Abstract
Background
Current estimates suggest that 75% of children diagnosed with a central nervous system (CNS) tumor will become five-year survivors. However, survivors of childhood CNS tumors are at increased risk for long-term morbidity.
Methods
To determine neuropsychological and socioeconomic (SES) long-term outcomes, adult survivors of pediatric low grade gliomas (n=181) in the Childhood Cancer Survivor Study and an age-, sex-frequency matched sibling comparison group (N=105) completed a comprehensive battery of standardized neuropsychological tests and SES assessment. Multivariable regression models compared treatment-specific groups for neuropsychological and SES outcomes and evaluated association with tumor location, age at diagnosis, sex and age at evaluation.
Results
In adjusted models, survivors (median age at diagnosis 8 years; median age at assessment 40 years) treated with surgery+radiotherapy performed lower than survivors treated with surgery-only, who performed lower than siblings on Estimated IQ (surgery+radiotherapy=93.9 vs. surgery-only=101.2 vs. siblings=108.5; all p-values <0.0001). Survivors diagnosed at younger ages had low scores on all outcomes (p<0.05) except attention/processing speed. For SES outcomes, survivors treated with surgery+radiotherapy had lower occupation (OR 2.6, 95% CI 1.1 to 5.9) and income (OR 2.6, 95% CI 1.3 to 5.0) and education (OR 2.1, 95% CI 1.1 to 4.0) compared to those treated with surgery-only.
Conclusions
Decades after treatment, survivors treated with radiotherapy and at younger ages had poorer neuropsychological and SES outcomes. Life-long surveillance of survivors of pediatric low grade glioma may be warranted as life events, stages and transitions (employment, family, aging) present new challenges and risks.
Keywords: Brain Tumors, Neuropsychological, Childhood Astrocytoma
Precise:
Neuropsychological and SES outcomes of 181 adult survivors of pediatric low grade gliomas were compared to those of 105 siblings. Decades after treatment, survivors treated with radiotherapy and at younger ages had poorer neuropsychological and SES outcomes, supporting the need for life-long surveillance.
Introduction
Current estimates suggest that 75% of children diagnosed with a central nervous system (CNS) tumor will become five-year survivors. As a result, in 2015 there were an estimated 66,798 people in the U.S. alive following diagnosis of a pediatric CNS tumor.1,2,3 However, survivors of childhood CNS tumors are at increased risk for long-term morbidity, mortality, neurocognitive impairment, psychological distress, stroke, and recurrent stroke that can have significant impact across their lifespan.4,5,6,7,8
The existing literature on neurocognitive outcomes primarily addresses survivors of the most aggressive/malignant tumors who often receive multimodal neurotoxic therapies (surgery, craniospinal radiotherapy, chemotherapy),9 with less attention to low grade tumors receiving less toxic treatment exposures (surgery alone or combined with focal radiotherapy). Historically, it has often been assumed that these tumors, particularly those treated with surgery alone, are associated with fewer long-term effects.10 Yet, neurobehavioral morbidity in children following surgery only for low grade astrocytoma has been identified.11 Turner et al12 also reported significant functional morbidity in older adolescents and young adults treated as children for low grade tumors with surgery only. Thus, even patients with histologically “benign” conditions receiving the least toxic treatment are at risk. Low grade gliomas are the most common CNS tumor of childhood and therefore it is imperative to follow this population into adulthood to more fully characterize the long-term societal burden due to life-long disability, psychosocial adversity, and under-employment.
Survivors of pediatric low grade glioma participating in the Childhood Cancer Survivor Study (CCSS), and a sibling comparison group, were invited to one of 16 study sites for direct, comprehensive neurocognitive assessment. Our primary aims were to ascertain the nature of neuropsychological and socioeconomic outcomes in adults treated with and without radiation for childhood low grade glioma compared to siblings, and to identify disease, treatment, and survivor-specific factors related to poor outcome.
Methods
CCSS is a retrospective cohort of 14,370 five-year survivors of childhood cancer diagnosed between 1970 and 1986, and over 4,000 siblings.13 Survivors eligible for the current study included CCSS participants active as of 2010 who were previously treated for WHO Grades I or II14 glioma, including Pilocytic Astrocytoma, Fibrillary Astrocytoma, Subependymal Giant Cell Astrocytoma, Oligodendroglioma NOS, Subependymal Glioma, and Astrocytoma NOS. Two participants also had a diagnosis of neurofibromatosis. Comparison subjects were recruited from the CCSS sibling population and frequency matched to the combined case group on current age and sex.
Participant Recruitment.
