Abstract
Prenatal cocaine exposure (PCE) has ramifications for feedback processing. Measuring neural oscillatory dynamics (during electroencephalography provides insight into the time signatures and neural processes of feedback processing in adolescents with PCE. We measured spectral power in alpha and theta frequency bands while 49 adolescents with PCE and 34 non-drug exposed (NDE) performed a task with win/no-win feedback. Compared to NDE individuals, those with PCE showed reduced alpha power and increased theta power in response to no-win feedback. These findings suggest altered reactivity in PCE adolescents.
Keywords: Prenatal cocaine exposure, oscillations, electroencephalography, feedback processing, reward
Introduction
Adolescence is a high-risk period for engagement in risky behaviors, and risk-taking tendencies may also be influenced by prenatal cocaine exposure (PCE) (Allen et al., 2014; Min et al., 2016). Adolescents with PCE demonstrate increased likelihoods of behavioral and psychiatric problems, including aggression and externalizing behaviors (Linares et al., 2006), and early initiation of substance use (Delaney-Black et al., 2011; Minnes et al., 2014; Richardson et al., 2013). Many of these behaviors are linked to impaired reward-feedback processing (Gao et al., 2016; Vila-Ballo et al., 2015). The capacity to correctly identify feedback and use this information to update and monitor performance has repercussions for controlled behavior—impairments in feedback processing may manifest in impulsivity and poorer behavioral control (Krakowski et al., 2016; Schmidt et al., 2017). Exactly how neural and physiological correlates of feedback processing may be affected in PCE remains to be fully understood.
A useful methodology for examining feedback processing is with electroencephalography (EEG). Important aspects of the EEG signal, reflected in oscillatory dynamics, can yield distinct event-related information about cognitive control and feedback processing (van Noordt and Segalowitz, 2012). Oscillatory frequencies are related to specific processes, and these relationships can be understood by using specific tasks to identify the specific brain network(s) from which task-related performances arise. Oscillatory frequency power can be measured using event-related spectral perturbation (ERSP), an event-locked spectral power change in the EEG signal (Makeig et al., 2004). ERSPs may reflect the integrity of neuronal firing and serve as temporally sensitive indicators of neural processes. In addition, oscillations arising from different regions of the scalp may reflect different neuronal populations and thus have different functional bases (Cavanagh et al., 2010).
In terms of feedback processing, potentially relevant frequency spectra are within the alpha band (8–12 hz), and the theta band (4–8Hz). Alpha and theta power can arise from different neural sources and may reflect different processes depending upon the paradigm employed (Michel et al., 1993). These frequencies are useful as a potential measure of feedback and reward processing (van Driel, 2012). Indeed, both alpha and theta power were found to increase during loss conditions in a gambling task, and changes in theta power and alpha power during that task corresponded to measures of motor impulsivity and sensation-seeking, respectively (Leicht et al., 2013).
The theta frequency accounts for a large portion of the EEG signal in the N2, feedback-related negativity (FRN) and error-related negativity (ERN) event-related potential (ERP) responses associated with feedback processing (Cavanagh et al., 2012). In two-choice feedback tasks, similar to the task we use, theta may reflect continuous monitoring of expected outcome (Cavanagh and Frank, 2014). Thus, the increase in theta power in response to losses may reflect comparisons of actual outcomes with the expected outcome, similar to the process which is thought to underlie the feedback related negativity (Cohen et al., 2007). Theta additionally has been shown to be enhanced during losses in many kinds of feedback paradigms in which reward is involved. Increased theta power is seen during losses in a probabilistic learning task (Cohen et al., 2007). Theta has also been demonstrated to be enhanced during losses in gambling tasks (Leicht et al., 2013; Marco-Pallares et al., 2008) and flanker tasks designed to elicit errors (Trujillo and Allen, 2007). This enhancement of theta power has been associated with activation in a fronto-parietal cognitive control network during simultaneous fMRI and EEG acquisition during a two-choice gambling task (Andreou et al., 2017).
Alpha has also been associated with reward and feedback processing. Increases in alpha correspond to low-value, non-rewarding targets during performance of reward-processing tasks (Heuer et al., 2017). Alpha is also relevant to reward learning, as has been demonstrated by reductions in alpha power in the ventral striatum in response to small wins or losses of money (Cohen et al., 2009) or to wins or losses during a video game (Lega et al., 2011).
The alpha and theta frequency bands may constitute good indicators of impairments in the feedback-processing system in substance-related disorders. In adults with alcoholism performing a Go/No-Go task (Pandey et al., 2016), increased alpha power during No-Go trials was linked to poorer task performance, though overall the alcoholic group showed decreased alpha power in parietal and central regions in response to Go and No-Go conditions when compared to control subjects. Studies in adult cocaine users have demonstrated decreased left hemisphere alpha power (and decreased beta and theta power) in response to disadvantageous choices (more immediate, riskier rewards) during a decision-making task (Balconi and Finocchiaro, 2015). Decreased alpha power in this context suggests that they may have paid closer attention to these disadvantageous choices. Poor suppression of distracting information and increased attention to high-risk choices may underlie impaired feedback processing and decision-making often observed in individuals who use cocaine.
Adolescents may show increased engagement in high-risk behaviors including initiation of substance use (Geier, 2013), and such tendencies appear elevated in adolescents with PCE (Delaney-Black et al., 2011; Minnes et al., 2014). However, oscillations related to reward-feedback processes have yet to be examined in adolescents with PCE relative to those without prenatal drug exposure (NDE). There are, however, two existing studies that have examined oscillations in children with PCE. Though these studies are not focused on reward or feedback processing, they are relevant to understanding how PCE may affect development. Prichep and colleagues (Prichep et al., 1995) collected EEG data in children with PCE while the children sat quietly with eyes closed (resting EEG). The authors reported increased alpha power and decreased theta power, a pattern that was remarkably similar to that seen in cocaine-using adults. It should be noted that this study compared children with PCE to normative age-expected values, and a control group was not directly examined. In another study, Jones and colleagues examined oscillatory power in a cohort of PCE children while they sat quietly for three minutes (Jones et al., 2004). The authors found greater asymmetry (defined as the difference in power in one hemisphere versus the other) in right-frontal areas that corresponded to poorer behavioral performance on a cooperative task (solving a puzzle with their mothers) that was administered after the EEG recording. While these two tasks suggest that there are alterations in processing in PCE individuals that are detectable in theta and alpha oscillations in childhood, it is an open question whether or not adolescents with PCE show differences in theta and alpha oscillatory dynamics during feedback processing.
