Abstract
Previous research indicates that loneliness and social isolation may contribute to behavioral disorders and neurobiological dysfunction. Environmental enrichment (EE), including both cognitive and physical stimulation, may prevent some behavioral, endocrine, and cardiovascular consequences of social isolation; however, specific neural mechanisms for these benefits are still unclear. Therefore, the current study examined potential neuroendocrine protective effects of both EE and exercise. Adult female prairie voles were randomly assigned to one of 4 experimental conditions: paired control, social isolation/sedentary, social isolation/EE, and social isolation/voluntary exercise. All isolated animals were housed individually for 8 weeks, while paired animals were housed with their respective sibling for 8 weeks. Animals in the EE and voluntary exercise conditions received EE items (including a running wheel) and a running wheel only, respectively, at week 4 of the isolation period. At the end of the experiment, plasma and brains were collected from all animals for corticosterone and FosB and delta FosB (FosB/ΔFosB)–immunoreactivity in stress-related brain regions. Overall, social isolation increased neuroendocrine stress responses, as reflected by the elevation of corticosterone levels and increased FosB/ΔFosB-immunoreactivity in the basolateral amygdala compared to paired animals; EE and voluntary exercise attenuated these increases. EE and exercise also increased FosB/ΔFosB-immunoreactivity in the medial prefrontal cortex compared to other conditions. Limbic structures statistically mediated hypothalamic immunoreactivity in EE and exercise animals. This research has translational value for socially isolated individuals by informing our understanding of neural mechanisms underlying responses to social stressors.
Keywords: Environmental Enrichment, Voluntary Exercise, Delta FosB, Prairie Vole, Social Isolation, Corticosterone
Introduction
Social stressors produce emotional disturbances, physical disorders, poor adaptation to environmental demands, and premature mortality (see Cacioppo, Cacioppo, Capitanio, & Cole, 2015; Hare, Toukhsati, Johansson, & Jaarsma, 2014; Hawkley and Cacioppo, 2010). Animal models also demonstrate several consequences of social stressors, including depressive- and anxiety-related behaviors, physiological dysfunction, and increased neuroendocrine reactivity to stressors (Carnevali et al., 2012; Kalinichev, Easterling, Plotsky, & Holtzman, 2002).
Social and environmental stressors activate neuroendocrine processes that mediate stress and emotion, producing elevated corticosterone and altered activity of the hypothalamic paraventricular nucleus (PVN), basolateral amygdala (BLA), and bed nucleus of stria terminalis (BNST; Benite-Ribeiro, Santos, & Duarte, 2014; Flak, Solomon, Jankord, Krause, & Herman, 2012; Garrido et al., 2013; Hawley and Leasure, 2012; Herman and Tasker, 2016; Hostinar, Sullivan, & Gunnar, 2014; Laine et al., 2017; Perrotti et al., 2004). However, stimulation from the environment in the form of environmental enrichment (EE) and/or physical exercise may ameliorate the negative effects of social stressors (e.g., Campeau et al., 2010; Grippo et al., 2014; Lehmann and Herkenham, 2011; Lindsay-Smith, Banting, Eime, O’Sullivan, & van Uffelen, 2017; Normann et al., 2018; Watanasriyakul et al., 2018). EE involves exposure to species-specific items and often physical activity, promoting cognitive, sensory, and neural stimulation (van Praag, Kempermann, & Gage, 2000). For instance, EE and exercise can prevent dysfunction in the PVN, BLA, and BNST of animals exposed to chronic stressors (Koe, Ashokan, & Mitra, 2016; Lehmann and Herkenham, 2011; Lin et al., 2015; Nader et al., 2014; Zheng, Sharma, Liu, & Patel, 2012), and may alter hypothalamic-pituitary-adrenal (HPA) activity via actions in the hippocampus and medial prefrontal cortex (mPFC; see Hostinar et al., 2014).
Because EE paradigms often include a physical activity component (Cao et al., 2017; Garrido et al., 2013; Nader et al., 2014), previous studies have attempted to dissociate the effects of EE and physical exercise alone on central processes. Several lines of evidence indicate that EE and exercise have some overlapping mechanisms in the brain, but certain processes may be dissociated. For example, voluntary exercise significantly increased cell proliferation in the hippocampal dentate gyrus compared to standard and enriched housing; however, both voluntary exercise and EE equally promoted cell survival (van Praag, Kempermann, & Gage, 1999). Both exercise and EE promoted neurogenesis, with the majority of these newborn cells being oligodendrocytic precursors (Klaissle et al., 2012), suggesting that EE and exercise may enhance the neural maintenance process in the central nervous system (Olson, Eadie, Ernst, & Christie, 2006). Voluntary exercise and EE may promote neurogenesis through different mechanisms such that voluntary exercise may increase neurogenesis through increased cerebral blood flow using β-endorphin, brain-derived neurotrophic factor (BDNF), and serotonin; whereas EE may increase neurogenesis through cortical restructuring using BDNF and nerve growth factor (Olson et al., 2006). Taken together, both EE and exercise directly affect the hippocampus to promote adaptive neural functions. It is possible that similar changes may also influence central stress-related communication, given inhibitory hippocampal effects on HPA-related regions (Herman, Ostrander, Mueller, & Figueiredo, 2005).
A valuable model for investigating the differential influence of EE and physical exercise alone on neural responses to social stress is the socially monogamous prairie vole (Carter and Keverne, 2002; Young, Wang, & Insel, 1998). This rodent species engages with the surrounding social context similar to humans, by developing long-term social bonds, exhibiting bi-parental care of offspring, and displaying altered behavior and neurobiology to both short- and long-term social stressors (e.g., Bosch, Nair, Ahern, Neumann, & Young, 2009; Carter and Keverne, 2002; Grippo et al., 2007; Smith and Wang, 2014; Young et al., 1998). Further, both EE (including an exercise component) and exercise alone were protective against some behavioral, physiological, and neuroendocrine consequences of social isolation in the prairie vole model (Grippo et al., 2014; Jarcho et al., in press; Normann et al., 2018; Watanasriyakul et al., 2018).
Therefore, the current study examined potential neuroprotective effects of EE (including an exercise component), versus exercise alone, against social isolation in prairie voles. The neuroendocrine mechanisms of EE and exercise were examined using FosB and delta FosB (FosB/ΔFosB) immunohistochemistry as an index of long-term neural activation. FosB/ΔFosB are transcription factors generated by alternative splicing of the FosB gene; FosB/ΔFosB have long half-lives and are protected from rapid degradation unlike other proteins in the Fos family (Nestler, Barrot, & Self, 2001). It has been shown that FosB/ΔFosB proteins remain in the neurons several weeks after cessation of stress (Nestler, 2015). Therefore, the current study examined FosB/ΔFosB expression in the PVN, anterior BNST (aBNST), BLA, hippocampal subregions, and mPFC; and circulating corticosterone levels were examined as an index of basal HPA function. We hypothesized that: (a) social isolation would increase plasma corticosterone levels and influence FosB/ΔFosB-immunoreactivity in the PVN, aBNST, and BLA; and (b) both EE and exercise alone would be effective at attenuating these neuroendocrine disruptions. We used exploratory analyses to test the additional hypothesis that the beneficial effects of EE and exercise on neuroendocrine function in the PVN would be mediated through specific limbic structure communication.
Methods
Animals
Sixty-three adult, sexually naïve, female prairie voles were used in the current study. At the beginning of the study, animals had a mean (± standard error of the mean; SEM) age of 90 ± 3 days and a body weight of 35 ± 0.7 grams. All animals were maintained on a 14/10 h light/dark cycle, with lights on at 0630 h; mean ambient temperature and relative humidity were 25 ± 2°C and 40 ± 5%, respectively. Food (Purina rabbit chow) and water were available ad libitum. Animals were weaned at post-natal day 21 and housed in same-sex sibling pairs until the beginning of the study. For all procedures described, only one animal from each sibling pair was studied. All procedures were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were also approved by the Northern Illinois University Institutional Animal Care and Use Committee.
Female prairie voles were chosen for the current study for several reasons. First, the female population continues to be understudied in neuroscience and biomedical research (Beery and Zucker, 2011); therefore, the current design will contribute valuable knowledge regarding female responses to social stress. Second, females may be more sensitive to perceived social isolation compared to their male counterparts in both human and animal models (e.g., Dong and Chen, 2017; Grippo et al., 2007). Third, the study of females here allows us to discuss the association of these results with previous EE and exercise studies using the prairie vole model (e.g., Grippo et al., 2014; Jarcho et al., in press; Normann et al., 2018; Watanasriyakul et al., 2018). Finally, female prairie voles do not exhibit spontaneous puberty; the physical presence of a male is required to induce ovulation in this species (Carter, Witt, Schneider, Harris, & Volkening, 1987). Therefore, this allowed us to examine intact animals without having to control for potential estrous cycle confounds.
General Experimental Design
Animals were randomly assigned to one of 4 experimental conditions: paired control (n = 15), social isolation/sedentary (n = 15), social isolation/EE (n = 16), and social isolation/voluntary exercise (n = 17). Animals in the 3 social isolation conditions were removed from their respective siblings and housed individually for 8 weeks (without visual, olfactory, or auditory cues from the previously paired sibling), while paired control animals remained paired with their respective sibling for 8 weeks. After 4 weeks of social isolation, animals in the EE and voluntary exercise conditions were given access to enrichment items and a running wheel, respectively, for the final 4 weeks of social isolation. These delayed intervention paradigms were chosen in the current study based on previous findings showing that both immediate and delayed environmental interventions were effective in decreasing depressive- and anxiety-like behaviors in socially isolated prairie voles (Grippo et al., 2014). Additionally, the delayed intervention paradigm reflects a commonly observed phenomenon in the clinical population, such that individuals who suffer from mental or behavioral health issues (such as depression, anxiety, or other problems associated with social stressors) often do not seek help until these problems negatively impact their work, home, and/or social relationships (Ando et al., 2018; Rickwood, Deane, & Wilson, 2007), which adds to the translational value of the present study design. Handling, cage changing, and body weight measurement were matched among all conditions. Following 8 weeks in the experimental or control conditions, plasma, brains, hearts, and adrenal glands were collected from all animals for further analysis, as described below.
Experimental Conditions
Paired control:
Animals in the paired control condition were continually housed with a same-sex sibling for the duration of the experiment (8 weeks), in a standard cage (12 × 18 × 28 cm) with food, water and bedding.
