compared with 1.1% of the unstimulated (Fig. 3D), similar results were detected in the mREC (Supplemental Fig. S1D). Taken together, this indicates that IL-17A plays a role in capillary degeneration and retinal endothelial cell death, which are the hallmarks for capillary non-perfusion and the onset of non-proliferative diabetic retinopathy.
Figure 3. IL-17A elicits apoptosis in human retinal endothelial cells.
Quantification of flow cytometry analysis (n=6) of retinal endothelial cell death (CD144+/7-AAD+) 18h after murine retinal endothelial cells were co-cultured with bone marrow cells from non-diabetic (ND) and diabetic (DB) C57BL/6 (black) or IL17A−/− (grey) mice (A), or of human retinal endothelial cells stimulated with recombinant human IL-17A (B). C) Representative microscopy of unstimulated (top) and IL-17A stimulated (bottom) human retinal endothelial cells (hREC) 48h after incubation; dead red cells are Propidium iodide (PI) positive and viable green cells retain the FITC cytoplasmic membrane stain (scale bars of all images = 450μm). D) PI quantification of flow cytometry analysis of 6 separate unstimulated (grey) and IL-17 stimulated (black) hREC samples. Representative flow cytometry analysis of unstimulated (black) and IL-17A stimulated (red) hRECs, as well as the quantified percent of apoptotic cells using Active Caspase 8 (E), Active Caspase 3 (F), and Annexin V assays (G) 6h after stimulation. Numbers above overlay peaks represent percent positive cells of 30,000 events, and the graph displays the percentage of Caspase 8 or 3, and Annexin V positive cells in six separate samples. *=p<0.01 per unpaired student’s t-test. Data are representative of three separate experiments with similar results.
3.3. IL-17A activates Caspase-3 and Caspase-8 in retinal endothelial cells.
To determine if IL-17A induces apoptosis in retinal endothelial cells, we stimulated the cells for 6 hours with 100 ng/ml of rIL17A, and quantified apoptotic cysteine proteases, cleaved-active caspase 8 and caspase 3. Compared with unstimulated hREC, 59.7% and 31.5% of the IL-17A stimulated hREC had active caspase 8 and 3 (Fig. 3E–F) respectively.
We also analyzed early stage apoptosis in IL-17A stimulated hREC through Annexin V analysis. We found that 37.4% of IL-17A stimulated hREC were Annexin V positive compared to 1.3% of unstimulated hREC (Fig. 3G). Similar results were discovered in IL-17A-stimulated mREC (Supplemental Fig. S1E–F). This indicates that programmed IL-17A-dependent retinal endothelial cell death is caspase mediated.
3.4. Silencing Act1 in retinal endothelial cells inhibits IL-17A dependent cell death.
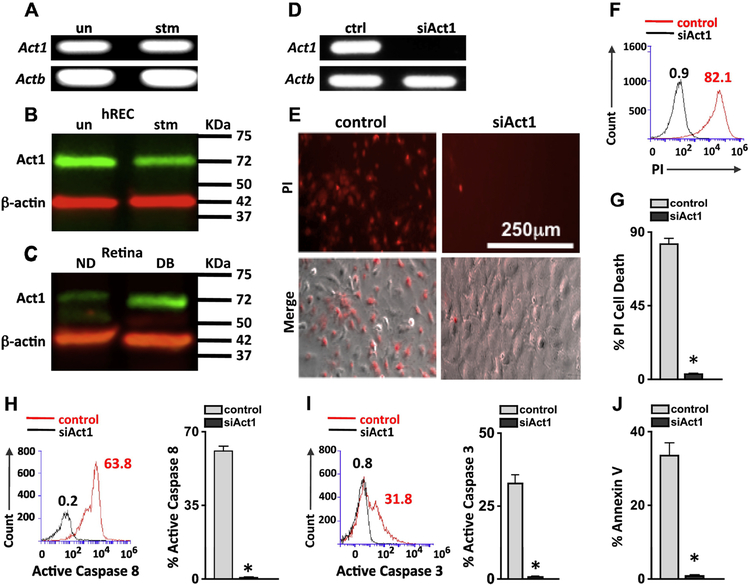
The receptors for IL-17A can signal through the Act1 adaptor13,14, therefore we examined if retinal endothelial cells express Act1. Constitutive gene expression of Act1 in hREC (Fig. 4A) and in mREC (Supplemental Fig. S2A) were detected by qPCR. Similarly, Act1 protein in unstimulated and rIL-17A stimulated hREC (Fig. 4B) and mREC (Supplemental Fig. S2B) were detected by immunoblot analysis. Additionally, Act1 protein was detected in the retinas of non-diabetic mice, which increased in the retinas of diabetic mice (Fig. 4C).