The goal of the current study was to examine event-related EEG oscillations related to feedback processing in adolescents with PCE and NDE. We employed the same reward-feedback task as previously in (Crowley et al, 2014), which examined theta power in healthy adolescents at three different age ranges. This study extends work by (Morie et al., 2018) that considered ERPs and feedback within the same task and sample. We used a high-density (128 channel) electrode array, allowing for regional specificity of event-related spectral perturbation (ERSP) measures. Given the previous reports using the reward-feedback task in adolescents, we hypothesized that complex wavelet frequency decomposition would reveal enhanced theta power during a no-win feedback condition (i.e., non-reward) compared to a reward-feedback condition. Moreover, given previous findings in children with PCE (Prichep, 1995) and cocaine-using adults (Balconi, 2015), we hypothesized that compared to NDE adolescents, adolescents with PCE would show reduced theta power, suggesting poor feedback-processing tendencies. We also hypothesized adolescents with PCE would show relatively increased alpha power for both no-win and win conditions compared to controls, suggesting impaired reward processing and control over’s one’s response to rewarding feedback, as is evident in the literature demonstrating impulsivity in PCE individuals (Ackerman et al., 2010).
Methods
Participants
In brief, participants were recruited from a cohort of 563 individuals participating in a larger study examining the effects of PCE. The cohort has been followed since birth, with assessments taken bi-annually (Mayes et al., 2005). Mothers were originally enrolled over a 5-year time period from the Women’s Center at a large urban hospital setting. Maternal cocaine use was determined based on maternal self-report, on urine toxicology during pregnancy or following delivery, and on meconium toxicology. From the originally recruited longitudinal cohort, at the time this data was collected, contact was maintained with 78% with no selective attrition between the cocaine-exposed (21.4% lost) and nondrug-exposed (24.4% lost). Recently, this cohort was examined in study concerning language processing (Landi et al., 2017), during which reward-feedback processing was assessed. Forty-nine adolescents (average age 17.6 years) with PCE and 34 NDE adolescents (average age 16.8 years) were enrolled. Participants were the same subset as those in our previous report which examined ERP responses to feedback processing (Morie et al., 2018). Among PCE individuals, in the 30 days pre-delivery, 61% of mothers reported using cocaine, 63% reported marijuana, 63% reported alcohol, and 79% reported tobacco use. Among NDE individuals, in the 30 days pre-delivery, no mothers reported using any illegal substance, and 3 of the mothers reported some tobacco use. No mothers within the NDE sample reported alcohol use. Demographics for the sample are presented in Table 1.
Table 1.
Demographics of the sample
| NDE (N=34) | PCE (N=49) | F or chi square | p | |
|---|---|---|---|---|
| Age | 17.1 (1.9) | 17.6(2.0) | 1.01 | 0.30 |
| Gender (M) | 19(15) | 21(28) | 0.63 | 0.56 |
| Mother completed HS (No) | 17(7) | 37(11) | .326 | .57 |
| Ethnicity | 1.55 | 0.22 | ||
| African American | 23 | 44 | ||
| Hispanic/Latino | 2 | 1 | ||
| Caucasian | 9 | 3 | ||
| Other | 0 | 1 | ||
| Substance Initiation by subject; N(%) | ||||
| Alcohol | 11(32) | 34(69) | 11.09 | 0.002 |
| Marijuana | 13(38) | 31(63) | 5.048 | 0.028 |
| tobacco | 8(23) | 16(32) | 0.72 | 0.463 |
| Any | 18(52) | 38(77) | 5.7 | 0.031 |
Task
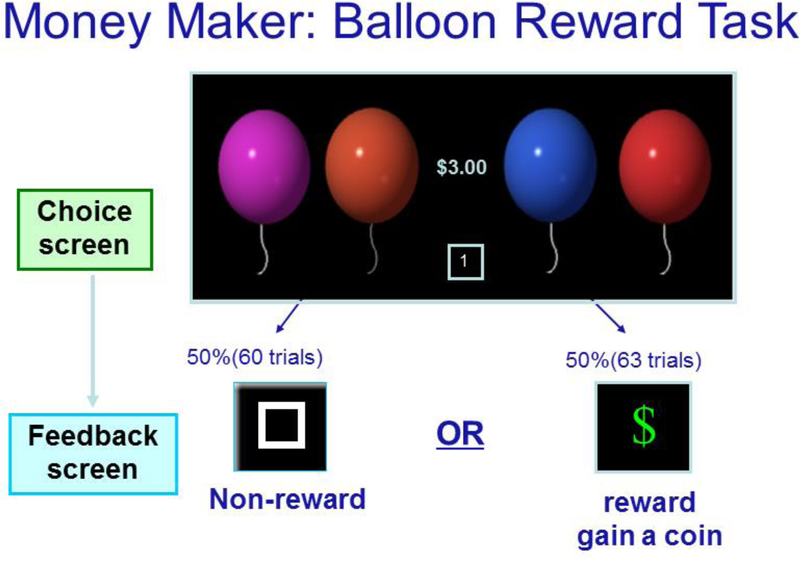
The task was presented to participants as a game called “Money Maker” (see Figure 1). Stimuli were presented on a 17-inch Dell CRT monitor. Participants were instructed that they would be selecting balloons to maximize winning money that they would receive at game’s end. At the beginning of each trial, participants were presented with four balloon images of different colors (e.g., red, green, orange, blue) that randomly appeared in different serial positions along a row centered on the screen. Participants responded with their right and left middle and index fingers on a four-button response pad. After the participant made their selection, all the balloons disappeared, and either a green dollar sign (indicating a reward of 10 cents, a win condition) or a white square (indicating a non-reward, a no-win condition) appeared. Feedback stimuli were delayed 1 to 1.2 seconds after balloon selection and lasted 1,000 msec. After the feedback, a 1,000–1,200 msec crosshair was presented, followed by a 100 msec blank screen, and then the balloons reappeared. Participants made balloon choices at self-paced intervals. Participant earnings were displayed numerically on the screen throughout each trial, centered between the middle two balloons.