Social isolation/sedentary:
Animals in the social isolation/sedentary condition were housed alone for the duration of the experiment (8 weeks), in a standard cage, with food, water and bedding.
Social isolation/EE:
Animals in the social isolation/EE condition were housed alone in a standard cage, with food, water and bedding, during the first 4 weeks of the isolation period; they were then housed in a larger cage (25 × 45 × 60 cm) and also received enrichment items continuously during the last 4 weeks of the isolation period. Enrichment items were based on previous studies that included a physical activity component and several items of varying textures, colors, and sizes (Cao et al., 2017; Garrido et al., 2013; Nader et al., 2014), including: a 4-in diameter running wheel (Super Pet Mouse Silent Spinner Mini Exercise Wheel, Model #100079369, Elk Grove Village, IL), a piece of cotton nesting material (Mountain Mist, Cincinnati, OH), a wood block, a rubber die, a wood jack chew toy, a mini straw hat, a cardboard toilet paper roll, a tin foil ball, a plastic bowl with small food pallets, two plastic toys (one hanging from cage top and one inside the cage), two flat-bottomed marbles, and a plastic igloo house. All items other than the running wheel and cotton nesting material were purchased commercially at a craft store, pet store, or supermarket. Items were placed randomly inside the cage and were sanitized or replaced each week. Daily distance traveled (km/day) and daily maximum speed reached (km/hr) in the running wheel were monitored via an odometer adapted for use with the running wheel (Bell F12 Cyclocomputer, Model #7001115, Van Nuys, CA).
Social isolation/voluntary exercise:
Animals in the social isolation/voluntary exercise condition were housed alone in a standard cage during the first 4 weeks of the isolation period; they were then housed in a larger cage (25 × 45 × 60 cm) and received a 4-in diameter running wheel during the last 4 weeks of the isolation period. Daily distance traveled (km/day) and daily maximum speed (km/hr) in the running wheel were monitored.
Collection of Blood
All animals remained in their respective housing conditions until the time of blood collection. At the end of the 8-week social isolation/pairing period, blood samples were collected from all animals for circulating plasma corticosterone analysis. All samples were collected between 1030 h and 1400 h, to minimize circadian variations of hormone levels. Each animal was anesthetized with a combination of ketamine (67 mg/kg, sc; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg, sc; NLS Animal Health, Owings Mills, MD). Animals were anesthetized within 1 minute of being removed from the housing room, to standardize the potential influence of short-term disruptions on circulating hormone levels. Blood was sampled within 2 minutes of the anesthetic injection from the periorbital sinus via a heparinized capillary tube, and was collected during a period not exceeding 1.5 minutes, to minimize the influence of short-term anesthesia on circulating hormone levels. The blood was placed immediately on ice, and centrifuged at 4° C, at 3500 rpm, for 15 minutes to obtain plasma. Plasma aliquots were stored at −80° C until assayed.
Enzyme-Linked Immunosorbent Assay
Plasma levels of corticosterone were determined using a commercially available enzyme-linked immunosorbent assay kit (Enzo Life Sciences, Farmingdale, NY), which has been validated previously by our laboratory for use in prairie voles (McNeal et al., 2014). The plasma was diluted in assay buffer (1:500) to give results reliably within the linear portion of the standard curve. The minimum detection limits for this assay is .027 ng/ml; and inter- and intra-assay coefficients of variation are both < 5 %. Cross-reactivity with other steroids or peptides is less than 1.7%.
Collection of Hearts, Adrenal Glands, & Brains
Immediately following blood collection, prairie voles were euthanized under anesthesia via rapid decapitation. Heart and adrenal glands were immediately dissected and weighed for the analyses of adrenal-to-body weight ratio and heart-to-body weight ratio, respectively. Brains were immediately dissected for the analysis of FosB/ΔFosB-immunoreactivity. The brains were processed with a passive perfusion (i.e., spin immersion) technique that has been previously validated for use in prairie voles (e.g., Cushing, Yamamoto, Hoffman, & Carter, 2003). Briefly, brains were immersed in a fixative solution consisting of 4% paraformaldehyde containing 5% acrolein (Sigma-Aldrich, St. Louis, MO) and gently agitated for a total of 4 hours. Brains were postfixed in 4% paraformaldehyde at 4º C for 24 hours and then submerged in 25% sucrose solution. Brains were stored in 25% sucrose solution at 4º C until sectioned at 40 µm coronally on a cryostat. Brain sections were sliced, stored, and distributed serially among 6 wells of a well-plate; as such, one well contained a representation of the entire brain. Brain sections were stored in cryoprotectant antifreeze solution (30% sucrose and 30% ethylene glycol) at −20º C until assayed for FosB/ΔFosB-immunoreactivity in various brain regions.
FosB/ΔFosB Immunohistochemistry
Serial brain slices (40 µm) from approximately 1/3 of the brain were assayed for immunoreactivity of FosB/ΔFosB as an index of long-term neural activation (Nestler, 2015; Nestler et al., 2001) using standard double-label avidin:biotinylated enzyme complex (ABC) immunohistochemistry, according to procedures described previously in the prairie vole (Hostetler and Bales, 2012). Anti-FosB (Santa Cruz Biotechnology Inc., Santa Cruz, CA; rabbit polyclonal IgG; Lot A0814) was used at a concentration of 1:2,500, and the target was visualized using nickel-diaminobenzadine (Ni-DAB) dissolved in 0.1 M Tris Buffer. This primary antibody detects both the ΔFosB protein (that peaks its production at 12 hours post-activation) and FosB protein (that peaks its production at 6 hours post-activation; Nestler et al., 2001).
All immunohistochemical procedures were performed at room temperature, unless otherwise noted. Free-floating sections were rinsed 3 times during a 30-minute period with potassium phosphate buffered saline (KPBS). Sections were then incubated in 3% hydrogen peroxide for 15 minutes. After 3 washes in KPBS, sections were incubated in KPBS + 0.3% Triton X-100 (Sigma-Aldrich Co., St. Louis, MO) + 3% normal goat serum (Vector Laboratories, Burlingame, CA) for 1 hour. Sections were then incubated in primary antibody for FosB/ΔFosB (1:2,500) diluted in KPBS + blocking reagent (Roche Diagnostics Co., Mannheim, Germany) + 0.3% Triton X-100 + 1% normal goat serum at 4º C for 72 hours. Following this incubation period, tissues were rinsed 3 times during a 30-minute period with KPBS, and then tissues were incubated in biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA, anti-rabbit polyclonal IgG BA-1000) diluted in KPBS + 0.05% Triton X-100 for 1.5 hours. Sections were washed 3 times in KPBS and then incubated in A/B solution (45 µl A, 45 µl B per 10 ml KPBS + 0.4% Triton X-100; Vectastain Elite PK-6100; Vector Laboratories, Burlingame, CA) for 1 hour. Following this incubation period, tissues were rinsed twice in KPBS and then twice in 0.1 M Tris buffer (pH 7.5). FosB/ΔFosB was visualized by incubation in DAB solution with nickel intensification (168 µl buffer stock solution, 200 µl DAB stock solution, 160 µl, H2O2 solution, 120 µl nickel solution per 10 ml distilled water; DAB Peroxidase Substrate Kit SK-4100; Vector Laboratories, Burlingame, CA) for 7 minutes, after which tissues were washed 3 times in KPBS.
Stained sections were mounted on electrostatically-charged slides (Fisher Scientific International Inc., Hampton, NH), air-dried, dehydrated in a series of ethanol dilutions, cleared with Histoclear (National Diagnostics, Atlanta, GA), and then protected with coverslips using Histomount mounting medium (National Diagnostics, Atlanta, GA).
Quantification of FosB/ΔFosB-Immunoreactivity
Images were captured using a Nikon Eclipse E 800 microscope, Sensi-cam camera and IPLab software (Scanalytics, Inc., Fairfax, VA). For all brain regions, the density of FosB/ΔFosB-immunoreactive cell bodies was determined in with a 10x objective; cells were considered to be FosB/ΔFosB-positive cells if they exhibited black and round/oval shape characteristics demonstrated in previous studies (Lehmann and Herkenham, 2011; Nishijima, Kawakami, & Kita, 2013; Perrotti et al., 2004). For all brain regions, the Paxinos and Watson (2006) rat atlas was used to determine the location.
PVN.
The PVN is approximately Bregma −1.56 to −1.80 mm and was further characterized by the medial-lateral position of the fornix (relative to the third ventricle) and medial and dorsal location of the optic tract (relative to more central and ventral position in more rostral sections). In ImageJ (National Institutes of Health, Bethesda, MD), a standardized oval shape (area: 16,700 pixels per hemisphere) was used to determine the density reading of the PVN.
Dorsal Hippocampus.
The location of the dorsal hippocampus was approximately Bregma −2.64 to −3.00 mm, more specifically the immediate area ventral to the corpus callosum. Regions of interest within the dorsal hippocampus included CA1, CA3, dorsal DG (dDG), and ventral DG (vDG). An average reading of 3 standardized squares (area: 2500 pixels) were used to determine the density for the dorsal hippocampus.
aBNST.
The location of the aBNST was approximately Bregma 0.12 to −0.12 mm. Specifically, the aBNST is located dorsally to the anterior commissure and adjacent to the internal capsule. This includes the oval, juxtacapsular, anteromedial, and anterolateral nuclei. An average reading of 3 standardized squares (area: 2500 pixels) were used to determine the density for the aBNST.
BLA.
The location of the BLA was approximately Bregma −2.64 to −3.00 mm and is located adjacent to the external capsule, in a medial fashion. An average reading of 3 standardized squares (area: 2500 pixels) were used to determine the density for the BLA.
mPFC.
The location of the mPFC was approximately Bregma 3.24 to 2.52 mm. More specifically, the mPFC is the medial area of the section and is adjacent to the forceps minor of the corpus callosum. An average reading of 3 standardized squares (area: 2500 pixels) were used to determine the density for the mPFC. It should be noted that the current analysis did not differentiate between the prelimbic and the infralimbic subregions of the mPFC; both areas were combined as one mPFC region for the analyses.
Corpus callosum.
The location of the corpus callosum was approximately Bregma −2.64 to −3.00 mm, specifically the immediate area dorsal to the hippocampus and ventral to the retrosplenial dysgranular cortex. An average reading of 3 standardized squares (area: 2500 pixels) were used to determine the density for the corpus callosum.