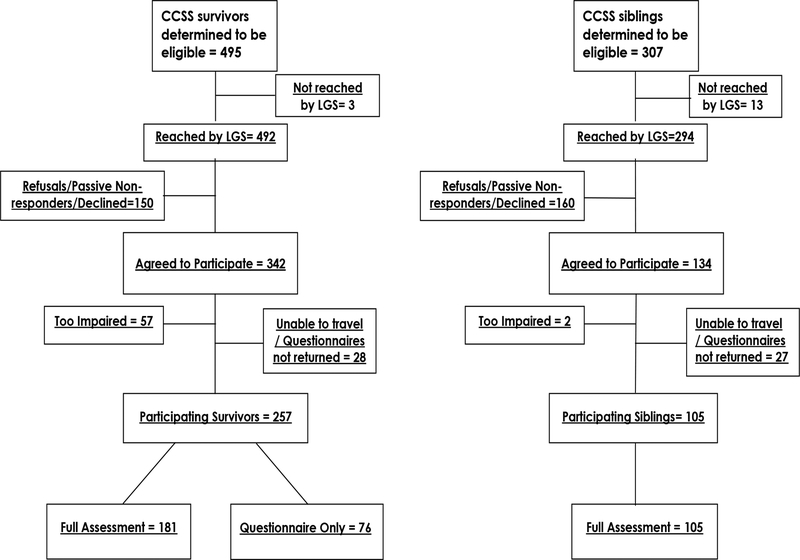
This study was approved by the Institutional Review Board at Baylor College of Medicine and IRBs at 16 participating institutions (see Acknowledgements). Eligible participants were screened by telephone and confirmed willingness to participate in the Low Grade Study (LGS). Of the 495 eligible survivors, 257 participated (181 completed full assessments while 76 elected not to travel to a study site but completed questionnaire outcomes only; Figure 1). Three hundred and seven siblings were eligible of which 105 participated. The current analysis reports results for only those completing full neurocognitive assessments.
Figure 1.
CONSORT Diagram for survivors and siblings. LGS=Low-grade study
Demographic, Diagnosis and Treatment Characteristics
Chemotherapy, surgery and radiotherapy were abstracted from medical records at the original treating institution. For analyses, survivors were categorized as treated with surgery-only or treated with surgery and radiotherapy (surgery+RT). Additional information was obtained from a background questionnaire completed by participants (Table 1).
Table 1.
Demographic and treatment characteristics of survivors of pediatric low grade glioma and siblings†
| Variable | Surgery-only (N=85) | Surgery+RT (N=96) | Siblings (N=105) |
|---|---|---|---|
| Sex N (%)† | |||
| Male | 42 (49.4%) | 43 (44.8%) | 38 (36.2%) |
| Female | 43 (50.6%) | 53 (55.2%) | 67 (63.8%) |
| Race/Ethnicity N (%) | |||
| Non-Hispanic White | 79 (92.9%) | 88 (91.7%) | 82 (84.5%) |
| Non-Hispanic Black | 0 (0%) | 1 (1%) | 3 (3.1%) |
| Hispanic | 0 (0%) | 0 (0%) | 3 (3.1%) |
| Other | 6 (7.1%) | 7 (7.3%) | 9 (9.3%) |
| Age-at-diagnosis, Median years (range) |
8.0 (0–18) | 7.0 (0–19) | - |
| Age-at-testing Median years (range) |
40.0 (27–58) | 41(27–58) | 40 (24–60) |
| Tumor location N (%) | |||
| Supratentorial | 26 (37.7%) | 47 (49%) | - |
| Infratentorial | 43 (62.3%) | 49 (51%) | - |
| Chemotherapy N, (%) | 0 | 15 (15.6%) | - |
| Median radiation dose (min,max)‡ | - | 52.5 (36,72) Gy | - |
| Socioeconomic status of the family of origin (Duncan) Median (range)§ |
68.8 (28.2–97.2) | 55.3 (29.4–92.3) | 65.2 (27.5 – 92.8) |
| Participant Occupational Prestige (Duncan) Median (range) | 64.8 (11.1– 97.1) | 37.3 (11.1–93.1) | 63.9 (11.1–93.7) |
| N (%) | |||
| 11.1–48.9 | 26 (38.8%) | 42 (66.7%) | 23 (25%) |
| >48.9–62.9 | 6 (9%) | 8 (12.7%) | 23 (25%) |
| >62.9–79.9 | 19 (28.4%) | 8 (12.7%) | 23 (25%) |
| >79.9–93.7 | 16 (23.9%) | 5 (7.9%) | 23 (25%) |
| No score | 18 (Disabled=8) |
33 (Disabled=19) |
13 (Disabled=0) |
| Participant Income¶ N (%) | |||
| Under $20,000 | 27 (32.1%) | 56 (58.3%) | 28 (26.7%) |
| $20–59,999 | 33 (39.3%) | 31 (32.3%) | 29 (27.6%) |
| ≥$60,000 | 24 (28.6%) | 9 (9.4%) | 48 (45.7%) |
| Unknown | 1 | 0 | 0 |
| Participant Education N (%) | |||
| ≤ High School (HS) | 13 (15.3%) | 19 (19.8%) | 3 (2.9%) |
| ≥ HS to < Bachelor’s Degree | 23 (27.1%) | 42 (43.8%) | 37 (35.2%) |
| Bachelor’s Degree | 27 (31.8%) | 22 (22.9%) | 33 (31.4%) |
| Any Graduate Studies | 22 (25.9%) | 13 (13.5%) | 32 (30.5%) |
All percents are evaluated using N for non-missing values as the denominator.