Figure 1:
The Money Maker task. Participants choose a balloon color and can either win money (win condition) or fail to gain money (no win condition).
Although there were four options (balloons) on a given trial, feedback was programmed such that the probabilities of win and no-win outcomes were approximately 50% across the task (60 no-wins and 63 wins). While feedback was random, participants were instructed that some people may “figure out a pattern some of the time.” This instruction was given to ensure participant attention. Participants maintained central fixation throughout each block.
There were four blocks of trials with approximately 30 trials in each block. Four distinct, novel balloons appeared in each block. After each block, a clear glass coin jar appeared to reflect cumulative winnings to that point. Realistic dime images appeared in the jar, one by one, each followed by a coin-clinking sound. Prior to beginning the game, there were 3 practice trials, which introduced the coin jar. A total of 120 trials (60 per condition of win or no-win) were administered. Three additional trials were added such that the total winnings were $6.30 for each participant. Participants received this payment as part of a larger compensation ($70) for a study on language.
EEG recording and signal analysis
Participants were seated 1 m before an LCD screen. Each participant’s head was measured to determine the appropriate EEG net size. ERPs were acquired from a 128-channel sensor net of Ag/Cl electrodes. The nets were soaked in a potassium chloride solution for 10 minutes beforehand. Recording was performed at a sampling rate of 250 Hz using Netstation v4.4 software and high-impedance amplifiers (EGI, Inc. Series 200 amplifier). All electrodes were referenced to Cz for recording and then re-referenced offline for data analysis. All impedances were determined to be under 40 kohms before recording began.
ERSP measures were examined with EEGLab version 11.0.4.3b, MATLab version R2011a, with statistical analyses performed in R1.15.1. Frequencies of 4–8 hz and 8–12 hz were used for theta and alpha, respectively. The average reference was used for analysis, as it is considered a better representation of true zero (Junghofer et al., 1999). Our data processing pipeline involved computing ERSP values in EEGLab, using algorithms for implementing time-frequency spectrograms (Delorme & Makeig, 2004). Specifically, we relied on the EEGLab function “newtimef” to calculate average ERSP across our regions of interest, including the medial frontal areas, fronto-central areas, posterior-occipital areas, and left-frontal areas. ERSP calculations relied on both fast Fourier Transform (FFT) (at the lowest frequency) and wavelet decomposition (at the highest frequency). Using the standard setting with EEGLab (cycles were set as [3, 0.5]), cycles increase linearly with frequency from 0 for FFT (same window width at all frequencies) to 1 for wavelet (same number of cycles at all frequencies). Specifically, the software uses 3 cycles at lowest frequency to 11.25 cycles at highest. The time–frequency decomposition yields a time-frequency transform with a complex number for every time point, frequency, and trial. Head-plots were generated using MATLAB 2011a.
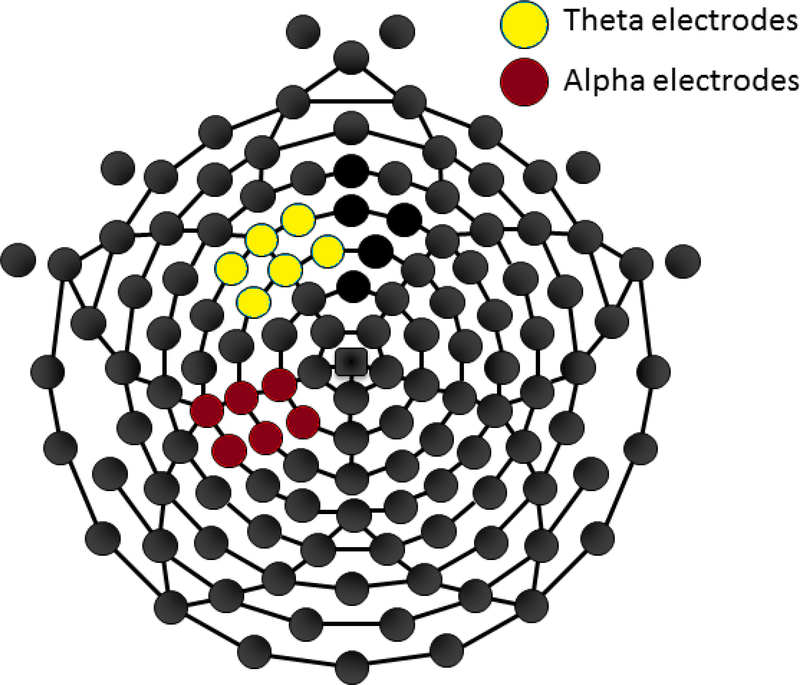
As this sample, consisting of a long-followed PCE sample and a similarly long-followed NDE sample in order ensure NDE status on the part of the controls, is relatively unique, we employed a data-driven approach to electrode selection. Head-plots were examined, and clusters showing the highest power in alpha power (for alpha analyses) or theta power (for theta analyses) were selected, with alpha power being examined in the left-posterior region and theta power being examined in the left-frontal region. Figure 2 demonstrates the regions chosen for our primary analyses.
Figure 2:
A headmap of electrode locations. Blue indicates electrodes used for the theta analysis. Red indicates electrodes used for the alpha analysis. The additional analysis using FCz overlapped slightly with the electrodes used for the initial theta analysis. These include electrodes 11,16, 19, 12, 6, 4 and 5.
By request of a reviewer, theta power was also examined in the fronto-central region, corresponding to electrodes typically used to examine the FRN (electrodes 11,16, 19, 12, 6, 4 and 5). This is the same region chosen for previous examinations of theta power in a large sample of healthy control adolescents in (Crowley et al., 2014).
SPSS analyses for EEG power consisted of repeated measures analyses of covariance (RM ANCOVAs) with factors of condition and group, with covariates of age, gender and substance-use initiation. Substance-use initiation was determined by participant report of ever having tried alcohol, marijuana, or tobacco. If the answer to any of these questions was affirmative, participants were considered as having initiated.