Using ImageJ, FosB/ΔFosB-immunoreactivity was determined with a mean optical density method described previously (Watanasriyakul et al., 2018). For each animal, corpus callosum readings were used as the background, and these numbers were subtracted from the density readings obtained from each region to determine the final FosB/ΔFosB-immunoreactivity. The optical density values were relative to the gray values associated with the sample area (white: 0; black: 255). Density measurements were taken from sections matched in rostral-caudal orientation to minimize variability. Three to four brain sections were analyzed from each animal, and results were averaged across sections. Density measurements were performed separately for each hemisphere, and the results were averaged between hemispheres. For all animals, density measures were conducted by two trained, experimentally-blind raters, and the results were averaged between raters. Therefore, density measures were averaged across multiple brain slices, hemispheres, and raters to provide an accurate estimation of optical density for all brain regions. Damaged sections were excluded from the analyses.
Data Analyses
Primary hypothesis-driven analyses.
All data analyses were performed using IBM SPSS Statistics 24.0. Data are presented as means ± SEM for all analyses and figures. Body weight data were analyzed with a mixed-design analysis of variance (ANOVA), with housing condition as the independent factor and time as the repeated measures factor. Behavioral data from running wheel usage (distance traveled and maximum speed) were analyzed with a single factor (one-way) ANOVA, with condition (EE v. exercise) as the independent factor. Physiological data from basal corticosterone and FosB/ΔFosB-immunoreactivity in all brain regions were analyzed with one-way ANOVA, with housing condition as the independent factor. Follow-up pairwise comparisons were conducted using Fisher’s LSD analyses, with an additional Bonferroni adjustment for all multiple comparisons. Heart and adrenal weights were normalized to body weight for each animal; and heart-to-body ratio and adrenals-to-body ratio were compared with single-factor ANOVAs. A value of p < .05 was considered to be statistically significant.
Exploratory correlational and mediation analyses.
To further understand how social isolation and the current interventions (EE and voluntary exercise) influenced HPA-associated neural circuitry, we performed exploratory correlational analyses. Since previous findings indicate that EE and physical activity promote neurogenesis in the hippocampus (Trivino-Paredes, Patten, Gil-Mohapel, & Christie, 2016; van Praag et al., 2000), we were primarily interested in hippocampal immunoreactivity in relations to PVN immunoreactivity. Using multiple regression analyses, all subregions of the hippocampus were chosen to be the predictors, with the PVN as the outcome. Each experimental condition was analyzed individually.
Using PROCESS with SPSS 2.16.3 (Hayes, 2013), mediation analyses were performed to examine whether limbic regions mediated PVN activity of animals in the current experimental conditions (Model 6 in PROCESS). Animals with missing data values (e.g., due to damaged tissue) were excluded, leading to a subsection of animals included in these analyses (n = 45). PVN activity was entered as the dependent variable, with BLA, aBNST, CA3, and dDG as potential mediators. The PVN was chosen as the outcome variable given that the PVN initiates the HPA cascade; and integrates several physiological and endocrine signals (Herman and Tasker, 2016). Only the CA3 and dDG subregions were chosen to represent the hippocampus due to (a) all hippocampal subregions were highly intercorrelated (multicollinearity); therefore, they are not statistically unique predictors; (b) previous findings have found changes in CA3 and dDG due to EE and/or exercise (e.g., Nishijima et al., 2013; van Praag et al., 2000). Because a categorical independent variable cannot be used in Model 6, housing conditions were recoded as a dichotomous variable. This allowed us to compare two groups in each analysis. Therefore, both environmental intervention conditions were collapsed as one group (n = 31), given prior research showing that access to physical activity is a common component of EE protocols (e.g., Cao et al., 2017; Garrido et al., 2013; Nader et al., 2014). Three analyses were performed: (a) the intervention condition was compared with the paired control condition (n = 13); (b) the paired control condition was compared with the social isolation/sedentary condition (n = 13); (c) the social isolation/environmental intervention condition was compared with social isolation/sedentary condition. Model 6 first performed a path-by-path analysis using multiple linear regression with housing condition and brain regions as predictors in a progressive fashion; this is similar to hierarchical regression analysis. Then, Model 6 performed full path analyses by examining direct and indirect effects with selected brain regions as mediators using all possible pathways. The number of bootstrap samples for bias corrected bootstrap confidence intervals (CI) was 5000.
Additional mediation analyses were conducted in an attempt to detect differences in neural patterns between EE and voluntary exercise conditions. Using Model 6 in PROCESS, PVN activity was again chosen as the outcome, with BLA, aBNST, CA3, and dDG immunoreactivity chosen as mediators, and housing conditions were chosen as predictors; EE and voluntary exercise conditions were analyzed separately. Five analyses were performed: (a) the social isolation/EE condition (n = 16) was compared with the paired control condition (n = 13); (b) the social isolation/EE condition was compared with the social isolation/sedentary condition (n = 13); (c) the social isolation/voluntary exercise condition (n = 14) was compared with the paired control condition; (d) the social isolation/voluntary exercise condition was compared with the social isolation/sedentary condition; and (e) the social isolation/EE condition was compared with the social isolation/voluntary exercise condition. The number of bootstrap samples for bias corrected bootstrap CI was 5000.
Results
Body Weight.
Animals in all housing conditions gained body weight at a similar rate throughout the study. A mixed-design ANOVA yielded a significant main effect of time [F(2, 58) = 29.989, p < .001], but no significant main effect of group (p > .05; data not shown), and no significant housing condition x time interaction (p > .05; data not shown).
Heart-to-Body & Adrenal-to-Body Ratios.
Housing condition did not influence heart-to-body weight ratio or adrenal-to-body weight ratio. For heart-to-body weight ratio, a single-factor ANOVA showed that there was no significant effect of housing condition (p > .05; data not shown). For adrenal-to-body weight ratio, a single factor ANOVA also did not yield a significant effect of housing condition (p > .05; data not shown).
Physical Activity.
Average daily distance (km/day), total distance ran (km), and maximum daily speed (km/hr) were used as markers for levels of physical activity in the social isolation/EE and social isolation/voluntary exercise conditions. Overall, animals in the social isolation/voluntary exercise condition were more physically active compared to animals in the social isolation/EE condition. Examining mean daily distance traveled in the running wheel, a single factor ANOVA showed that animals in the social isolation/voluntary exercise condition ran greater daily distances (4.24 ± .950 km/day) compared to animals in the social isolation/EE condition [.46 ± .238 km/day; F(1, 31) = 14.061, p < .01]. Examining total distance traveled across the entire 4-week period, animals in the social isolation/voluntary exercise condition (115.54 ± 29.27 km total) also ran significantly more compared to those in the social isolation/EE condition [27.94 ± 10.65 km total; F(1, 31) = 15.99, p < .001]. Lastly, examining mean daily maximum speed, animals in the social isolation/voluntary exercise condition also ran at a significantly higher speed (3.08 ± .307 km/hr) compared to animals in the social isolation/EE condition [1.28 ± .390 km/hr; F(1, 31) = 13.233, p < .01].
Corticosterone.
Corticosterone levels in the social isolation/sedentary condition were higher than all other housing conditions (Figure 1). A single factor ANOVA revealed that there was a significant effect of housing condition [F(3, 59) = 7.88, p < .001]. Post hoc Fisher’s LSD analyses with a Bonferroni adjustment showed that animals in the social isolation/sedentary condition had significantly higher circulating corticosterone levels compared to animals in the paired control, social isolation/EE, and social isolation/voluntary exercise conditions (each p < .01). Corticosterone levels did not differ among paired control, social isolation/EE, and social isolation/voluntary exercise conditions (each p > .05).
Figure 1.
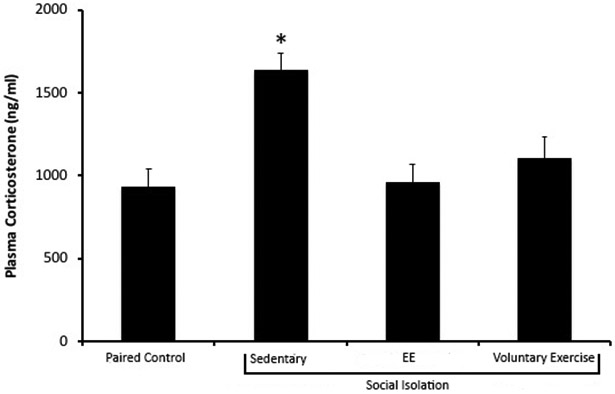
Mean (+ SEM) circulating plasma corticosterone levels of female prairie voles following 8 weeks of social isolation (or paired control conditions), with unlimited access to EE items and a running wheel from weeks 4-8 for social isolation/EE and social isolation/voluntary exercise groups, respectively. The social isolation/sedentary condition exhibited the highest basal corticosterone levels compared to other housing conditions. * denotes p < .05 compared to all other housing conditions.
Optical Density of FosB/ΔFosB-immunoreactivity.
General Results:
Table 1 displays mean optical density values for all brain regions of all housing conditions. EE and voluntary exercise resulted in comparable changes in FosB/ΔFosB-immunoreactivity in the BLA and mPFC, but housing condition did not influence FosB/ΔFosB-immunoreactivity in the PVN, hippocampus, or aBNST.
Table 1.
Optical density readings (pixels) of FosB/ΔFosB-immunoreactivity in all brain regions in female prairie voles as a function of social isolation and environmental interventions.
| PVN | CA1 | CA3 | dDG | vDG | aBNST | BLA | mPFC | |
|---|---|---|---|---|---|---|---|---|
| Paired Control | 18.39 ± 1.68 | 25.12 ± 1.53 | 28.04 ± 1.77 | 34.01 ± 2.08 | 35.51 ± 2.41 | 15.47 ± 2.17 | 9.96 ± 1.31 | 13.27 ± 1.58 |
| Social Isolation/Sedentary | 24.29 ± 2.71 | 28.63 ± 2.34 | 32.57 ± 2.71 | 37.73 ± 2.61 | 41.71 ± 3.19 | 16.55 ± 2.83 | 15.55 ± 0.53a | 13.52 ± 1.81 |
| Social Isolation/EE | 25.48 ± 2.39 | 32.15 ± 2.40 | 33.57 ± 2.21 | 40.57 ± 2.37 | 40.25 ± 2.41 | 16.24 ± 1.52 | 13.37 ± 1.66 | 21.60 ± 2.65b |
| Social Isolation/Voluntary Exercise | 23.60 ± 2.39 | 29.81 ± 1.81 | 32.51 ± 1.61 | 40.46 ± 2.27 | 44.27 ± 3.16 | 13.56 ± 1.62 | 12.54 ± 0.68 | 23.89 ± 1.58b, c |
Note: Values represent means ± SEM.
denotes p < .05 when compared to all other housing conditions.
denotes p < .05 when compared to the social isolation/sedentary condition,
denotes p < .05 when compared to the paired control condition.