Based upon N=86 for which CCSS has records.
Duncan Occupational Prestige is an unequal interval scale with scores ranging from 17 (e.g. laborers, operators) to over 90 (e.g. higher professions and specialty occupations). Occupation category cut points were defined by the quartiles for the sibling population.
Income categories are divided at the cut points for the lower two quartiles of siblings, with upper two quartiles combined due to low numbers among survivors.
Outcomes
Neuropsychological Outcomes:
Participants were individually administered standardized, age-normed neuropsychological tests with well-established reliability and validly that are in widespread clinical and research use (Supplemental Table 1). For the primary outcome measures, a Composite Neuropsychological Index (average of the Verbal, Visual-Spatial, Memory, Attention/Processing Speed, Motor, and Executive domains) captured the broad neuropsychological impact in this population. Estimated IQ facilitated comparison of our findings with the extant literature.
Socioeconomic Status (SES) Outcomes:
We used three indexes of SES (see Appendix 1 for explanation of our approach):15,16 (1) Educational Attainment, (2) Income, and (3) Occupational Prestige from Duncan’s Socioeconomic Index.
Statistical Methods
Representativeness of participants versus non-participants was assessed among eligible subjects by testing subject demographic and cancer-related (for survivors) characteristics in logistic regression models with participation as a binary outcome variable. For each neuropsychological and SES outcome, multivariable regression models evaluated age- and sex-adjusted comparisons 1) between survivors and siblings, 2) between survivor treatment groups (surgery-only and surgery+RT) and siblings, and 3) among survivors, between survivor treatment groups, and evaluating association with tumor location (supratentorial and infratentorial) and age at brain tumor diagnosis.
Multivariable linear regression models were used for the Neuropsychological Composite and Domain and IQ scores. We report adjusted means, 95% Confidence Intervals (CI), and p-values for comparisons of survivors and siblings, and adjusted mean differences (β), 95% CI, and p-values for impact of subject-specific characteristics. SES outcomes were descriptively summarized as categorical variables and were analyzed in multivariable logistic regression models based on dichotomized versions of these variables—occupation (lowest quartile), income (<$20,000), and education (<Bachelor’s Degree) estimated odds ratios (ORs) and 95% CI for associations with survivor and sibling groups and subject-specific characteristics. All p-values were two sided and those less than 0.05 are considered significant.
RESULTS
Race/ethnicity, age at evaluation, family of origin socioeconomic status and sex distributions were similar across all groups (Table 1), though siblings had a higher percent of female participants. Among survivors, age-at-diagnosis was similar between treatment groups but survivors treated with surgery-only were more likely to have an infratentorial tumor (62%) versus supratentorial location (38%).
Representativeness of the Sample
Survivors and siblings who were eligible but did not participate were compared with those who participated (Supplemental Tables 2 and 3). Eligible survivors were more likely to participate if they were older at diagnosis (11–15 vs. ≤ 5 years, p=0.002), had lower education (education through high school vs. post-high school education, p=0.011) or higher household annual income (40–59,999 vs 0–19,999 p=0.010). Eligible survivors were less likely to participate if they were 21–30 years compared to over 40 years of age at most recent follow-up (21–20 vs. >40 years, p=0.015). Female siblings were more likely to participate than male siblings (p=0.004). Of the 342 survivors identified as eligible for the study, 57 were too impaired to be tested (Figure 1). The relative risk of being untestable was 2.7 times higher for survivors who received surgery+RT as compared to surgery-only (95% CI 1.5, 5.3, p=0.002).