Results
Participants
Of the 34 NDE and 49 PCE participants, there were no differences between groups in either age (ͼ=1.01, p=.30), socioeconomic status as assessed with the proxy measures of maternal education (Mother did or did not complete high school, with information not available for 11 participants) (ͼ=.326, p=.57), or gender distribution (ͼ=.013, p=.9). Adolescent participants also reported their own substance-use histories at the time of recruitment. Initiation status for a substance was determined based upon participant’s self-report of use in the past three months and how often they reported using. PCE participants were more likely to report initiation of substance use (ͼ =5.7, p<.04), including alcohol (ͼ =11.09.0, p<.01) and marijuana (ͼ=5.048, p<.03), but not tobacco (ͼ =.72, p>.46).
ERSP Analyses--alpha
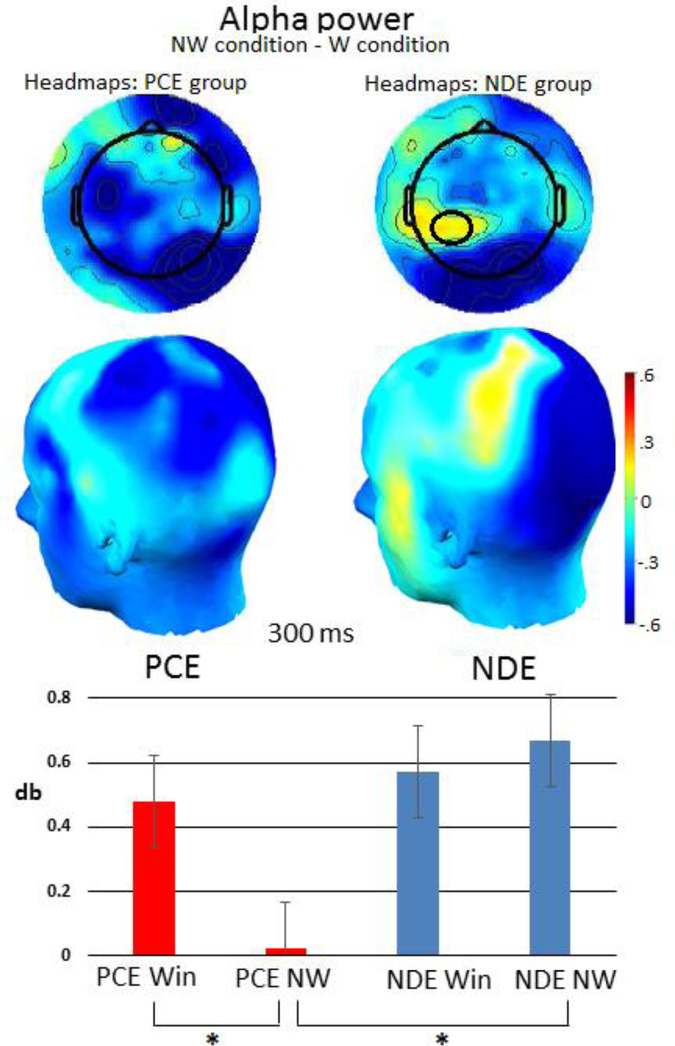
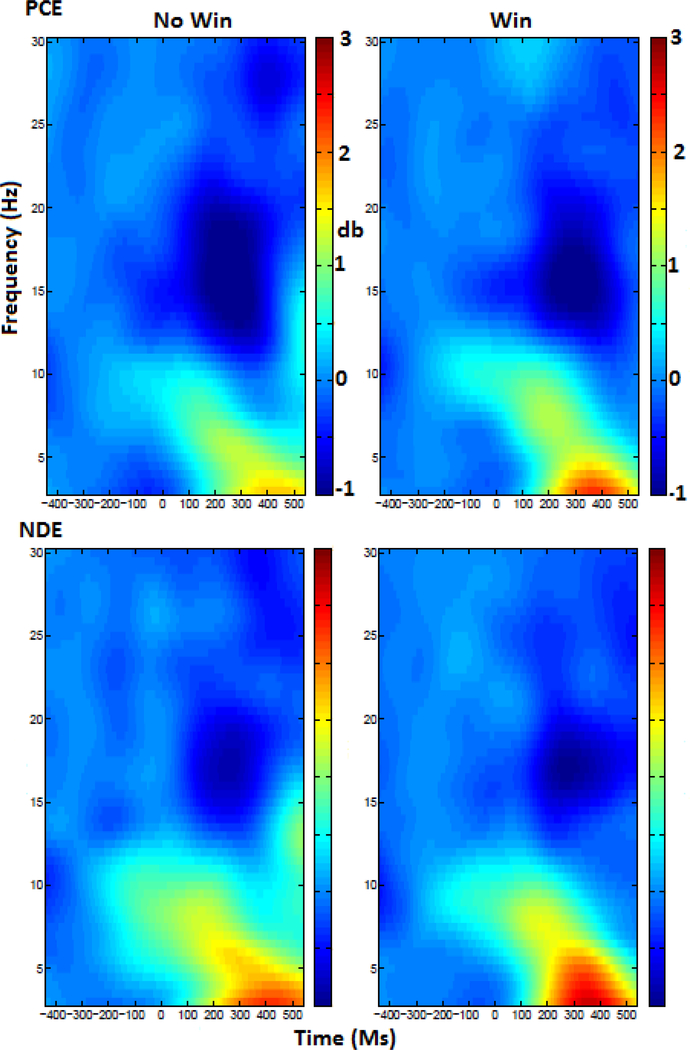
Alpha power was derived from electrodes located in the left posterior region between 200–300 ms. A 2X2 (condition x group) ANOVA analysis of mean spectral power in the alpha band revealed no main effect of condition (F1,82=.54, p=.46) or group (F1,82=1.8, p=.17), but did reveal an interaction of condition by group (F1,82=5.7, p<.02). We examined the condition x group interaction with post-hoc tests that consisted of paired samples t-tests of the condition for each group separately. In these post hoc tests for condition, we applied Bonferroni correction. NDE individuals did not show a significant difference between win or loss conditions (t = .571, p = .572). PCE individuals showed a significant condition difference (t = 12.35, p < .01) that survived Bonferroni correction (p < .01), indicating that individuals with PCE had reduced alpha power during the no-win condition relative to their win condition. In addition, we examined each condition by group. Multiple comparison corrections were not applied in this case as there were fewer than three groups. Alpha power during the no-win condition was significantly different between groups (F = 5.22, p < .03) while alpha power during the win condition was not (F = .082, p = .77). The graph in figure 3 illustrates alpha power across groups and conditions. Brackets indicate comparisons that were significant. The head maps illustrate the difference in alpha power between no-win and win conditions for both groups, plotted across the scalp. The spectrograms in figure 3 illustrate power in all frequencies across time in each condition for the left-posterior region.