PVN:
A single-factor ANOVA did not show a significant effect of housing condition on FosB/ΔFosB-immunoreactivity in the PVN (p > .05).
Dorsal Hippocampus:
Four subregions within the dorsal hippocampus were examined: CA1, CA3, dDG, and vDG. There were no significant effects of housing condition for any hippocampal sub-region (each p > .05).
aBNST:
A single-factor ANOVA did not yield a significant effect of housing condition (p > .05).
BLA:
A single-factor ANOVA yielded a significant effect of housing condition [F(3, 55) = 3.025, p < .05; Figure 2A & 2B]. Post hoc Fisher’s LSD analyses with a Bonferroni adjustment indicated that FosB/ΔFosB-immunoreactivity of social isolation/sedentary animals was significantly higher than FosB/ΔFosB-immunoreactivity of paired control, social isolation/EE, and social isolation/voluntary exercise conditions (each p < .05). FosB/ΔFosB-immunoreactivity did not differ among paired/control, social isolation/EE, and social isolation/voluntary exercise conditions (each p > .05).
Figure 2.
Representative images and group data showing FosB/ΔFosB-immunoreactivity in the BLA and mPFC of animals in paired control, social isolation/sedentary, social isolation/EE, and social isolation/voluntary exercise conditions. Panel A represents the FosB/ΔFosB-immunoreactivity in one hemisphere of the BLA, with a graphical representation of mean (+ SEM) density in Panel B (* denotes p < .05 when compared to all other housing conditions). Panel C represents FosB/ΔFosB-immunoreactivity of the mPFC, with a graphical representation of mean (+ SEM) density in Panel D ($ denotes p < .05 when compared to the paired control condition, and & denotes p < .05 when compared to the social isolation/sedentary condition). Oval and rectangle shapes indicate regions of interest of the respective brain regions. Images were captured at 10X magnification.
mPFC:
A single-factor ANOVA yielded a significant effect of housing condition [F(3, 24) = 9.591, p < .001; Figure 2C & 2D]. Post hoc Fisher’s LSD analyses with a Bonferroni adjustment revealed that FosB/ΔFosB-immunoreactivity in the social isolation/EE condition and social isolation/voluntary exercise conditions were significantly higher than that of the social isolation/sedentary condition (each p < .05). Animals in the social isolation/voluntary exercise condition had significantly higher FosB/ΔFosB-immunoreactivity compared to those in the paired control condition (p < .01). No group differences in immunoreactivity were found between (a) social isolation/EE v. paired control; (b) paired control v. social isolation/sedentary; (c) social isolation/EE v. social isolation/voluntary exercise (each p > .05).
Exploratory Mediation Analyses
Predicting PVN Activity with Hippocampal Subregions as Predictors.
While there were no group differences in FosB/ΔFosB-immunoreactivity in the PVN, there appear to be differences among the experimental conditions in the relationship between the hippocampus and the PVN, suggesting that social and environmental changes may impact hippocampal-PVN connectivity. For paired control animals, multiple regression analysis showed that FosB/ΔFosB-immunoreactivity of hippocampal subregions did not predict FosB/ΔFosB-immunoreactivity of the PVN (p > .05; Table 2A). For social isolation/sedentary animals, multiple regression analysis showed that FosB/ΔFosB-immunoreactivity in the hippocampus did not predict FosB/ΔFosB-immunoreactivity in the PVN [F(4, 10) = 2.625, p > .05; Table 2B]. For social isolation/EE animals, multiple regression analysis showed that hippocampal FosB/ΔFosB-immunoreactivity did not predict PVN FosB/ΔFosB-immunoreactivity [F(4, 10) = 3.061, p > .05; Table 2C]. For social isolation/voluntary exercise animals, hippocampal FosB/ΔFosB levels significantly predicted PVN FosB/ΔFosB-immunoreactivity [F(4, 10) = 15.886, p < .001; Table 2D]; however, no particular subregion appeared to solely drive this effect.
Table 2.
Multiple regression analyses in each condition separately, with FosB/ΔFosB-immunoactivity of hippocampal subregions as predictors and FosB/ΔFosB-immunoactivity of PVN as the predicted outcome.
| A. Paired control | |||
|---|---|---|---|
| R2 = .424 | |||
| Adjusted R2 = .193 | |||
| Brain Regions | B | SE B | ϐ |
| CA1 | 1.102 | 0.911 | 1.21 |
| CA3 | −1.449 | 0.792 | −1.829 |
| dDG | 0.098 | 0.922 | 0.106 |
| vDG | 0.668 | 0.67 | 0.996 |
| B. Social isolation/Sedentary | |||
| R2 = .512 | |||
| Adjusted R2 = .317 | |||
| Brain Regions | B | SE B | ϐ |
| CA1 | 0.738 | 0.553 | 1.333 |
| CA3 | −0.271 | 0.715 | −0.380 |
| dDG | 0.335 | 0.973 | 0.344 |
| vDG | 0.019 | 0.766 | 0.025 |
| C. Social isolation/EE | |||
| R2 = .424 | |||
| Adjusted R2 = .193 | |||
| Brain Regions | B | SE B | ϐ |
| CA1 | −0.036 | 0.625 | −0.058 |
| CA3 | 1.613 | 0.857 | 1.882 |
| dDG | −1.201 | 1.101 | −1.091 |
| vDG | 0.509 | 0.395 | 1.290 |
| D. Social isolation/Voluntary exercise | |||
| R2 = .527 | |||
| Adjusted R2 = .355* | |||
| Brain Regions | B | SE B | ϐ |
| CA1 | 0.513 | 0.392 | 1.310 |
| CA3 | −0.075 | 0.500 | −0.150 |
| dDG | 0.160 | 0.467 | 0.342 |
| vDG | 0.369 | 0.353 | 1.045 |
Note:
denotes correlational significance of p < .05.
Predicting PVN Activity with Multiple Brain Regions as Mediators.
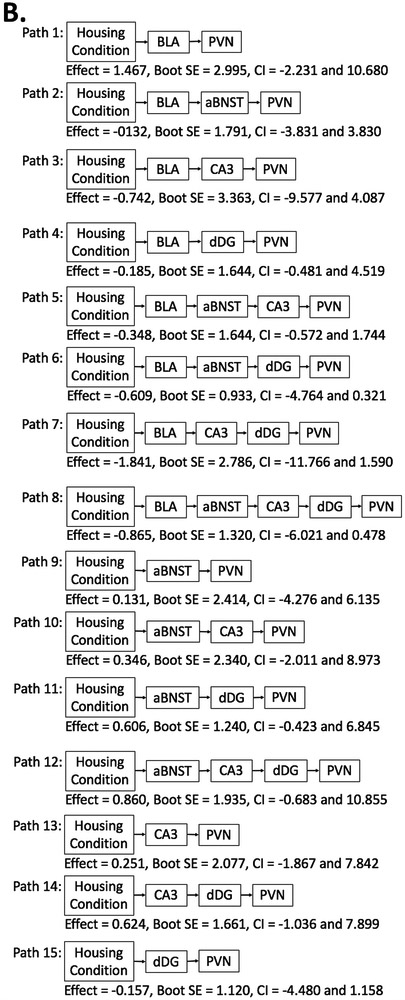
Mediation analyses (Model 6 in PROCESS) were performed to examine whether differences in FosB/ΔFosB-immunoreactivity in the PVN between paired control animals and social isolation/intervention animals (EE and physical activity combined) were due to potential mediation by limbic regions (i.e., BLA, aBNST, CA3, dDG). The complete results of path-by-path analyses are displayed in Table 3A. Full path analyses were also performed; Figure 3A represents the complete results. Briefly, there was no significant direct effect of housing condition predicting FosB/ΔFosB-immunoreactivity in the PVN (Effect = −1.228, Boot SE = 2.378, CI = −6.045 and 3.580). However, there was a significant indirect full path effect (Effect = −0.260, Boot SE = 0.250, CI = −1.553 to −0.015); this suggests that housing condition predicted FosB/ΔFosB-immunoreactivity in the PVN indirectly via the mediation by BLA, aBNST, CA3, and dDG (Figure 3A Indirect Path 8).
Table 3.
Path-by-path mediation analyses using PROCESS Model 6
| A. Paired control v. social isolation/intervention conditions (EE and voluntary exercise combined) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Outcome | ||||||||||||||
| BLA | aBNST | CA3 | dDG | PVN | |||||||||||
| B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | |
| Housing Condition | −2.483 | 1.834 | −1.354 | 1.746 | 2.125 | 0.822 | −2.875 | 1.960 | −1.467 | −1.965 | 1.426 | −1.378 | −1.228 | 2.378 | −0.517 |
| BLA | 0.497* | 0.177 | 2.805 | 0.410* | 0.180 | 2.312 | −0.038 | 0.134 | −0.280 | 0.231 | 0.218 | 1.058 | |||
| BNST | 0.401* | 0.150 | 2.775 | 0.259* | 0.112 | 2.314 | −0.162 | 0.195 | −0.830 | ||||||
| CA3 | 0.896* | 0.113 | 7.902 | −0.007 | 0.300 | −0.023 | |||||||||
| dDG | 0.586* | 0.264 | 2.222 | ||||||||||||
| R2 | 0.043 | 0.165* | 0.396* | 0.396* | 0.420* | ||||||||||
| B. Paired control v. social isolation/sedentary conditions | |||||||||||||||
| Predictor | Outcome | ||||||||||||||
| BLA | aBNST | CA3 | dDG | PVN | |||||||||||
| B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | |
| Housing Condition | −5.202* | 1.486 | −3.500 | −4.69 | 4.225 | 1.110 | 1.417 | 2.810 | 0.505 | −0.319 | 1.410 | −0.226 | −6.192 | 3.877 | −1.597 |
| BLA | 0.907 | 0.472 | 1.920 | 0.803* | 0.330 | 3.084 | −0.073 | 0.185 | −0.392 | −0.282 | 5.108 | −0.552 | |||
| BNST | 0.416* | 0.140 | 3.084 | 0.263* | 0.081 | 3.264 | −0.028 | 0.272 | 0.103 | ||||||
| CA3 | 0.898* | 0.107 | 8.444 | 1.780 | 0.612 | 0.290 | |||||||||
| dDG | 0.491 | 0.599 | 0.819 | ||||||||||||
| R2 | 0.338* | 0.128 | 0.542* | 0.916* | 0.449* | ||||||||||
| C. Social isolation/intervention (EE and voluntary exercise combined) v. social isolation/sedentary conditions | |||||||||||||||
| Predictor | Outcome | ||||||||||||||
| BLA | aBNST | CA3 | dDG | PVN | |||||||||||
| B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | |
| Housing Condition | 2.719 | 1.646 | 1.520 | −0.260 | 2.507 | −0.100 | −2.205 | 2.220 | −0.994 | −1.749 | 1.398 | −1.252 | 2.854 | 2.452 | 1.163 |
| BLA | 0.294 | 0.230 | 1.274 | 0.389 | 0.210 | 1.870 | −0.037 | 0.135 | −0.273 | 0.113 | 0.232 | 0.487 | |||
| BNST | 0.437 | 0.140 | 3.126* | 0.195 | 0.097 | 2.006 | −0.165 | 0.176 | −0.934 | ||||||
| CA3 | 0.941* | 0.100 | 9.450 | 0.443 | 0.314 | 1.412 | |||||||||
| dDG | 0.410 | 0.279 | 1.468 | ||||||||||||
| R2 | 0.062 | 0.040 | 0.296* | 0.806* | 0.484* | ||||||||||
Note:
denotes correlational significance of p < .05.