Neuropsychological Outcomes
Brain Tumor Survivors Compared to Siblings
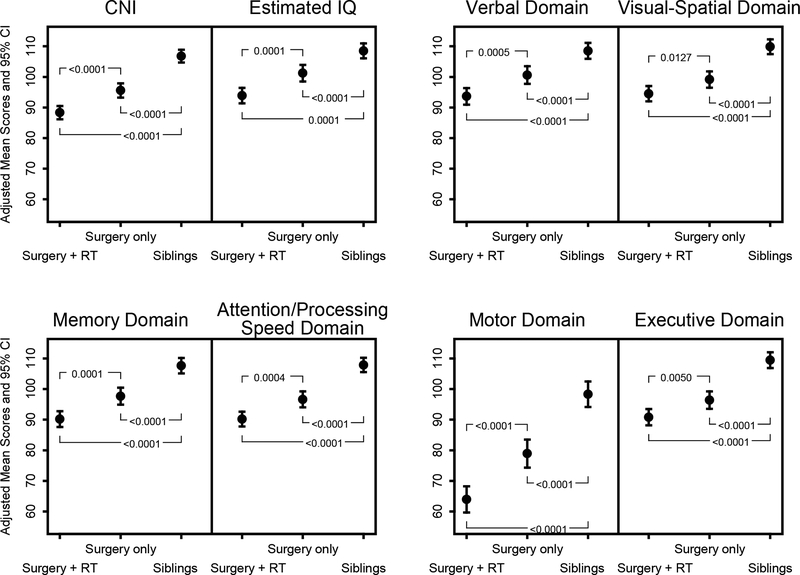
In age- and sex-adjusted linear models, siblings had higher mean scores than survivors treated with surgery+RT or surgery-only across all neuropsychological outcomes, including Composite Neuropsychological Index (CNI; siblings 106.8; surgery-only 95.6; surgery+RT: 88.3, all P-values ≤0.0001) and Estimated IQ: (siblings: 108.5, surgery-only: 101.2, surgery+RT: 93.9; all p-values <0.0001; Figure 2). Adjusted mean scores were particularly low for survivors on Motor (siblings: 98.3, surgery-only: 78.9, surgery+RT: 64.0; all P-values ≤0.0001).
Figure 2.
Composite Neuropsychological Index (CNI), Estimated IQ, and domain-specific neuropsychological outcomes for survivors by treatment exposure compared to siblings
Multivariable Analyses among Brain Tumor Survivors
In multivariable models adjusted for sex, age-at-testing, age-at-diagnosis and tumor location, survivors who received surgery-only had significantly higher neuropsychological scores compared to survivors with surgery+RT, except for Visual-Spatial and Executive (Table 2, Supplemental Table 2). When compared to survivors treated with surgery+RT, surgery-only survivors had higher mean CNI scores (5.8 points, p=0.005) and Estimated IQ scores (5.6 points, p=0.010) with the largest difference seen in Motor (12.8 points, p=0.001). Verbal, Memory and Attention/Processing Speed all had significant differences of approximately 5–6 points higher for surgery-only compared to surgery+RT (all p<0.03). Only Verbal was significantly associated with tumor location, with survivors with an infratentorial tumor location scoring 4.7 points (p=0.040) higher than survivors with supratentorial tumor location. Older age-at-diagnosis was associated with statistically higher neuropsychological scores, except for Attention/Processing Speed. Association of age-at-diagnosis with scores was lowest in Visual-Spatial, which increased by 3.5 points (p=0.034) for every 5-years increase in age-at-diagnosis. It was highest in Motor, which increased 7.1 points (p=0.012) for every 5-year increase in age-at-diagnosis. We did not detect significant effects of age-at-testing in any of the scores. Females on average scored 8 points higher than males in Motor (p=0.033). No other significant differences in scores between males and females were detected. We also examined the following interaction terms in these models: age-at-diagnosis x treatment group, age-at-diagnosis x tumor location, and treatment group x tumor location. After adjustment for multiple comparisons, we did not detect any significant interactions.
Table 2.