Figure 3:
The headmap shows the difference between the no-win and win conditions in alpha power across the scalp. The graph shows alpha power in each condition and group. Alpha power during a no-win condition was significantly reduced in adolescents with PCE when compared to alpha power in PCE individuals during a win condition, and when compared to NDE individuals during a no-win condition. These significant comparisons are indicated with brackets and an asterisk (*).
ERSP Analyses: Theta
Theta power was derived from electrodes located in the left frontal region between 250–350 ms. A 2X2 (condition x group) ANOVA analysis of mean spectral power in the theta band revealed no main effect of condition (F1,82=1.8 p=.17), and no main effect of group (F1,82=.318, p=.574), but did reveal an interaction of condition by group (F1,82=8.5, p< .02). As with our alpha analyses, we examined the condition x group interaction with post-hoc tests that consisted of paired samples t-tests of the condition for each group separately. In these post hoc tests for condition, we applied Bonferroni correction. NDE individuals showed a non-significant reduction in theta power during the no-win condition relative to the win condition (F = 2.9, p = .095), while PCE individuals showed a significant increase in theta power during the no-win condition relative to the win condition (F= 6.8, p < .03), which survived Bonferroni correction.
In addition, we examined each condition by group. Multiple comparison corrections were not applied in this case as there were fewer than three groups. There were no differences in the win condition between groups (F = 1.7, p = .19) and there were no differences in the no-win condition between groups (F = .753, p = .38)
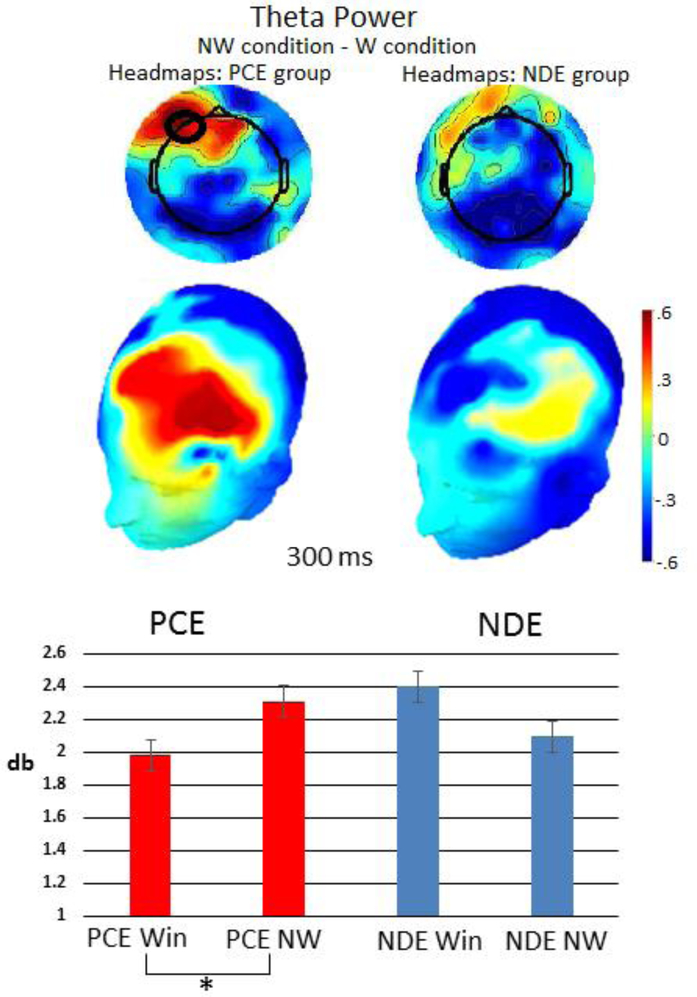
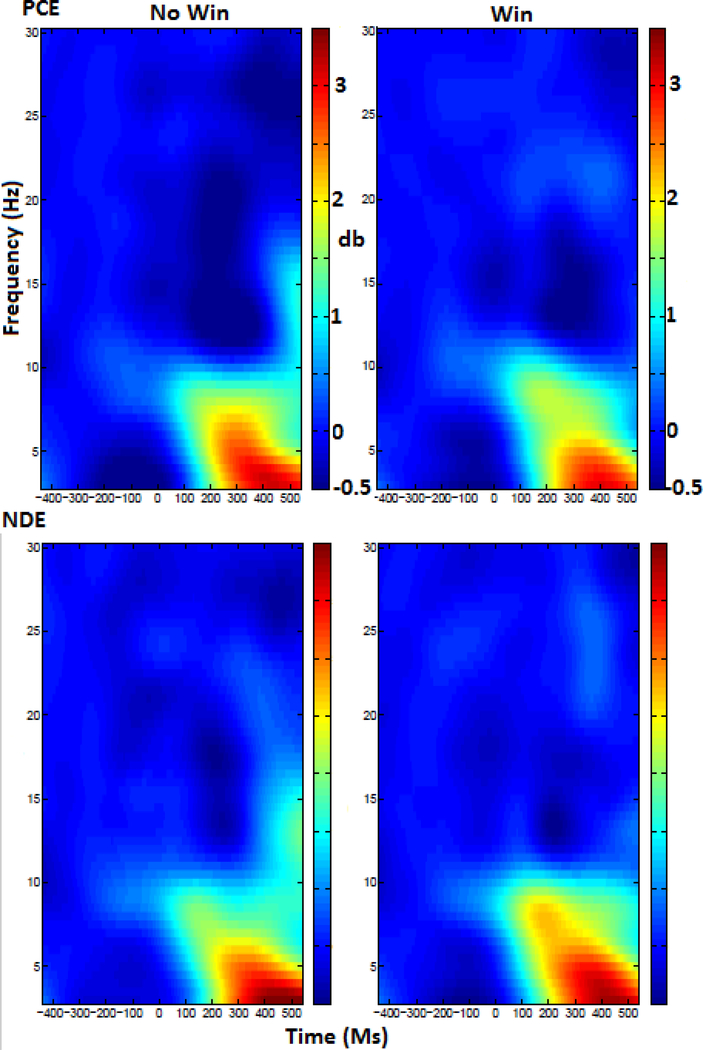
The graph in figure 5 illustrates theta power across groups and conditions. Figure 5 also illustrates the difference in theta power between no-win and win conditions for both groups, plotted across the scalp. The spectrograms in figure 6 illustrate power in all frequencies across time in each condition for the left-frontal region.