Figure 4. Act1 dependent retinal endothelial cell death.
A) Qualitative qPCR analysis of Act1 expression in mRNA of unstimulated and IL-17A stimulated hREC. Actb was used as a loading control. Immunoblot analysis of Act1 in protein lysates of unstimulated (un) or IL-17A stimulated (stm) hREC cultures (B), and non-diabetic (ND) and diabetic (DB) C57BL/6 mouse retinas (C); protein was collected 2-months post-diabetes or 6h post-stimulation. β-actin was used as a loading control. D) Act1 analysis by RT-PCR verifying Act1 gene silencing (siAct1) in hREC cultures; ctrl samples were transfected with scrambled sequences. E) Representative images of IL-17A stimulated control and siAct1 hREC viability; 48h after stimulation. Dead cells were examined using Propidium Iodide (PI) and Phase (Merge (PI + Phase)) microscopy analysis (scale bars for all images = 250 μm). Representative overlay of PI flow cytometry analysis in scrambled control (red) and siAct1 (black) hREC (F), and percentage of scramble control (grey) and siAct1 (black) PI+ cells (G). Representative flow cytometry analysis (left) and quantification (right) of 6 separate samples of active Caspase 8 (H) and active Caspase 3 (I) in IL-17A stimulated control and siAct1 hREC cultures 6h after incubation. Numbers represent percent positive cells of 30,000 events. J) Percentage of apoptotic Annexin V positive cells per flow cytometry analysis of 6 samples/group. *=p<0.01 per unpaired student’s t-test. Data are representative of two separate experiments with similar results.
Because IL-17A stimulation induces apoptosis in retinal endothelial cells, we investigated whether this was Act1 mediated. Unstimulated hREC were transfected with lentiviral particles containing a scrambled sequence that did not interfere in Act1 expression (ctrl) or an Act1 sequence that silenced Act1 (siAct1) expression. After gene silencing of Act1 was confirmed (Fig. 4D), we stimulated the control and siAct1 hREC with rIL-17A, stained the cells with Propidium iodide (PI), and examined membrane permeability. We found that ~82% of the control hREC were PI positive. In contrast, <1% of the siAct1 hREC had died (Fig. 4E–G). An average of 63% and 31% of the Act1-expressing hREC (ctrl) exhibited active caspase 8 and 3 positivity respectively, but there was no active caspase 8 or 3 detected in the siAct1 hREC (Fig. 4H–I). Finally, ~38% of all control hREC were Annexin V+ that was significantly lowered in the siAct1 hREC (Fig. 4J). Similar results were found in siAct1 mREC (Supplemental Fig. S2C–H). This suggests that IL-17A initiates apoptosis in retinal endothelial cells through Act1.
3.5. Act1 mediates FADD dependent apoptosis in retinal endothelial cells.
In previous studies of EAE, it was discovered that Act1 interacted with FADD to induce apoptosis in glial cells14. To examine Act1-FADD interactions in retinal endothelial cells, FADD protein was analyzed in control hREC or mREC or siAct1 knockdown hREC or mREC cells by immunoblot analysis. As shown in Fig. 5A, FADD was detected in IL-17A stimulated hREC when Act1 is expressed (ctrl), but was barely detected following Act1 knockdown (siAct1).
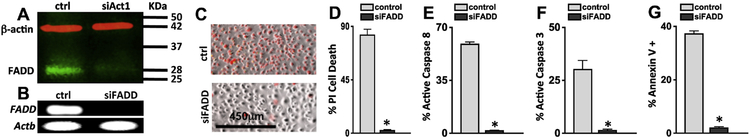
Figure 5. Act1-FADD dependent retinal endothelial cell death.