Multivariable regression of the effects of age-at-diagnosis, age-at-testing, sex, tumor location, and treatment on neuropsychological outcomes among survivors of low grade tumors
| Outcome | Independent Variable | Category | Adjusted Mean Difference [β] (95% CI) | P-value |
|---|---|---|---|---|
| Composite Neuropsychological Index | Age-at-Diagnosis | 5-year increase | 3.9 (1 ,6.9) | 0.010 |
| Age-at-Testing | 5-year increase | −1.7 (−3.8 ,0.5) | 0.13 | |
| Sex | Female | Ref | ||
| Male | −1.8 (−5.8 ,2.2) | 0.37 | ||
| Tumor Location | Infratentorial | Ref | ||
| Supratentorial | −2.9 (−7 ,1.3) | 0.18 | ||
| Treatment | Surgery-only | Ref | ||
| Surgery+RT | −5.8 (−9.8 ,−1.8) | 0.005 | ||
| Estimated IQ | Age-at-Diagnosis | 5-year increase | 4.5 (1.4 ,7.6) | 0.005 |
| Age-at-Testing | 5-year increase | −1.4 (−3.6 ,0.8) | 0.21 | |
| Sex | Female | Ref | ||
| Male | 0.4 (−3.8 ,4.5) | 0.86 | ||
| Tumor Location | Infratentorial | Ref | ||
| Supratentorial | −3.9 (−8.3 ,0.4) | 0.073 | ||
| Treatment | Surgery-only | Ref | ||
| Surgery+RT | −5.6 (−9.8 ,−1.3) | 0.010 | ||
| Verbal Domain | Age-at-Diagnosis | 5-year increase | 4.3 (1.1 ,7.5) | 0.009 |
| Age-at-Testing | 5-year increase | −1.8 (−4.1 ,0.5) | 0.12 | |
| Sex | Female | Ref | ||
| Male | −0.6 (−4.9 ,3.7) | 0.79 | ||
| Tumor Location | Infratentorial | Ref | ||
| Supratentorial | −4.7 (−9.1 ,−0.2) | 0.040 | ||
| Treatment | Surgery-only | Ref | ||
| Surgery+RT | −5 (−9.4 ,−0.6) | 0.024 | ||
| Visual-Spatial Domain | Age-at-Diagnosis | 5-year increase | 3.5 (0.3 ,6.7) | 0.034 |
| Age-at-Testing | 5-year increase | −0.5 (−2.8 ,1.8) | 0.66 | |
| Sex | Female | Ref | ||
| Male | 1.6 (−2.8 ,5.9) | 0.48 | ||
| Tumor Location | Infratentorial | Ref | ||
| Supratentorial | −3.1 (−7.6 ,1.5) | 0.18 | ||
| Treatment | Surgery-only | Ref | ||
| Surgery+RT | −4 (−8.4 ,0.4) | 0.076 | ||
| Memory Domain | Age-at-Diagnosis | 5-year increase | 3.7 (0.4 ,7) | 0.029 |
| Age-at-Testing | 5-year increase | −2 (−4.4 ,0.4) | 0.097 | |
| Sex | Female | Ref | ||
| Male | −3.2 (−7.6 ,1.3) | 0.16 | ||
| Tumor Location | Infratentorial | Ref | ||
| Supratentorial | −2.3 (−6.9 ,2.3) | 0.32 | ||
| Treatment | Surgery-only | Ref | 0.008 | |
| Surgery+RT | −6.1 (−10.6 ,−1.6) | |||
| Attention/Processing Speed Domain | Age-at-Diagnosis | 5-year increase | 2.2 (−1.2 ,5.6) | 0.20 |
| Age-at-Testing | 5-year increase | −0.6 (−3 ,1.8) | 0.64 | |
| Sex | Female | Ref | ||
| Male | 1.2 (−3.4 ,5.7) | 0.62 | ||
| Tumor Location | Infratentorial | Ref | ||
| Supratentorial | 0 (−4.7 ,4.6) | 0.99 | ||
| Treatment | Surgery-only | Ref | ||
| Surgery+RT | −5.3 (−9.9 ,−0.7) | 0.025 | ||
| Motor Domain | Age-at-Diagnosis | 5-year increase | 7.1 (1.6 ,12.5) | 0.012 |
| Age-at-Testing | 5-year increase | −3.1 (−7 ,0.8) | 0.12 | |
| Sex | Female | Ref | ||
| Male | −8 (−15.4 ,−0.6) | 0.033 | ||
| Tumor Location | Infratentorial | Ref | ||
| Supratentorial | −3.4 (−11 ,4.2) | 0.38 | ||
| Treatment | Surgery-only | Ref | ||
| Surgery+RT | −12.8 (−20.3 ,−5.3) | 0.001 | ||
| Executive Domain | Age-at-Diagnosis | 5-year increase | 4.3 (0.7 ,7.9) | 0.020 |
| Age-at-Testing | 5-year increase | −1.1 (−3.7 ,1.5) | 0.40 | |
| Sex | Female | Ref | ||
| Male | −1.3 (−6.2 ,3.6) | 0.60 | ||
| Tumor Location | Infratentorial | Ref | ||
| Supratentorial | −4.8 (−9.9 ,0.3) | 0.063 | ||
| Treatment | Surgery-only | Ref | ||
| Surgery+RT | −3.7 (−8.7 ,1.3) | 0.14 |
Ref=Reference group
In a subset analysis among survivors who received surgery and radiation, we compared outcomes between those who did and did not receive chemotherapy. After adjusting for age-at-testing, age-at-diagnosis, sex, and tumor location, those with chemotherapy scored significantly lower compared to survivors who received surgery and radiation without chemotherapy in CNI (−8.0, 95% CI: −15.0, −1.0), Estimated IQ (−7.6 95% CI: −15.0, −0.2) , Visual-Spatial (−10.2, 95% CI: −18.4, −2.0), and Memory (−10.3 95% CI: −18.3, −2.3).