Figure 5:
The headmap shows the difference between the no-win and win conditions in theta power across the scalp. The graph shows theta power in each condition and group. The pattern of theta power was different between the win and no-win conditions in PCE participants. These significant comparisons are indicated with brackets and an asterisk (*).
Figure 6:
A spectrogram displaying theta power in each condition and group. Time is on the x axis and frequency on the Y, with the colorbar denoting the power.
By request of a reviewer, we also examined theta power derived from electrodes located in the front-central region between 250–350 ms. This region revealed no effect of condition (F = .182, p = .71), no effect of group (F = .327, p = .569) and no interaction (F = 2.4, p = .12).
Induced Theta power
The condition-by-group interaction was in an unexpected direction. Specifically, theta was anticipated to be greater during no-win conditions wherein reward is not gained or lost, a pattern that would have been consistent with prior findings regarding theta power during feedback tasks (Luft et al., 2013). However, in NDE participants, theta was reduced in the no-win condition. To investigate further, we examined induced theta power, which is the theta power independent of the evoked potential (in this case, the FRN). This was achieved by performing the same oscillation analysis as described above after subtracting the averaged FRN from each single trial. This analysis results in systematically smaller theta power amplitudes that are independent of the induced response. This analysis, including all covariates as before, revealed similar findings to the non-evoked analysis. There was no main effect of condition (F1,82=1.9, p=.171,) or group (F1,82=.368, p=.54), but the interaction of condition-by-group (F1,82=3.7, p=.056) approached statistical significance. Like the evoked analyses, when the interaction was investigated with paired-samples t-tests, there was a different pattern of responding between groups. NDE individuals showed a non-significant reduction in theta power during the no-win condition relative to the win condition (t = −.91, p = .37), while PCE individuals showed a significant increase in theta power during the no-win condition relative to the win condition (t = 2.07, p < .05).
Discussion
We examined oscillatory dynamics of feedback processing in individuals with PCE, with a focus on reward and feedback processing. Use of high-density electrophysiological mapping revealed that individuals with PCE, relative to NDE individuals, had reduced alpha power during a no-win condition and increased theta power during a no-win condition. These findings raise the possibility of alterations in continuous monitoring ability and comparison of expected or unexpected outcomes, as suggested by theta differences, and in reward and arousal regulation, as suggested by alpha differences, in response to feedback in adolescents with PCE. However, additional direct examination of these possibilities is warranted.
Adolescents with PCE showed within-group reductions in the left posterior region in alpha power during a no-win condition relative to a win condition. This finding is inconsistent with our initial hypothesis of increased alpha power. Instead, this finding may suggest increased reactivity to what was perceived as an incorrect choice, and suggest alterations in feedback processing in PCE. Reductions in alpha power have been seen in performance monitoring tasks, where incorrect responses on a Stroop task were associated with alpha reductions (Carp and Compton, 2009) that were correlated with post-error changes in behavior. It is possible that the alpha reductions seen in our sample of PCE individuals reflected changes in cortical arousal related to preparation for the next choice. Alterations in alpha power in the left hemisphere have been related to loss processing and higher reactivity to reward and risky decision-making in cocaine-using adults (Balconi and Finocchiaro, 2015). These authors found that cocaine-using adults performing a gambling task made riskier choices (selecting more immediate, riskier rewards), and showed decreased alpha power in the left hemisphere in response to higher-risk choices. In that our PCE sample did not report initiation of cocaine use and yet showed similar reductions in alpha in the left hemisphere to loss outcomes suggests that prenatal exposure to cocaine has consequences for feedback processing. Future studies that directly measure behavior in PCE individuals could further examine this relationship between alpha power and decision-making behavior in this population.
The reduction in alpha to a non-winning outcome may suggest greater attentional engagement to potentially emotional stimuli. Changes in alpha power in this case may also reflect changes in affect, including frustration associated with a loss. Greater reductions in left-parietal alpha (where we observed the reduction in our PCE individuals) have been seen in response to emotional video clips, and the amount of alpha reduction correlated with self-reports of emotional arousal (Simons et al., 2003). This finding illustrates the importance of alpha as reflective of potential changes in affect. While emotional response was not directly measured in our study, data suggest increased reactivity in PCE individuals, both in childhood (Jones et al., 2004) and in adolescence (Chaplin et al., 2010). The Jones et al. paper also showed alterations in alpha band power in the left hemisphere, similar to our data. Other work using functional connectivity techniques suggests increased reactivity, evidenced by increased amygdala activation, in adolescents with PCE when they were exposed to emotional distractors during a working memory task (Li et al., 2013). As a “no-win” in our study represented what may be considered a loss, it is possible that the noted sharp reduction in alpha power reflects alterations in arousal regulation in PCE adolescents to what is perceived as a losing outcome.