A) Immunoblot analysis of FADD expression in protein lysates of IL-17A stimulated scrambled control (Act1 expressing (ctrl)) or siAct1 (Act1 gene silenced) hREC cultures; protein was collected 6h post-stimulation. β-actin was used as a loading control. B) FADD expression by RT-PCR verifying gene silencing (siFADD) in hREC cultures; ctrl samples were transfected with scrambled sequences. Representative images of cellular death in IL-17A stimulated control (FADD expressing) and siFADD (FADD knockdown) hREC (scale bars of all images = 450μm) examined by microscopy (C) and quantified by flow cytometry (D) using Propidium Iodide (PI). Quantification of percent positive cells per flow cytometry analysis (n=6) of active Caspase 8 (E), Caspase 3 (F), and Annexin V (G) in IL-17A stimulated control (grey) and IL-17A stimulated siFADD (black) hREC cultures 6h after incubation. *=p<0.01 per unpaired student’s t-test. Data are representative of two separate experiments with similar results.
To examine the role of FADD in IL-17A-dependent endothelial cell death, hREC were transfected with lentiviral particles containing a silencing FADD sequence or a scrambled control sequence (FADD expressing). Knockdown of FADD was confirmed by RT-PCR (Fig. 5B). Cells were then stimulated with 100 ng/ml of rIL-17A, stained with Propidium iodide (PI), and imaged 48h after stimulation. Control hREC were PI positive, but IL-17A stimulated siFADD hREC were viable (Fig. 5C–D). Additionally, an average of ~65% caspase 8 and ~30% caspase 3 were detected in the IL-17A-stimulated control hREC, while ~2% of active caspase 8 and 3 were detected in the siFADD hREC (Fig. 5E–F). Annexin V was also significantly decreased in siFADD hREC (Fig. 5G). FADD expression was also required for apoptotic signaling and cell death to occur in murine retinal endothelial cells (Supplemental Fig. S3). Overall these data provide evidence that IL-17A-mediated apoptosis in retinal endothelial cells occurs through an Act1-FADD signaling pathway.
4. Discussion
Diabetic retinopathy is associated with chronic, low-grade retinal inflammation15,16. Inhibition of multiple inflammatory processes in animal models have been shown to decrease retinal pathologies that lead to diabetic retinopathy17,18. Although there is extensive evidence that inflammatory mediators and cytokines play a pivotal role in the development of diabetic retinopathy, the signaling pathways involved in initiating capillary non-perfusion in the early stages of diabetic retinopathy are still unclear. In the current study several notable observations emerged. First, the pathologic role of IL-17A in the development of capillary non-perfusion was determined. Also, a novel mechanistic pathway by which IL-17A induces retinal endothelial cell death, capillary degeneration, and the onset of non-proliferative diabetic retinopathy was identified.
IL-17A has been identified as an important cytokine in the promotion of diabetes and the progression of diabetic complications4,19,20. Further, previous studies have demonstrated a role for IL-17A in retinal neural cell pathogenesis at the 3-month time point in diabetic mice. It was determined that diabetic conditions induced Muller glia to produce IL-17A. In addition to stimulating IL-17A production, hyperglycemia also enhanced the expression of the IL-17RA subunit of the IL-17A receptor in Muller glia. Muller glia were determined to enhance neuronal apoptosis, vascular leukostasis, and vascular permeability in the retina through an autocrine signaling cascade in Muller glia5,6. These findings identified an IL-17-dependent mechanism of diabetic macular oedema during diabetic retinopathy in short-term diabetic murine models. However, the role of IL-17A in the vasoregressive process of retinal capillary degeneration was still unknown. Thus, this is the first study to examine the role of IL-17A in long-term diabetic murine models; wherein capillary cell death, degeneration, and non-perfusion associated with clinical ischaemic maculopathy of non-proliferative diabetic retinopathy could be observed. Additionally, the IL-17A-dependent apoptotic signaling cascade in retinal endothelial cells was examined, whereas a novel IL-17A/IL-17R →Act1/FADD signaling pathway that initiates caspase-mediated apoptosis in vascular retinal endothelial cells was discovered. These data provide evidence that diabetes-mediated IL-17A enhances capillary degeneration and nonperfusion in the retina.