Socioeconomic Status Outcomes
Occupation.
Occupation varied across groups (Table 1). In multivariable models adjusted for age-at-testing and sex, surgery+RT was associated with a 7.7-fold (p<0.001) higher risk of having an occupation in the lowest sibling quartile (<48.9) compared to siblings. Surgery-only survivors had a 2.8-fold (p=0.007) higher risk than siblings (Table 3, Supplemental Figure 1, Supplemental Table 1). Analyses among survivors demonstrated that surgery+RT was associated with a 2.6-fold (p=0.027) increased risk of low occupation compared to surgery-only survivors. Among 51 unemployed survivors who did not receive an occupation score, odds that this was due to disability did not differ significantly between the surgery+RT and surgery-only groups after adjusting for sex, age-at-testing, age-at-diagnosis, tumor location (p=0.35). Reasons for disability were most often related to visual, hearing or motor impairments.
Table 3.
Multivariable logistic regressions for lower occupational prestige score (lowest quartile [<48.92]), income (<$20,000), and education (<Bachelor’s Degree) between survivor groups and siblings and among survivors.
| Model Population | Variable | Category | Occupational Prestige Score <48.92 |
Income <$20,000 |
Education <Bachelor’s Degree |
|||
|---|---|---|---|---|---|---|---|---|
| OR† (95% CI) | P-value | OR* (95% CI) | P-value | OR* (95% CI) | P-value | |||
| Survivors and Siblings | Treatment Group | Siblings | 1 | . | 1 | 1 | . | |
| Survivors: Surgery-only | 2.8 (1.3, 5.8) | 0.007 | 1.5 (0.8, 2.8) | 0.24 | 1.2 (0.7, 2.2) | 0.50 | ||
| Survivors: Surgery+RT | 7.7 (3.6,16.4) | <0.001 | 4.5 (2.4, 8.3) | <0.001 | 2.9 (1.6, 5.2) | <0.001 | ||
| Survivors Only | Age-at-testing | 5 year increase | 1.4 (0.9, 2.2) | 0.16 | 0.9 (0.6,1.2) | 0.45 | 1.4 (1.0, 2.0) | 0.076 |
| Age-at-diagnosis | 5 year increase | 0.5 (0.2, 0.9) | 0.022 | 0.9 (0.6,1.5) | 0.74 | 0.5 (0.3,0.8) | 0.006 | |
| Sex | Male | 1 | . | 1 | . | 1 | . | |
| Female | 3.9 (1.7, 8.8) | 0.001 | 2.4 (1.2, 4.8) | 0.010 | 1.2 (0.6,2.2) | 0.66 | ||
| Tumor Location | Infratentorial | 1 | . | 1 | . | 1 | . | |
| Supratentorial | 2.5 (1.0, 6.0) | 0.045 | 1.9 (1.0,3.9) | 0.063 | 1.9 (0.9,3.7) | 0.078 | ||
| Treatment Group | Surgery-only | 1 | . | 1 | . | 1 | . | |
| Surgery+RT | 2.6 (1.1, 5.9) | 0.027 | 2.6 (1.3, 5.0) | 0.007 | 2.1 (1.1, 4.0) | 0.030 | ||
OR= Odds Ratio. Survivor and sibling model also adjusted for age-at-testing and sex. Survivor only model includes all variables shown
Younger age at diagnosis was associated with lower occupation, such that a 5-year increase in age at diagnosis conferred a 50% reduction in the odds of lower occupation (p=0.022). Supratentorial tumor location was associated with a higher risk of low occupation compared to Infratentorial tumors (p=0.045). Female survivors were more likely to have low occupation than male survivors (p=0.001).
Income.
Reported income varied across groups (Table 1). Compared to siblings, surgery+RT was associated with a 4.5-fold (p<0.001) risk of an annual income of <$20,000 while surgery-only survivors’ risk did not significantly differ from siblings. (Table 3, Supplemental Figure 1, Supplemental Table 2). Among survivors, surgery+RT was associated with a 2.6-fold higher risk than surgery-only (p=0.007). While not statistically significant, risk of an annual income of <$20,000 was somewhat higher for supratentorial tumor locations (p=0.063). Female sex was significantly associated, with female survivors having a 2.4-fold (p=0.010) higher risk compared to male survivors.
Education.