Theta power has been associated with executive function, including decision-making and adjustment of behavior after receiving the outcome of a choice (Cavanagh et al., 2010). There were no group differences detected in overall theta power, implying that adolescents with PCE do not show an overall decrement in feedback processing that is often associated with theta frequencies (Marco-Pallares et al., 2008). However, the pattern of theta power did differ from controls within conditions. Within groups, theta power was reduced during win conditions in PCE individuals, while no significant differences for win or loss was found in NDE adolescents, for both induced and evoked theta power. This finding is contrary to our hypotheses with respect to published reports of theta power during reward-feedback tasks, which typically finds greater theta power in non-clinical individuals during a loss/no-win condition (Luft et al., 2013). A possible explanation for this seeming inconsistency is that the losses in the task we employed were less salient to the NDE individuals, as the win and no-win feedback occurred at equal probabilities and at equal magnitudes. A study by (Cavanagh et al., 2010) which employed a probabilistic learning task to examine theta responses to feedback at different probabilities has illustrated that theta power reflects the degree of negative prediction error. In a task with nearly 50% probability like the one we employed, the lack of prediction error may have reduced the effect of negative feedback on theta power in the NDE group. Another study by (Marco-Pallares et al., 2008) utilized a gambling task with random feedback and illustrated no effect at all of wins or losses on theta power in healthy individuals. It is also possible that the data-driven approach resulted in the selection of electrodes that were not ideal for detecting the expected increase in the no-win condition in the NDE group. However, our additional analyses using electrodes from the FCz region also failed to detect significant differences in this group or in the PCE group. The PCE group, however, still showed increased theta power to no-win feedback when the left-temporal region was examined, suggesting that the perceived loss may be salient to this group, even without the additional effect of violation of a prediction error.
Our findings add to the literature on the potential ramifications of PCE. Our findings suggest that PCE adolescents may be highly sensitive to feedback, and this may be reflected in their increased theta power and decreased alpha power to no-win outcomes. Future work should examine if this heightened sensitivity to feedback is associated with actual reports or indices of frustration, which would be consistent with previous findings in PCE children (Jones et al., 2004).
The strengths of this study include the fact that participants have been followed since birth and their PCE or NDE status is well documented. This study includes limitations. Many individuals were prenatally exposed to substances other than cocaine, including tobacco, marijuana and alcohol, making it difficult to specify if findings relate to prenatal cocaine exposure specifically. Another limitation is the prevalence of substance initiation by subjects, especially those with PCE. The high percentage of substance-use initiation in our sample makes it difficult to determine if differences observed in feedback processing are a result of intrauterine exposure or are a result of substance use later in life or other factors. In addition, it would have been helpful to have more data about participants’ substance usage patterns, including how often or how much they use specific substances. To consider possible influences of initiation, we used substance-use-initiation data as covariates in our analyses. Another limitation is the limited information present on the environment faced in our sample. While both NDE and PCE individuals were from low-income backgrounds, having more information could help identify potential factors contributing to alterations in feedback processing. In addition, while there were no significant differences between groups in ethnicity, the groups were still somewhat skewed in ethnic distribution, which may have had subtle effects on our data.
In summary, PCE status was associated with reduced alpha power during no-win conditions, and an increase in theta power in response to no-win conditions, compared to NDE individuals. This work adds to the literature on the possible long-term neural correlates of PCE and the oscillatory dynamics of feedback processing. Alterations in feedback processing could underlie some of the behavioral differences seen in this population, including increased externalization in childhood (Linares, 2006). In addition, increased reactivity to non-winning feedback may lead to sensation-seeking, and ultimately, to increased initiation of use of substances, though this is speculative. Future work should examine feedback processing and how it relates to sensation-seeking behaviors and impulsivity more closely in this population.
Figure 4:
A spectrogram displaying alpha power in each condition and group. Time is on the x axis and frequency on the Y, with the colorbar denoting the power.
Acknowledgments
DISCLOSURES
Role of the Funding Source
Funding for this work included National Institute of Health grants K01DA042937, K01 DA034125 (MJC), T32 MH018268 (MJC), P50 DA09241, UL1-DE19586, RL1 AA017539, R01 DA006025, R01 DA017863, K05 DA020091; T32 DA007238 and R21 DA030665. KPM receives support from MH018268–31 and from K01DA042937. MNP was supported by R01 DA035058, R01 DA039136, the National Center for Responsible Gaming, the Connecticut Council on Problem Gambling, and the Connecticut Department of Mental Health and Addiction Services. Beyond funding, the funding agencies had no further role in the writing of the report or in the decision to submit the paper for publication.
Dr. Potenza has consulted for and advised Shire, Rivermend Health, Opiant/Lightlake Therapeutics and Jazz Pharmaceuticals; received research support (to Yale) from the Mohegan Sun Casino and the National Center for Responsible Gaming; participated in surveys, mailings, or telephone consultations related to drug addiction, impulse control disorders or other health topics; consulted for and/or advised legal and gambling entities on issues related to impulse control and addiction; provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; given academic lectures in grand rounds, CME events and other clinical/scientific venues; and generated books or chapters for publishers of mental health texts. The other authors report no financial relationships with commercial interests.
Footnotes
Conflicts of Interest
The authors report no conflict of interest with respect to the content of this manuscript.