Figure 3.
Full path analyses using Model 6 by PROCESS with housing condition as the predictor and FosB/ΔFosB-immunoreactivity in the PVN as the outcome, with FosB/ΔFosB-immunoreactivity in the BLA, aBNST, CA3, dDG as mediators. Panel A represents paired control v. social isolation/intervention conditions (EE and voluntary exercise conditions combined;). Panel B represents paired control v. social isolation/sedentary conditions. Panel C represents social isolation/intervention (EE and voluntary exercise conditions combined) v. social isolation/sedentary conditions. * denotes a significant mediation effect for that pathway.
Mediation analyses were performed to examine differences between the paired control and social isolation/sedentary conditions. The complete results of the path-by-path analyses are displayed in Table 3B. Figure 3B represents the complete results of the full path analyses. Briefly, there was no significant direct effect of housing condition predicting FosB/ΔFosB–immunoreactivity in the PVN (Effect = −6.192, Boot SE = 3.877, CI = −14.279 to 1.895), and there was no significant indirect effect for mediation (CI for each indirect path contains zero).
Lastly, mediation analyses were performed to examine differences between the social isolation/environmental intervention and social isolation/sedentary conditions. The complete results of the path-by-path analyses are displayed in Table 3C. Figure 3C represents the complete results of the full path analyses. Briefly, there was no significant direct effect of housing condition predicting FosB/ΔFosB–immunoreactivity in the PVN (Effect = 2.852, Boot SE = 2.452, CI = −2116 to 7.821), and there was also no significant indirect effect for mediation (CI for each indirect path contains zero).
Potential Differences between EE & Exercise through Mediation Analyses.
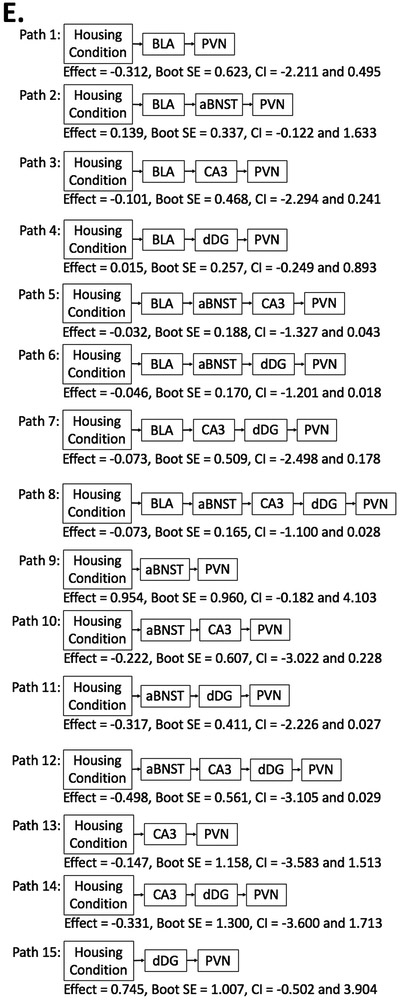
Follow-up mediation analyses (Model 6 in PROCESS) were conducted to detect potential differences in neural patterns between EE and exercise. First, mediation analyses were performed to examine differences between the social isolation/EE and paired control conditions. The complete results of the path-by-path analyses are displayed in Table 4A. Figure 4A represents the complete results of the full path analyses. Briefly, there was no significant direct effect of housing condition predicting FosB/ΔFosB–immunoreactivity in the PVN (Effect = 3.059, Boot SE = 3.003, CI = −3.152 to 9.271), and there was also no significant indirect effect for mediation (CI for each indirect path contains zero).
Table 4.
Follow-up path-by-path mediation analyses using PROCESS Model 6
| A. Social isolation/EE v. paired control conditions | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Outcome | ||||||||||||||
| BLA | aBNST | CA3 | dDG | PVN | |||||||||||
| B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | |
| Housing Condition | 3.109 | 2.222 | 1.399 | −0.971 | 2.464 | −0.394 | 2.941 | 2.424 | 1.213 | 1.157 | 1.114 | −1.038 | 3.059 | 3.003 | 1.019 |
| BLA | 0.604* | 0.206 | 2.932 | 0.425 | 0.233 | 1.823 | 0.079 | 0.111 | 0.713 | 0.372 | 0.295 | 1.260 | |||
| BNST | 0.511* | 0.192 | 2.654 | 0.285* | 0.097 | 2.925 | −0.049 | 0.299 | −0.163 | ||||||
| CA3 | 0.849* | 0.089 | 9.503 | 0.134 | 0.514 | 0.261 | |||||||||
| dDG | 0.287 | 0.538 | 1.019 | ||||||||||||
| R2 | 0.068 | 0.252* | 0.493* | 0.922* | 0.436* | ||||||||||
| B. Social isolation/EE v. social isolation/sedentary conditions | |||||||||||||||
| Predictor | Outcome | ||||||||||||||
| BLA | aBNST | CA3 | dDG | PVN | |||||||||||
| B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | |
| Housing Condition | −2.092 | 1.919 | −1.090 | 1.620 | 3.051 | 0.531 | 2.446 | 2.784 | 0.879 | 1.476 | 1.062 | 1.390 | −0.529 | 3.121 | −0.170 |
| BLA | 0.354 | 0.300 | 1.182 | 0.459 | 0.279 | 1.645 | 0.104 | 0.110 | 0.9433 | 0.274 | 0.318 | 0.862 | |||
| BNST | 0.529* | 0.178 | 2.973 | 0.204* | 0.078 | 2.623 | −0.021 | 0.250 | −0.082 | ||||||
| CA3 | 0.920* | 0.075 | 12.248 | 1.034 | 0.572 | 1.808 | |||||||||
| dDG | −0.272 | 0.577 | −0.471 | ||||||||||||
| R2 | 0.042 | 0.054 | 0.375* | 0.931* | 0.504* | ||||||||||
| C. Social isolation/voluntary exercise v. paired control conditions | |||||||||||||||
| Predictor | Outcome | ||||||||||||||
| BLA | aBNST | CA3 | dDG | PVN | |||||||||||
| B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | |
| Housing Condition | 1.766 | 1.847 | 0.956 | −3.157 | 2.610 | −1.209 | 2.270 | 1.829 | 1.241 | 3.110 | 1.950 | 1.595 | 0.169 | 2.520 | 0.067 |
| BLA | 0.577* | 0.278 | 2.079 | 0.280 | 0.139 | 2.016 | −0.241 | 0.234 | −1.027 | 0.124 | 0.294 | 0.421 | |||
| BNST | 0.467* | 0.139 | 2.016 | 0.317 | 0.156 | 2.041 | −0.105 | 0.208 | −0.507 | ||||||
| CA3 | 0.927* | 0.215 | 4.307 | −0.424 | 0.358 | −1.185 | |||||||||
| dDG | 0.789* | 0.261 | 3.023 | ||||||||||||
| R2 | 0.035 | 0.173 | 0.428* | 0.685* | 0.414* | ||||||||||
| D. Social isolation/voluntary exercise v. social isolation/sedentary conditions | |||||||||||||||
| Predictor | Outcome | ||||||||||||||
| BLA | aBNST | CA3 | dDG | PVN | |||||||||||
| B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | |
| Housing Condition | −3.435* | 1.402 | −2.450 | −2.099 | 3.504 | −0.599 | 1.741 | 2.611 | 0.667 | 1.479 | 2.010 | 0.736 | −5.650 | 2.867 | −1.971 |
| BLA | 0.019 | 0.449 | 0.043 | 0.358 | 0.332 | 1.077 | −0.312 | 0.260 | −0.265 | −0.265 | 0.377 | −0.701 | |||
| BNST | 0.365* | 0.151 | 2.426 | 0.185 | 0.129 | 1.431 | −0.195 | 0.190 | −1.024 | ||||||
| CA3 | 0.980* | 0.159 | 6.161 | 0.472 | 0.370 | 1.276 | |||||||||
| dDG | 0.530 | 0.300 | 1.765 | ||||||||||||
| R2 | 0.194* | 0.019 | 0.235 | 0.736* | 0.565* | ||||||||||
| E. Social isolation/EE v. social isolation/voluntary exercise conditions | |||||||||||||||
| Predictor | Outcome | ||||||||||||||
| BLA | aBNST | CA3 | dDG | PVN | |||||||||||
| B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | B | SE B | t | |
| Housing Condition | −1.343 | 2.124 | −0.632 | −2.658 | 2.102 | −1.264 | −0.653 | 2.427 | −0.269 | 1.377 | 1.737 | 0.793 | −3.019 | 2.482 | −1.216 |
| BLA | 0.288 | 0.186 | 1.550 | 0.334 | 0.217 | 1.535 | −0.021 | 0.162 | −0.130 | 0.232 | 0.229 | 1.013 | |||
| BNST | 0.370 | 0.216 | 1.714 | 0.221 | 0.163 | 1.356 | −0.359 | 0.238 | −1.508 | ||||||
| CA3 | 0.936* | 0.140 | 6.680 | 0.226 | 0.330 | 0.684 | |||||||||
| dDG | 0.541 | 0.282 | 1.915 | ||||||||||||
| R2 | 0.014 | 0.143 | 0.242 | 0.732* | 0.506* | ||||||||||
Note:
denotes correlational significance of p < .05.