After IL-17A initiates receptor signaling, constitutively expressed Act1 adaptor on the retinal endothelial cells recruits the Fas-activated death domain (FADD). IL-17A-induced Act1-FADD interactions then direct the activation of caspase 8 and caspase 3, whereas apoptosis of the vascular endothelial cells elicit capillary degeneration. However, the development of diabetic retinopathy in patients develops much slower than displayed in our ex vivo studies, we speculate that the acute responses observed were due to increased IL-17A concentrations required to examine the apoptotic signaling pathways within the life span of the cells studied in vitro. Still, we established that capillary cell death and degeneration was significantly decreased in IL17A−/− diabetic mice, and the deletion of Act1 and FADD was sufficient to inhibit apoptosis in retinal endothelial cells. Hence, we conclude that the IL-17A-dependent apoptotic signaling pathway identified in vitro, is the diabetes-mediated IL-17A-signaling mechanism that enhances capillary cell death and degeneration observed in vivo. Taken together, these studies provide evidence that IL-17A is involved in capillary degeneration through this apoptotic-signaling pathway in endothelial cells that quite possibly occurs throughout the systemic vasculature. Future studies are required to determine the role of IL-17A in other diabetic complications, like neuropathy, nephropathy, and heart disease.
We further identified FADD, an adaptor protein that contains a death domain, as a critical component to IL-17A-IL-17R-Act1 mediated apoptosis. FADD has been previously shown to interact with the IL-17E and IL-17R-Act1 SEFIR domain to activate caspase-mediated apoptosis in glial and cancer cells14,21. Consistent with these findings, IL-17A stimulation of retinal endothelial cells induced proteolytic cleavage of caspase 8 and 3 that mediated apoptosis, and this was negated in Act1 and FADD deficient cells. Moreover, FADD expression in IL-17A stimulated retinal endothelial cells was abrogated in Act1 deficient cells. This IL-17A signaling pathway establishes a novel IL-17A-dependent mechanism that is involved in retinal vascular impairment, which leads to the onset of non-proliferative diabetic retinopathy.
Here it was determined that IL-17A has a pro-apoptotic affect on retinal endothelial cells, which induce vasoregression. In response to this vasoregression, angiogenesis of retina vessels can be later elicited12,22,23. This suggests that IL-17A plays an indirect role in the angiogensis of retina vessels. However, it has been previously shown that IL-17A plays a direct role in angiogenesis of cancer cells, glia, and astrocytes by enhancing VEGF production24,25. Additionally, IL-17A enhanced VEGF production in retinal microglia, Muller glia, and ganglion cells in oxygen-induced retinopathy26. Since Muller glia have been identified as a predominant source of VEGF during diabetic retinopathy23,27, it is possible that IL-17A induces Muller glia to produce elevated levels of VEGF during early stage diabetic retinopathy. This potential IL-17A-dependent VEGF will be examined in future studies.
Collectively, our findings indicate a crucial link between Act1, FADD, and caspase-mediated apoptosis that implicates the IL-17R-Act1-FADD axis as the IL-17A-dependent pathway for microvascular retinal endothelial cell death and capillary degeneration in the retina during diabetes. Notably, these are two clinically meaningful abnormalities that characterize diabetic retinopathy in patients, and these novel findings may provide new therapeutic targets for non-proliferative diabetic retinopathy.
Supplementary Material
Highlights.
Diabetes induces IL-17A production that can be detected in the retina
IL-17A enhances retinal capillary degeneration
IL-17A induces retinal endothelial cell death in an Act1 dependent manner
Act1 mediates FADD dependent apoptosis in retinal endothelial cells
IL-17A-Act1-FADD dependent apoptosis in retinal endothelial cells induce retinal capillary degeneration and the onset of non-proliferative diabetic retinopathy
Acknowledgements
We would like to thank Heather Butler, John Denker, Catherine Doller, Anthony Gardella, Scott Howell, Denice Major, and Kathryn Zongolowicz for outstanding technical assistance. We also thank Dr. Nader Sheibani for providing the murine retinal endothelial cells.