References
- Ackerman JP, Riggins T, Black MM, 2010. A Review of the Effects of Prenatal Cocaine Exposure Among School-Aged Children. Pediatrics 125, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JWP, Bennett DS, Carmody DP, Wang YP, Lewis M, 2014. Adolescent risk-taking as a function of prenatal cocaine exposure and biological sex. Neurotoxicol Teratol 41, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C, Frielinghaus H, Rauh J, Mussmann M, Vauth S, Braun P, Leicht G, Mulert C, 2017. Theta and high-beta networks for feedback processing: a simultaneous EEG-fMRI study in healthy male subjects. Transl Psychiatry 7, e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi M, Finocchiaro R, 2015. Decisional impairments in cocaine addiction, reward bias, and cortical oscillation “unbalance”. Neuropsychiatr Dis Treat 11, 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Compton RJ, 2009. Alpha power is influenced by performance errors. Psychophysiology 46, 336–343. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, 2014. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 18, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJ, 2010. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. NeuroImage 49, 3198–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Freiburger MB, Mayes LC, Sinha R, 2010. Prenatal cocaine exposure, gender, and adolescent stress response: a prospective longitudinal study. Neurotoxicol Teratol 32, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE, 2009. Good vibrations: cross-frequency coupling in the human nucleus accumbens during reward processing. J Cogn Neurosci 21, 875–889. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C, 2007. Reward expectation modulates feedback-related negativity and EEG spectra. NeuroImage 35, 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, van Noordt SJ, Wu J, Hommer RE, South M, Fearon RM, Mayes LC, 2014. Reward feedback processing in children and adolescents: medial frontal theta oscillations. Brain and cognition 89, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Partridge RT, Ager J, Sokol RJ, 2011. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol Teratol 33, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Chen H, Jia H, Ming Q, Yi J, Yao S, 2016. Dysfunctional feedback processing in adolescent males with conduct disorder. Int J Psychophysiol 99, 1–9. [DOI] [PubMed] [Google Scholar]
- Geier CF, 2013. Adolescent cognitive control and reward processing: Implications for risk taking and substance use. Horm Behav 64, 333–342. [DOI] [PubMed] [Google Scholar]
- Heuer A, Wolf C, Schutz AC, Schubo A, 2017. The necessity to choose causes reward-related anticipatory biasing: Parieto-occipital alpha-band oscillations reveal suppression of low-value targets. Sci Rep 7, 14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Field T, Davalos M, Hart S, 2004. Greater right frontal EEG asymmetry and nonemphathic behavior are observed in children prenatally exposed to cocaine. Int J Neurosci 114, 459–480. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Braun C, 1999. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clin Neurophysiol 110, 1149–1155. [DOI] [PubMed] [Google Scholar]
- Krakowski MI, De Sanctis P, Foxe JJ, Hoptman MJ, Nolan K, Kamiel S, Czobor P, 2016. Disturbances in Response Inhibition and Emotional Processing as Potential Pathways to Violence in Schizophrenia: A High-Density Event-Related Potential Study. Schizophr Bull 42, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Avery T, Crowley MJ, Wu J, Mayes L, 2017. Prenatal Cocaine Exposure Impacts Language and Reading Into Late Adolescence: Behavioral and ERP Evidence. Dev Neuropsychol 42, 369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega BC, Kahana MJ, Jaggi J, Baltuch GH, Zaghloul K, 2011. Neuronal and oscillatory activity during reward processing in the human ventral striatum. Neuroreport 22, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht G, Troschutz S, Andreou C, Karamatskos E, Ertl M, Naber D, Mulert C, 2013. Relationship between Oscillatory Neuronal Activity during Reward Processing and Trait Impulsivity and Sensation Seeking. PloS one 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S, Hu XP, 2013. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiat Res-Neuroim 213, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MYO, Hussey P, Minnes S, 2006. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol 31, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft CD, Nolte G, Bhattacharya J, 2013. High-learners present larger mid-frontal theta power and connectivity in response to incorrect performance feedback. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A, 2004. Mining event-related brain dynamics. Trends Cogn Sci 8, 204–210. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Cucurell D, Cunillera T, Garcia R, Andres-Pueyo A, Munte TF, Rodriguez-Fornells A, 2008. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia 46, 241–248. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Molfese DL, Key AP, Hunter NC, 2005. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol 27, 797–813. [DOI] [PubMed] [Google Scholar]
- Michel CM, Henggeler B, Brandeis D, Lehmann D, 1993. Localization of Sources of Brain Alpha-Theta-Delta-Activity and the Influence of the Mode of Spontaneous Mentation. Physiol Meas 14, A21–A26. [DOI] [PubMed] [Google Scholar]
- Min MO, Minnes S, Lang A, Albert JM, Kim JY, Singer LT, 2016. Pathways to adolescent sexual risk behaviors: Effects of prenatal cocaine exposure. Drug and alcohol dependence 161, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer L, Min MO, Wu MAP, Lang A, Yoon S, 2014. Effects of prenatal cocaine/polydrug exposure on substance use by age 15. Drug and alcohol dependence 134, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie KP, Wu J, Landi N, Potenza MN, Mayes LC, Crowley MJ, 2018. Feedback processing in adolescents with prenatal cocaine exposure: an electrophysiological investigation. Dev Neuropsychol, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Manz N, Chorlian DB, Stimus A, Porjesz B, 2016. Delta, theta, and alpha event-related oscillations in alcoholics during Go/NoGo task: Neurocognitive deficits in execution, inhibition, and attention processing. Prog Neuropsychopharmacol Biol Psychiatry 65, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichep LS, Kowalik SC, Alper K, de Jesus C, 1995. Quantitative EEG characteristics of children exposed in utero to cocaine. Clin Electroencephalogr 26, 166–172. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Larkby C, Goldschmidt L, Day NL, 2013. Adolescent Initiation of Drug Use: Effects of Prenatal Cocaine Exposure. J Am Acad Child Psy 52, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Holroyd CB, Debener S, Hewig J, 2017. I can’t wait! Neural reward signals in impulsive individuals exaggerate the difference between immediate and future rewards. Psychophysiology 54, 409–415. [DOI] [PubMed] [Google Scholar]
- Simons RF, Detenber BH, Cuthbert BN, Schwartz DD, Reiss JE, 2003. Attention to television: Alpha power and its relationship to image motion and emotional content. Media Psychol 5, 283–301. [Google Scholar]
- Trujillo LT, Allen JJ, 2007. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol 118, 645–668. [DOI] [PubMed] [Google Scholar]
- van Driel J, Ridderinkhof R, Cohen M, 2012. Not All Errors Are Alike: Theta and Alpha EEG Dynamics Relate to Differences in Error-Processing Dynamics. The Journal of Neuroscience 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noordt SJR, Segalowitz SJ, 2012. Performance monitoring and the medial prefrontal cortex: a review of individual differences and context effects as a window on self-regulation. Frontiers in human neuroscience 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Ballo A, Cunillera T, Rostan C, Hdez-Lafuente P, Fuentemilla L, Rodriguez-Fornells A, 2015. Neurophysiological correlates of cognitive flexibility and feedback processing in violent juvenile offenders. Brain Res 1610, 98–109. [DOI] [PubMed] [Google Scholar]