Figure 4.
Full path analyses using Model 6 by PROCESS with housing condition as the predictor and FosB/ΔFosB-immunoreactivity in the PVN as the outcome, with FosB/ΔFosB-immunoreactivity in the BLA, aBNST, CA3, dDG as mediators. Panel A represents social isolation/EE v. paired control conditions. Panel B represents social isolation/EE v. social isolation/sedentary conditions. Panel C represents social isolation/voluntary exercise v. paired control conditions. Panel D represents social isolation/voluntary exercise v. social isolation/sedentary conditions. Panel E represents social isolation/EE v. social isolation/voluntary exercise conditions. * denotes a significant mediation effect for that pathway.
Second, mediation analyses were performed to examine differences between the social isolation/EE and social isolation/sedentary conditions. The complete results of the path-by-path analyses are displayed in Table 4B. Figure 4B represents the complete results of the full path analyses. Briefly, there was no significant direct effect of housing condition predicting FosB/ΔFosB–immunoreactivity in the PVN (Effect = −0.529, Boot SE = 3.121, CI = −6.985 to 5.927), and there was also no significant indirect effect for mediation (CI for each indirect path contains zero).
Third, mediation analyses were performed to examine differences between the social isolation/voluntary exercise and paired control conditions. The complete results of the path-by-path analyses are displayed in Table 4C. Figure 4C represents the complete results of the full path analyses. Briefly, there was no significant direct effect of housing condition predicting FosB/ΔFosB–immunoreactivity in the PVN (Effect = 0.169, Boot SE = 2.520, CI = −5.072 to 5.411); however, there was a significant indirect effect for mediation for dDG (Effect = 2.452, Boot SE = 1.794, CI = 0.078 to 8.199), suggesting that housing condition predicted PVN-immunoreactivity through dDG (Figure 3C Indirect Path 15).
Fourth, mediation analyses were performed to examine differences between the social isolation/voluntary exercise and social isolation/sedentary conditions. The complete results of the path-by-path analyses are displayed in Table 4D. Figure 4D represents the complete results of the full path analyses. Briefly, there was no significant direct effect of housing condition predicting FosB/ΔFosB–immunoreactivity in the PVN (Effect = −5.650, Boot SE = 2.867, CI = −11.611 to 0.312), and there was also no significant indirect effect for mediation (CI for each indirect path contains zero).
Lastly, mediation analyses were performed to examine differences between the social isolation/EE and social isolation/voluntary exercise conditions. The complete results of the path-by-path analyses are displayed in Table 4E. Figure 4E represents the complete results of the full path analyses. Briefly, there was no significant direct effect of housing condition predicting FosB/ΔFosB–immunoreactivity in the PVN (Effect = −3.019, Boot SE = 2.482, CI = −8.143 to 2.104), and there was also no significant indirect effect for mediation (CI for each indirect path contains zero).
Discussion
Social isolation may contribute to physiological and psychological decline (Cacioppo et al., 2015; Hawkley and Cacioppo, 2010), via changes in brain circuitry associated with stress, emotion, and physiological regulation (Grippo et al., 2007; Ruscio, Sweeny, Hazelton, Suppatkul, & Carter, 2007). EE and exercise may be useful environmental interventions against social stressors, promoting adaptive cognitive processes, physical fitness, and improvements in neural functions (Campeau et al., 2010; Lehmann and Herkenham, 2011; Nishijima et al., 2013). Therefore, the current study examined whether EE, relative to voluntary exercise alone, produced differential neuroendocrine buffering effects against social isolation in prairie voles, and further statistically examined how these environmental interventions may have influenced the relationships among HPA-related brains regions. Both EE (which included an exercise component) and voluntary exercise alone similarly protected against an elevation of corticosterone levels and altered neural activity in brain regions associated with stress, emotion, and behavior.
Social isolation increased basal plasma corticosterone levels compared to paired conditions. Both EE and physical exercise attenuated this corticosterone elevation to the same degree as paired housing. The current findings support previous studies demonstrating that EE and physical activity buffer the effects of chronic stress at the endocrine level (Benite-Ribeiro et al., 2014; Garrido et al., 2013). For example, voluntary exercise protected against corticosterone reactivity to an acute stressor in prairie voles (Watanasriyakul et al., 2018), and EE protected against corticosterone elevations to foot shock in rats (Ronzoni, Anton, Mora, Segovia, & Del Arco, 2016). Taken together, EE and exercise may protect against hypercortisolism as a function of social isolation in addition to protecting against short-term adrenocortical response to acute stressors. However, it is not clear how short-term anesthesia may have influenced circulating corticosterone. Although anesthesia may influence circulating hormone levels, as suggested previously (Arnold and Langhans, 2010; Zardooz, Rostamkhani, Zaringhalam, & Faraji Shahrivar, 2010), significant plasma corticosterone elevations were not observed in mice anesthetized with ketamine/xylazine compared to unanesthetized mice (Arnold and Langhans, 2010). It is also notable that social isolation and the environmental interventions did not influence overall adrenal and cardiac structure in the current study. These findings are somewhat contradictory to previous studies, which have demonstrated that some social environmental manipulations influence either adrenal or heart weights (Carnevali et al., 2012; Grippo, Trahanas, Zimmerman, Porges, & Carter, 2009; Jarcho et al., in press; Watanasriyakul et al., 2018). Given the dissociation between corticosterone levels and organ weights in the present study, it is possible that the heart and adrenal weights are reflective of long-term changes, in which the animals may have already adapted to the new social and environmental settings; whereas circulating corticosterone may reflect acute responses to social and environmental changes. Further studies will benefit from investigating repeated corticosterone levels along with long-term organ responsiveness, as well as considering how short-term influences (such as anesthesia or recent use of the EE items or running wheel) may influence circulating corticosterone (e.g., Nishijima, Kamidozono, Ishiizumi, Amemiya, & Kita, 2017).
To further investigate the effects of EE and physical exercise, FosB/ΔFosB-immunoreactivity was used as a marker of long-term neural activation in brain regions that are relevant to stress, emotion, social behavior, and neuroendocrine communication. First, we investigated neural activation in regions that are associated with stress activation, including the PVN, BLA, and aBNST (Herman and Tasker, 2016; Hostinar et al., 2014). The PVN is a crucial region for HPA signaling, endocrine-autonomic interactions, and behavior (Herman and Tasker, 2016). Further, amygdaloid regions have indirect communication with the PVN via the aBNST, and the BLA specifically has been associated with emotional processing, fear conditioning, and stress-induced anxiety (Herman et al., 2005; Herman and Tasker, 2016; Hostinar et al., 2014; Lebow and Chen, 2016; McEwen, 2017). Chronic stress may elevate FosB/ΔFosB-immunoreactivity in the PVN, BLA, and aBNST (Flak et al., 2012; Hawley and Leasure, 2012; Laine et al., 2017; Perrotti et al., 2004). Elevations of FosB/ΔFosB-immunoreactivity were observed in the BLA in sedentary socially isolated prairie voles, relative to paired animals, but changes were not observed in the PVN or aBNST. It is possible that the BLA is especially sensitive to social isolation. For instance, pyramidal neural excitability was increased in the BLA of mice following social isolation for 2 weeks (Lin et al., 2018). Previous studies also indicate that EE and voluntary exercise may attenuate hyperactivity in these stress-responsive regions (Koe et al., 2016; Lehmann and Herkenham, 2011; Lin et al., 2015; Novaes et al., 2017; Zheng et al., 2012). In the present study, this attenuation effect was observed only in the BLA, with animals in both environmental intervention conditions exhibiting lower FosB/ΔFosB-immunoreactivity compared to animals in the sedentary social isolation condition. It is possible that EE or voluntary exercise may have influenced the down-regulation of circulating corticosterone through altered glucocorticoid receptor signaling. This hypothesis is supported by findings demonstrating that EE protects against stress-related anxiety in male rats, by preventing glucocorticoid receptor nucleus translocation in the BLA (Novaes et al., 2017).
Aside from stress activation mechanisms, we also investigated FosB/ΔFosB-immunoreactivity in brain regions related to stress inhibition, specifically the mPFC and hippocampus. Generally, the prefrontal cortex is involved in the interpretation of a stimulus in order to respond accordingly (see Arruda-Carvalho and Clem, 2015). In doing so, the mPFC can interact with stress systems at the level of the PVN and BLA to initiate or suppress the fight-or-flight response (see McKlveen, Myers, & Herman, 2015). Group differences in FosB/ΔFosB-immunoreactivity were observed in the mPFC in prairie voles, with EE and exercise alone producing similar increases in FosB/ΔFosB-immunoreactivity in the mPFC, compared to the sedentary isolation condition. The current findings support previous research reporting that EE and voluntary exercise increase mPFC function and promote dendritic branching (Eddy and Green, 2017; Lehmann and Herkenham, 2011; Ronzoni et al., 2016). Additionally, the mPFC is known to inhibit the BLA during fear memory extinction (Giustino and Maren, 2015); EE and exercise may influence similar neural pathways in the context of social isolation. In addition to cortical communication, the hippocampus plays an important role in initiating negative feedback on the HPA axis (Herman et al., 2005; Hostinar et al., 2014), and chronic stress has been associated with the loss of hippocampal volume, decreased neurogenesis, and psychiatric symptoms (McEwen, 2017). FosB/ΔFosB-immunoreactivity was not altered in the hippocampus as a function of either social isolation or environmental interventions. These findings are contrary to previous research demonstrating that EE promotes cell proliferation and decreases FosB/ΔFosB-immunoreactivity in the dorsal hippocampus (Lopes et al., 2018; Tanti, Rainer, Minier, Surget, & Belzung, 2012), and long-term exercise promotes neurogenesis, enhances synaptic plasticity, and increases FosB/ΔFosB-immunoreactivity in the hippocampus (Nishijima et al., 2013; Trivino-Paredes et al., 2016). It is possible that species or methodological differences underlie the differential effects observed in the present study, relative to previous research on hippocampal function.