Education varied across groups (Table 1). Surgery+RT was associated with significantly increased risk (p<0.001) of education lower than a Bachelor’s degree compared to siblings, while surgery-only was not (p=0.50). Among survivors, risk of attained education less than a Bachelor’s degree was significantly higher for those who received surgery+RT (p=0.030) compared to surgery-only (Table 3, Supplemental Figure 1, Supplemental Table 2). Having a supratentorial tumor was not significantly associated with an increased risk of low education (p=0.078) compared to an infratentorial tumor. Younger age-at-diagnosis was associated with risk of low education, with each 5-year increase in age-at-diagnosis being associated with a 50% reduction in risk (p=0.006). Survivor sex was not related educational. There was no significant difference in occupation, income, and education between survivors who received surgery, radiation and chemotherapy and survivors who received only surgery and radiation (no chemotherapy).
DISCUSSION
To our knowledge, this is the first study providing direct (i.e. not survey based) neuropsychological and SES long-term outcomes for a diagnostically homogeneous group of adult survivors of pediatric low grade glioma. Within a broad gradient of risk based on treatment exposure, adult survivors who were testable tended to have “average range” outcomes (i.e. IQs 90–110; Wechsler, 2008).17 These results replicate, though in an aging adult survivor population now decades from exposure, risk factors documented in younger survivors, particularly the deleterious effects associated with radiotherapy at an early age. In the CCSS cohort, survivors of pediatric low grade gliomas had lower scores in all neuropsychological domains compared to siblings. As a group all domains were affected identifying no distinct pattern of strengths and weaknesses (i.e. neuropsychological phenotype) that characterize long-term survivors of childhood glioma. In contrast, late effects research in medulloblastoma has established a pattern of preserved (verbal) and vulnerable (processing speed, working memory, executive) functions.18 At least three factors could account for this difference. First, differences in measurement approaches (i.e. tests, constructs), could account for inconsistencies in outcome across studies. Second, over time an “evening-out” of neuropsychological functioning may result from diminished brain and cognitive reserve capacity consequent to early brain injury.19 That is to say, compromised reserve capacity prospectively attenuates a broad range of cognitive skills. Third, this was a heterogeneous sample in terms of tumor location and treatment, and so more diverse individual outcomes would be expected. Also, no participant was treated with craniospinal radiation, which has been associated with the risk pattern for medulloblastoma noted above.
Consistent with the neuropsychological outcomes, survivors were less educated, earned lower incomes, and had lower status occupations than siblings. The difference in occupational outcomes between survivors and siblings is actually underestimated by these analysis since 26 survivors reported that they were disabled and so were not included in these analyses of occupation.
Survivors with supratentorial tumors had lower Verbal scores, consistent with previous literature on children treated for low grade gliomas.11 While tumor location was a weak predictor of outcome, it is acknowledged that such a broad distinction (infratentorial vs. supratentorial) may obscure more specific structure-function relationships discernable only through the study of larger samples.
Radiotherapy was associated with the most robust and general effects on all neuropsychological and SES measures, and this was most apparent in Motor. While not surprising, it should be kept in mind that these patients received only focal/restricted-field and not whole brain or craniospinal radiotherapies, which have been associated with the most deleterious effects in the treatment of malignant disease.9 Patients who were younger at the time of diagnosis were at significantly increased risk of low education and occupation, and with the exception of Attention/Processing Speed, poorer neuropsychological outcomes than older treated patients. The correlational nature of these data and inherent confounding of predictors precludes firm conclusions. Still, taken together with a growing body of late-effects research on radiotherapy, these findings suggest that modern approaches to treatment that eliminate or delay radiotherapy, particularly in young children, may well reduce risk for cognitive injury in this population.
Although sex was generally not associated with increased risk of neuropsychological dysfunction, females were at increased risk for low occupation and income, similar to the historic disparities between males and females in the general population.20 This is not consistent with reports of greater vulnerability of females to neuropsychological late-effects in pediatric cancer.21, 22 However, it is consistent with other research that failed to find a robust sex effect in pediatric gliomas.9,11
The major limitation of the study was the potential for selection bias through differential participation. It is worth noting that 57 eligible cases, a disproportionate number of whom were treated with RT, were not included in this study sample because they were too impaired to complete testing, in most of these cases because of severe visual or motor impairment, often secondary to stroke, second malignancy, or other event subsequent to the initial diagnosis and treatment for low grade glioma. Also, this study spanned decades of diagnostic, treatment, and supportive care advancements that may contribute to improved outcomes. Therefore, caution must be exercised in generalizing from this cohort to more recently treated cohorts of survivors. For more contemporary survivors, cautious observation after surgery, or use of chemotherapeutic approaches in younger patients to delay or eliminate radiotherapy may reduce risk for poor long-term outcomes. As such, results from this study among survivors who received surgery-only may have broadened applicability in the modern era. While only 15 survivors received chemotherapy, results suggest addition risk associated with combined RT and chemotherapy.