Funding
This work was supported by the following grants: VA BX003403 (PRT), RO1 EY022938 (TSK), R24 EY024864 (TSK), VA BX002117 (TSK), P30 EY011373 (CWRU), the Research to Prevent Blindness Foundation (PRT), and the Ohio Lions Eye Research Foundation (PRT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011; 60: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kern TS, Tang J, Berkowitz BA. Validation of structural and functional lesions of diabetic retinopathy in mice. Mol Vis. 2010; 16: 2121–2131. [PMC free article] [PubMed] [Google Scholar]
- 3.Rasta SH, Nikfarjam S, Javadzadeh A. Detection of retinal capillary nonperfusion in fundus fluorescein angiogram of diabetic retinopathy. Bioimpacts. 2015; 5: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baharlou R, Ahmadi-Vasmehjani A, Davami MH, Faraji F, Atashzar MR, Karimipour F, Sadeghi A, Asadi MA, Khoubyari M. Elevated Levels of T-helper 17-associated Cytokines in Diabetes Type I Patients: Indicators for Following the Course of Disease. Immunol Invest. 2016; 45: 641–651. [DOI] [PubMed] [Google Scholar]
- 5.Qiu AW, Bian Z, Mao PA, Liu QH. IL-17A exacerbates diabetic retinopathy by impairing Muller cell function via Act1 signaling. Exp Mol Med. 2016; 48: e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu AW, Liu QH, Wang JL. Blocking IL-17A Alleviates Diabetic Retinopathy in Rodents. Cell Physiol Biochem. 2017; 41: 960–972. [DOI] [PubMed] [Google Scholar]
- 7.Veenstra A, Liu H, Lee CA, Du Y, Tang J, Kern TS. Diabetic Retinopathy: Retina-Specific Methods for Maintenance of Diabetic Rodents and Evaluation of Vascular Histopathology and Molecular Abnormalities. Curr Protoc Mouse Biol. 2015; 5: 247–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech. 2012; 5: 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonade D, Liu H, Palczewski K, Kern TS. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia. 2017; 60: 2111–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003; 9: 171–178. [PubMed] [Google Scholar]
- 11.Li G, Veenstra AA, Talahalli RR, Wang X, Gubotosi-Klug RA, Sheibani N, Kern TS. Marrow-derived cells regulate the development of early diabetic retinopathy and tactile allodynia in mice. Diabetes. 2012; 61: 3294–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bresnick GH, Davis MD, Myers FL, de Venecia G. Clinicopathologic correlations in diabetic retinopathy. II. Clinical and histologic appearances of retinal capillary microaneurysms. Arch Ophthalmol. 1977; 95: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Zhong M, Liang L, Gu F, Peng H. Interleukin-17 induces angiogenesis in human choroidal endothelial cells in vitro. Invest Ophthalmol Vis Sci. 2014; 55: 6968–6975. [DOI] [PubMed] [Google Scholar]
- 14.Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, Li X. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci. 2013; 16: 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011; 30: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangasamy S, McGuire PG, Das A. Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol. 2012; 19: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallejo S, Palacios E, Romacho T, Villalobos L, Pairo C, Sanchez-Ferrer CF. The interleukin-1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2014; 13: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Verma A, Han PY, Nakagawa T, Johnson RJ, Grant MB, Campbell-Thompson M, Jarajapu YP, Lei B, Hauswirth WW. Diabetic eNOS-knockout mice develop accelerated retinopathy. Invest Ophthalmol Vis Sci. 2010; 51: 5240–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fores JP, Crisostomo LG, Orii NM, Santos AS, Fukui RT, Matioli SR, de Moraes Vasconcelos D, Silva MERD. Th17 pathway in recent-onset autoimmune diabetes. Cell Immunol. 2018; 324: 8–13. [DOI] [PubMed] [Google Scholar]
- 20.Marwaha AK, Crome SQ, Panagiotopoulos C, Berq KB, Qin H, Ouyang Q, Xu L, Priatel JJ, Levings MK, Tan R. Cutting edge: Increased IL-17-secreting T cells in children with new-onset type 1 diabetes. J Immunol. 2010; 185: 3814–3818. [DOI] [PubMed] [Google Scholar]
- 21.Furuta S, Jeng YM, Zhou L, Huang L, Kuhn I, Bissell MJ, Lee WH. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci Transl Med. 2011; 3: 78ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017; 2(14): e93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le YZ. VEGF production and signaling in Muller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vision Res. 2017; 139: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You T, Yihui B, Jun L, Mingkai Z, Xuezhou C, Keke Z, Jun L. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci Rep. 2017; 7: 41779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin S, Cao S, Dehai C, Lui F, Yu Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep. 2015; 5: 16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talia DM, Deliyanti D, Agrotis A, Wilkinson-Berka JL. Inhibition of the Nuclear Receptor RORgamma and Interleukin-17A Suppresses Neovascular Retinopathy: Involvement of Immunocompetent Microglia. Arterioscler Thromb Vasc Biol. 2016; 36: 1186–1196. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010; 59: 2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.