Considered together with previous evidence, the current findings suggest that both EE and voluntary exercise alone may protect against negative endocrine effects of social isolation in the prairie vole model by enhancing mPFC function to suppress subcortical stress-related functions, including in the BLA. These findings support the view that specific communication among stress- and emotion-mediated circuitry may be influenced by social and environmental stimuli. Exploratory mediation analyses demonstrated that, while there was a slight difference in FosB/ΔFosB activity in the PVN between paired control and sedentary social isolation conditions, a full mediation was not observed through the BLA, aBNST, CA3, and dDG. In contrast, a full mediation was observed through these limbic regions when comparing the difference in PVN activity between paired control and the intervention conditions (both EE and exercise combined). Therefore, EE and voluntary exercise may influence PVN activity through BLA-aBNST-CA3-dDG communication. Additional mediation analyses were conducted to determine whether the effects of EE were dissociable from voluntary exercise alone. A direct comparison between the two intervention conditions (EE v. voluntary exercise) did not show a significant difference of housing condition in predicting PVN activity through a BLA-aBNST-CA3-dDG pathway. Interestingly, when comparing voluntary exercise to paired control conditions, a significant effect of housing condition was observed, such that PVN activity was predicted by voluntary exercise through dDG activity. Additionally, multiple regression analyses using the activity in hippocampal subregions to predict PVN activity also showed significant correlations in socially isolated and physically active animals; the same significant correlations were not observed in the other housing conditions. Taken together, the current findings suggest that while EE and voluntary exercise may equally attenuate corticosterone elevations and BLA hyperactivation, voluntary exercise alone may promote hippocampal-PVN communication better than EE.
Limitations
The current study provides insight into the neuroendocrine effects of prolonged social isolation as well as the protective effects of EE and voluntary exercise; however, some questions remain. First, the EE paradigm included an exercise component, based on previous research with EE paradigms in rodents (e.g., Cao et al., 2017; Garrido et al., 2013; Nader et al., 2014; van Praag et al., 2000). Therefore, we cannot specifically dissociate the effects of exercise from other EE items. The present mediation analyses suggest that voluntary exercise alone may promote hippocampal-PVN connectivity better than the full EE paradigm, but whether these neural changes translate directly into improved behavioral or physiological stress reactivity remains to be determined. The findings from our mediation analyses are correlational in nature, and interpreting these findings as causation should be cautioned. However, previous evidence focused on this line of questioning is mixed. For example, Rogers et al. (2016) reported that EE – but not voluntary exercise – protects against anxiety-related behavior; while voluntary exercise – but not EE – facilitates hippocampal-dependent learning in pair-housed and unstressed mice. Additionally, preliminary research from our laboratory indicates that depressive- and anxiety-related behaviors in socially isolated prairie voles are similarly protected by a sedentary form of EE (that is, a paradigm that does not include a running wheel), compared to EE plus exercise (Cox et al., 2018). Future studies will benefit from a direct investigation of the complexity of items used in EE paradigms, to determine whether this complexity has important protective neural, physiological, and/or behavioral effects that are separate from physical exercise.
Additionally, we observed an attenuation of corticosterone levels in both intervention conditions versus sedentary isolation conditions. However, this buffering effect of HPA activity was not reflected in organ responsiveness or PVN activation, with animals in both EE and exercise conditions exhibiting elevated levels of FosB/ΔFosB in the PVN (comparable to the sedentary isolated condition). Because FosB/ΔFosB is indicative of general neural activation, and the PVN is also a major site of neural integration for multiple systems (e.g., glucocorticoid, oxytocin, noradrenergic), it is possible that social isolation or the environmental interventions differentially influenced PVN neuronal subpopulations. Future studies may improve our understanding of the potential benefits of EE and physical exercise by investigating specific cell types in the PVN such as oxytocin or corticotropin-releasing hormone neurons.
Conclusions
The current study indicates that both EE and exercise attenuate corticosterone elevations due to chronic social isolation in the prairie vole model. EE and exercise also buffer against hyperactivation of the BLA, and are associated with increased activation of the mPFC, suggesting that the mPFC may be recruited as a part of this protective effect. Correlational mediation analyses suggest that the beneficial effects of EE and exercise may involve the limbic regions such as the BLA, aBNST, and hippocampal subregions working in concert to influence functions in the PVN. Additional studies involving translational animal models can further increase our understanding of specific mechanisms through which EE and exercise influence central and peripheral processes. This research can inform clinical practice, suggesting benefits of incorporating cognitive stimulation and physical activity into strategies to protect against the consequences of social stress.
Highlights.
Prolonged social isolation increased basal corticosterone levels and basolateral amygdala immunoreactivity
Environmental enrichment and exercise buffered corticosterone elevations and basolateral amygdala hyperactivity
Protective effects of environmental enrichment and exercise may be mediated by medial prefrontal cortex and limbic structures
Acknowledgements
We would like to thank the following individuals for their assistance with data collection: Sarah Ciosek, Miranda Cox, Nicole Holzapfel, Meredith McCormick, Cassidy Padal, Cynthia Sanchez-Vazquez, and Samantha Sujet. We would like to thank Amber Ballard for her assistance with animal care. Lastly, we would like to thank Dr. Brad Sagarin for his assistance with statistical analyses.
Role of The Funding Source
This research was supported by the National Institutes of Health (HL112350).
Footnotes
Conflicts of Interest
The authors report no potential conflicts of interest.
References
- Ando S, Nishida A, Usami S, Koike S, Yamasaki S, Kanata S, … Kasai K (2018). Help-seeking intention for depression in early adolescents: Associated factors and sex differences. J Affect Disord, 238, pp. 359–365. doi: 10.1016/j.jad.2018.05.077 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29908475 [DOI] [PubMed] [Google Scholar]
- Arnold M, & Langhans W (2010). Effects of anesthesia and blood sampling techniques on plasma metabolites and corticosterone in the rat. Physiol Behav, 99(5), pp. 592–598. doi: 10.1016/j.physbeh.2010.01.021 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20152845 [DOI] [PubMed] [Google Scholar]
- Arruda-Carvalho M, & Clem RL (2015). Prefrontal-amygdala fear networks come into focus. Front Syst Neurosci, 9, p 145. doi: 10.3389/fnsys.2015.00145 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26578902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, & Zucker I (2011). Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev, 35(3), pp. 565–572. doi: 10.1016/j.neubiorev.2010.07.002 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20620164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benite-Ribeiro SA, Santos JM, & Duarte JA (2014). Moderate physical exercise attenuates the alterations of feeding behaviour induced by social stress in female rats. Cell Biochem Funct, 32(2), pp. 142–149. doi: 10.1002/cbf.2984 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23740556 [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, & Young LJ (2009). The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology, 34(6), pp. 1406–1415. doi: 10.1038/npp.2008.154 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18923404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Capitanio JP, & Cole SW (2015). The neuroendocrinology of social isolation. Annu Rev Psychol, 66, pp. 733–767. doi: 10.1146/annurev-psych-010814-015240 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25148851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Kryskow EM, Herlihy L, Masini CV, … Day HE (2010). Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. J Neuroendocrinol, 22(8), pp. 872–888. doi: 10.1111/j.1365-2826.2010.02007.x Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20406350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Pu T, Wang L, Marshall C, He H, Hu G, & Xiao M (2017). Early enriched physical environment reverses impairments of the hippocampus, but not medial prefrontal cortex, of socially-isolated mice. Brain Behav Immun, 64, pp. 232–243. doi: 10.1016/j.bbi.2017.04.009 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28412142 [DOI] [PubMed] [Google Scholar]
- Carnevali L, Mastorci F, Graiani G, Razzoli M, Trombini M, Pico-Alfonso MA, … Sgoifo A (2012). Social defeat and isolation induce clear signs of a depression-like state, but modest cardiac alterations in wild-type rats. Physiol Behav, 106(2), pp. 142–150. doi: 10.1016/j.physbeh.2012.01.022 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22330326 [DOI] [PubMed] [Google Scholar]
- Carter CS, & Keverne EB (2002). The neurobiology of social affiliation and pair bonding. Hormones & Behavior, 1, pp. 299–337. [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, & Volkening D (1987). Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster). Horm Behav, 21(1), pp. 74–82. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3549517 [DOI] [PubMed] [Google Scholar]
- Cox M, Normann MC, Watanasriyakul WT, Akinbo OI, Sujet S, Ciosek S, … Grippo AJ (2018) Investigating a sedentary form of environmental enrichment in a prairie vole model of social stress. Paper presented at the Society for Neuroscience, San Diego, CA. [Google Scholar]
- Cushing BS, Yamamoto Y, Hoffman GE, & Carter CS (2003). Central expression of c-Fos in neonatal male and female prairie voles in response to treatment with oxytocin. Brain Res Dev Brain Res, 143(2), pp. 129–136. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12855184 [DOI] [PubMed] [Google Scholar]
- Dong X, & Chen R (2017). Gender differences in the experience of loneliness in U.S. Chinese older adults. J Women Aging, 29(2), pp. 115–125. doi: 10.1080/08952841.2015.1080534 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27441601 [DOI] [PubMed] [Google Scholar]
- Eddy MC, & Green JT (2017). Running wheel exercise reduces renewal of extinguished instrumental behavior and alters medial prefrontal cortex neurons in adolescent, but not adult, rats. Behav Neurosci, 131(6), pp. 460–469. doi: 10.1037/bne0000218 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29083204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Solomon MB, Jankord R, Krause EG, & Herman JP (2012). Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur J Neurosci, 36(4), pp. 2547–2555. doi: 10.1111/j.1460-9568.2012.08161.x Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22789020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido P, De Blas M, Ronzoni G, Cordero I, Anton M, Gine E, … Mora F (2013). Differential effects of environmental enrichment and isolation housing on the hormonal and neurochemical responses to stress in the prefrontal cortex of the adult rat: relationship to working and emotional memories. J Neural Transm (Vienna), 120(5), pp. 829–843. doi: 10.1007/s00702-012-0935-3 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23254925 [DOI] [PubMed] [Google Scholar]
- Giustino TF, & Maren S (2015). The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci, 9, p 298. doi: 10.3389/fnbeh.2015.00298 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26617500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, & Carter CS (2007). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology, 32(8–10), pp. 966–980. doi: 10.1016/j.psyneuen.2007.07.004 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17825994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Ihm E, Wardwell J, McNeal N, Scotti MA, Moenk DA, … Preihs K (2014). The effects of environmental enrichment on depressive and anxiety-relevant behaviors in socially isolated prairie voles. Psychosom Med, 76(4), pp. 277–284. doi: 10.1097/PSY.0000000000000052 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24804886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, Porges SW, & Carter CS (2009). Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology, 34(10), pp. 1542–1553. doi: 10.1016/j.psyneuen.2009.05.017 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19553027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare DL, Toukhsati SR, Johansson P, & Jaarsma T (2014). Depression and cardiovascular disease: a clinical review. European Heart Journal, 35(21), pp. 1365–1372. doi: 10.1093/eurheartj/eht462 Retrieved from 10.1093/eurheartj/eht462 [DOI] [PubMed] [Google Scholar]