Further work is needed to ascertain risk profiles in contemporaneously-treated patients as well as interventions (e.g. educational and occupational services, cognitive rehabilitation) to mitigated late-effects. Nevertheless, in the context of survivorship, these findings are more directly relevant to understanding and addressing the degree and nature of the challenges confronted by this growing (and aging) population. There are three main implications of this study: (1) although ostensibly at lower risk than some other brain tumor survivors (e.g. Medulloblastoma), life-long surveillance of survivors of pediatric low grade glioma may be warranted as life events, stages and transitions (employment, family, aging) present new challenges and risks; (2) surveillance and intervention strategies cannot be based on a particular neuropsychological “phenotype” or risk profile considering the broad range of outcomes found; and (3) of the neuropsychological domains measured, fine-motor speed and dexterity (i.e. Motor) stood out as most impaired in survivors, consistent with infratentorial location of over half of the tumors and the associated risk to the integrity of motor systems in the cerebellum and brain stem. Together with the findings of Spiegler et al,18 it appears that such abilities are impacted early and persist. Further research into related graphomotor competence and educational and occupational implications of this core liability is needed.
Supplementary Material
Funding
This work was supported by the National Cancer Institute (CA132899 M.D. Ris Principal Investigator and CA55727, G.T. Armstrong, Principal Investigator). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
No conflicts of interest to report
REFERENCES
- 1.Porter KR, McCarthy BJ, Freels S, et al. : Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro-Oncology 12:520–527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CBTRUS (2011). CBTRUS Statistical Report: Primary brain and central nervous system tumors diagnosed in the United States in 2004-2007. Source: Central Brain Tumor Registry of the United States, Hinsdale, IL: http://www.cbtrus.org [Google Scholar]
- 3.Phillips SM, Padgett LS, Leisenring WM, et al. : Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015. April;24(4):653–63. doi: 10.1158/1055-9965EPI-14–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Liu Q, Yasui Y, et al. : Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009. July 1;101(13):946–58. doi: 10.1093/jnci/djp148 Epub 2009 Jun 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller S, Fullerton HJ, Stratton K, et al. : Radiation atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys 86:649–655, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fullerton HJ, Stratton K, Mueller S, et al. : Recurrent stroke in childhood cancer survivors. Neurology 85:1056–1064, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad PK, Hardy KK, Zhang N, et al. : Psychosocial and neurocognitive outcomes in adult survivors of adolescent and early adult cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 33:2545–2552, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong GT M.D., Chen Y, Yasui Y, et al. : Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 374:833–842, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ris MD, Walsh K, Wallace D, et al. : Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediat Blood Cancer 60: 1350– 1357, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pompili A, Caperle M, Pace A, et al. : Quality-of-life assessment in patients who had been surgically treated for cerebellar pilocytic astrocytomas in childhood. J Neurosurg 96:229–234, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Ris MD, Beebe DW, Armstrong D, et al. : Cognitive and adaptive outcome in extracerebellar low grade brain tumors in children from a national collaborative study (CCG9891/POG9130). J Clin Oncol 19:3470–3476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner CD, Chordas CA, Liptak CC, et al. : Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer 53P:417–423, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Robison LL, Armstrong GT, Boice JD, et al. : The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009. May 10;27(14):2308–18. doi: 10.1200/JCO.2009.22.3339 Epub 2009 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleihues P World Health Organization classification of Tumours, in Kleihues P & Cauance WK (eds), Pathology and Genetics of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer Press, 2000. pp 208–241 [Google Scholar]
- 15.Duncan GJ, Daly MC, McDonough P, et al. : Optimal indicators of socioeconomic status for health research. American Journal of Public Health 92:1151–1157, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger N, Williams DR, & Moss NE. Measuring social class in US public health research: Concepts, methodologies and guidelines. Annual Review of Public Health 18 :341–78, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D Wechsler Adult Intelligence Scale (ed.4). San Antonio, TX NCS Pearson, 2008. [Google Scholar]
- 18.Spiegler BJ, Bouffet E, Greenberg ML, et al. : Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol 22: 706–713, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Ris MD & Hiscock M: Modeling cognitive aging following CNS injury: Reserve and the Flynn Effect, in Baron IS & Rey-Casserly C (eds) Pediatric Neuropsychology: Medical Advances and Lifespan Outcomes. New York, NY, Oxford University Press, 2013, pp 395–421 [Google Scholar]
- 20.Brown A & Patton E: The narrowing, but persistent, gender gap in pay. Pew Research Center, April 14, 2015 [Google Scholar]
- 21.Fouladi M, Gilger E, Kocak M, et al. : Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J Clin Oncol 23:7152–7160 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lahteenmaki PM, Harila-Saari A, Pukkala EI, et al. : Scholastic achievements of children with brain tumors at the end of comprehensive education - A nationwide, register-based study. Neurology 69:296–305.2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.