- Hawkley LC, & Cacioppo JT (2010). Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med, 40(2), pp. 218–227. doi: 10.1007/s12160-010-9210-8 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20652462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley DF, & Leasure JL (2012). Region-specific response of the hippocampus to chronic unpredictable stress. Hippocampus, 22(6), pp. 1338–1349. doi: 10.1002/hipo.20970 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21805528 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression Based Approach New York, NY: Guilford Publications, Inc. [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, & Figueiredo H (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry, 29(8), pp. 1201–1213. doi: 10.1016/j.pnpbp.2005.08.006 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16271821 [DOI] [PubMed] [Google Scholar]
- Herman JP, & Tasker JG (2016). Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol (Lausanne), 7, p 137. doi: 10.3389/fendo.2016.00137 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27843437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, & Bales KL (2012). DeltaFosB is increased in the nucleus accumbens by amphetamine but not social housing or isolation in the prairie vole. Neuroscience, 210, pp. 266–274. doi: 10.1016/j.neuroscience.2012.03.019 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22450232 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull, 140(1), pp. 256–282. doi: 10.1037/a0032671 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23607429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, McNeal N, Colburn W, Normann MC, Watanasriyakul WT, & Grippo AJ (in press). Wheel access has opposing effects on stress physiology depending on social environment in female prairie voles (Microtus ochrogaster). Stressdoi: 10.1080/10253890.2018.1553948 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30628521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, & Holtzman SG (2002). Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav, 73(1), pp. 131–140. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12076732 [DOI] [PubMed] [Google Scholar]
- Klaissle P, Lesemann A, Huehnchen P, Hermann A, Storch A, & Steiner B (2012). Physical activity and environmental enrichment regulate the generation of neural precursors in the adult mouse substantia nigra in a dopamine-dependent manner. BMC Neurosci, 13(132), pp. 1–15. doi: 10.1186/1471-2202-13-132 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23110504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe AS, Ashokan A, & Mitra R (2016). Short environmental enrichment in adulthood reverses anxiety and basolateral amygdala hypertrophy induced by maternal separation. Transl Psychiatry, 6(e729), pp. 1–7. doi: 10.1038/tp.2015.217 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26836417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine MA, Sokolowska E, Dudek M, Callan SA, Hyytia P, & Hovatta I (2017). Brain activation induced by chronic psychosocial stress in mice. Sci Rep, 7(15061), pp. 1–7. doi: 10.1038/s41598-017-15422-5 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29118417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, & Chen A (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry, 21(4), pp. 450–463. doi: 10.1038/mp.2016.1 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26878891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, & Herkenham M (2011). Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci, 31(16), pp. 6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21508240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Li X, Chen YH, Gao F, Chen H, Hu NY, … Gao TM (2018). Social isolation during adolescence induces anxiety behaviors and enhances firing activity in BLA pyramidal neurons via mGluR5 upregulation. Mol Neurobiol, 55(6), pp. 5310–5320. doi: 10.1007/s12035-017-0766-1 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28914419 [DOI] [PubMed] [Google Scholar]
- Lin TW, Shih YH, Chen SJ, Lien CH, Chang CY, Huang TY, … Kuo YM (2015). Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol Learn Mem, 118, pp. 189–197. doi: 10.1016/j.nlm.2014.12.005 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25543023 [DOI] [PubMed] [Google Scholar]
- Lindsay-Smith G, Banting L, Eime R, O’Sullivan G, & van Uffelen JGZ (2017). The association between social support and physical activity in older adults: a systematic review. Int J Behav Nutr Phys Act, 14(1), p 56. doi: 10.1186/s12966-017-0509-8 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28449673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes DA, Souza TMO, de Andrade JS, Silva MFS, Antunes HKM, Sueur-Maluf LL, … Viana MB (2018). Environmental enrichment decreases avoidance responses in the elevated T-maze and delta FosB immunoreactivity in anxiety-related brain regions. Behav Brain Res, 344, pp. 65–72. doi: 10.1016/j.bbr.2018.02.012 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29448033 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks), 1doi: 10.1177/2470547017692328 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28856337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, & Herman JP (2015). The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol, 27(6), pp. 446–456. doi: 10.1111/jne.12272 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25737097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti MA, Wardwell J, Chandler DL, Bates SL, Larocca M, … Grippo AJ (2014). Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton Neurosci, 180, pp. 9–16. doi: 10.1016/j.autneu.2013.10.001 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24161576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J, Claudia C, El Rawas R, Favot L, Jaber M, Thiriet N, & Solinas M (2014). Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology, 39(3), p 780. doi: 10.1038/npp.2013.303 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24430915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2015). FosB: a transcriptional regulator of stress and antidepressant responses. Eur J Pharmacol, 753, pp. 66–72. doi: 10.1016/j.ejphar.2014.10.034 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25446562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, & Self DW (2001). DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A, 98(20), pp. 11042–11046. doi: 10.1073/pnas.191352698 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11572966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T, Kamidozono Y, Ishiizumi A, Amemiya S, & Kita I (2017). Negative rebound in hippocampal neurogenesis following exercise cessation. Am J Physiol Regul Integr Comp Physiol, 312(3), pp. R347–R357. doi: 10.1152/ajpregu.00397.2016 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28052868 [DOI] [PubMed] [Google Scholar]
- Nishijima T, Kawakami M, & Kita I (2013). Long-term exercise is a potent trigger for DeltaFosB induction in the hippocampus along the dorso-ventral axis. PLOS ONE, 8(11), p e81245. doi: 10.1371/journal.pone.0081245 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24282574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann MC, McNeal N, Dagner A, Ihm E, Woodbury M, & Grippo AJ (2018). The influence of environmental enrichment on cardiovascular and behavioral responses to social stress. Psychosom Med, 80(3), pp. 271–277. doi: 10.1097/PSY.0000000000000558 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29360667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaes LS, Dos Santos NB, Batalhote RFP, Malta MB, Camarini R, Scavone C, & Munhoz CD (2017). Environmental enrichment protects against stress-induced anxiety: Role of glucocorticoid receptor, ERK, and CREB signaling in the basolateral amygdala. Neuropharmacology, 113(Pt A), pp. 457–466. doi: 10.1016/j.neuropharm.2016.10.026 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27815155 [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, & Christie BR (2006). Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus, 16(3), pp. 250–260. doi: 10.1002/hipo.20157 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16411242 [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2006). The Rat Brain in Stereotaxic Coordinates (sixth ed.) New York: Elservier. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, & Nestler EJ (2004). Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci, 24(47), pp. 10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15564575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickwood DJ, Deane FP, & Wilson CJ (2007). When and how do young people seek professional help for mental health problems? Med J Aust, 187(7 Suppl), pp. S35–39. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17908023 [DOI] [PubMed] [Google Scholar]
- Rogers J, Vo U, Buret LS, Pang TY, Meiklejohn H, Zeleznikow-Johnston A, … Renoir T (2016). Dissociating the therapeutic effects of environmental enrichment and exercise in a mouse model of anxiety with cognitive impairment. Transl Psychiatry, 6, p e794. doi: 10.1038/tp.2016.52 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27115125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzoni G, Anton M, Mora F, Segovia G, & Del Arco A (2016). Infralimbic cortex controls the activity of the hypothalamus-pituitary-adrenal axis and the formation of aversive memory: Effects of environmental enrichment. Behav Brain Res, 297, pp. 338–344. doi: 10.1016/j.bbr.2015.10.037 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26518332 [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, & Carter SC (2007). Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Hormones and Behavior, 51(1), pp. 54–61. doi: 10.1016/j.yhbeh.2006.08.004 Retrieved from 10.1016/j.yhbeh.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Smith AS, & Wang Z (2014). Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry, 76(4), pp. 281–288. doi: 10.1016/j.biopsych.2013.09.017 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24183103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti A, Rainer Q, Minier F, Surget A, & Belzung C (2012). Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology, 63(3), pp. 374–384. doi: 10.1016/j.neuropharm.2012.04.022 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22561281 [DOI] [PubMed] [Google Scholar]
- Trivino-Paredes J, Patten AR, Gil-Mohapel J, & Christie BR (2016). The effects of hormones and physical exercise on hippocampal structural plasticity. Front Neuroendocrinol, 41, pp. 23–43. doi: 10.1016/j.yfrne.2016.03.001 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26989000 [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, & Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci, 2(3), pp. 266–270. doi: 10.1038/6368 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10195220 [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, & Gage FH (2000). Neural consequences of environmental enrichment. Nat Rev Neurosci, 1(3), pp. 191–198. doi: 10.1038/35044558 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11257907 [DOI] [PubMed] [Google Scholar]
- Watanasriyakul TW, Wardwell J, McNeal N, Schultz R, Woodbury M, Dagner A, … Grippo AJ (2018). Voluntary physical exercise protects against behavioral and endocrine reactivity to social and environmental stressors in the prairie vole. Social Neuroscience, 13(5), pp. 602–615. doi: 10.1080/17470919.2017.1365761 Retrieved from 10.1080/17470919.2017.1365761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z, & Insel TR (1998). Neuroendocrine bases of monogamy. Trends Neurosci, 21(2), pp. 71–75. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9498302 [DOI] [PubMed] [Google Scholar]
- Zardooz H, Rostamkhani F, Zaringhalam J, & Faraji Shahrivar F (2010). Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and CO2 in male rats. Physiol Res, 59(6), pp. 973–978. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20533863 [DOI] [PubMed] [Google Scholar]
- Zheng H, Sharma NM, Liu X, & Patel KP (2012). Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: role of angiotensin II. Am J Physiol Regul Integr Comp Physiol, 303(4), pp. R387–394. doi: 10.1152/ajpregu.00046.2012 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22718804 [DOI] [PMC free article] [PubMed] [Google Scholar]